Abstract
(1) Background: Countercurrent chromatography (CCC) is liquid-liquid partition chromatography without using a solid support matrix. This technique requires further improvement of peak resolution and shortening of separation time. (2) Methods: The long-pressed locular multilayer coils with and without mixer glass beads were developed for the separation of proteins and 4-methylumbelliferyl sugar derivatives using a small-scale cross-axis coil planet centrifuge. (3) Results: Proteins were separated from each other and the separation was improved when the flow rate of the mobile phase was decreased from 0.8 to 0.4 mL/min. On the other hand, 4-methylumbelliferyl sugar derivatives were separated at the resolution of almost over 1.5 in short separation time under satisfactory stationary phase retention when the flow rate of the mobile phase was increased from 1.0 to 1.4 mL/min. (4) Conclusion: Better peak resolutions over the previous results were achieved using the long-pressed locular multilayer coil for proteins with aqueous two-phase systems (ATPS) and for 4-methylumbelliferyl sugar derivatives with organic-aqueous two-phase solvent systems by inserting a glass bead into each locule.
1. Introduction
Countercurrent chromatography (CCC) is liquid-liquid partition chromatography which eliminates the solid support matrix for stationary phase [1,2,3,4]. In the past, various types of CCC instruments have been developed to achieve satisfactory retention of the liquid stationary phase in the column. Among them, the type-J multilayer coil planet centrifuge (type-J CCC) and the cross-axis coil planet centrifuge (cross-axis CCC) have proven most useful for effective CCC separations. The type-J CCC is useful for separations mainly using organic-aqueous two-phase solvent systems [1,2,3,4], while the cross-axis CCC can be used for the separation of biopolymers with aqueous two-phase systems (ATPS) having low-interfacial tension and high viscosity.
The cross-axis CCC has a distinctive feature that the coiled column revolves around the vertical central axis of the centrifuge while it rotates about its horizontal axis at the same angular velocity [5,6]. This centrifuge instrument permits flow tubes to rotate without twisting so that the rotating column can be continuously eluted with the mobile phase without a risk of leakage which is often observed in centrifuge instruments with a rotary seal. In our laboratory, the floor model of the cross-axis CCC has been built and successfully applied to the protein separation using ATPS [7]. Recently, a new small-scale cross-axis CCC has been further developed in our laboratory to improve the theoretical plates and peak resolution [8,9]. This small-scale cross-axis CCC is a compact model where four coiled columns of similar weight are mounted symmetrically around the rotary frame to achieve stable balancing of the centrifuge even under a high revolution speed.
The peak resolution and the stationary phase retention of this cross-axis CCC varies depending on the configuration of the separation column such as conventional multilayer coil [8,9,10], eccentric coil [7,11,12], toroidal coil [11,12], spiral coil [13], and so forth. Our previous studies revealed that the locular multilayer coil prepared by compressing a long piece of polytetrafluoroethylene (PTFE) tubing with a pair of hemostats at regular intervals was useful for protein separation with ATPS [14]. It has also been reported that the spiral disk mounted by the cross-pressed tubing for the type-J planet centrifuge was useful for separation of proteins with ATPS [15]. Recently, Englert et al. reported the tubing modifications using the crimping tool to improve the separation efficiency for CCC separation of organic-aqueous two-phase solvent system by the type-J CCC [16,17]. As described above, the cross-axis CCC is useful for the separation of various compounds having a wide range of hydrophobicity from fatty acids to proteins with organic-aqueous and aqueous-aqueous two-phase solvent systems, respectively. In order to enhance the mixing of the two phases in each locule of the locular tubing, the extension of the compression length to increase the linear velocity of the mobile phase and the insertion of a bead into each locule of the long-pressed locular tubing may lead to improved separations over the previous studies [14]. The present paper describes the improved separations of proteins with ATPS for the long-pressed locular multilayer coil and of 4-methylumbelliferyl sugar derivatives for the long-pressed locular multilayer coil containing a glass bead mixer in each locule using the small-scale cross-axis CCC.
2. Materials and Methods
2.1. Apparatus
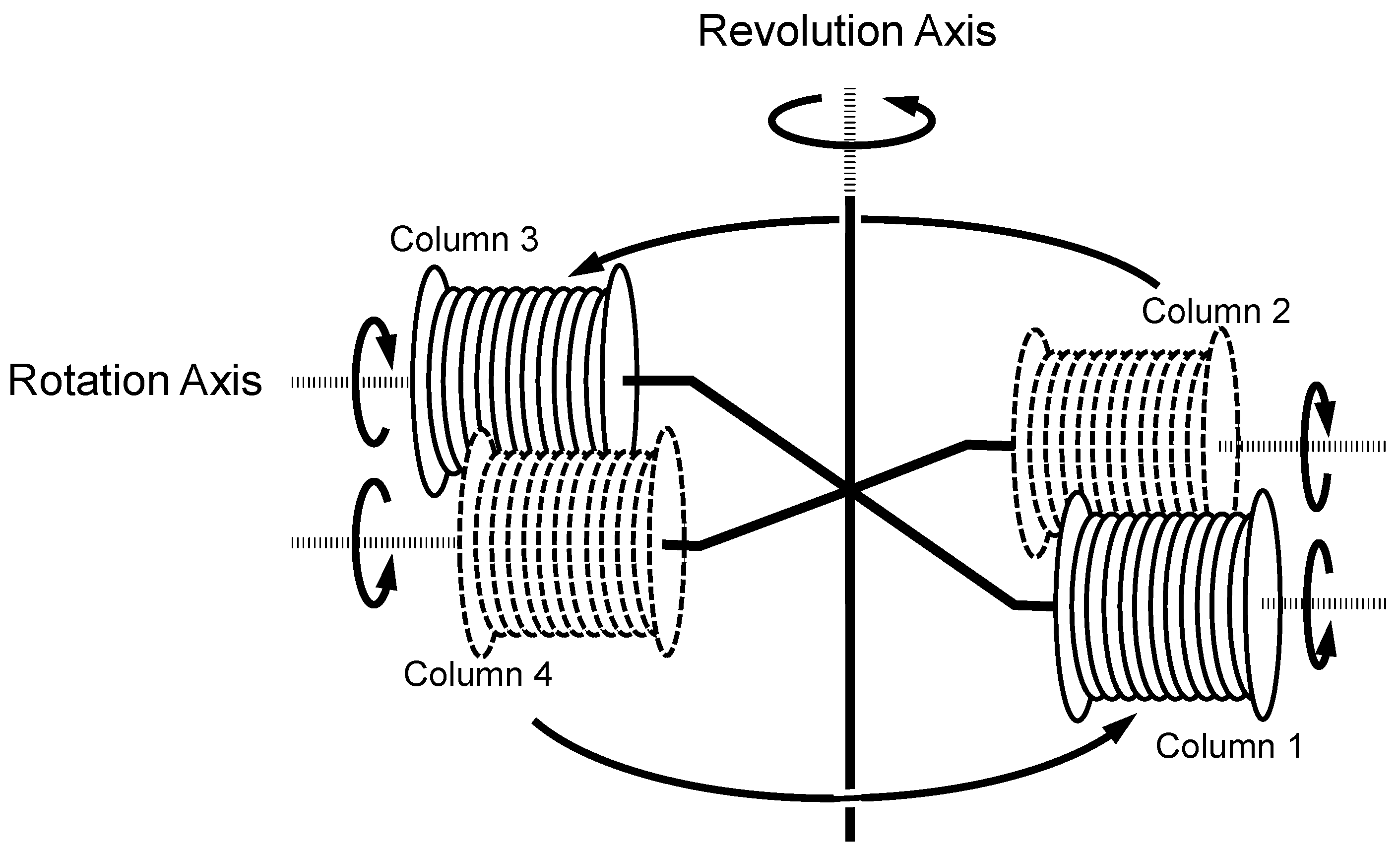
The small-scale cross-axis CCC employed in the present study was constructed at the Machining Technology Center of Nihon University (Chiba, Japan). The design and fabrication of the apparatus and the column configuration were previously described in detail [8,9]. Figure 1 illustrates the schematic drawing of the small-scale cross-axis CCC used in the present study. Four coiled columns are distributed at a constant distance from the central revolution axis. The neighboring columns rotate in the opposite direction to each other while revolving in a horizontal plane around the vertical axis of the centrifuge as indicated by a set of arrows.
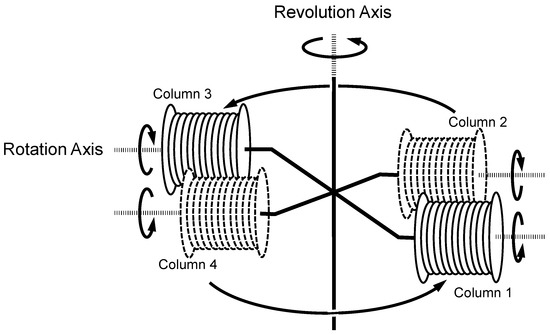
Figure 1.
Schematic illustration of the small-scale cross-axis CCC used in the present study.
2.2. Preparation of the Long-Pressed Locular Tubing and the Bead Embedded Locular Tubing
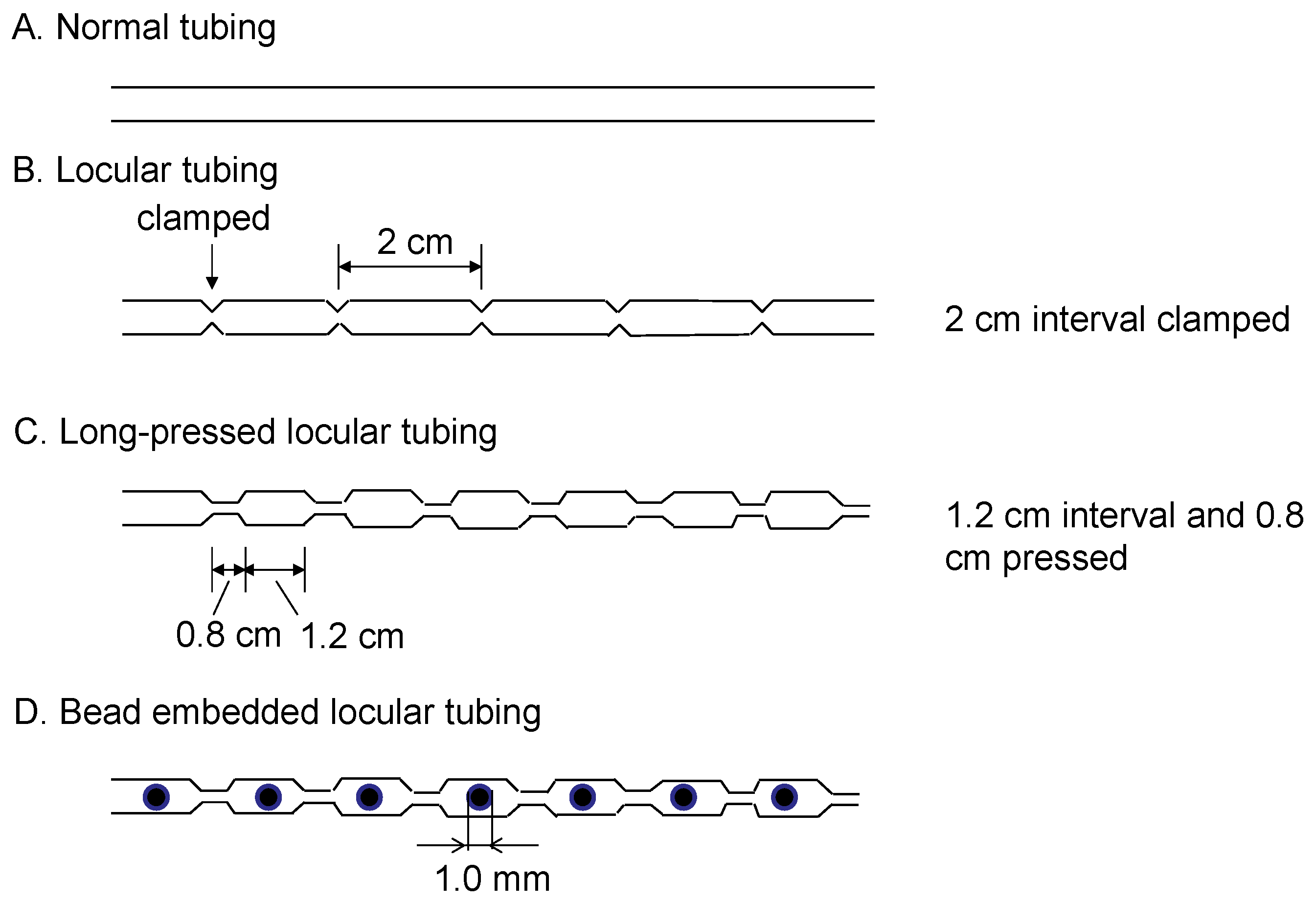
The newly designed long-pressed locular tubing was prepared from a single piece of 1.5 mm I.D., 2.5 mm O.D. PTFE (polytetrafluoroethylene) tubing (Flon Kogyo, Tokyo, Japan) by compressing it with a pair of tongs (0.8 cm wide) for pressing at 1.2 cm intervals to form a number of segments called locules. The bead embedded locular tubing was prepared by adding a glass ball bead (1 mm I.D., Toshinriko, Inc., Tokyo, Japan) in each locule for enhancing the mixing of two phases. Figure 2 illustrates the schematic drawing of the long-pressed locular tubing with and without glass beads.
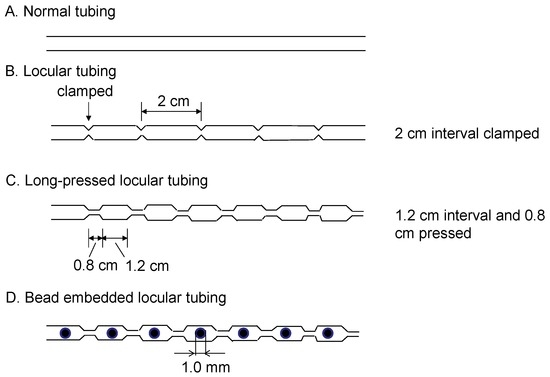
Figure 2.
Schematic illustration of the locular tubing with and without a glass bead mixer.
2.3. Preparation of Coiled Column Assembly
Each multilayer coil was prepared by tightly winding a long piece of the aforementioned PTFE tubing around the holder hub of 3 cm in diameter, forming five tight coiled layers between a pair of flanges spaced 5 cm apart. Each coiled column was prepared according to the following procedure: The tubing was directly wound onto the holder hub starting on the proximal side, which is close to the center of revolution. After each coil layer was completed, the layer was wrapped with an adhesive tape and the tubing was straightly returned to the other side to resume winding in the same direction. It results in a multilayer coil assembly composed of either entirely right- or left-handed coils, which is different from that commonly used in the type-J CCC. Two columns of left-handed coils were subjected to the forward rotation; and right-handed coils, the backward rotation. Two pairs of right- and left-handed coil assemblies were connected in series with flow tubes in such a way that the distal terminal of the first column assembly is connected to the proximal terminal, which is close to the center of revolution, of the second column assembly, and so on. Four coil assemblies were symmetrically mounted on the rotary frame for balancing the centrifuge instrument.
2.4. Reagents
Polyethylene glycol (PEG) 1000 (MW 1000), cytochrome C (horse heart) (MW 12,384), myoglobin (horse skeletal muscle) (MW 17,800), and lysozyme (chicken egg) (MW 13,680) were purchased from Sigma (St. Louis, MO, USA). Dibasic potassium phosphate was obtained from Wako Pure Chemicals (Osaka, Japan).
4-Methylumbelliferyl sugar derivatives used as test samples were purchased as follows: 4-Methylumbelliferyl-α-l-fucopyranoside (α-l-Fuc), 4-methylumbelliferyl-β-d-fucopyranoside (β-d-Fuc), 4-methylumbelliferyl-β-d-glucopyranoside (Glc), 4-methylumbelliferyl-β-d-galactopyranoside (Gal), and 4-methylumbelliferyl-β-d-cellobioside (Cel) were purchased from BIOSYNTH AG (Staad, Switzerland). 4-Methylumbelliferyl-α-d-mannopyransoide (Man) was obtained from Wako Pure Chemicals.
All other reagents were of reagent grade.
2.5. Preparation of Two-Phase Solvent Systems and Sample Solutions
An ATPS composed of 12.5% (w/w) PEG 1000 and 12.5% (w/w) dibasic potassium phosphate was prepared by dissolving 125 g of PEG 1000 and 125 g of dibasic potassium phosphate (anhydrous) in 750 g of distilled water.
The organic-aqueous two-phase solvent system used in the present studies was composed of ethyl acetate/1-butanol/water (3:2:5, v/v) for the separation by the lower mobile phase and (1:4:5, v/v) for the upper mobile phase [18].
Each solvent mixture was thoroughly equilibrated in a separatory funnel at room temperature. The two phases were separated after the two layers were formed.
The sample solutions were prepared by dissolving the standard sample mixture with equal volumes of each phase of the two-phase solvent system used for separation.
2.6. CCC Separation
Each separation was initiated by completely filling the column with the stationary phase, followed by injection of the sample solution through the flow tube leading into the head of CCC column by a syringe. Then, the mobile phase was pumped into the column at a suitable flow rate using a reciprocating pump (Model LC-6A, Shimadzu Corporation, Kyoto, Japan), while the column was rotated at 1000 rpm of revolution speed. The effluent from the column outlet was collected into test tubes using a fraction collector (Model CHF 100AA, Advantec, Tokyo, Japan).
2.7. Analysis of CCC Fractions
Each collected fraction was diluted with an aliquot of distilled water for proteins and of methanol for 4-methylumbelliferyl sugar derivatives and the absorbance measured at 280 nm for proteins, 540 nm for myoglobin, and 318 nm for 4-methylumbelliferyl sugar derivatives with a spectrophotometer (Model UV-1600, Shimadzu).
2.8. Evaluation of Theoretical Plate Number and Peak Resolution
The efficiencies in separations in the present studies were computed from the chromatogram and expressed in terms of theoretical plate number (N) and peak resolution (Rs) each according to the following conventional formula.
3. Results
3.1. Proteins
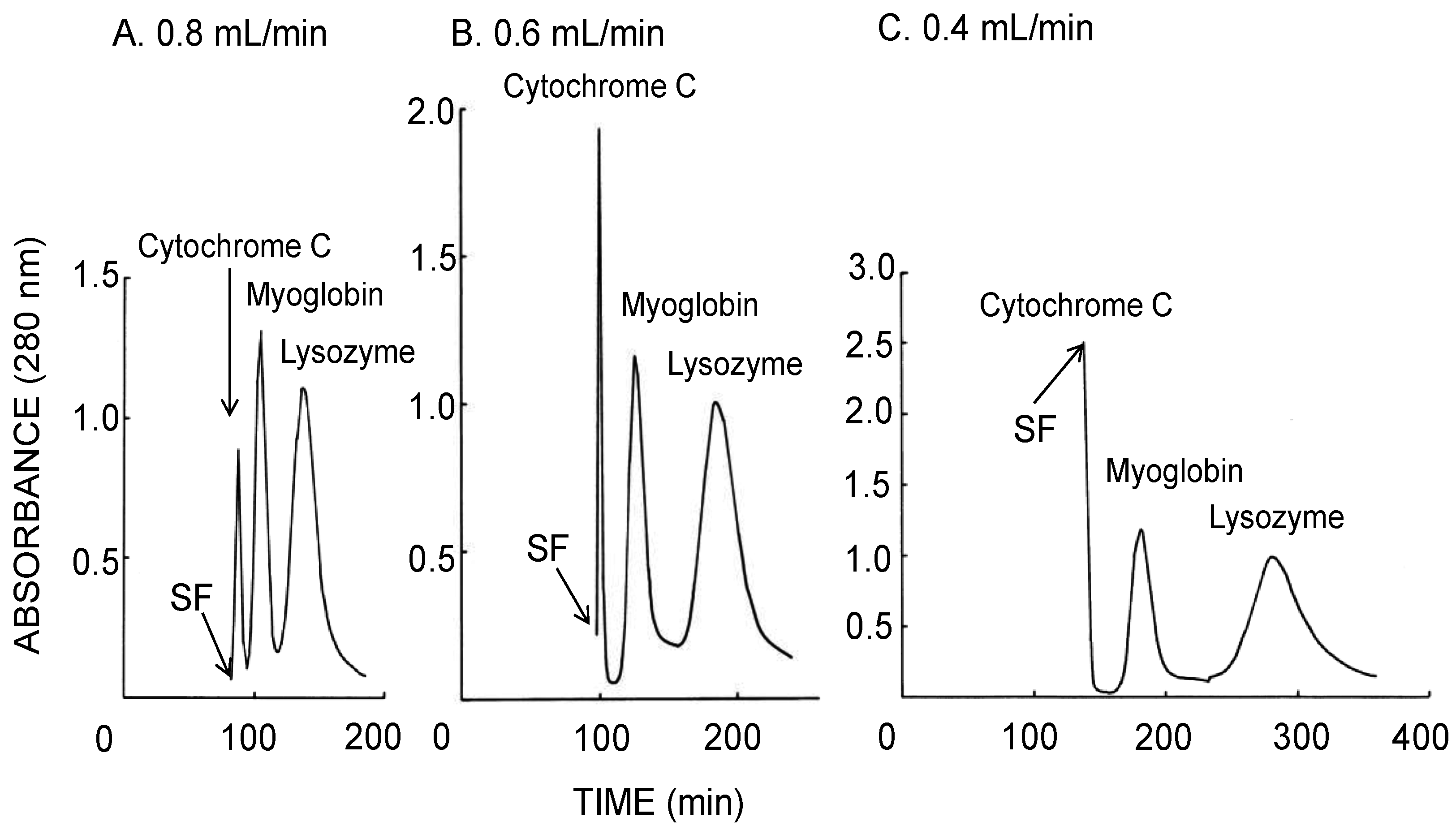
Figure 3 illustrates the CCC separation of stable proteins obtained using the long-pressed locular multilayer coil with the lower mobile phase of the 12.5% (w/w) PEG 1000–12.5% (w/w) dibasic potassium phosphate system. The decrease of the flow rate produced better resolution of cytochrome C, myoglobin, and lysozyme peaks apparently due to the increased stationary phase retention in the column. Table 1 (upper side) summarizes the analytical values calculated from each chromatogram shown in Figure 3. Remarkable increase in the peak resolution was observed by a lower flow rate with increased retention of the stationary phase while the theoretical plate number calculated from the myoglobin peak was almost unchanged.
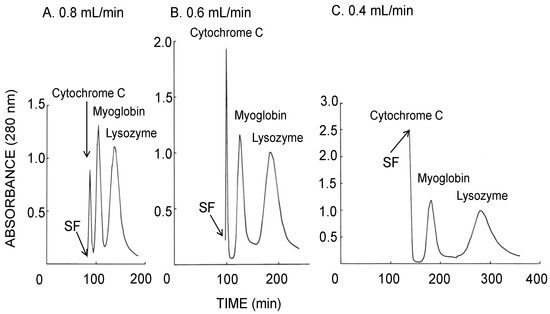
Figure 3.
CCC separation of proteins obtained by the long-pressed locular multilayer coiled columns (without glass beads) with the lower mobile phase at three different flow rates. Experimental conditions: apparatus: small-scale cross-axis CCC with four long-pressed locular multilayer coil assemblies, 1.5 mm I.D. and 90 mL total capacity; sample: cytochrome C (2 mg), myoglobin (8 mg), and lysozyme (10 mg); solvent system: 12.5% (w/w) PEG 1000–12.5% (w/w) dibasic potassium phosphate; mobile phase: lower phase (outward elution); revolution: 1000 rpm; revolution direction: counterclockwise; fractions collected at 2 min/tube. SF = solvent front.

Table 1.
Analytical values obtained by CCC separations of proteins with the long-pressed locular multilayer columns at three different flow rates.
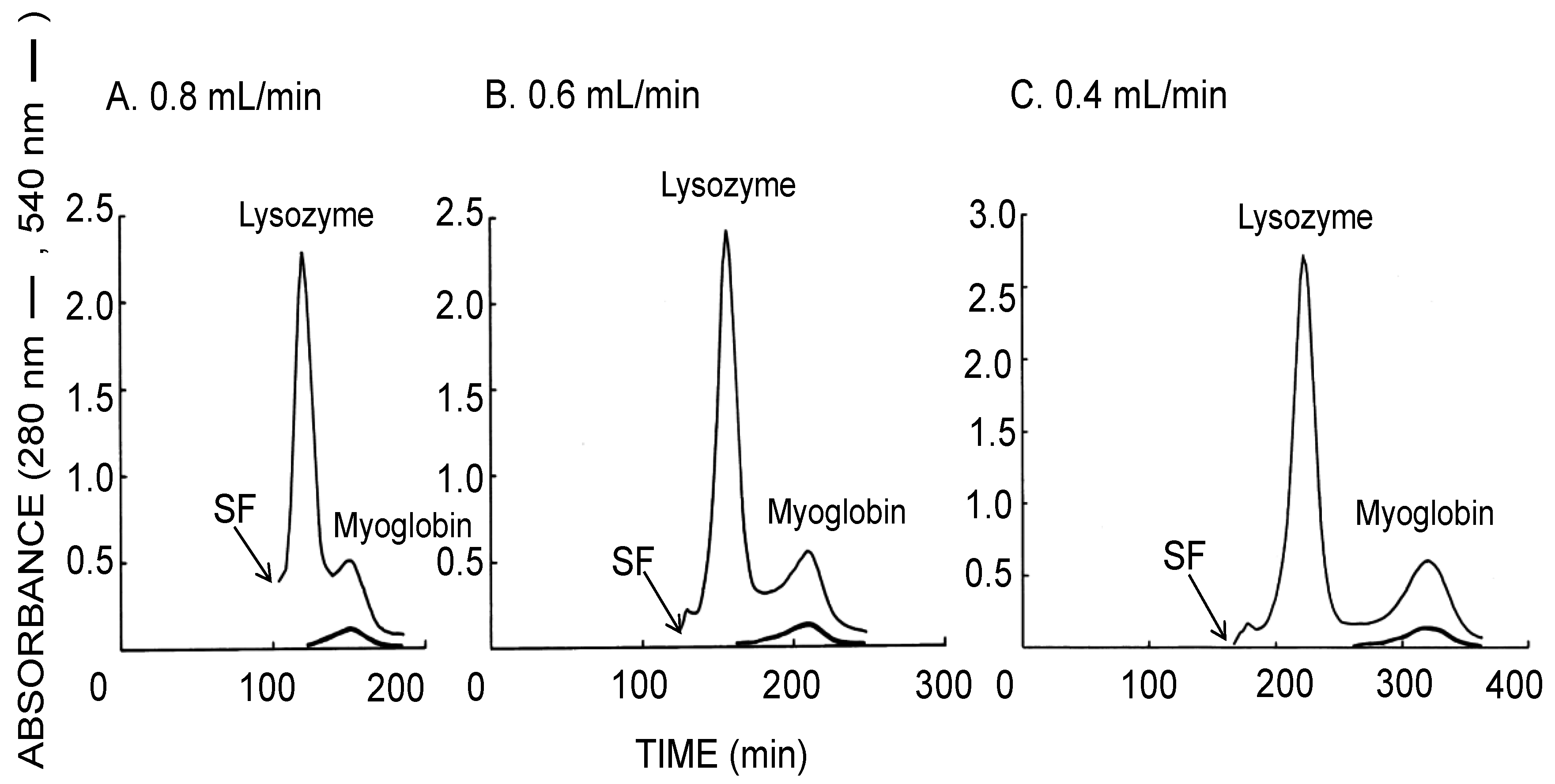
Figure 4 similarly illustrates the CCC separation of proteins obtained using the long-pressed locular multilayer coil (without glass beads) with the upper mobile phase. The decrease of the flow rates also improved the resolution between the lysozyme and the myoglobin peaks while their separation times were increased. As summarized in Table 1 (lower side), both of theoretical plate number of the lysozyme peak and stationary phase retention were increased by decreased flow rate of the upper mobile phase.
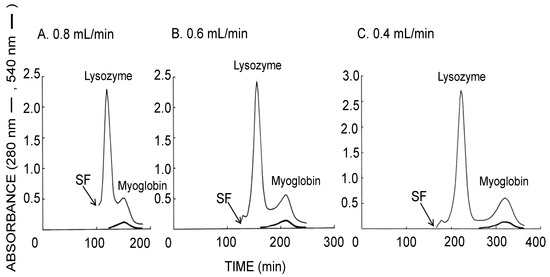
Figure 4.
CCC separation of proteins obtained by the long-pressed locular multilayer coiled columns with the upper mobile phase at three different flow rates. Experimental conditions: apparatus: small-scale cross-axis CCC with four long-pressed locular multilayer coil assemblies, 1.5 mm I.D. and 90 mL total capacity; sample: lysozyme (10 mg) and myoglobin (8 mg); solvent system: 12.5% (w/w) PEG 1000–12.5% (w/w) dibasic potassium phosphate; mobile phase: upper phase (inward elution); revolution: 1000 rpm; revolution direction: clockwise; fractions collected at 2 min/tube. SF = solvent front.
3.2. Sugar Derivatives
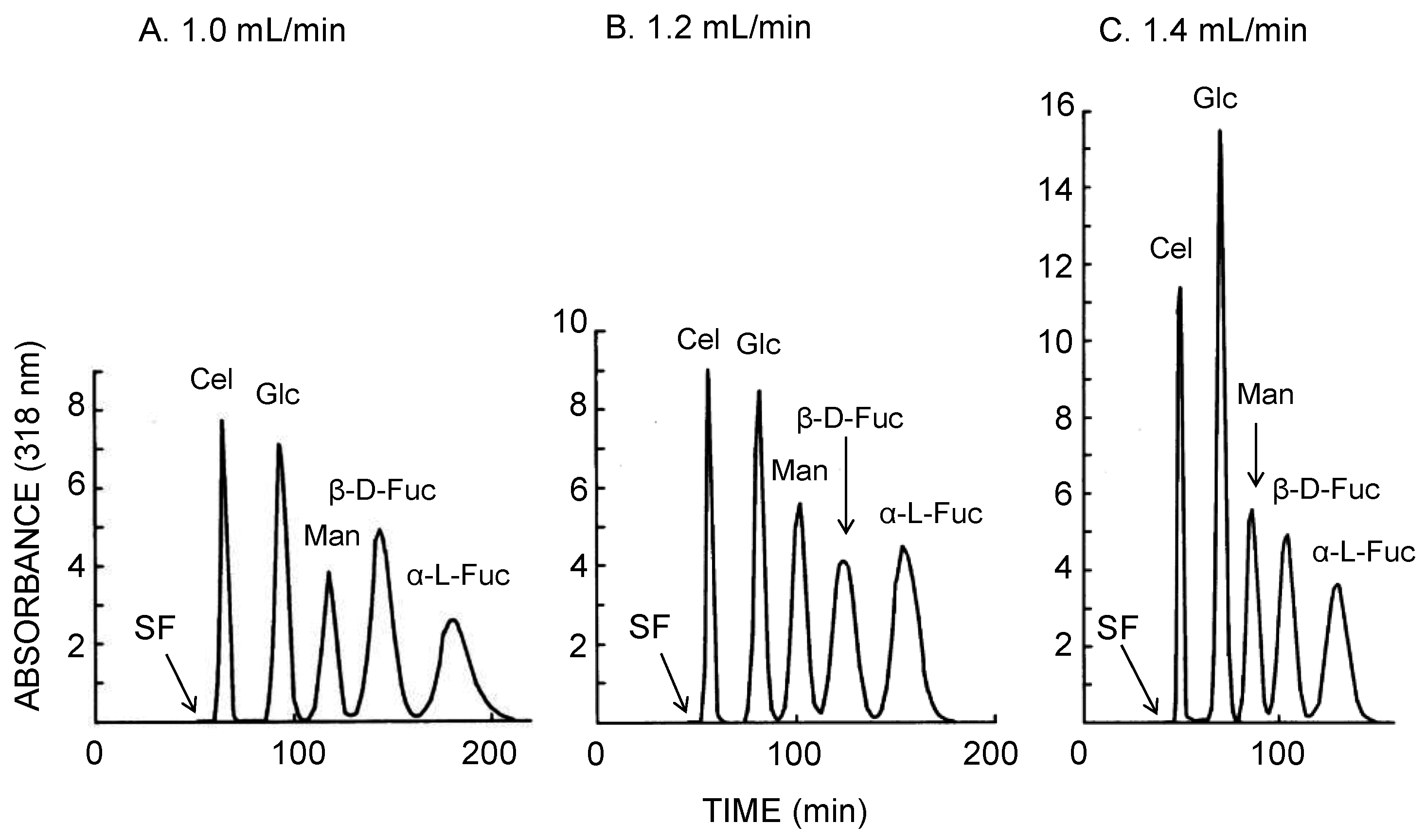
In order to achieve further increase of peak resolution in protein separation, a glass bead was inserted into each locule of the long-pressed locular tubing as shown in Figure 2D. The inclusion of a glass bead into each locule was expected to enhance mixing of two phases in the rotating column. However, at the flow rate of 0.6 and 0.4 mL/min, the retention of stationary phase substantially decreased along with peak resolution. At the flow rate of 0.8 mL/min, no stationary phase was retained in the column. It seems that the glass bead enhances mixing but does not allow enough settling to provide for significant stationary phase retention. It was predicted that improved separation would be obtained using an organic-aqueous two-phase solvent system with higher interfacial tension than of the ATPS. Figure 5 illustrates the CCC separation of five 4-methylumbelliferyl sugar derivatives obtained using the bead embedded locular multilayer coil with the lower mobile phase. While retaining the satisfactory volume of stationary phase in CCC separation, faster flow rate revealed the baseline peak resolution between each sugar derivative peak. At the flow rate of 1.4 mL/min, five sugar derivatives were separated in almost 150 min with baseline peak resolution.
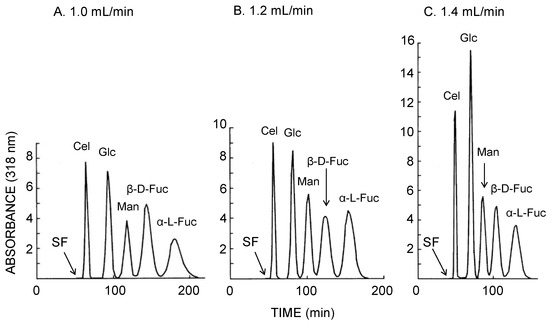
Figure 5.
CCC separation of 4-methylumbelliferyl sugar derivatives obtained by the bead embedded locular multilayer coiled columns with the lower mobile phase at three different flow rates. Experimental conditions: apparatus: small-scale cross-axis CCC with four bead embedded locular multilayer coil assemblies, 1.5 mm I.D. and 90 mL total capacity; sample: 4-methylumbelliferyl derivative of β-d-cellobioside (1 mg), β-d-glucopyranoside (1 mg), α-d-mannopyranoside (1 mg), β-d-fucopyranoside (1 mg), and α-l-fucopyranoside (1 mg); solvent system: ethyl acetate/1-butanol/water (3:2:5, v/v); mobile phase: lower phase (outward elution); revolution: 1000 rpm; revolution direction: counterclockwise; fractions collected at 2 min/tube. SF = solvent front.
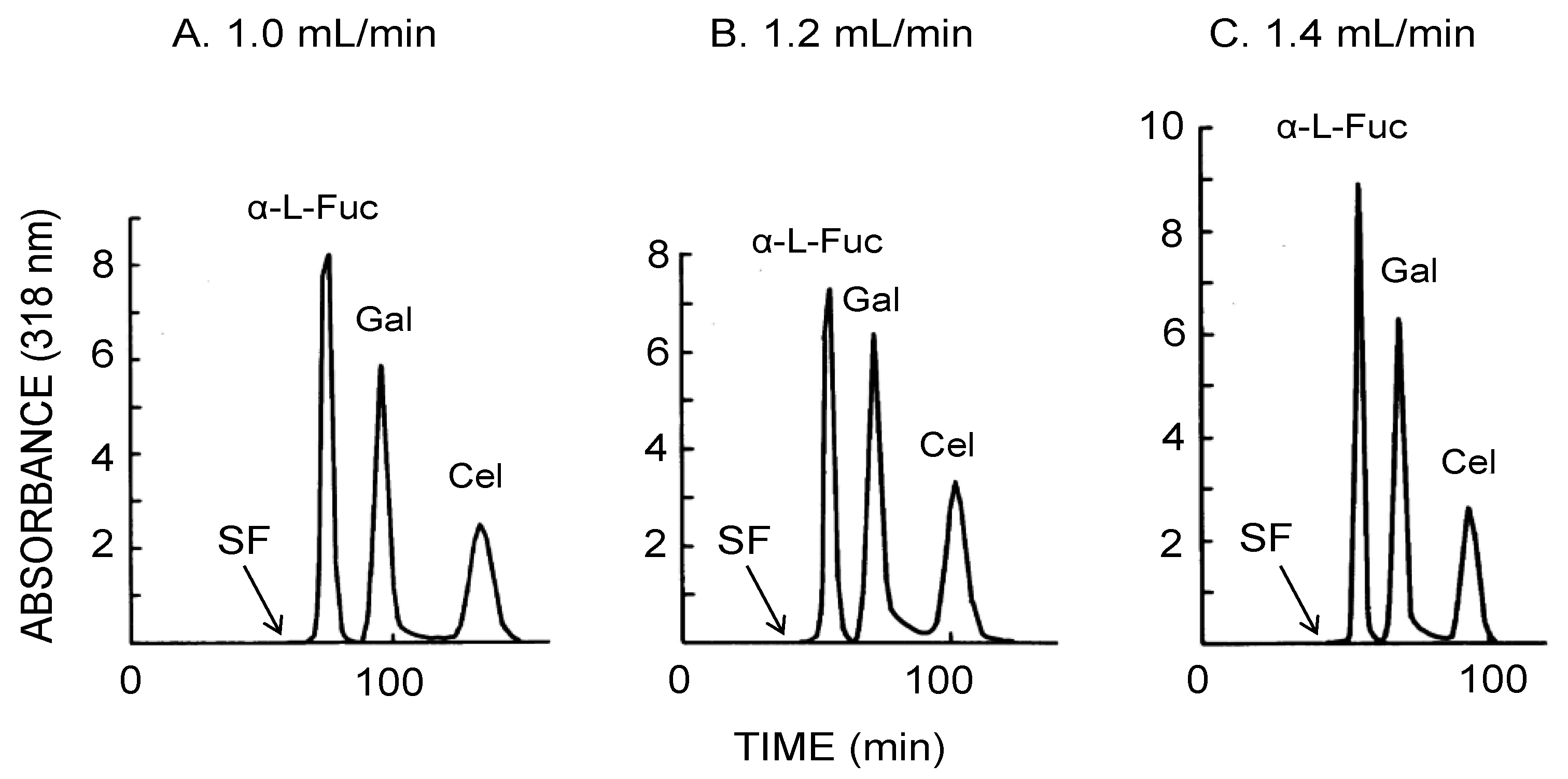
Figure 6 illustrates the CCC separation of three 4-methylumbelliferyl sugar derivatives obtained using the bead embedded locular multilayer coil with the upper mobile phase. Increased flow rates produced shorter separation times within 100 min at the flow rate of 1.4 mL/min where three sugar derivatives baseline separated from each other at peak resolution ratio of over 2.0 as summarized in Table 2.
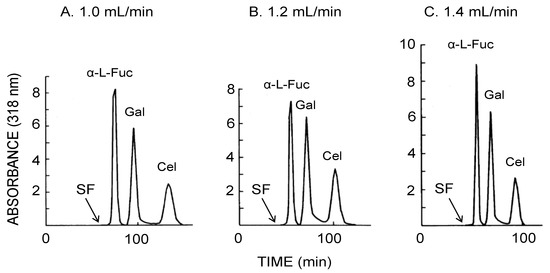
Figure 6.
CCC separation of 4-methylumbelliferyl sugar derivatives obtained by the bead embedded locular multilayer coiled columns with the upper mobile phase at three different flow rates. Experimental conditions: apparatus: small-scale cross-axis CCC with four bead embedded locular multilayer coil assemblies, 1.5 mm I.D. and 90 mL total capacity; sample: 4-methylumbelliferyl derivative of α-l-fucopyranoside (1 mg), β-d-galactopyranoside (1 mg) and β-d-cellobioside (1 mg); solvent system: ethyl acetate/1-butanol/water (1:4:5, v/v); mobile phase: upper phase (inward elution); revolution: 1000 rpm; revolution direction: clockwise; fractions collected at 2 min/tube. SF = solvent front.

Table 2.
Analytical values obtained by CCC separations of 4-methylumbelliferyl sugar derivatives with the bead embedded locular multilayer coiled column at three different flow rates.
4. Discussion
The cross-axis CCC is useful for separation of hydrophilic and hydrophobic compounds using both aqueous-aqueous and organic-aqueous two-phase solvent systems at a wide range of hydrophobicity. In the present study, improved separations of proteins were performed using the long-pressed locular tubing without glass beads and baseline separations of 4-methylumbelliferyl sugar derivatives were achieved using the bead embedded locular tubing for the small-scale cross-axis CCC.
As shown in Figure 3, sufficient partitioning of proteins at the resolution of over 1.2 between aqueous two immiscible phases in the rotating column was achieved even at a relatively high flow rate of the lower mobile phase of 0.8 mL/min, while the separation was further increased by a lower flow rate but with a longer separation time. Figure 4 also suggested that more sufficient partitioning of proteins at the resolution of over 1.5 is accomplished at a lower flow rate of 0.4 mL/min with the upper mobile phase. As summarized in Table 1, the stationary phase retention is inversely proportional to flow rate. Decreased flow rate of the mobile phase produced the increase of stationary phase retention and peak resolution. In general, higher N value does not follow higher Rs values. Faster flow rate decreases separation time of each analyte due to the decrease of the stationary phase retention. As described in the conventional formula, apparently both retention time and W decrease at faster flow rates. If N increases or decreases depend on their rate of decrease, smaller W value calculates higher N value while all analytes elute within shorter separation time.
Using the organic-aqueous two-phase solvent system with the lower mobile phase, 4-methylumbelliferyl sugar derivatives were baseline separated from each other at high theoretical plate number obtained from the second β-d-glucopyranoside peak (Figure 5). The results obtained using the upper mobile phase shown in Figure 6 also appear similar to those obtained using the lower mobile phase, indicating that a glass bead inserted into each locule promotes the partitioning of analytes between two phases. As described above, increased flow rate generally decreases stationary phase retention and peak resolution. However, in the present studies, baseline separations at their resolutions of almost over 1.5 were achieved by the faster flow rates whereas the stationary phase retention was gradually decreased as summarized in Table 2. In a series of these experiments, the long-pressed locular tubing inserted with a bead into every other locule and the long-pressed tubing inserted with a bead into every third locule were also applied to similar separations of 4-methylumbelliferyl sugar derivatives with almost equivalent column volumes. Better peak resolution with shorter separation time was obtained using the long-pressed locular tubing inserted with a bead into each locule than the other two kinds of the bead embedded locular tubing. This suggests that the beads increased mixing at higher flow rates which compensated for decrease in stationary phase retention. Similar studies were further performed using ATPS for protein separation. Better peak resolution and stationary phase retention was obtained in the long-pressed locular tubing without inserting with a bead into each locule more than three kinds of the bead embedded locular tubing, which was different from the result obtained using organic-aqueous two-phase solvent systems with the present small-scale cross-axis CCC instrument.
The long-pressed locular tubing and the bead embedded locular tubing used in the present CCC instrument successfully contributed to the improvement of CCC separation. It suggests that the compressed portion of the tubing increases the linear velocity of the mobile phase to enhance the mixing of the two phases in each locule.
5. Summary
The cross-axis CCC can be used for the effective separation and purification of various compounds at a wide range of hydrophobicity. Improved separation is accomplished using the long-pressed locular multilayer coil for highly hydrophilic compounds such as proteins with ATPS, and the long-pressed locular multilayer coil inserted with a glass bead into each locule for hydrophobic compounds with organic-aqueous two-phase solvent systems.
Acknowledgments
The authors would like to thank Motoki Umezawa, Manami Seki, and Jun Nitta for their technical assistance.
Author Contributions
Kazufusa Shinomiya designed, conducted, performed the experiments in the present study and wrote the paper; Kazumasa Zaima and Naoki Harikai analyzed the data; Yoichiro Ito discussed about the data and checked the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ito, Y. Principles and Instrumentation of Countercurrent Chromatography. In Countercurrent Chromatography: Theory and Practice; Mandava, N.B., Ito, Y., Eds.; Marcel Dekker: New York, NY, USA, 1988; pp. 79–442. [Google Scholar]
- Conway, W.D. The evolution of Countercurrent Chromatography. In Countercurrent Chromatography: Apparatus, Theory & Applications; VCH: Weinheim, Germany, 1990; pp. 37–115. [Google Scholar]
- Ito, Y. Principle, Application, and Methodology of High-Speed Countercurrent Chromatography. In High-Speed Countercurrent Chromatography; Ito, Y., Conway, W.D., Eds.; Wiley-Interscience: New York, NY, USA, 1996; pp. 3–44. [Google Scholar]
- Ito, Y.; Menet, J.-M. Coil Planet Centrifuge for High-Speed Countercurrent Chromatography. In Countercurrent Chromatography; Menet, J.-M., Thiebaut, D., Eds.; Marcel Dekker: New York, NY, USA, 1999; pp. 87–119. [Google Scholar]
- Ito, Y. Cross-axis synchronous flow-through coil planet centrifuge free of rotary seals for preparative countercurrent chromatography. Part I. Apparatus and analysis of acceleration. Sep. Sci. Technol. 1987, 22, 1971–1988. [Google Scholar] [CrossRef]
- Ito, Y. Cross-axis synchronous flow-through coil planet centrifuge free of rotary seals for preparative countercurrent chromatography. Part II. Studies on phase distribution and partition efficiency in coaxial coils. Sep. Sci. Technol. 1987, 22, 1989–2009. [Google Scholar] [CrossRef]
- Shinomiya, K.; Muto, M.; Kabasawa, Y.; Fales, H.M.; Ito, Y. Protein separation by improved cross-axis coil planet centrifuge with eccentric coil assemblies. J. Liq. Chromatogr. Relat. Technol. 1996, 19, 415–425. [Google Scholar] [CrossRef]
- Shinomiya, K.; Yanagidaira, K.; Ito, Y. New small-scale cross-axis coil planet centrifuge: The design of the apparatus and its application to counter-current chromatographic separation of proteins with aqueous-aqueous polymer phase systems. J. Chromatogr. A 2006, 1104, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Shinomiya, K.; Kobayashi, H.; Inokuchi, N.; Kobayashi, K.; Oshima, H.; Kitanaka, S.; Yanagidaira, K.; Sasaki, H.; Muto, M.; Okano, M.; et al. New small-scale cross-axis coil planet centrifuge: Partition efficiency and application to purification of bullfrog ribonuclease. J. Chromatogr. A 2007, 1151, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Shinomiya, K.; Menet, J.-M.; Fales, H.M.; Ito, Y. Studies on a new cross-axis coil planet centrifuge for performing counter-current chromatography. I. Design of the apparatus, retention of the stationary phase, and efficiency in the separation of proteins with polymer phase systems. J. Chromatogr. A 1993, 644, 215–229. [Google Scholar] [CrossRef]
- Shinomiya, K.; Kabasawa, Y.; Ito, Y. Protein Separation by Cross-Axis Coil Planet Centrifuge with Two Different Types of Coiled Columns. J. Liq. Chromatogr. Relat. Technol. 1998, 21, 111–120. [Google Scholar] [CrossRef]
- Shinomiya, K.; Kabasawa, Y.; Ito, Y. Effect of Elution Modes on Protein Separation by Cross-Axis Coil Planet Centrifuge with Two Different Types of Coiled Columns. Prep. Biochem. Biotechnol. 1999, 29, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Shinomiya, K.; Kabasawa, Y.; Ito, Y. Protein Separation by Cross-Axis Coil Planet Centrifuge with Spiral Column Assemblies. J. Liq. Chromatogr. Relat. Technol. 2002, 25, 2665–2678. [Google Scholar] [CrossRef]
- Shinomiya, K.; Ito, Y. Partition efficiency of newly designed locular multilayer coil for countercurrent chromatographic separation of proteins using small-scale cross-axis coil planet centrifuge with aqueous-aqueous polymer phase systems. J. Liq. Chromatogr. Relat. Technol. 2009, 32, 1096–1106. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y. Spiral column configuration for protein separation by high-speed countercurrent chromatography. Chem. Eng. Process. 2010, 49, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Englert, M.; Vetter, W. Tubing modifications for countercurrent chromatography (CCC): Stationary phase retention and separation efficiency. Anal. Chim. Acta 2015, 884, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Englert, M.; Vetter, W. Tubing modifications for countercurrent chromatography: Investigation of geometrical parameters. J. Liq. Chromatogr. Relat. Technol. 2016, 39, 445–452. [Google Scholar] [CrossRef]
- Shinomiya, K.; Sato, K.; Yoshida, K.; Tokura, K.; Maruyama, H.; Yanagidaira, K.; Ito, Y. Partition efficiencies of newly fabricated universal high-speed counter-current chromatograph for separation of two different types of sugar derivatives with organic-aqueous two-phase solvent systems. J. Chromatogr. A 2013, 1322, 74–80. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).