The Simultaneous Determination of Silicic, Boric and Carbonic Acids in Natural Water via Ion-Exclusion Chromatography with a Charged Aerosol Detector
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Apparatus
2.3. Columns
2.4. Natural Water Samples
3. Results
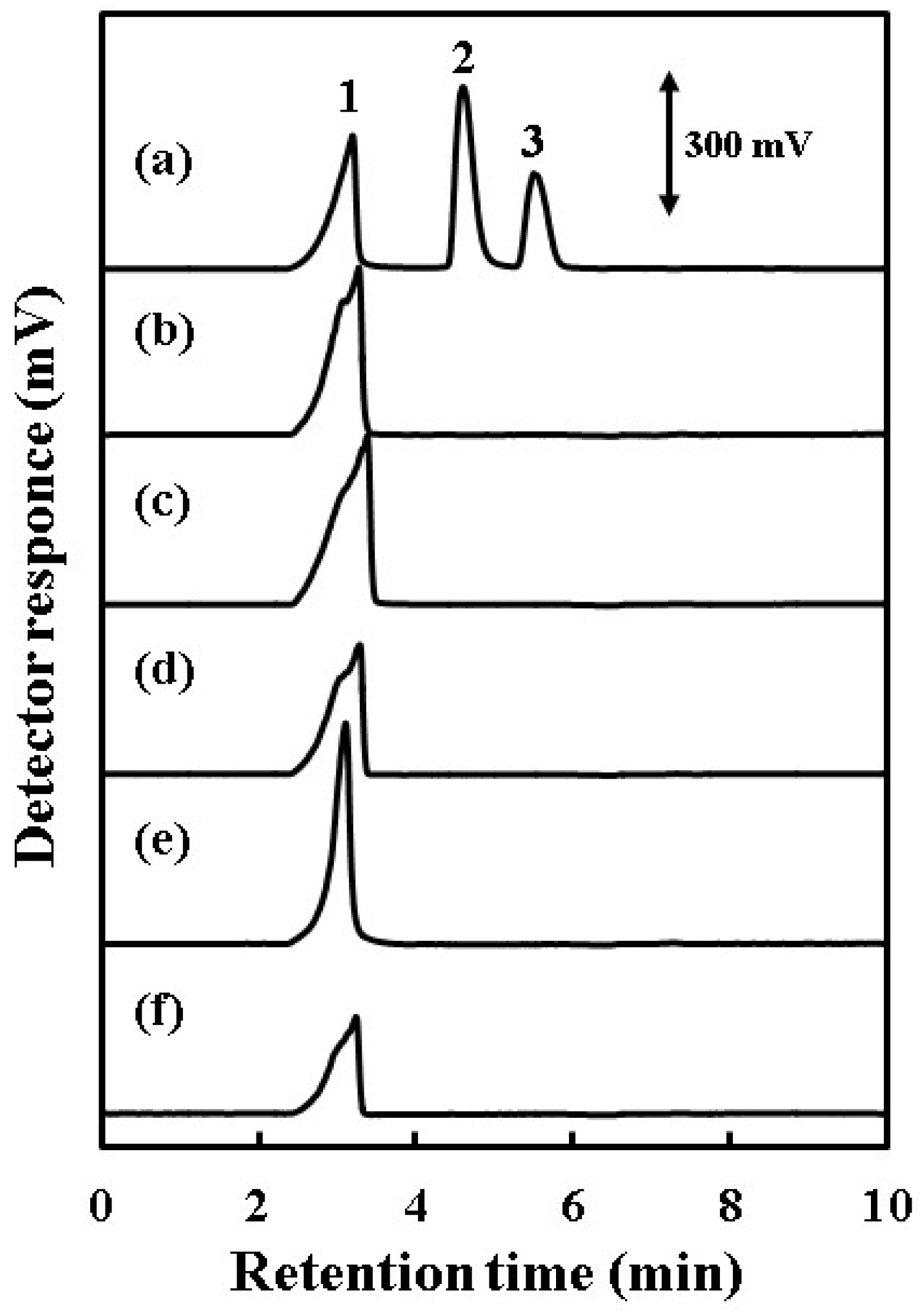
3.1. Ion-Exclusion Chromatography with the C-CAD of Weak Inorganic Acids with a Water Eluent
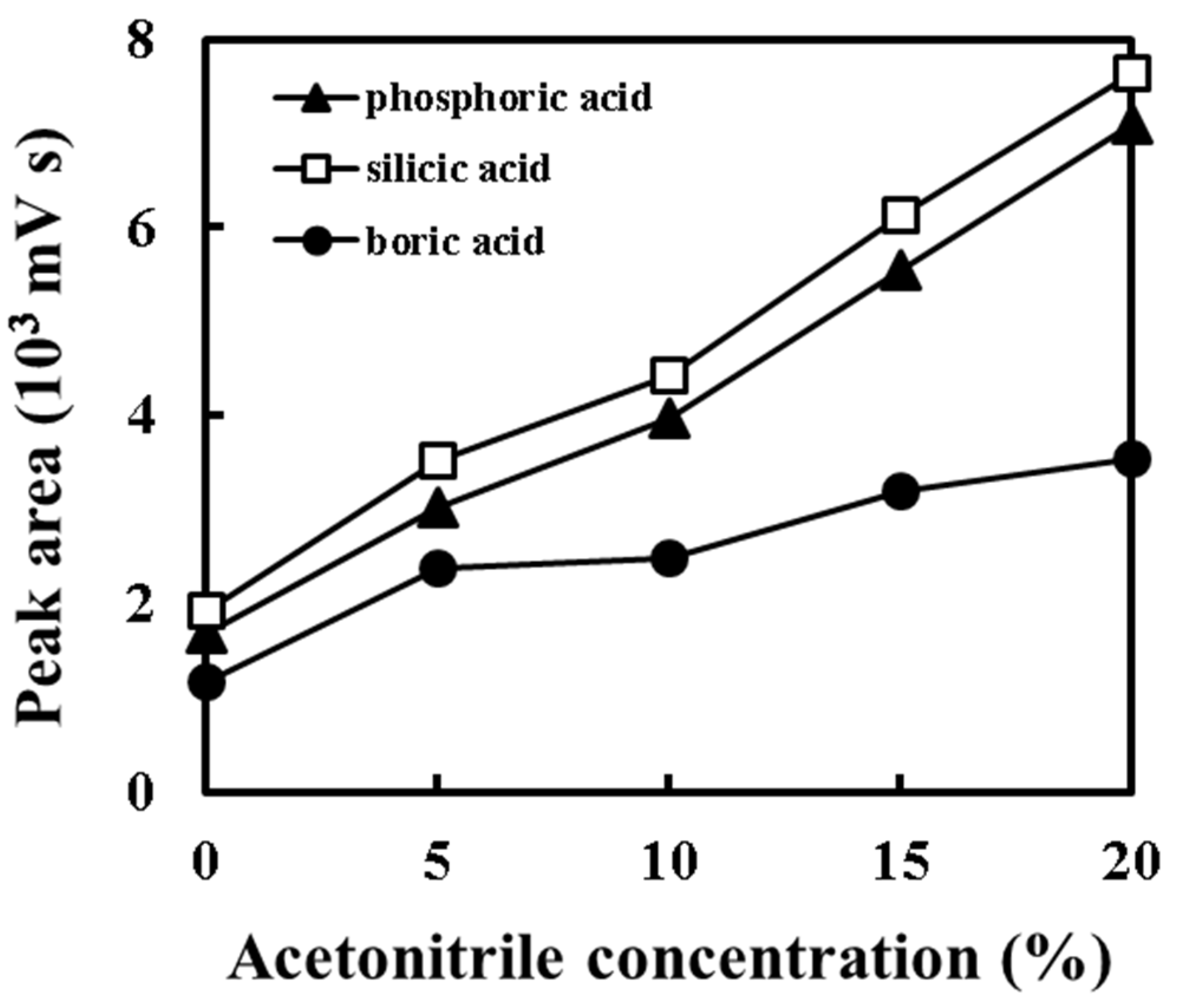
3.2. Effect of Acetonitrile Addition to a Water Eluent on the Detector Response of Weak Inorganic Acids
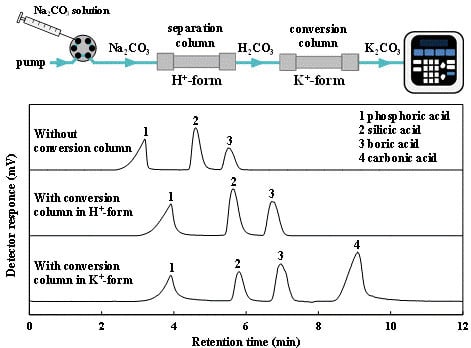
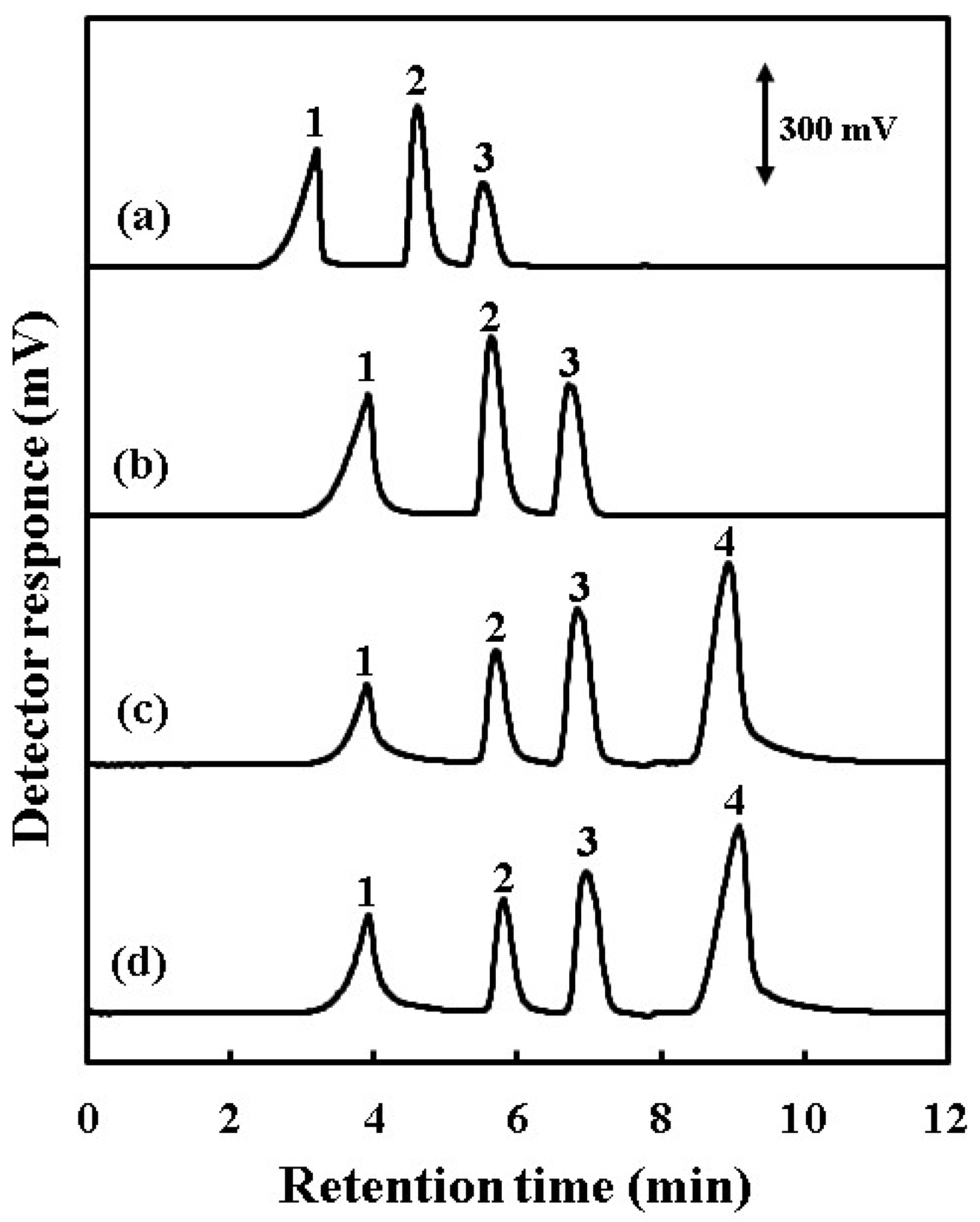
3.3. Effect of Conversion Column for Simultaneous Determination of Silicic, Boric and Carbonic Acids
3.4. Analytical Performance Using IEC with the C-CAD
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Subranabuan, V.; Ittekkot, V.; Unger, D.; Madhavan, N. Silicate Weathering in South Asian Tropical River Basins. In The Silicon Cycle: Human Perturbations and Impacts on Aquatic Systems; Ittekkot, V., Unger, D., Humborg, C., An, N.T., Eds.; Island Press: Washington DC, USA, 2006; pp. 3–12. [Google Scholar]
- Davila, L.P.; Risbud, S.H.; Shackelford, J.F. Quartz and Silicas. In Ceramic and Glass Materials. Structure, Properties and Processing; Shackelford, J.F., Doremus, R.H., Eds.; Springer Scence+Business Media, LLC: New York, NY, USA, 2008; pp. 71–86. [Google Scholar]
- Park, H.; Schlesinger, W.H. Global biogeochemical cycle of boron. Global Biogeochem. Cycles 2002. [Google Scholar] [CrossRef]
- Schlesinger, W.H. An Overview of the Carbon Cycle. In Soils and Global Change; Lal, R., Kimble, J., Levine, E., Stewart, B.A., Eds.; Lewis Publishers: Boca Raton, FL, USA, 1995; pp. 9–26. [Google Scholar]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters, 3rd ed.; Wiley/Interscience: New York, NY, USA, 1996. [Google Scholar]
- Eaton, A.D.; Clesceri, L.S.; Rice, E.W.; Greenberg, A.E. Standard Methods for the Examination of Water & Wastewater, 21st ed.; APHA-AWWA-WFE: Washington DC, USA, 2005. [Google Scholar]
- Abe, K.; Watanabe, Y. Determination of silicate in seawater by inductivity coupled plasma atomic emission spectrometry. J. Oceanogr. 1992, 48, 283–292. [Google Scholar] [CrossRef]
- Farhat, F.; Ahmad, F.; Arafat, H. Analytical techniques for boron quantification supporting desalination processes: A review. Desalination 2013, 310, 9–17. [Google Scholar] [CrossRef]
- Fritz, J.; Gjerde, D.T. Ion-Exclusion Chromatography. In Ion Chromatography, 4th ed.; Wiley-VCH: Weinheim, Germany, 2009; pp. 207–237. [Google Scholar]
- Li, H.-B.; Chen, F. Determination of silicate in water by ion exclusion chromatography with conductivity detection. J. Chromatogr. A 2000, 874, 143–147. [Google Scholar] [CrossRef]
- Wilshire, J.P.; Brown, W.A. Determination of boric oxide by ion chromatography and ion chromatography exclusion. Anal. Chem. 1982, 54, 1647–1650. [Google Scholar] [CrossRef]
- Tanaka, K.; Fritz, J.S. Determination of bicarbonate by ion-exclusion chromatography with ion-exchange enhancement of conductivity detection. Anal. Chem. 1987, 59, 708–712. [Google Scholar] [CrossRef]
- Ikedo, M.; Mori, M.; Kurachi, K.; Hu, W.; Tanaka, K. Selective and simultaneous determination of phosphate and silicate ions in leaching process waters for ceramics glaze raw materials of natural origin by ion-exclusion chromatography coupled with UV-detection after postcolumn derivatization. Anal. Sci. 2006, 22, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, N.; Kozaki, D.; Masuda, W.; Nakagoshi, N.; Hasebe, K.; Mori, M.; Tanaka, K. Simultaneous spectrophotometric determination of phosphate and silicate ions in river water by using ion-exclusion chromatographic separation and post-column derivatization. Anal. Chim. Acta 2008, 619, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, N.; Masuda, W.; Kozaki, D.; Goto, R.; Nakagoshi, N.; Mori, M.; Hasebe, K.; Tanaka, K. Simultaneous spectrophotometric determination of orthophosphate and silicate ions in river water using ion-exclusion chromatography with an ascorbate solution as both eluent and reducing agent, followed by postcolumn derivatization with molybdate. Anal. Sci. 2009, 25, 379–383. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Inoue, Y.; Date, Y. Determination of boron using ion-exclusion chromatography with postcolumn derivatization method. Bunseki Kagaku 1994, 43, 365–370. [Google Scholar] [CrossRef]
- Vehovec, T.; Obreza, A. Review of operating principle and applications of the charged aerosol detector. J. Chromatogr. A 2010, 1217, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Ligor, M.; Studzińska, S.; Horna, A.; Buszewski, B. Corona-charged aerosol detection: An analytical approach. Crit. Rev. Anal. Chem. 2013, 43, 64–78. [Google Scholar] [CrossRef]
- Magnusson, L.-E.; Risley, D.S.; Koropchak, J.A. Aerosol-based detectors for liquid chromatography. J. Chromatogr. A 2015, 1421, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Almeling, S.; Ilko, D.; Holzgrabe, U. Charged aerosol detection in pharmaceutical analysis. J. Pharm. Biomed. Anal. 2012, 69, 50–63. [Google Scholar] [CrossRef] [PubMed]
- Crafts, C.; Bailey, B.; Plante, M.; Acworth, I. Evaluation of methods for the simultaneous analysis of cations and anions using HPLC with charged aerosol detection and a zwitterionic stationary phase. J. Chromatogr. Sci. 2009, 47, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Richards, M.A.; Zha, Y.; Francis, R.; Lozano, R.; Ruan, J. Determination of inorganic pharmaceutical counterions using hydrophilic interaction chromatography coupled with Corona® CAD detector. J. Pharm. Biomed. Anal. 2009, 50, 809–814. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Dai, L.; Chetwyn, N.P. Simultaneous determination of positive and negative pharmaceutical counterions using mixed-mode chromatography coupled with charged aerosol detector. J. Chromatogr. A 2010, 1217, 5776–5784. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.; Sagara, K.; Arai, K.; Nakatani, N.; Ohira, S.; Toda, K.; Itabashi, H.; Kozaki, D.; Sugo, Y.; Watanabe, S.; et al. Simultaneous analysis of silicon and boron dissolved in water by combination of electrodialytic salt removal and ion-exclusion chromatography with corona charged aerosol detection. J. Chromatogr. A 2016, 1431, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K. Study on water quality monitoring by advanced ion-exclusion chromatography. Bunseki Kagaku 2006, 55, 275–289. [Google Scholar] [CrossRef]
- Nakatani, N.; Kozaki, D.; Mori, M.; Hasebe, K.; Nakagoshi, K.; Tanaka, K. Ion-exclusion/cation-exchange chromatography with dual detection of the conductivity and spectrophotometry for the simultaneous determination of common inorganic anionic species and cations in river and wastewater. Anal. Sci. 2011, 27, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, N.; Kozaki, D.; Mori, M.; Tanaka, K. Recent progress and applications of ion-exclusion/ion-exchange chromatography for simultaneous determination of inorganic anions and cations. Anal. Sci. 2012, 28, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Ishizuka, T. Elution behavior of acids in ion-exclusion chromatography using a cation-exchange resin. J. Chromatogr. 1979, 174, 153–157. [Google Scholar] [CrossRef]
- Glòd, B.K. Ion exclusion chromatography: Parameters influencing retention. Neurochem. Res. 1997, 22, 1237–1248. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Ohta, K.; Fritz, J.S.; Matsushita, S.; Miyanaga, A. Simultaneous ion-exclusion chromatography -cation-exchange chromatography with conductimetric detection of anions and cations in acid rain waters. J. Chromatogr. A 1994, 671, 239–248. [Google Scholar] [CrossRef]
- Mori, M.; Itabashi, H.; Helaleh, M.I.H.; Kaczmarski, K.; Glòd, B.K.; Kowalska, T.; Xu, Q.; Ikedo, M.; Hu, W.; Tanaka, K. Vacancy ion-exclusion chromatography of inorganic acids on a weakly acidic cation-exchange resin column. J. Chromatogr. A 2006, 1118, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.; Tanaka, K.; Taoda, H.; Ikedo, M.; Hu, W. Ion-exclusion chromatography of silicate ion in sea and river water samples by an ion-exchange enhancement of conductivity detection. Bunseki Kagaku 2004, 53, 1481–1486. [Google Scholar] [CrossRef]
- Mori, M.; Ikedo, M.; Hu, W.; Helaleh, M.I.H.; Xu, Q.; Itabashi, H.; Tanaka, K. High-speed ion-exclusion chromatography of dissolved carbon dioxide on a small weakly acidic cation-exchange resin column with ion-exchange enhancement columns of conductivity detection. J. Chromatogr. A 2005, 1092, 250–253. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.; Itabashi, H.; Ikedo, M.; Tanaka, K. Ion-exclusion chromatography with the direct UV detection of non-absorbing inorganic cations using an anion-exchange conversion column in the iodide-form. Talanta 2006, 70, 174–177. [Google Scholar] [CrossRef] [PubMed]
- Karu, N.; Dicinoski, G.W.; Haddad, P.R. Use of suppressors for signal enhancement of weakly-acidic analytes in ion chromatography with universal detection methods. Trends Anal. Chem. 2012, 40, 119–132. [Google Scholar] [CrossRef]
- Morris, J.; Fritz, J.S. Eluent modifiers for the liquid chromatographic separation of carboxylic acids using conductivity detection. Anal. Chem. 1994, 66, 2390–2396. [Google Scholar] [CrossRef]

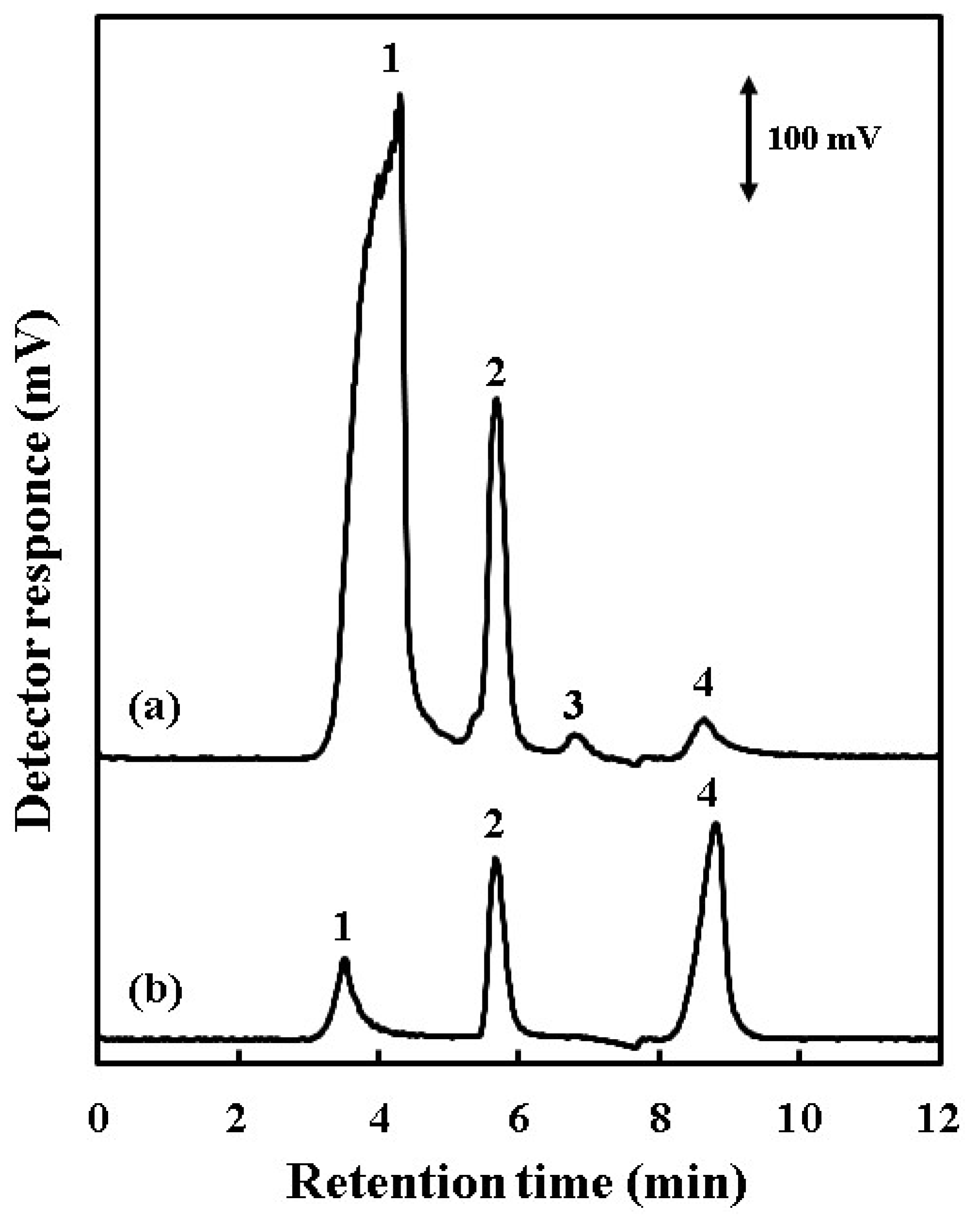
| Acid | Retention Time (%) | Peak Area (%) |
|---|---|---|
| Silicic acid | 1.03 | 3.11 |
| Boric acid | 1.55 | 3.40 |
| Carbonic acid | 0.39 | 3.08 |
| Acid | Linear Range (mg·L−1) | r2 | Detection Limit (mg·L−1) | Recovery * (%) (min.–max.) |
|---|---|---|---|---|
| Silicic acid | 0.5–10 | 0.996 | 0.03 | 101.4 ± 2.7 (98.2–104.6) |
| Boric acid | 10–100 | 0.998 | 0.40 | 99.3 ± 3.7 (94.3–103.5) |
| Carbonic acid | 1.3–21 | 0.993 | 0.08 | 98.4 ± 4.0 (92.2–102.8) |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otsuka, Y.; Nakatani, N.; Takahashi, T.; Kozaki, D.; Mori, M.; Tanaka, K. The Simultaneous Determination of Silicic, Boric and Carbonic Acids in Natural Water via Ion-Exclusion Chromatography with a Charged Aerosol Detector. Separations 2016, 3, 9. https://doi.org/10.3390/chromatography3010009
Otsuka Y, Nakatani N, Takahashi T, Kozaki D, Mori M, Tanaka K. The Simultaneous Determination of Silicic, Boric and Carbonic Acids in Natural Water via Ion-Exclusion Chromatography with a Charged Aerosol Detector. Separations. 2016; 3(1):9. https://doi.org/10.3390/chromatography3010009
Chicago/Turabian StyleOtsuka, Yu, Nobutake Nakatani, Takuya Takahashi, Daisuke Kozaki, Masanobu Mori, and Kazuhiko Tanaka. 2016. "The Simultaneous Determination of Silicic, Boric and Carbonic Acids in Natural Water via Ion-Exclusion Chromatography with a Charged Aerosol Detector" Separations 3, no. 1: 9. https://doi.org/10.3390/chromatography3010009
APA StyleOtsuka, Y., Nakatani, N., Takahashi, T., Kozaki, D., Mori, M., & Tanaka, K. (2016). The Simultaneous Determination of Silicic, Boric and Carbonic Acids in Natural Water via Ion-Exclusion Chromatography with a Charged Aerosol Detector. Separations, 3(1), 9. https://doi.org/10.3390/chromatography3010009