Recent Trends in Solid-Phase Microextraction for the Monitoring of Drugs of Abuse in Wastewater
Abstract
1. Introduction
2. Materials and Methods
3. Solid-Phase Microextraction Techniques
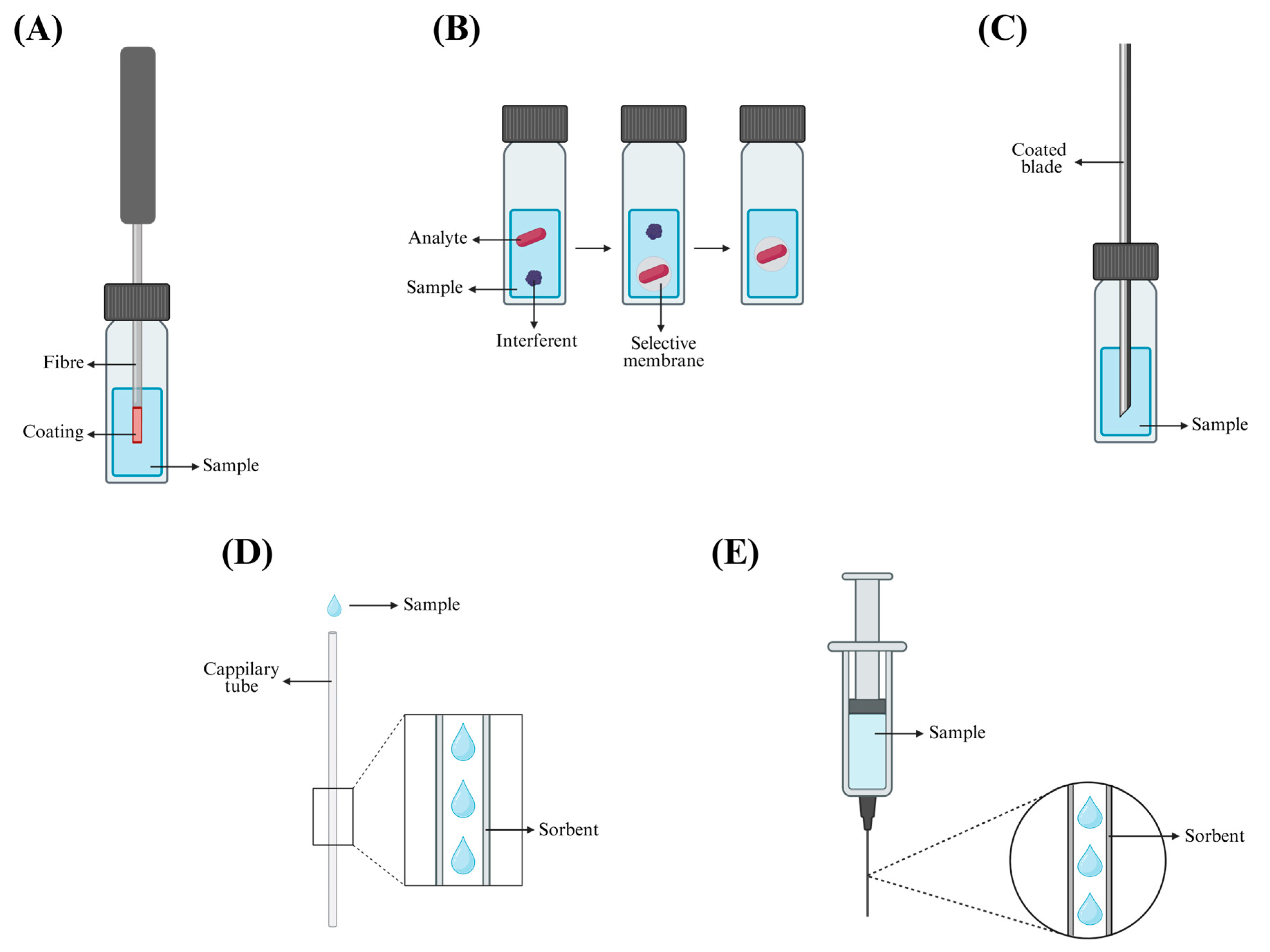
3.1. Solid-Phase Microextraction
- Membrane-protected SPME: Similarly to DI-SPME, but the fibre is encased in a selective membrane to block interfering substances [62].
3.2. Micro Solid-Phase Extraction
3.3. Magnetic Solid-Phase Extraction
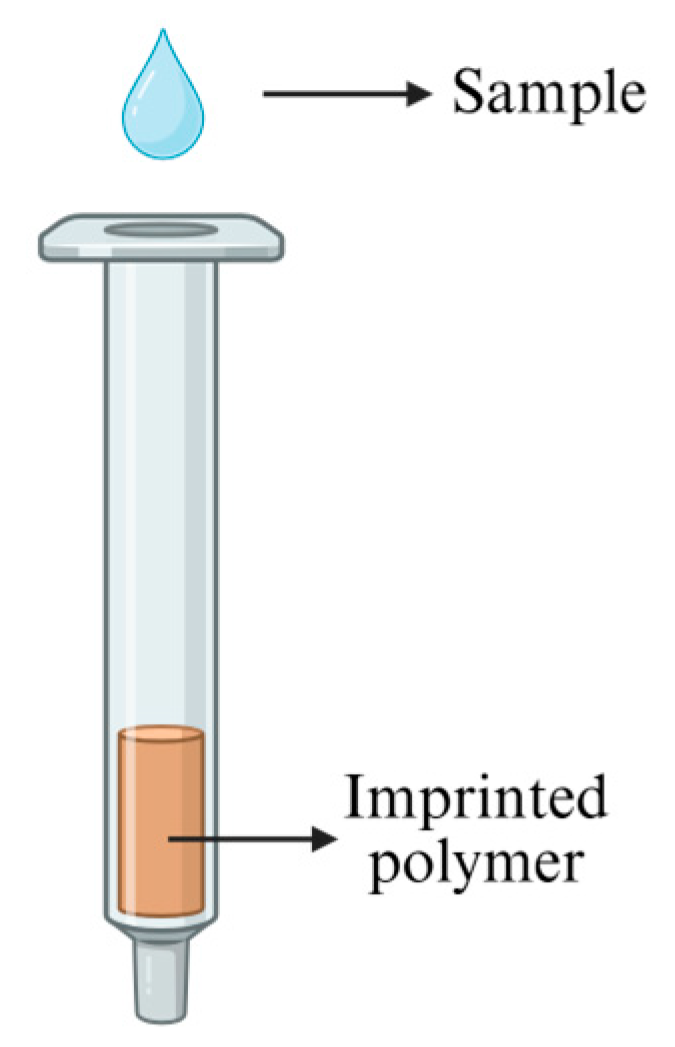
3.4. Molecularly Imprinted Polymers
4. Comparative Overview of Solid-Phase Microextraction Techniques and Their Integration into WBE
5. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Castiglioni, S.; Salgueiro-González, N.; Bijlsma, L.; Celma, A.; Gracia-Lor, E.; Beldean-Galea, M.S.; Mackuľak, T.; Emke, E.; Heath, E.; Kasprzyk-Hordern, B.; et al. New Psychoactive Substances in Several European Populations Assessed by Wastewater-Based Epidemiology. Water Res. 2021, 195, 116983. [Google Scholar] [CrossRef]
- Gracia-Lor, E.; Castiglioni, S.; Bade, R.; Been, F.; Castrignanò, E.; Covaci, A.; González-Mariño, I.; Hapeshi, E.; Kasprzyk-Hordern, B.; Kinyua, J.; et al. Measuring Biomarkers in Wastewater as a New Source of Epidemiological Information: Current State and Future Perspectives. Environ. Int. 2017, 99, 131–150. [Google Scholar] [CrossRef] [PubMed]
- Daghir, E.; Markuszewski, M.J. Disposition of Drugs of Abuse and Their Metabolites in Wastewater as a Method of the Estimation of Drug Consumption. Curr. Drug Metab. 2010, 11, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.K.; Short, M.D.; Aryal, R.; Gerber, C.; van den Akker, B.; Saint, C.P. Occurrence of Illicit Drugs in Water and Wastewater and Their Removal during Wastewater Treatment. Water Res. 2017, 124, 713–727. [Google Scholar] [CrossRef]
- Janusz, A.; Kirkbride, K.P.; Scott, T.L.; Naidu, R.; Perkins, M.V.; Megharaj, M. Microbial Degradation of Illicit Drugs, Their Precursors, and Manufacturing by-Products: Implications for Clandestine Drug Laboratory Investigation and Environmental Assessment. Forensic Sci. Int. 2003, 134, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Boles, T.H.; Wells, M.J.M. Analysis of Amphetamine and Methamphetamine as Emerging Pollutants in Wastewater and Wastewater-Impacted Streams. J. Chromatogr. A 2010, 1217, 2561–2568. [Google Scholar] [CrossRef]
- Logarinho, F.; Rosado, T.; Lourenço, C.; Barroso, M.; Araujo, A.R.T.S.; Gallardo, E. Determination of Antipsychotic Drugs in Hospital and Wastewater Treatment Plant Samples by Gas Chromatography/Tandem Mass Spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016, 1038, 127–135. [Google Scholar] [CrossRef]
- Langa, I.M.; Ribeiro, A.R.L.; Ratola, N.; Gonçalves, V.M.F.; Tiritan, M.E.; Ribeiro, C. Amphetamine-like Substances and Synthetic Cathinones in Portuguese Wastewater Influents: Enantiomeric Profiling and Role of Suspended Particulate Matter. Forensic Sci. Int. 2024, 361, 112128. [Google Scholar] [CrossRef]
- Zuccato, E.; Castiglioni, S. Illicit Drugs in the Environment. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2009, 367, 3965–3978. [Google Scholar]
- Baker, D.R.; Očenášková, V.; Kvicalova, M.; Kasprzyk-Hordern, B. Drugs of Abuse in Wastewater and Suspended Particulate Matter—Further Developments in Sewage Epidemiology. Environ. Int. 2012, 48, 28–38. [Google Scholar] [CrossRef]
- Bade, R.; Abdelaziz, A.; Nguyen, L.; Pandopulos, A.J.; White, J.M.; Gerber, C. Determination of 21 Synthetic Cathinones, Phenethylamines, Amphetamines and Opioids in Influent Wastewater Using Liquid Chromatography Coupled to Tandem Mass Spectrometry. Talanta 2020, 208, 120479. [Google Scholar] [CrossRef]
- Castiglioni, S.; Thomas, K.V.; Kasprzyk-Hordern, B.; Vandam, L.; Griffiths, P. Testing Wastewater to Detect Illicit Drugs: State of the Art, Potential and Research Needs. Sci. Total Environ. 2014, 487, 613–620. [Google Scholar] [CrossRef]
- Lai, F.Y.; O’Brien, J.; Bruno, R.; Hall, W.; Prichard, J.; Kirkbride, P.; Gartner, C.; Thai, P.; Carter, S.; Lloyd, B.; et al. Spatial Variations in the Consumption of Illicit Stimulant Drugs across Australia: A Nationwide Application of Wastewater-Based Epidemiology. Sci. Total Environ. 2016, 568, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Tscharke, B.J.; Chen, C.; Gerber, J.P.; White, J.M. Trends in Stimulant Use in Australia: A Comparison of Wastewater Analysis and Population Surveys. Sci. Total Environ. 2015, 536, 331–337. [Google Scholar] [CrossRef]
- Zuccato, E.; Chiabrando, C.; Castiglioni, S.; Bagnati, R.; Fanelli, R. Estimating Community Drug Abuse by Wastewater Analysis. Environ. Health Perspect. 2008, 116, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- European Union Drugs Agency Wastewater Analysis and Drugs—A European Multi-City Study. Available online: https://www.euda.europa.eu/publications/html/pods/waste-water-analysis_en (accessed on 10 June 2025).
- SCORE Sewage Analysis CORe Group—Europe. Available online: https://score-network.eu/ (accessed on 14 June 2025).
- Thomas, K.V.; Bijlsma, L.; Castiglioni, S.; Covaci, A.; Emke, E.; Grabic, R.; Hernández, F.; Karolak, S.; Kasprzyk-Hordern, B.; Lindberg, R.H.; et al. Comparing Illicit Drug Use in 19 European Cities through Sewage Analysis. Sci. Total Environ. 2012, 432, 432–439. [Google Scholar] [CrossRef]
- Van Nuijs, A.L.N.; Lai, F.Y.; Been, F.; Andres-Costa, M.J.; Barron, L.; Baz-Lomba, J.A.; Berset, J.D.; Benaglia, L.; Bijlsma, L.; Burgard, D.; et al. Multi-Year Inter-Laboratory Exercises for the Analysis of Illicit Drugs and Metabolites in Wastewater: Development of a Quality Control System. TrAC—Trends Anal. Chem. 2018, 103, 34–43. [Google Scholar] [CrossRef]
- European Monitoring Centre for Drugs and Drug Addiction. Assessing Illicit Drugs in Wastewater: Advances in Wastewater-Based Drug Epidemiology; Publications Office of the European Union: Luxembourg, 2016. [Google Scholar]
- Gao, Z.; Li, P.; Lin, H.; Lin, W.; Ren, Y. Biomarker Selection Strategies Based on Compound Stability in Wastewater-Based Epidemiology. Environ. Sci. Pollut. Res. 2023, 30, 5516–5529. [Google Scholar] [CrossRef]
- Van Nuijs, A.L.N.; Abdellati, K.; Bervoets, L.; Blust, R.; Jorens, P.G.; Neels, H.; Covaci, A. The Stability of Illicit Drugs and Metabolites in Wastewater, an Important Issue for Sewage Epidemiology? J. Hazard. Mater. 2012, 239–240, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Senta, I.; Krizman, I.; Ahel, M.; Terzic, S. Assessment of Stability of Drug Biomarkers in Municipal Wastewater as a Factor Influencing the Estimation of Drug Consumption Using Sewage Epidemiology. Sci. Total Environ. 2014, 487, 659–665. [Google Scholar] [CrossRef]
- Davies, B.; Paul, R.; Osselton, D.; Woolley, T. Stability of New Psychoactive Substances in Crude Wastewater. Forensic Sci. Med. Pathol. 2025, 21, 478–486. [Google Scholar] [CrossRef]
- Lin, X.; Choi, P.M.; Thompson, J.; Reeks, T.; Verhagen, R.; Tscharke, B.J.; O’Malley, E.; Shimko, K.M.; Guo, X.; Thomas, K.V.; et al. Systematic Evaluation of the In-Sample Stability of Selected Pharmaceuticals, Illicit Drugs, and Their Metabolites in Wastewater. Environ. Sci. Technol. 2021, 55, 7418–7429. [Google Scholar] [CrossRef]
- European Union Council Directive 91/271/EEC of 21 May 1991 Concerning Urban Waste-Water Treatment. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:31991L0271 (accessed on 20 August 2025).
- European Union Directive (EU) 2024/3019 of the European Parliament and of the Council of 27 November 2024 Concerning Urban Wastewater Treatment. Available online: https://eur-lex.europa.eu/eli/dir/2024/3019/oj (accessed on 20 August 2025).
- European Union Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 Establishing a Framework for Community Action in the Field of Water Policy. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02000L0060-20141120 (accessed on 20 August 2025).
- International Organization for Standardization ISO/IEC 17025:2017. General Requirements for the Competence of Testing and Calibration Laboratories. Available online: https://www.iso.org/ISO-IEC-17025-testing-and-calibration-laboratories.html (accessed on 20 August 2025).
- European Union Commission Directive 2009/90/EC of 31 July 2009 Laying down, Pursuant to Directive 2000/60/EC of the European Parliament and of the Council, Technical Specifications for Chemical Analysis and Monitoring of Water Status. Available online: https://eur-lex.europa.eu/eli/dir/2009/90/oj/eng (accessed on 20 August 2025).
- SCORE Ethical Research Guidelines for Wastewater-Based Epidemiology and Related Fields. Available online: https://www.euda.europa.eu/drugs-library/ethical-research-guidelines-wastewater-based-epidemiology-and-related-fields_en (accessed on 20 August 2025).
- Centazzo, N.; Frederick, B.M.; Jacox, A.; Cheng, S.Y.; Concheiro-Guisan, M. Wastewater Analysis for Nicotine, Cocaine, Amphetamines, Opioids and Cannabis in New York City. Forensic Sci. Res. 2019, 4, 152–167. [Google Scholar] [CrossRef]
- Ning, H.; Fan, Y.; Liu, H.; Huang, Z.; Ke, X.; Xu, Y.; She, Y. Preparation and Application of Polystyrene-Divinylbenzene Sorbent with Weak Cation-Exchange Character for the Selective Extraction of Illicit Drugs in Environmental Water. J. Chromatogr. A 2022, 1671, 462994. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, A.F.B.; de Melo Vieira, A.; Santos, J.M. Trends and Challenges in Analytical Chemistry for Multi-Analysis of Illicit Drugs Employing Wastewater-Based Epidemiology. Anal. Bioanal. Chem. 2023, 415, 3749–3758. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wu, X.; Luan, T.; Jiang, R.; Ouyang, G. Sample Preparation and Instrumental Methods for Illicit Drugs in Environmental and Biological Samples: A Review. J. Chromatogr. A 2021, 1640, 461961. [Google Scholar] [CrossRef]
- Causanilles, A.; Baz-Lomba, J.A.; Burgard, D.A.; Emke, E.; González-Mariño, I.; Krizman-Matasic, I.; Li, A.; Löve, A.S.C.; McCall, A.K.; Montes, R.; et al. Improving Wastewater-Based Epidemiology to Estimate Cannabis Use: Focus on the Initial Aspects of the Analytical Procedure. Anal. Chim. Acta 2017, 988, 27–33. [Google Scholar] [CrossRef]
- Benaglia, L.; Udrisard, R.; Bannwarth, A.; Gibson, A.; Béen, F.; Lai, F.Y.; Esseiva, P.; Delémont, O. Testing Wastewater from a Music Festival in Switzerland to Assess Illicit Drug Use. Forensic Sci. Int. 2020, 309, 110148. [Google Scholar] [CrossRef] [PubMed]
- Daglioglu, N.; Guzel, E.Y.; Atasoy, A.; Gören, İ.E. Comparison of Community Illicit Drug Use in 11 Cities of Turkey through Wastewater-Based Epidemiology. Environ. Sci. Pollut. Res. 2020, 28, 15076–15089. [Google Scholar] [CrossRef]
- Sulej-Suchomska, A.M.; Klupczynska, A.; Dereziński, P.; Matysiak, J.; Przybyłowski, P.; Kokot, Z.J. Urban Wastewater Analysis as an Effective Tool for Monitoring Illegal Drugs, Including New Psychoactive Substances, in the Eastern European Region. Sci. Rep. 2020, 10, 4885. [Google Scholar] [CrossRef]
- Löve, A.S.C.; Ásgrímsson, V.; Ólafsdóttir, K. Illicit Drug Use in Reykjavik by Wastewater-Based Epidemiology. Sci. Total Environ. 2022, 803, 149795. [Google Scholar] [CrossRef]
- Bade, R.; Bijlsma, L.; Sancho, J.V.; Baz-Lomba, J.A.; Castiglioni, S.; Castrignanò, E.; Causanilles, A.; Gracia-Lor, E.; Kasprzyk-Hordern, B.; Kinyua, J.; et al. Liquid Chromatography-Tandem Mass Spectrometry Determination of Synthetic Cathinones and Phenethylamines in Influent Wastewater of Eight European Cities. Chemosphere 2017, 168, 1032–1041. [Google Scholar] [CrossRef]
- Croft, T.L.; Huffines, R.A.; Pathak, M.; Subedi, B. Prevalence of Illicit and Prescribed Neuropsychiatric Drugs in Three Communities in Kentucky Using Wastewater-Based Epidemiology and Monte Carlo Simulation for the Estimation of Associated Uncertainties. J. Hazard. Mater. 2020, 384, 121306. [Google Scholar] [CrossRef]
- Luo, L.; Wang, C.; Wu, Y.; Zhang, H.; Xu, F.; Tang, K.; Yu, J. Rapid Screening of Illicit Drugs in Wastewater Based On-Site Automated Solid Phase Extraction and Portable Mass Spectrometry Techniques. J. Chromatogr. A 2025, 1753, 466011. [Google Scholar] [CrossRef]
- Wang, J.; Qi, L.; Hou, C.; Zhang, T.; Chen, M.; Meng, H.; Su, M.; Xu, H.; Hua, Z.; Wang, Y.; et al. Automatic Analytical Approach for the Determination of 12 Illicit Drugs and Nicotine Metabolites in Wastewater Using On-Line SPE-UHPLC-MS/MS. J. Pharm. Anal. 2021, 11, 739–745. [Google Scholar] [CrossRef]
- Yao, B.; Lian, L.; Pang, W.; Yin, D.; Chan, S.A.; Song, W. Determination of Illicit Drugs in Aqueous Environmental Samples by Online Solid-Phase Extraction Coupled to Liquid Chromatography–Tandem Mass Spectrometry. Chemosphere 2016, 160, 208–215. [Google Scholar] [CrossRef]
- Mastroianni, N.; Bleda, M.J.; de Alda, M.L.; Barceló, D. Occurrence of Drugs of Abuse in Surface Water from Four Spanish River Basins: Spatial and Temporal Variations and Environmental Risk Assessment. J. Hazard. Mater. 2016, 316, 134–142. [Google Scholar] [CrossRef] [PubMed]
- López-García, E.; Mastroianni, N.; Postigo, C.; Barceló, D.; de Alda, M.L. A Fully Automated Approach for the Analysis of 37 Psychoactive Substances in Raw Wastewater Based on On-Line Solid Phase Extraction-Liquid Chromatography-Tandem Mass Spectrometry. J. Chromatogr. A 2018, 1576, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zhang, Y.; Hu, Y.; Shui, F.; Tang, J.; Yu, Y.; Zheng, G.; Zeng, J. Development of a Highly Sensitive Field-Amplified Capillary Electrophoresis Tandem Triple Quadrupole Mass Spectrometry for the Quantification of Trace Methamphetamine in Sewage. J. Chromatogr. A 2025, 1750, 465920. [Google Scholar] [CrossRef] [PubMed]
- Perkons, I.; Tomsone, L.E.; Sukajeva, V.; Neilands, R.; Kokina, K.; Pugajeva, I. Qualitative Fingerprinting of Psychoactive Pharmaceuticals, Illicit Drugs, and Related Human Metabolites in Wastewater: A Year-Long Study from Riga, Latvia. J. Environ. Chem. Eng. 2022, 10, 108110. [Google Scholar] [CrossRef]
- Hajeb, P.; Zhu, L.; Bossi, R.; Vorkamp, K. Sample Preparation Techniques for Suspect and Non-Target Screening of Emerging Contaminants. Chemosphere 2022, 287, 132306. [Google Scholar] [CrossRef]
- Kokosa, J.M.; Przyjazny, A. Green Microextraction Methodologies for Sample Preparations. Green Anal. Chem. 2022, 3, 100023. [Google Scholar] [CrossRef]
- Soares, S.; Rosado, T.; Barroso, M.; Gallardo, E. Solid Phase-Based Microextraction Techniques in Therapeutic Drug Monitoring. Pharmaceutics 2023, 15, 1055. [Google Scholar] [CrossRef]
- Psillakis, E.; Pedersen-Bjergaard, S.; Ozkan, S.A. Separation Science: The State of the Art: Analytical Chemistry: There Is No Green Like More Green. LCGC Europe 2022, 35, 438–439. [Google Scholar] [CrossRef]
- Barroso, M.; Moreno, I.; Da Fonseca, B.; Queiroz, J.A.; Gallardo, E. Role of Microextraction Sampling Procedures in Forensic Toxicology. Bioanalysis 2012, 4, 1805–1826. [Google Scholar] [CrossRef]
- Rosendo, L.M.; Brinca, A.T.; Pires, B.; Catarro, G.; Rosado, T.; Guiné, R.P.F.; Araújo, A.R.T.S.; Anjos, O.; Gallardo, E. Miniaturized Solid Phase Extraction Techniques Applied to Natural Products. Processes 2023, 11, 243. [Google Scholar] [CrossRef]
- Nascimento, M.M.; Nascimento, M.L.; dos Anjos, J.P.; Cunha, R.L.; da Rocha, G.O.; dos Santos, I.F.; Pereira, P.A.d.P.; de Andrade, J.B. A Green Method for the Determination of Illicit Drugs in Wastewater and Surface Waters-Based on a Semi-Automated Liquid-Liquid Microextraction Device. J. Chromatogr. A 2023, 1710, 464230. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Bartual, M.; Garrigues, S.; Esteve-Turrillas, F.A. Micro-Solid Phase Extraction of Pharmaceuticals and Illicit Drugs from Wastewaters Using Monolith-Coated Syringe Filters. Microchem. J. 2024, 200, 110484. [Google Scholar] [CrossRef]
- Ghorbani, M.; Mohammadi, P.; Keshavarzi, M.; Ziroohi, A.; Mohammadi, M.; Aghamohammadhasan, M.; Pakseresht, M. Developments of Microextraction (Extraction) Procedures for Sample Preparation of Antidepressants in Biological and Water Samples, a Review. Crit. Rev. Anal. Chem. 2023, 53, 1285–1312. [Google Scholar] [CrossRef]
- Zheng, J.; Li, S.; Wang, Y.; Li, L.; Su, C.; Liu, H.; Zhu, F.; Jiang, R.; Ouyang, G. In Situ Growth of IRMOF-3 Combined with Ionic Liquids to Prepare Solid-Phase Microextraction Fibers. Anal. Chim. Acta 2014, 829, 22–27. [Google Scholar] [CrossRef]
- Reyes-Garcés, N.; Gionfriddo, E.; Gómez-Ríos, G.A.; Alam, M.N.; Boyacl, E.; Bojko, B.; Singh, V.; Grandy, J.; Pawliszyn, J. Advances in Solid Phase Microextraction and Perspective on Future Directions. Anal. Chem. 2018, 90, 302–360. [Google Scholar] [CrossRef]
- Arthur, C.L.; Pawliszyn, J. Solid Phase Microextraction with Thermal Desorption Using Fused Silica Optical Fibers. Anal. Chem. 1990, 62, 2145–2148. [Google Scholar] [CrossRef]
- Pawliszyn, J. Theory of Solid-Phase Microextraction. J. Chromatogr. Sci. 2000, 38, 270–278. [Google Scholar] [CrossRef]
- Kabir, A.; Locatelli, M.; Ulusoy, H.I. Recent Trends in Microextraction Techniques Employed in Analytical and Bioanalytical Sample Preparation. Separations 2017, 4, 36. [Google Scholar] [CrossRef]
- Gonçalves, A.; Gallardo, E.; Barroso, M. Variations in Headspace Microextraction Procedures and Current Applications in Bioanalysis. Bioanalysis 2015, 7, 2235–2240. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, H. Current Developments and Future Trends in Solid-Phase Microextraction Techniques for Pharmaceutical and Biomedical Analyses. Anal. Sci. 2011, 27, 893–905. [Google Scholar] [CrossRef] [PubMed]
- Racamonde, I.; Villaverde-de-Sáa, E.; Rodil, R.; Quintana, J.B.; Cela, R. Determination of Δ9-Tetrahydrocannabinol and 11-nor-9-Carboxy-Δ9-Tetrahydrocannabinol in Water Samples by Solid-Phase Microextraction with on-Fiber Derivatization and Gas Chromatography-Mass Spectrometry. J. Chromatogr. A 2012, 1245, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Racamonde, I.; Rodil, R.; Quintana, J.B.; Cela, R. In-Sample Derivatization-Solid-Phase Microextraction of Amphetamines and Ecstasy Related Stimulants from Water and Urine. Anal. Chim. Acta 2013, 770, 75–84. [Google Scholar] [CrossRef]
- Bruheim, I.; Liu, X.; Pawliszyn, J. Thin-Film Microextraction. Anal. Chem. 2003, 75, 1002–1010. [Google Scholar] [CrossRef]
- Olcer, Y.A.; Tascon, M.; Eroglu, A.E.; Boyacı, E. Thin Film Microextraction: Towards Faster and More Sensitive Microextraction. TrAC—Trends Anal. Chem. 2019, 113, 93–101. [Google Scholar] [CrossRef]
- Chen, X.; Liu, S.; Jiang, R.; Luan, T.; Ouyang, G. Rapid Detection and Speciation of Illicit Drugs via a Thin-Film Microextraction Approach for Wastewater-Based Epidemiology Study. Sci. Total Environ. 2022, 842, 156888. [Google Scholar] [CrossRef]
- Hu, K.; Zhou, W.; Yang, C.; Wang, Y.; Jiang, R.W.; Zhang, Z.; Pawliszyn, J. Rapid Screening of Drugs in Environmental Water Using Metal Organic Framework/Ti3C2Tx Composite Coated Blade Spray-Mass Spectrometry. J. Hazard. Mater. 2024, 472, 134609. [Google Scholar] [CrossRef]
- Zhou, W.; Hu, K.; Wang, Y.; Jiang, R.W.; Pawliszyn, J. Embedding Mixed Sorbents in Binder: Solid-Phase Microextraction Coating with Wide Extraction Coverage and Its Application in Environmental Water Analysis. Environ. Sci. Technol. 2024, 58, 771–779. [Google Scholar] [CrossRef]
- Kataoka, H. In-Tube Solid-Phase Microextraction: Current Trends and Future Perspectives. J. Chromatogr. A 2021, 1636, 461787. [Google Scholar] [CrossRef]
- Zhao, L.; Qin, M.; Zheng, T.; Wu, G.; Lu, T. Carboxyl Hybrid Monolithic Column In-Tube Solid-Phase Microextraction Coupled with UPLC-QTRAP MS/MS for the Determination of Amphetamine-Type Stimulants. J. Chromatogr. A 2024, 1737, 465464. [Google Scholar] [CrossRef]
- Zhao, L.-Y.; Qin, M.; Wu, G.-P.; Zhou, Y.-T.; Zhu, J.-X.; Peng, H. Quantitative Determination of Amphetamine-Type Stimulants in Sewage and Urine by Hybrid Monolithic Column Solid-Phase Microextraction Coupled with UPLC-QTRAP MS/MS. Talanta 2024, 269, 125437. [Google Scholar] [CrossRef] [PubMed]
- Vuckovic, D. High-Throughput Solid-Phase Microextraction in Multi-Well-Plate Format. TrAC—Trends Anal. Chem. 2013, 45, 136–153. [Google Scholar] [CrossRef]
- Hamidi, S.; Taghvimi, A.; Mazouchi, N. Micro Solid Phase Extraction Using Novel Adsorbents. Crit. Rev. Anal. Chem. 2021, 51, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Płotka-Wasylka, J.; Szczepańska, N.; de la Guardia, M.; Namieśnik, J. Miniaturized Solid-Phase Extraction Techniques. TrAC—Trends Anal. Chem. 2015, 73, 19–38. [Google Scholar] [CrossRef]
- Naing, N.N.; Tan, S.C.; Lee, H.K. Micro-Solid-Phase Extraction. In Solid-Phase Extraction; Poole, C., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 443–471. ISBN 9780128169070. [Google Scholar]
- Spietelun, A.; Marcinkowski, Ł.; de la Guardia, M.; Namieśnik, J. Recent Developments and Future Trends in Solid Phase Microextraction Techniques towards Green Analytical Chemistry. J. Chromatogr. A 2013, 1321, 1–13. [Google Scholar] [CrossRef]
- Baz-Lomba, J.A.; Löve, A.S.C.; Reid, M.J.; Ólafsdóttir, K.; Thomas, K.V. A High-Throughput Solid-Phase Microextraction and Post-Loop Mixing Large Volume Injection Method for Water Samples. J. Chromatogr. A 2018, 1531, 32–38. [Google Scholar] [CrossRef]
- Šafaříková, M.; Šafařík, I. Magnetic Solid-Phase Extraction. J. Magn. Magn. Mater. 1999, 194, 108–112. [Google Scholar] [CrossRef]
- Capriotti, A.L.; Cavaliere, C.; La Barbera, G.; Montone, C.M.; Piovesana, S.; Laganà, A. Recent Applications of Magnetic Solid-Phase Extraction for Sample Preparation. Chromatographia 2019, 82, 1251–1274. [Google Scholar] [CrossRef]
- Ansari, S. Application of Magnetic Molecularly Imprinted Polymer as a Versatile and Highly Selective Tool in Food and Environmental Analysis: Recent Developments and Trends. TrAC—Trends Anal. Chem. 2017, 90, 89–106. [Google Scholar] [CrossRef]
- Maya, F.; Cabello, C.P.; Frizzarin, R.M.; Estela, J.M.; Palomino, G.T.; Cerdà, V. Magnetic Solid-Phase Extraction Using Metal-Organic Frameworks (MOFs) and Their Derived Carbons. TrAC—Trends Anal. Chem. 2017, 90, 142–152. [Google Scholar] [CrossRef]
- Zhang, S.; Hua, Z.; Yao, W.; Ting, L.; Zhang, D.; Zhao, H. Utilization of Magnetic Pomelo Peel-Derived Biochar for Extraction and Liquid Chromatography-Mass Spectrometry Determination of Opioid Drugs in Wastewaters. J. Sep. Sci. 2022, 45, 4099–4106. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Qie, Y.; Hua, Z.; Zhang, H.; Wang, Y.; Di, B.; Su, M. Preparation of Poly(Methacrylic Acid-Co-Ethylene Glycol Dimethacrylate)-Functionalized Magnetic Polydopamine Nanoparticles for the Extraction of Six Cannabinoids in Wastewater Followed by UHPLC-MS/MS. Talanta 2023, 264, 124752. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Dong, T.; Jiang, X.; Li, J.; Di, B.; Yan, F. Preparation of Polydopamine-Functionalized Mesoporous Silica-Coated Core/Shell Magnetic Nanocomposite for Efficiently Extracting Five Amphetamine-Type Stimulants from Wastewater Followed by UPLC-MS/MS Determination. J. Hazard. Mater. 2022, 426, 128082. [Google Scholar] [CrossRef]
- Ning, H.; Fan, Y.; Chen, H.; Liu, H.; Huang, Z.; Ke, X.; Xu, Y.; She, Y. Preparation of Mixed-Mode Weak Cation Exchange Magnetic Solid-Phase Extraction Sorbent and Its Application in the Extraction of 21 Illicit Drugs from Wastewater. J. Hazard. Mater. 2024, 464, 133007. [Google Scholar] [CrossRef]
- Li, X.; Jiang, L.; Di, B.; Hu, C. Preparation of Amphiphilic Poly(Divinylbenzene-Co-N-Vinylpyrrolidone)-Functionalized Polydopamine Magnetic Nanoadsorbents for Enrichment of Synthetic Cannabinoids in Wastewater. Anal. Methods 2024, 16, 3968–3982. [Google Scholar] [CrossRef]
- Fontanals, N.; Marcé, R.M.; Borrull, F. Materials for Solid-Phase Extraction of Organic Compounds. Separations 2019, 6, 56. [Google Scholar] [CrossRef]
- Cao, R.; Chen, J.; Pang, N.; Li, S.; Chen, M.; Di, B.; Xiao, D. Simultaneous Enrichment and Ultra-High Sensitivity Detection of Multi-Structural Synthetic Cannabinoids in Large-Volume Wastewater. Microchim. Acta 2025, 192, 279. [Google Scholar] [CrossRef]
- Zhang, S.; Cui, H.R.; Zhao, Y.G.; Zhan, P.P. Preparation and Application of Nano Petal-Shaped Covalent Organic Frameworks Modified Polystyrene-Divinylbenzene-Glycidylmethacrylate Microspheres for the Extraction of Illicit Drugs from Wastewater. J. Chromatogr. A 2022, 1682, 463505. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, S.; Tian, J.; You, J.; Liu, Z.; Chen, Z. A Magnetic Solid Phase Extraction Based on DES/ZIF-MGO Coupled with UPLC-MS/MS for the Simultaneous Detection and Consumption Evaluation of Four Illicit Drugs. Microchem. J. 2024, 200, 110448. [Google Scholar] [CrossRef]
- Yang, F.; Ma, K.; Cao, Y.; Li, Z. Application of Magnetic Materials Combined with Echo® Mass Spectrometry System in Analysis of Illegal Drugs in Sewage. Molecules 2024, 29, 2060. [Google Scholar] [CrossRef]
- Yuan, S.; Xiang, Y.; Chen, L.; Xiang, P.; Li, Y. Magnetic Solid-Phase Extraction Based on Polydopamine-Coated Magnetic Nanoparticles for Rapid and Sensitive Analysis of Eleven Illicit Drugs and Metabolites in Wastewater with the Aid of UHPLC-MS/MS. J. Chromatogr. A 2024, 1718, 464703. [Google Scholar] [CrossRef]
- Zhu, R.; Cao, S.; Su, H.; Ming, D.; Tang, Y.; Chen, Z. Efficient Magnetic Solid-Phase Extraction, UPLC-MS/MS Detection, and Consumption Assessment of Five Trace Psychoactive Substances. Environ. Sci. Pollut. Res. 2024, 31, 31455–31466. [Google Scholar] [CrossRef]
- Zhou, J.; Ning, H.; Wang, H.; Zhao, S.; Xu, Y.; Fan, Y.; Huang, Z.; Gao, J. Determination of Four Cannabinoids in Wastewater Based on a Hyperbranched Mixed-Mode Anion Exchange Magnetic Solid-Phase Extraction Adsorbent Combined with UHPLC-MS/MS. Microchem. J. 2025, 210, 112960. [Google Scholar] [CrossRef]
- He, C.; Long, Y.; Pan, J.; Li, K.; Liu, F. Application of Molecularly Imprinted Polymers to Solid-Phase Extraction of Analytes from Real Samples. J. Biochem. Biophys. Methods 2007, 70, 133–150. [Google Scholar] [CrossRef]
- Belbruno, J.J. Molecularly Imprinted Polymers. Chem. Rev. 2019, 119, 94–119. [Google Scholar] [CrossRef]
- Tamayo, F.G.; Turiel, E.; Martín-Esteban, A. Molecularly Imprinted Polymers for Solid-Phase Extraction and Solid-Phase Microextraction: Recent Developments and Future Trends. J. Chromatogr. A 2007, 1152, 32–40. [Google Scholar] [CrossRef]
- Turiel, E.; Martín-Esteban, A. Molecularly Imprinted Polymers-Based Microextraction Techniques. TrAC—Trends Anal. Chem. 2019, 118, 574–586. [Google Scholar] [CrossRef]
- González-Mariño, I.; Quintana, J.B.; Rodríguez, I.; Rodil, R.; González-Peñas, J.; Cela, R. Comparison of Molecularly Imprinted, Mixed-Mode and Hydrophilic Balance Sorbents Performance in the Solid-Phase Extraction of Amphetamine Drugs from Wastewater Samples for Liquid Chromatography-Tandem Mass Spectrometry Determination. J. Chromatogr. A 2009, 1216, 8435–8441. [Google Scholar] [CrossRef]
- Xiong, J.; Wei, X.; Shen, X.; Zhu, W.; Yi, S.; Huang, C. Synthesis of Molecularly-Imprinted Polymers towards a Group of Amphetamine-Type Stimulants by Reflux Precipitation Polymerization with a Pseudo Template. J. Chromatogr. A 2023, 1688, 463738. [Google Scholar] [CrossRef]
- Sorribes-Soriano, A.; Arráez-González, R.; Esteve-Turrillas, F.A.; Armenta, S.; Herrero-Martínez, J.M. Development of a Molecularly Imprinted Monolithic Polymer Disk for Agitation-Extraction of Ecgonine Methyl Ester from Environmental Water. Talanta 2019, 199, 388–395. [Google Scholar] [CrossRef]
- El-Akaad, S.; De Saeger, S.; Beloglazova, N. Molecularly Imprinted Polymer Based Capacitive Sensing of a Specific Leuckart Marker 4-Methyl-5-Phenylpyrimidine in Wastewater. Sens. Actuators B Chem. 2021, 343, 130116. [Google Scholar] [CrossRef]
- Bujak, R.; Gadzała-Kopciuch, R.; Nowaczyk, A.; Raczak-Gutknecht, J.; Kordalewska, M.; Struck-Lewicka, W.; Markuszewski, M.J.; Buszewski, B. Selective Determination of Cocaine and Its Metabolite Benzoylecgonine in Environmental Samples by Newly Developed Sorbent Materials. Talanta 2016, 146, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Tan, D.; Wang, Y.; Yu, Z.; Sun, X.; Wang, D. Selective Extraction of Synthetic Cathinones New Psychoactive Substances from Wastewater, Urine and Cocktail Using Dummy Molecularly Imprinted Polymers. J. Pharm. Biomed. Anal. 2022, 215, 114765. [Google Scholar] [CrossRef]



| Sample (mL) | Analytes | Mode | Properties | Conditions | Instrument | LOD | Recovery | References |
|---|---|---|---|---|---|---|---|---|
| Wastewater (10) | THC THC-COOH | DI-SPME | DVB-CAR-PDMS fibre | Extraction: 60 min at 60 °C Derivatization with 50 µL of MSTFA (headspace for 10 min at 40 °C) Desorption: 3 min at 250 °C | GC-MS | 1.0 and 2.5 ng/L | 104 and 112% | [66] |
| Wastewater (100) | Amphetamine Methamphetamine MDA MDMA MDEA | DI-SPME | PDMS-DVB fibre | Extraction: 40 min at 60 °C Desorption: 3 min at 250 °C | GC-MS | 0.4–2 ng/L | 98–111% | [67] |
| Wastewater (19) | Methamphetamine Ketamine | TFME | DVB-PDMS membrane | Extraction: sample was stirred at 200 rpm for 120 min at room temperature Desorption: initially at 50 °C, ramped to 250 °C (700 °C/min) and held for 5 min | TDU-GC-MS | 5.5 and 2.0 ng/L | 95–111% | [70] |
| Wastewater (1.5) | Amphetamine Methamphetamine Codeine Heroin Morphine Fentanyl | Coated Blade SPME | Cu-TCPP/Ti3C2Tx blades | Preconditioning: 15 min in 1.5 mL of methanol/water (50:50, v/v) Extraction: 20 min Wash: 5 s in 1.5 mL of Milli-Q water Desorption: 8 µL of MeOH:water (95:5, v/v) + 0.1% FA and MS analysis after 18 s | CBS-MS/MS | 1.5–9.0 ng/L | 70.7–115.6% | [71] |
| Environmental water (1.5) | Amphetamine BE Cocaine Codeine Fentanyl Heroin LSD MDMA Methamphetamine Morphine | Coated Blade SPME | HLB + HLB-WCX + HLB-WAX with a PAN binder blades | Preconditioning: 30 min in 1.5 mL of MeOH:water (50:50, v/v) Extraction: 30 min Wash: 5 s in 1.5 mL of Milli-Q water Desorption: 10 min in 300 µL of 10 mM NH4Ac + ACN:MeOH:water (3:3:4, v/v/v) | LC-HRMS and LC-MS/MS | 0.002–0.020 ng/mL | 67.4–134.2% | [72] |
| Sewage (2.0) | Amphetamine Methamphetamine Cathinone Methcathinone MDA MDMA MDEA | IT-SPME | TMOS-co-CES hybrid monolithic column sorbent | Wash: 150 µL of water Elution: 200 µL of 0.1% FA + MeOH (1:1, v/v) Extraction flow rate: 150 µL/min | UHPLC-QTRAP MS/MS | 0.01–0.02 ng/mL | 86.1–114% | [74] |
| Sewage (1.5) | Amphetamine Methamphetamine Cathinone Methcathinone MDA MDMA MDEA | SPDE | Thiol hybrid monolithic column sorbent | Wash: 150 µL of water Elution: 150 µL of 0.1% FA + MeOH (1:1, v/v) Extraction flow rate: 150 µL/min | UHPLC-QTRAP MS/MS | 0.01–0.02 ng/mL | 85.4–114% | [75] |
| Sample (mL) | Analytes | µSPE Properties | Conditions | Instrument | LOD | Recovery | References |
|---|---|---|---|---|---|---|---|
| Wastewater (5) | Amphetamine Methamphetamine MDMA Cocaine BE Cocaethylene | HLB µElution plates, 30 µm | Conditioning: 1 mL of MeOH and 1 mL of ultrapure water Washing: 1 mL of ultrapure water Vacuum drying for 15 min Elution: 50 µL of 1% NH4OH in MeOH, 100 µL of MeOH and 50 µL of 1% FA in MeOH (200 µL extract) | PLM-LVI- UHPLC-MS/MS | 1.0–6.3 ng/L * | 92–110% | [81] |
| Wastewater (25) | Amphetamine Methamphetamine 2-Fluoroamphetamine 2-Fluoromethamphetamine Fenproporex Methylone 6-AM 3-MeO-PCE Deschloroketamine MDMA Butylone Ketamine α-PVP Pentylone PCP Cocaine Fenethylline α-PHP LSD Fentanyl 3-MeO-PCP | poly(MAA-co-EGDMA) monolith immobilised on a nylon membrane | Conditioning: 2 mL of MeOH (0.1% FA) and 2 mL deionised water Washing: 2 mL of deionised water Vacuum drying for 5 min Elution: 200 µL of MeOH (0.1% FA) | LC-MS/MS | 4–19 ng/L | 88–119% | [57] |
| Sample (mL) | Analytes | Magnetic Sorbent | MSPE Conditions | Instrument | LOD | Recovery | References |
|---|---|---|---|---|---|---|---|
| Wastewater (20) | Morphine Codeine 6-AM | Magnetic pomelo peel-derived biochar | 15 mg of adsorbent; Mixing for 14 min at 200 rpm; External magnet and supernatant decanted; 200 µL of MeOH and vortex for 4 min at 14,000 rpm; 0.22-µm membrane filtering. | LC-MS | 0.006–0.010 µg/L | 71.6–84.4% | [86] |
| Wastewater (50) | Amphetamine Methamphetamine Methcathinone MDMA MDA | Fe3O4@nSiO2@mSiO2@PDA | 50 mg of adsorbent; Mixing in 5 M NaOH solution for 15 min at room temperature in a PET bottle; External magnet and supernatant discarding; 5 mL of washing solution and vortex for 30 s; External magnet and supernatant discarding; 5 mL of desorption solution (95% ACN and 5% FA, v/v) and vortex for 3 min; Extracts drying at 50 °C under N2; Reconstitution with 100 µL water (0.1% FA). | UHPLC-MS/MS | 0.5–2.5 ng/L | 95.1–106.6% | [88] |
| Wastewater (100) | Amphetamine Codeine Morphine Ketamine Methamphetamine Cocaine 6-AM MDMA BE Norketamine MDA | NP-COF@Mag-PS/DVB/GMA | 30 mg of adsorbent; Mixing for 5 min; Magnetic field for 30 s; 2 mL of washing solution: MeOH:water (1:9, v/v); 1.5 mL of desorption solution: 95% MeOH:Ammonia (95:5, v/v); Extracts drying; Reconstitution with 1 mL mobile phase (water with 0.1% FA). | LC-MS/MS | 0.12–1.47 ng/L | 81.6–106% | [93] |
| Wastewater (100) | 5 F-EDMB-PINACA FUB-APINACA MDMB-4en-PINACA MDMB-FUBINACA PB-22 THC-COOH | Fe3O4@PDA@poly (MAA-co-EGDMA) | Prior filtration using a glass fibre filter membrane; pH adjustment to 2 by hydrochloric acid or sodium hydroxide in a PET bottle; Mixing for 10 min; External magnet and supernatant discarding; Elution with 200 µL of ACN and vortex for 1 min; Magnetic separation, centrifugation and mix with equal volume of deionised water. | UHPLC-MS/MS | 0.1–1.0 ng/L * | 64.01–124.0% | [87] |
| Wastewater (200) | Morphine 6-AM MDMA MDA Ketamine Norketamine Methamphetamine Amphetamine 4-Methylcathinone Methcathinone Cocaine BE Cotinine Codeine PMMA 4-ANPP Norfentayl NAFN Fentanyl | Fe3O4 @poly(ST/DVB/MA-COOH) | 50 mg of sorbent; Adsorption for 1 min; External magnet and supernatant discarding; Washing with 3 mL of ACN and vortex for 30 s; Elution with 4 mL of 4% TFA/MeOH and N2 drying at room temperature; Reconstitution with 200 µL of 0.2% HCOOH/MeOH and filtration with 0.22 µm organic phase membrane. | UHPLC-MS/MS | 0.03–0.67 ng/L | 93.4–118.0% | [89] |
| Wastewater (100) | Amphetamine Methamphetamine Methcathinone MDMA | DES/ZIF-MGO | 5 mg of adsorbent; Mixing for 30 min at 180 rpm; External magnet and supernatant discarding; Elution with 5 mL of MeOH:Ammonia (95:5, v/v) and mixing for 10 min; Magnetic separation and N2 drying at 50 °C; Reconstitution with 100 µL of mobile phase (2 mmol/L ammonium format with 0.1% FA) and filtration with 0.22 µm micropore membrane. | UHPLC-MS/MS | 0.02–1.55 µg/L | 92.1–100.9% | [94] |
| Wastewater (50) | Amphetamine Methamphetamine 6-AM Morphine Ketamine Norketamine Cocaine BE MDA MDMA Cathinone Methcathinone Fentanyl | Fe3O4@SiO2-MA@PLS | 20 mg of adsorbent; Sonication for 1 min and vortex for 10 min at room temperature; External magnet for 60 s and supernatant discarding; Elution with 3 mL of ACN by ultrasonic washing for 5 min and N2 drying at 60 °C; Reconstitution with 200 µL of MeOH:Water (2:8, v/v) and filtration through 0.22 µm filter membrane. | MS system | 1–2 ng/mL * | 44–100% | [95] |
| Wastewater (0.99) | Methamphetamine Amphetamine MDMA MDA Morphine 6-AM Codeine Cocaine BE Ketamine Norketamine | Fe3O4@PDA | 10 mg of adsorbent; Sonication of the adsorbent with 1 mL of MeOH for 5 min; MeOH discarding by magnetic separation; Sample introduction and vortex for 4 min at 1400 rpm; Magnetic rack and supernatant discarding; Washing with 1 mL of deionised water, vortexing for 2 min at 1400 rpm and supernatant discarding; Elution with 1 mL of MeOH:ACN (1:1, v/v) and vortex for 2 min at 1400 rpm; Drying under a N2 flow at 50 °C, reconstitution with 500 µL of deionised water and filtration through a PTFE membrane. | UHPLC-MS/MS | 2–5 ng/L | 27.81–98.29% | [96] |
| Wastewater (100) | Amphetamine Methamphetamine MDMA Methcathinone Mephedrone | DZMBC | Samples previous filtration with a 0.22 µm aqueous membrane; 5 mg of adsorbent; Mixing for 40 min at 25 °C under 155 rpm; External magnet and supernatant discarding; Desorption with 3 mL with MeOH (1% FA) for 20 min; Drying under N2 at 60 °C, reconstitution with 0.5 mL of ultrapure water and filtration through 0.22 µm film. | UHPLC-MS/MS | 1.0–4.75 ng/L | 96.2–106.1% | [97] |
| Wastewater (50) | MDMB-FUBINACA 4CN-CUMYL-BUTINACA 5F-MDMB-PICA MDMB-4en-PINACA ADB-4en-PINACA 5F-EMB-PICA AMB-FUBINACA ADB-BUTINACA 4F-MDMB-BUTICA | Fe3O4@PDA@poly(DVB-co-NVP) | pH adjustment to 9 by sodium hydroxide; 10 mg of adsorbent; Ultra-sound assisted extraction for 15 min; External magnet and supernatant discarding; Elution with 1 mL of 1% ammonia MeOH for 1 min; Drying under N2 and reconstitution with 100 µL mobile phase (0.1% FA aqueous solution) and centrifugation at 15,000 rpm for 5 min. | UHPLC-MS/MS | 0.01–1.0 ng/L * | 69.63–107.38% | [90] |
| Wastewater (300) | 5F-BZO-POXIZID Ethylphehethyl-FUBINCA BIM-018 4CN-CUMYL-BUTCZCA CH-FUBIATA CUMYL-NBMICA JWH-019 RCS-4 ACHMINACA BZO-HEXOXIZID BIM-2201 JWH-249 JWH-307 MDMB-CHMCZCA AFUB7AICA JWH-370 AB-001 JWH-030 | GO-Fe3O4 with an ionic liquid (ILs-GO-Fe3O4) | Samples previous filtration with a 1.2 µm glass filter membrane; 20 mg of adsorbent with 1 mL of ionic liquid solution (20 mg/mL); Ultrasonication for 20 s and mechanical mixing for 30 min; External magnet while stirring and supernatant discarding; Elution with 5 mL of ACN by vortexing for 5 min and magnetic separation; Drying under N2 and reconstitution with 100 µL of mobile phase (0.1% FA in water). | UHPLC-MS/MS | 10 pg/L | 72.6–97.8% | [92] |
| Wastewater (200) | THC CBD CBN THC-COOH | Fe3O4@poly(GMA/DVB-WAX) | pH adjustment to 7; 30 mg of adsorbent; 5 min of extraction; External magnet and supernatant discarding; Washing with 3 mL of 5% MeOH/Water and vortex for 30 s; Elution with 4 mL of 8% HCOOH/MeOH and drying under N2; Reconstitution with 200 µL of 0.2% HCOOH/MeOH and filtration through a 0.22 µm hydrophilic syringe filter. | UHPLC-MS/MS | 0.17–0.33 ng/L | 69.4–94.0% | [98] |
| Sample (mL) | Analytes | MIP Sorbent | Conditions | Instrument | LOD | Recovery | References |
|---|---|---|---|---|---|---|---|
| Wastewater (50) | Amphetamine Methamphetamine MDA MDMA MDEA | Commercial amphetamine class-selective MIP (25 mg) | Conditioning: 1 mL of MeOH and 1 mL of pH 8 Milli-Q water; Washing: 1 mL of pH 8 Milli-Q water, 1 mL of ACN/Water (6:4, v/v) and 1 mL of ACN (1% AA) in duplicate; Elution: 1 mL of MeOH (1% FA) in duplicate Drying under N2; Reconstitution: 100 µL of MeOH/Water (1:1, v/v) with 2% of NH3. | LC-MS/MS | 0.5–2.7 ng/L | 91.6–113.9% | [103] |
| Wastewater (100) | Cocaine BE | Scopolamine MIP with TFMAA (200 mg) | Conditioning: 3 mL of MeOH and 3 mL of water; Elution: 4 mL of MeOH/Ammonia (19:1, v/v); Drying under vacuum; Reconstitution with mobile phase (0.1% AA in deionised water). | HPLC-TOF-MS | N.P. | 83.6 and 72.1% | [107] |
| Wastewater (200) | EME | PTFE discs containing EME MIP with MAA | MIP-disc conditioning with 200 mL of deionised water for 5 min with magnetic stirrer mixing; Samples pH adjustment to 10 by 10 mL of carbonate buffer 0.1 M; MIP-disc introduction and mixing for 30 min; Washing: 50 mL deionised water for 1 min and drying with paper tissue; Elution: submerging the MIP-disk in 5 mL of MeOH (1% AA) for 30 min. | IMS and UHPLC-MS/MS | 75 and 13 ng/L | 100% | [105] |
| Wastewater (500) | 4M5PP | 4M5PP MIP with MAA and 2-VP on a gold electrode surface | Sample filtration through 0.22 µm filter membrane; | Electrochemical Sensor | 80µM | 93.3–101% | [106] |
| Wastewater (10) | Cathinone Methcathinone Mephedrone Methylone Ethylone MDPV | DMIP (200 mg) | Conditioning: 3 mL of MeOH and 3 mL of deionised water; Washing: 3 mL of deionised water; Drying under negative pressure; Rising with 3 mL of ACN and elution with 6 mL of MeOH (1% FA); Extracts drying with rotary evaporator and reconstitution with 500 µL of MeOH/water (50:50, v/v); Filtration through 0.22 µm membrane. | HPLC-MS/MS | 0.002–0.1 ng/mL | 84.1–97.7% | [108] |
| Wastewater (10) | Amphetamine Methamphetamine MDMA | MPEA-MIPs (50 mg) | Conditioning: 500 µL of 25 mM citrate buffer/ACN (50/50, v/v) Washing: 500 µL of ACN/Distilled water (3:7, v/v); Elution: 400 µL of a solution with 15% AA, 35% water and 50% MeOH; 50 µL of MeOH was added to 50 µL of the extract. | LC-MS/MS | 0.05–0.29 µg/L | 96–97% | [104] |
| Technique | Extraction Time | Solvent Consumption | Cost per Sample | Reusability | Advantages and Limitations |
|---|---|---|---|---|---|
| SPME | 30–60 min | None/Minimal | Low | Moderate (typically reusable for up to ~100 uses depending on matrix complexity and handling conditions) | Solvent-free; easy automation Limited for highly polar compounds |
| TFME | 20–40 min | None | Low | High | High sensitivity; large surface area Less robust; specialised setup |
| µSPE | 10–30 min | Low | Low | Low | Simple, fast, LC-MS/MS compatible Limited reuse; polymer activation needed |
| MSPE | 10–20 min | Minimal | Moderate | High | High selectivity; nanomaterials adaptable Nanoparticle synthesis complexity |
| MIPs | 30–60 min | Low | Moderate | High | Highly selective; reusablePolymer synthesis and validation time |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dinis, P.; Gallardo, E.; Margalho, C. Recent Trends in Solid-Phase Microextraction for the Monitoring of Drugs of Abuse in Wastewater. Separations 2025, 12, 256. https://doi.org/10.3390/separations12090256
Dinis P, Gallardo E, Margalho C. Recent Trends in Solid-Phase Microextraction for the Monitoring of Drugs of Abuse in Wastewater. Separations. 2025; 12(9):256. https://doi.org/10.3390/separations12090256
Chicago/Turabian StyleDinis, Pedro, Eugenia Gallardo, and Cláudia Margalho. 2025. "Recent Trends in Solid-Phase Microextraction for the Monitoring of Drugs of Abuse in Wastewater" Separations 12, no. 9: 256. https://doi.org/10.3390/separations12090256
APA StyleDinis, P., Gallardo, E., & Margalho, C. (2025). Recent Trends in Solid-Phase Microextraction for the Monitoring of Drugs of Abuse in Wastewater. Separations, 12(9), 256. https://doi.org/10.3390/separations12090256








