Development of an Efficient HPLC-MS/MS Method for the Detection of a Broad Spectrum of Hydrophilic and Lipophilic Contaminants in Marine Waters: An Experimental Design Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Instrumental Analysis
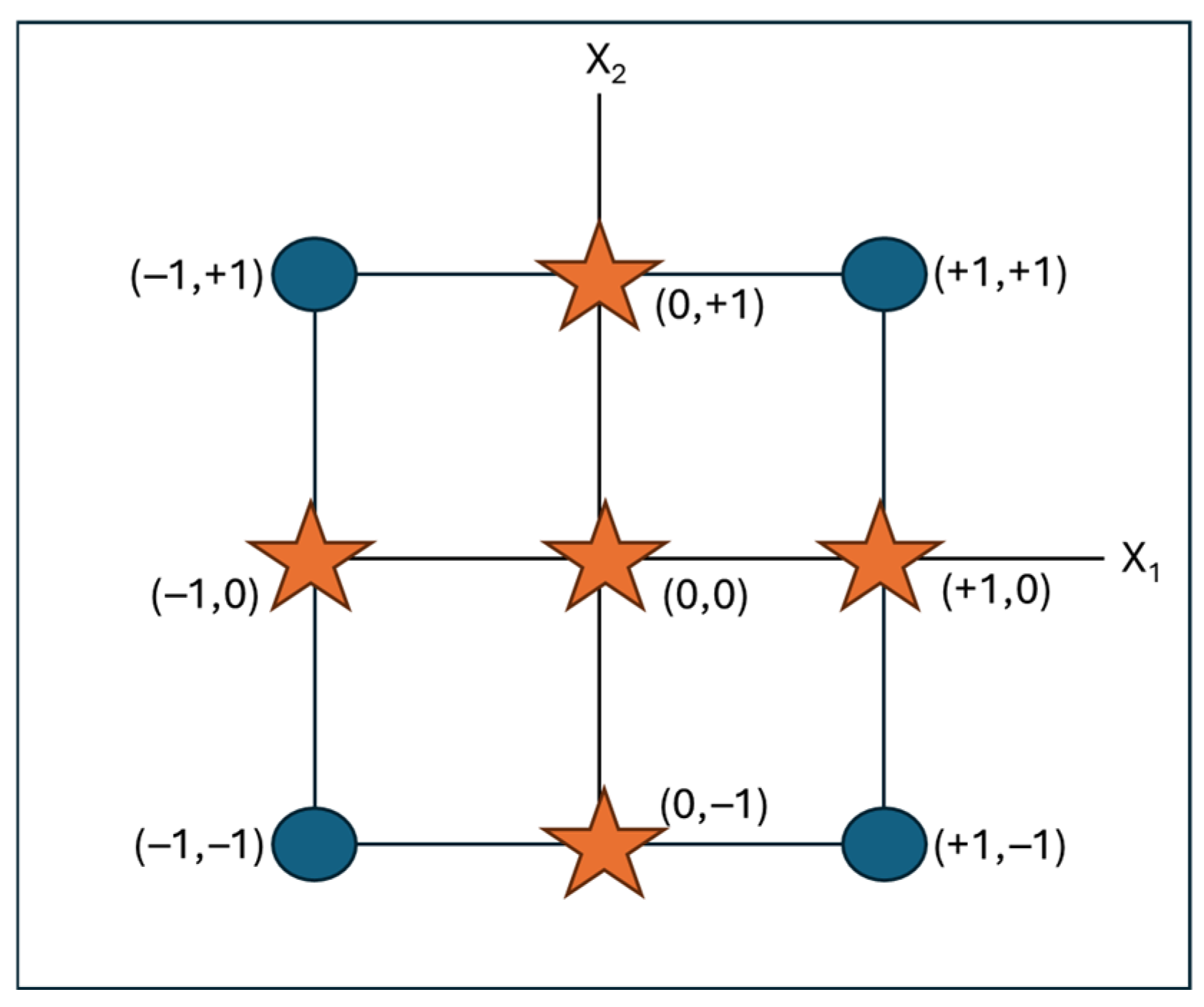
2.3. Design of Experiments: Face-Centered Design
2.4. Real Samples’ Processing and Sampling Sites
2.5. Method Performances
3. Results and Discussion
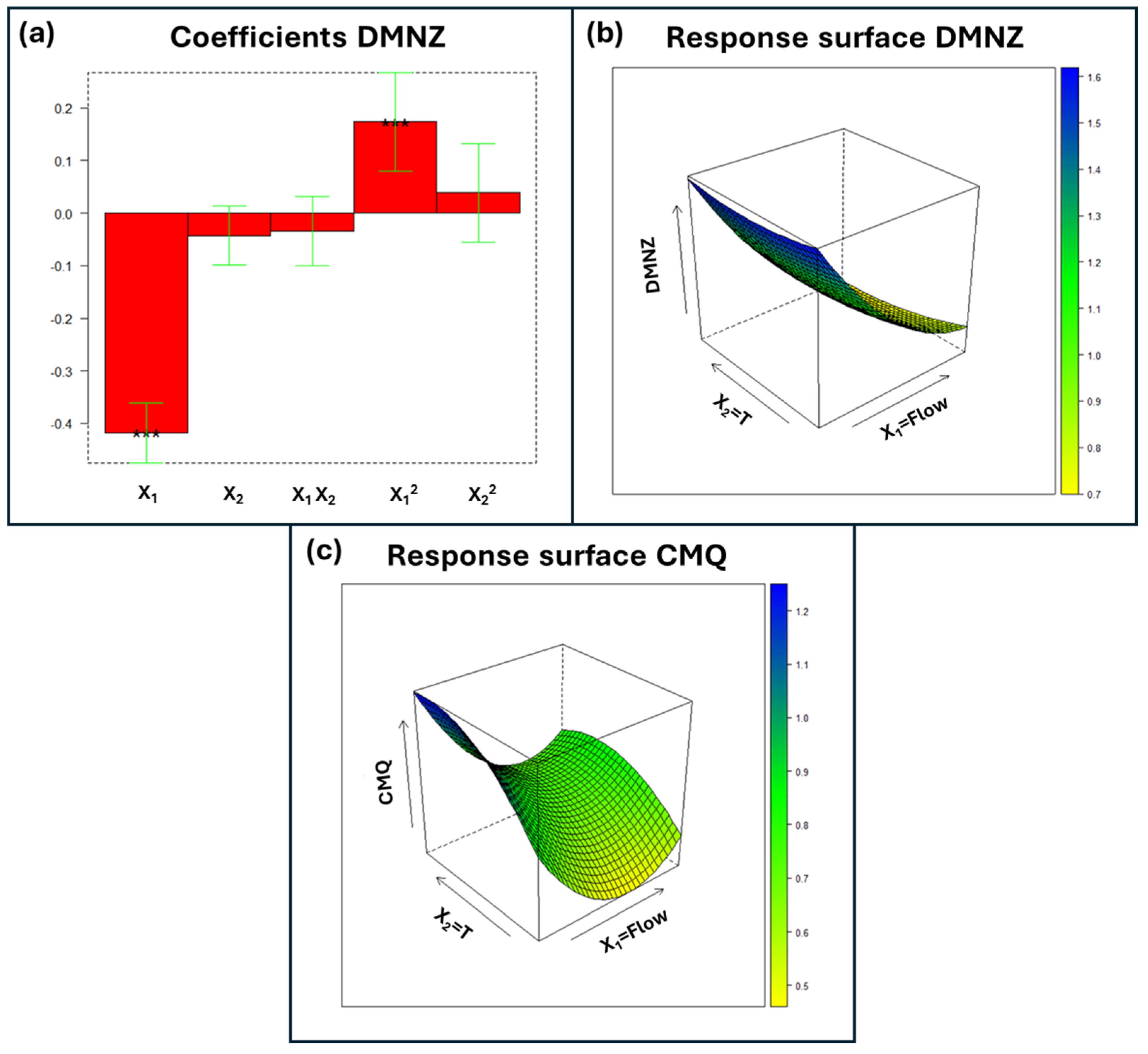
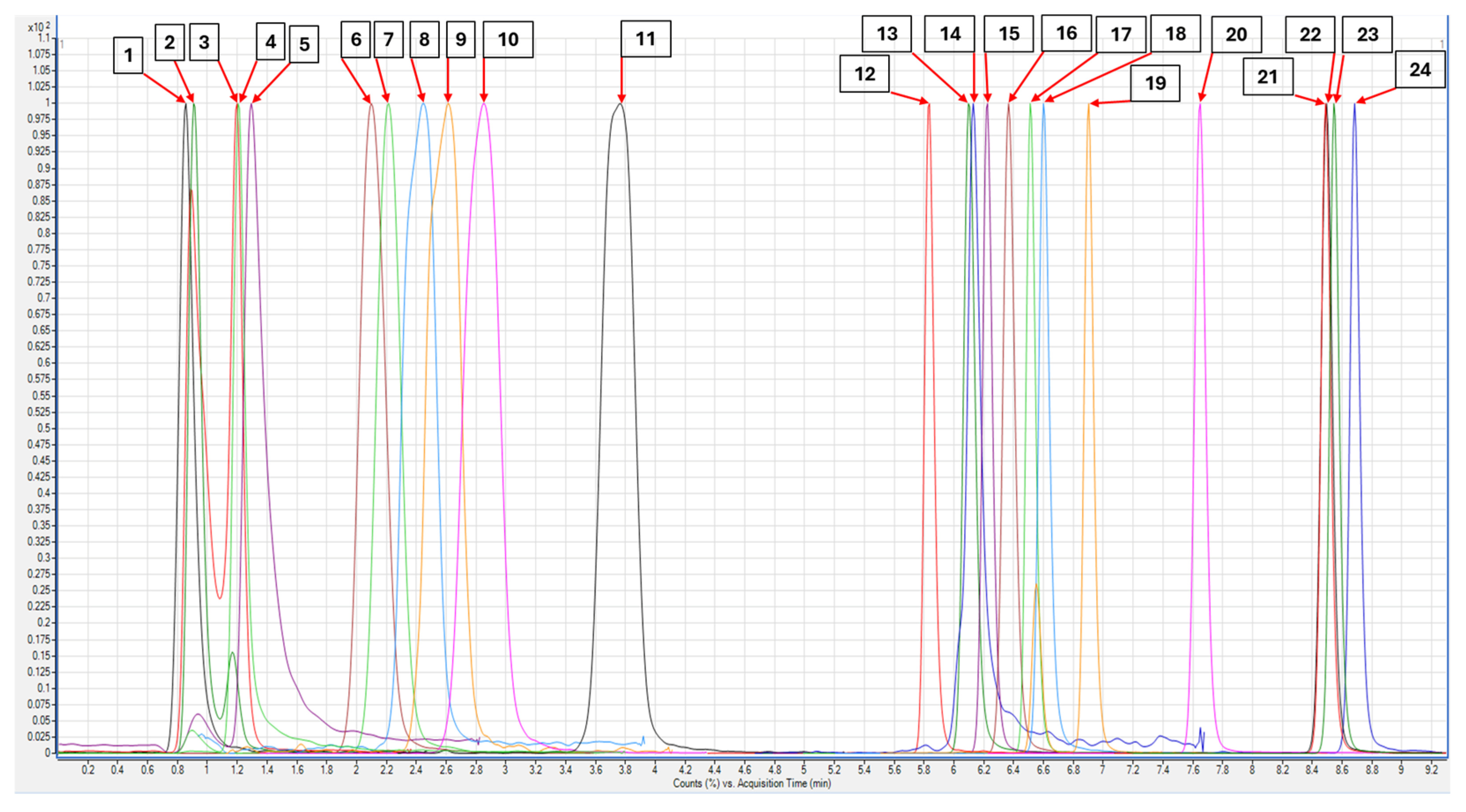
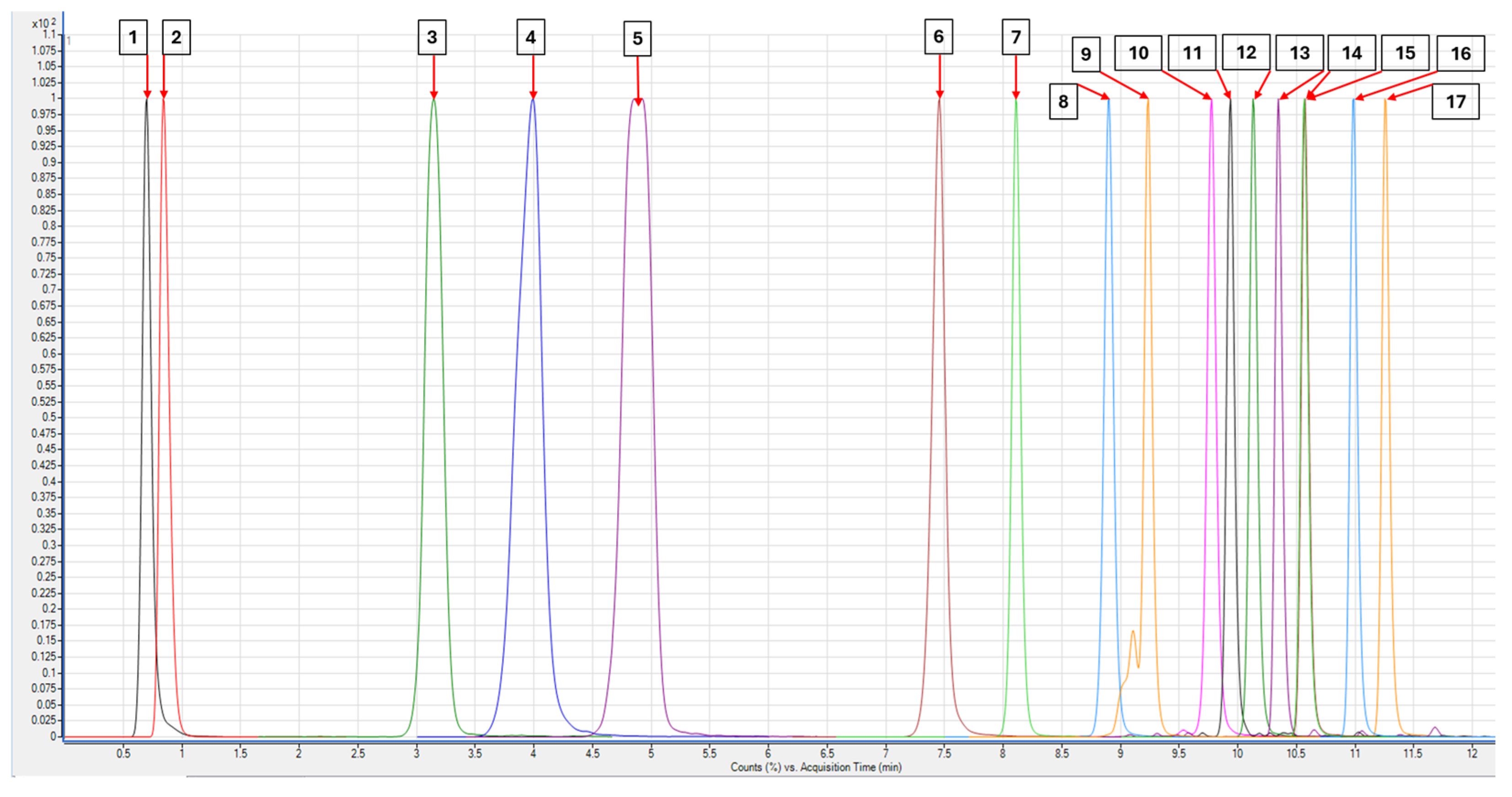
3.1. Multivariate Optimization of Chromatographic Separation
Modeling and Final Method Development
3.2. Method Performances: Results
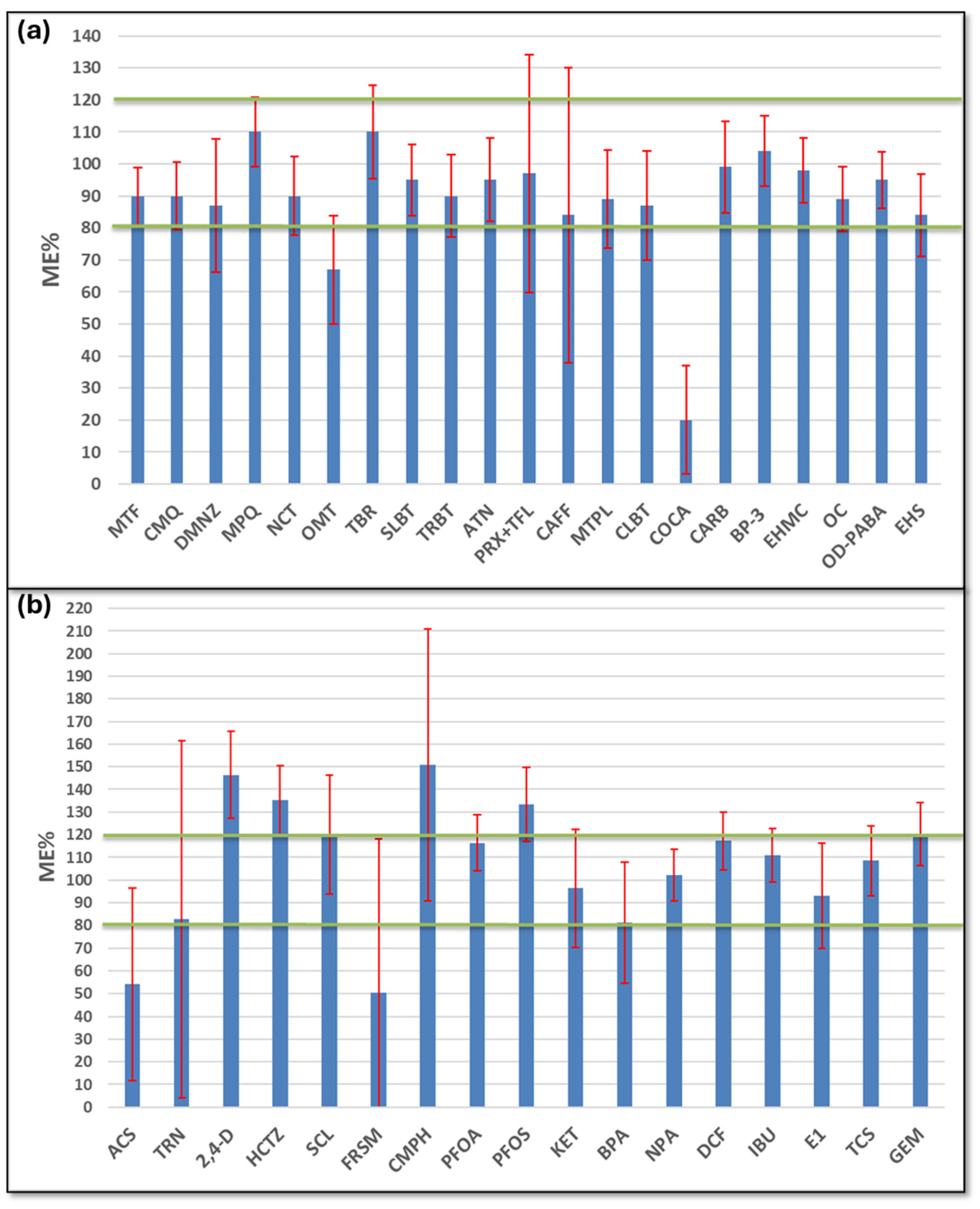
3.3. Matrix Effect in the Analyzed Samples
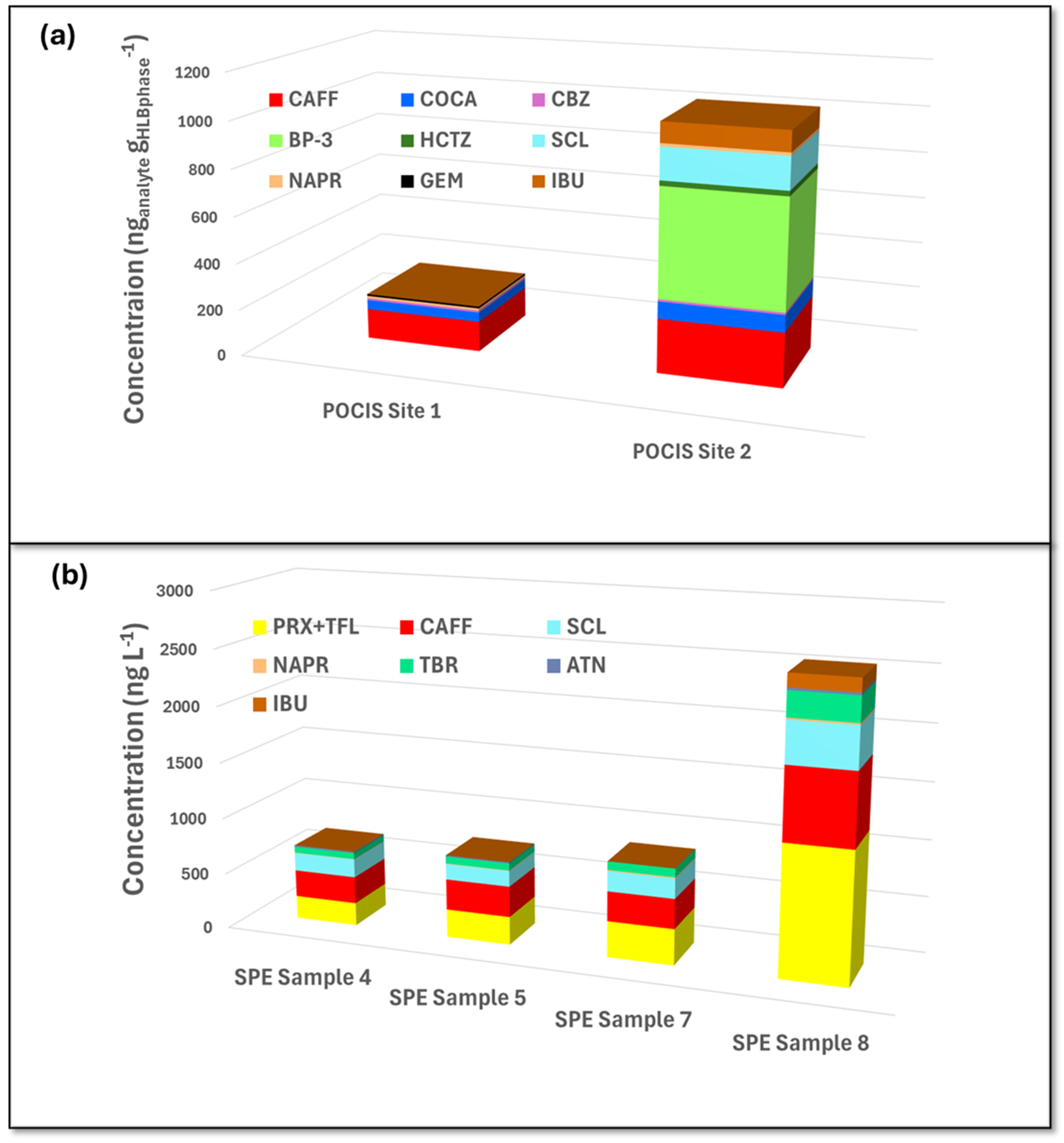
3.4. Method Application to Real Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Verlicchi, P.; Al Aukidy, M.; Zambello, E. Occurrence of Pharmaceutical Compounds in Urban Wastewater: Removal, Mass Load and Environmental Risk after a Secondary Treatment—A Review. Sci. Total Environ. 2012, 429, 123–155. [Google Scholar] [CrossRef]
- Li, X.; Shen, X.; Jiang, W.; Xi, Y.; Li, S. Comprehensive Review of Emerging Contaminants: Detection Technologies, Environmental Impact, and Management Strategies. Ecotoxicol. Environ. Saf. 2024, 278, 116420. [Google Scholar] [CrossRef]
- Lozano, I.; Pérez-Guzmán, C.J.; Mora, A.; Mahlknecht, J.; Aguilar, C.L.; Cervantes-Avilés, P. Pharmaceuticals and Personal Care Products in Water Streams: Occurrence, Detection, and Removal by Electrochemical Advanced Oxidation Processes. Sci. Total Environ. 2022, 827, 154348. [Google Scholar] [CrossRef]
- Méndez-Catalán, J.; Socas-Hernández, C.; Jiménez-Skrzypek, G.; Hernández-Borges, J.; González-Sálamo, J. Evaluation of the Presence of Emerging Contaminants in Wastewater and Seawater Using Automated Solid-Phase Extraction and Ultra-High-Performance Liquid Chromatography Coupled to Tandem Mass Spectrometry. J. Chromatogr. Open 2024, 6, 100178. [Google Scholar] [CrossRef]
- Robin, J.; Lefeuvre, S.; Guihenneuc, J.; Cambien, G.; Dupuis, A.; Venisse, N. Analytical Methods and Biomonitoring Results in Hair for the Assessment of Exposure to Endocrine-Disrupting Chemicals: A Literature Review. Chemosphere 2024, 353, 141523. [Google Scholar] [CrossRef]
- Bergman, A.; Heindel, J.; Jobling, S.; Kidd, K.; Zoeller, R.T. State-of-the-Science of Endocrine Disrupting Chemicals, 2012. Toxicol. Lett. 2012, 211, S3. [Google Scholar] [CrossRef]
- Colzani, L.; Forni, C.; Clerici, L.; Barreca, S.; Dellavedova, P. Determination of Pollutants, Antibiotics, and Drugs in Surface Water in Italy as Required by the Third EU Water Framework Directive Watch List: Method Development, Validation, and Assessment. Environ. Sci. Pollut. Res. 2024, 31, 14791–14803. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Wu, Y.; Wang, Z.; Ma, X. Detection of Trace Estrogens in Environmental Water by Multi-Template Molecularly Imprinted Polymers Based Magnetic Solid Phase Extraction Coupled Solidified Floating Organic Drop Microextraction and HPLC. Microchem. J. 2025, 213, 113875. [Google Scholar] [CrossRef]
- Mahdavijalal, M.; Petio, C.; Staffilano, G.; Mandrioli, R.; Protti, M. Innovative Solid-Phase Extraction Strategies for Improving the Advanced Chromatographic Determination of Drugs in Challenging Biological Samples. Molecules 2024, 29, 2278. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y. Recent Progress in the Fundamental Understanding of Hydrophilic Interaction Chromatography (HILIC). Analyst 2015, 140, 6452–6466. [Google Scholar] [CrossRef]
- Fernández-Fernández, V.; Ramil, M.; Cela, R.; Rodríguez, I. Solid-Phase Extraction and Fractionation of Multiclass Pollutants from Wastewater Followed by Liquid Chromatography Tandem-Mass Spectrometry Analysis. Anal. Bioanal. Chem. 2022, 414, 4149–4165. [Google Scholar] [CrossRef]
- Mirzaei, R.; Yunesian, M.; Nasseri, S.; Gholami, M.; Jalilzadeh, E.; Shoeibi, S.; Bidshahi, H.S.; Mesdaghinia, A. An Optimized SPE-LC-MS/MS Method for Antibiotics Residue Analysis in Ground, Surface and Treated Water Samples by Response Surface Methodology- Central Composite Design. J. Environ. Health Sci. Eng. 2017, 15, 21. [Google Scholar] [CrossRef]
- Gros, M.; Rodríguez-Mozaz, S.; Barceló, D. Rapid Analysis of Multiclass Antibiotic Residues and Some of Their Metabolites in Hospital, Urban Wastewater and River Water by Ultra-High-Performance Liquid Chromatography Coupled to Quadrupole-Linear Ion Trap Tandem Mass Spectrometry. J. Chromatogr. A 2013, 1292, 173–188. [Google Scholar] [CrossRef]
- Ramadan, L.; Ozturk-Ufuk, I.; Yuksel, E.; Topuz, E. Development of LC–MS/MS Method for the Simultaneous Detection of Emerging Contaminants in Aquatic Matrices. Water Air Soil Pollut. 2024, 235, 537. [Google Scholar] [CrossRef]
- Alvarez, D.A.; Petty, J.D.; Huckins, J.N.; Jones-Lepp, T.L.; Getting, D.T.; Goddard, J.P.; Manahan, S.E. Development of a Passive, in Situ, Integrative Sampler for Hydrophilic Organic Contaminants in Aquatic Environments. Environ. Toxicol. Chem. 2004, 23, 1640–1648. [Google Scholar] [CrossRef] [PubMed]
- MacKeown, H.; Magi, E.; Di Carro, M.; Benedetti, B. Unravelling the Role of Membrane Pore Size in Polar Organic Chemical Integrative Samplers (POCIS) to Broaden the Polarity Range of Sampled Analytes. Anal. Bioanal. Chem. 2022, 414, 1963–1972. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, G.; Alluvada, P.; Alemayehu, E.; Sherieff, S.H.M.; Addi, W.A.; Kwa, T.; Krishnamoorthy, J. Log D Analysis Using Dynamic Approach. Biochem. Biophys. Rep. 2018, 16, 1–11. [Google Scholar] [CrossRef]
- Metcalfe, C.D.; Bayen, S.; Desrosiers, M.; Muñoz, G.; Sauvé, S.; Yargeau, V. Methods for the Analysis of Endocrine Disrupting Chemicals in Selected Environmental Matrixes. Environ. Res. 2022, 206, 112616. [Google Scholar] [CrossRef]
- Meher, A.K.; Zarouri, A. Environmental Applications of Mass Spectrometry for Emerging Contaminants. Molecules 2025, 30, 364. [Google Scholar] [CrossRef] [PubMed]
- Heidorn, M. The Role of Temperature and Column Thermostatting in Liquid Chromatography; Thermo Fisher Scientific: Germering, Germany, 2016. [Google Scholar]
- Vanhoenacker, G.; Sandra, P. High Temperature and Temperature Programmed HPLC: Possibilities and Limitations. Anal. Bioanal. Chem. 2008, 390, 245–248. [Google Scholar] [CrossRef]
- Carr, P.; Wang, X.; Stoll, D. A Simple Approach to Performance Optimization in HPLC and Its Application in Ultrafast Separation Development. LCGC N. Am. 2010, 28, 932–942. [Google Scholar]
- Lakka, N.S.; Kuppan, C.; Lakka, N.S.; Kuppan, C. Principles of Chromatography Method Development. In Biochemical Analysis Tools—Methods for Bio-Molecules Studies; IntechOpen: London, UK, 2019; ISBN 978-1-78984-857-1. [Google Scholar]
- Saad, M.N.; Essam, H.M.; Elzanfaly, E.S.; Amer, S.M. A Two-Step Optimization Approach: Validated RP-HPLC Method for Determination of Gatifloxacin and Dexamethasone in Ophthalmic Formulation. J. Chromatogr. Sci. 2020, 58, 504–510. [Google Scholar] [CrossRef]
- Vilkickyte, G.; Raudone, L. Optimization, Validation and Application of HPLC-PDA Methods for Quantification of Triterpenoids in Vaccinium Vitis-Idaea L. Molecules 2021, 26, 1645. [Google Scholar] [CrossRef]
- Paun, I.; Pascu, L.F.; Iancu, V.I.; Pirvu, F.; Galaon, T.; Chiriac, F.L. Simultaneous Determination of 17 Phenolic Compounds in Surface Water and Wastewater Matrices Using an HPLC-DAD Method. Environments 2024, 11, 117. [Google Scholar] [CrossRef]
- Benedetti, B.; Caponigro, V.; Ardini, F. Experimental Design Step by Step: A Practical Guide for Beginners. Crit. Rev. Anal. Chem. 2022, 52, 1015–1028. [Google Scholar] [CrossRef] [PubMed]
- Nunes, M.J.; Paz, V.; Cordas, C.M.; Noronha, J.P.; Branco, L.C. LC-MS/MS Methodology Development and Validation for the Screening and Quantification of Five Antibiotics in Water. Anal. Methods 2022, 14, 935–948. [Google Scholar] [CrossRef] [PubMed]
- Carve, M.; Singh, N.; Askeland, M.; Allinson, G.; Shimeta, J. Salting-out Assisted Liquid–Liquid Extraction Combined with LC–MS/MS for the Simultaneous Determination of Seven Organic UV Filters in Environmental Water Samples: Method Development and Application. Environ. Sci. Pollut. Res. 2023, 30, 104870–104885. [Google Scholar] [CrossRef]
- Martin, P.J.; He, K.; Blaney, L.; Hobbs, S.R. Advanced Liquid Chromatography with Tandem Mass Spectrometry Method for Quantifying Glyphosate, Glufosinate, and Aminomethylphosphonic Acid Using Pre-Column Derivatization. ACS EST Water 2023, 3, 2407–2414. [Google Scholar] [CrossRef] [PubMed]
- Ikizoglu, B. PFOA and PFOS Pollution in Surface Waters and Surface Water Fish. Water 2024, 16, 2342. [Google Scholar] [CrossRef]
- Baglietto, M.; Benedetti, B.; Di Carro, M.; Magi, E. Polar Licit and Illicit Ingredients in Dietary Supplements: Chemometric Optimization of Extraction and HILIC-MS/MS Analysis. Anal. Bioanal. Chem. 2024, 416, 1679–1695. [Google Scholar] [CrossRef]
- Santasania, C.T.; Bell, D.S. Mechanisms of Interaction Responsible for Alternative Selectivity of Fluorinated Stationary Phases. LCGC Eur. 2016, 34, 86–92. [Google Scholar]
- Leardi, R.; Melzi, C.; Polotti, G. CAT (Chemometric Agile Tool), R version 3.1.2. 2023. Available online: https://gruppochemiometria.it/index.php/software (accessed on 2 January 2025).
- Baglietto, M.; MacKeown, H.; Benedetti, B.; Di Carro, M.; Magi, E. Increasing Chemical Coverage, Accuracy, and Reproducibility of the Processing Method for Polar Organic Chemical Integrative Samplers. Anal. Bioanal. Chem. 2025, 417, 1567–1580. [Google Scholar] [CrossRef]
- Benedetti, B.; Baglietto, M.; MacKeown, H.; Scapuzzi, C.; Di Carro, M.; Magi, E. An Optimized Processing Method for Polar Organic Chemical Integrative Samplers Deployed in Seawater: Toward a Maximization of the Analysis Accuracy for Trace Emerging Contaminants. J. Chromatogr. A 2022, 1677, 463309. [Google Scholar] [CrossRef] [PubMed]
- Dolan, J. Flow-Rate Adjustment and System Suitability. LCGC Eur. 2008, 21, 196–199. [Google Scholar]
- Linford, M.; Clark, J.; Teutenberg, T. Elevated Temperatures in Liquid Chromatography, Part I: Benefits and Practical Considerations. LCGC Eur. 2013, 2, 78–85. [Google Scholar]
- Ortiz, M.C.; Sarabia, L.A.; Sánchez, M.S.; Arroyo, D. Improving the Visualization of the Pareto-Optimal Front for the Multi-Response Optimization of Chromatographic Determinations. Anal. Chim. Acta 2011, 687, 129–136. [Google Scholar] [CrossRef]
- Sánchez, M.S.; Ortiz, M.C.; Sarabia, L.A.; Lletí, R. On Pareto-Optimal Fronts for Deciding about Sensitivity and Specificity in Class-Modelling Problems. Anal. Chim. Acta 2005, 544, 236–245. [Google Scholar] [CrossRef]
- Benedetti, B.; del Pulgar, J.S.; Di Lena, G.; Lombardi-Boccia, G. Simultaneous Analysis of 21 Bioactive Compounds in Biorefinery Oil: Multivariate Optimization of a Method Based on Liquid Chromatography, Atmospheric Pressure Chemical Ionization and Tandem Mass Spectrometry. Microchem. J. 2021, 170, 106761. [Google Scholar] [CrossRef]
- Benedetti, B.; Ceccardi, E.; MacKeown, H.; Di Carro, M.; Magi, E. Exploring the Potentialities of a Biodegradable Polymeric Film in Sample Preparation: An Optimized “White” Protocol to Extract and Quantify Emerging Contaminants in Water. Anal. Chim. Acta 2024, 1311, 342725. [Google Scholar] [CrossRef]
- Matuszewski, B.K.; Constanzer, M.L.; Chavez-Eng, C.M. Matrix Effect in Quantitative LC/MS/MS Analyses of Biological Fluids: A Method for Determination of Finasteride in Human Plasma at Picogram Per Milliliter Concentrations. Anal. Chem. 1998, 70, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Matuszewski, B.K.; Constanzer, M.L.; Chavez-Eng, C.M. Strategies for the Assessment of Matrix Effect in Quantitative Bioanalytical Methods Based on HPLC−MS/MS. Anal. Chem. 2003, 75, 3019–3030. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Analytical Quality Control and Method Validation Procedures for Pesticides Residues Analysis in Food and Feedm, SANTE 11312/2021 V2, Implemented by 01/01/2024. 2021. Available online: https://food.ec.europa.eu/system/files/2023-11/pesticides_mrl_guidelines_wrkdoc_2021-11312.pdf (accessed on 11 June 2025).
- Kruve, A.; Rebane, R.; Kipper, K.; Oldekop, M.-L.; Evard, H.; Herodes, K.; Ravio, P.; Leito, I. Tutorial Review on Validation of Liquid Chromatography–Mass Spectrometry Methods: Part II. Anal. Chim. Acta 2015, 870, 8–28. [Google Scholar] [CrossRef]
- Cadena-Aizaga, M.I.; Montesdeoca-Esponda, S.; Torres-Padrón, M.E.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J. Organic UV Filters in Marine Environments: An Update of Analytical Methodologies, Occurrence and Distribution. Trends Environ. Anal. Chem. 2020, 25, e00079. [Google Scholar] [CrossRef]
- Scapuzzi, C.; Benedetti, B.; Di Carro, M.; Chiesa, E.; Pussini, N.; Magi, E. Passive Sampling of Organic Contaminants as a Novel Approach to Monitor Seawater Quality in Aquarium Ocean Tanks. Appl. Sci. 2022, 12, 2951. [Google Scholar] [CrossRef]
| ESI+ | ESI− | ||||
|---|---|---|---|---|---|
| Flow (mL min−1) | Temperature (°C) | Flow (mL min−1) | Temperature (°C) | ||
| 0.3 | 37.5 | 0.3 | 25 | ||
| Gradient | Gradient | ||||
| Time (min) | H2O % | ACN % | Time (min) | H2O % | ACN % |
| 0 | 95 | 5 | 0 | 90 | 10 |
| 2 | 95 | 5 | 1 | 90 | 10 |
| 2.7 | 45 | 55 | 7 | 50 | 50 |
| 6 | 15 | 85 | 8 | 15 | 85 |
| 8 | 15 | 85 | 12 | 90 | 10 |
| 9.3 | 95 | 5 | |||
| Post-run | 4.7 min | Post-run | 3 min | ||
| ESI+ | ESI− | ||||||
|---|---|---|---|---|---|---|---|
| Factors | Levels | Factors | Levels | ||||
| −1 * | 0 | +1 | −1 | 0 | +1 | ||
| X1 = Flow (mL min−1) | 0.1 | 0.2 | 0.3 | X1 = Flow (mL min−1) | 0.2 | 0.25 | 0.3 |
| X2 = Temperature (°C) | 25 | 37.5 | 50 | X2 = Temperature (°C) | 25 | 37.5 | 50 |
| Y (Responses): retention time (RT); width at the base of the peak (W). | |||||||
| Experiment | Coded Values Flow (mL min−1) | Real Values Flow (mL min−1) | Coded Values Temperature (°C) | Real Values Temperature (°C) |
|---|---|---|---|---|
| 1 | 1 | 0.3 | 1 | 50 |
| 2 | −1 | 0.1 | 1 | 50 |
| 3 | 1 | 0.3 | −1 | 25 |
| 4 | −1 | 0.1 | −1 | 25 |
| 5 | 0 | 0.2 | 1 | 50 |
| 6 | 0 | 0.2 | −1 | 25 |
| 7 | 1 | 0.3 | 0 | 37.5 |
| 8 | −1 | 0.1 | 0 | 37.5 |
| 9 | 0 | 0.2 | 0 | 37.5 |
| 10 | 0 | 0.2 | 0 | 37.5 |
| 11 | 0 | 0.2 | 0 | 37.5 |
| Response: RT (ESI+) | ||||||
|---|---|---|---|---|---|---|
| Analytes | Explained variance % (min–max) | b1 | b2 | b12 | b11 | b22 |
| Group 1 1 | 99.22–99.99% | *** (−) | *** (−) | NS | *** (+) | NS |
| Group 2 2 | 99.81–99.82% | *** (−) | *** (+) | * (−) | *** (+) | * (−) |
| Group 3 3 | 89.96–99.98% | *** (−) | NS | NS | *** (+) | NS |
| MPQ | 99.88% | *** (−) | NS | *** (+) | *** (+) | ** (−) |
| Response: RT (ESI−) | ||||||
| Analytes | Explained variance % (min–max) | b1 | b2 | b12 | b11 | b2 |
| Group 4 4 | 99.54–99.89% | *** (−) | *** (−) | *** (+) | *** (+) | *** (+) |
| Group 5 5 | 99.78–99.99% | *** (−) | *** (−) | *** (+) | *** (+) | *** (−) |
| Group 6 6 | 99.96–99.99% | *** (−) | *** (−) | *** (+) | *** (+) | NS |
| ACS | 91.95% | *** (−) | *** (−) | NS | *** (+) | NS |
| TRN | 60.23% | *** (−) | NS | NS | NS | NS |
| Analyte | LOD (µg L−1) | LOQ (µg L−1) | RSD% | Analyte | LOD (µg L−1) | LOQ (µg L−1) | RSD% |
|---|---|---|---|---|---|---|---|
| MTF | 0.098 | 0.323 | 4 | EHS | 0.051 | 0.167 | 9 |
| CMQ | 0.015 | 0.049 | 6 | ACS | 0.013 | 0.044 | 7 |
| DMNZ | 0.357 | 1.179 | 8 | TRN | 0.005 | 0.016 | 10 |
| MPQ | 0.02 | 0.065 | 4 | 2,4-D | 0.073 | 0.242 | 7 |
| NCT | 0.269 | 0.886 | 7 | HCTZ | 0.013 | 0.045 | 10 |
| OMT | 0.066 | 0.218 | 15 | SCL | 0.244 | 0.806 | 8 |
| TBR | 0.206 | 0.68 | 11 | FRSM | 0.047 | 0.154 | 14 |
| SLBT | 0.041 | 0.136 | 5 | CMPH | 0.013 | 0.042 | 4 |
| TRBT | 0.063 | 0.206 | 5 | PFOA | 0.058 | 0.191 | 5 |
| ATN | 0.100 | 0.330 | 6 | PFOS | 0.025 | 0.083 | 5 |
| OFLO | 0.087 | 0.287 | 3 | KETO | 0.052 | 0.173 | 8 |
| PRX + TFL | 0.219 | 0.724 | 7 | BPA | 0.145 | 0.478 | 12 |
| CAFF | 0.05 | 0.165 | 10 | NAPR | 0.022 | 0.073 | 4 |
| MTPL | 0.041 | 0.136 | 5 | DCF | 0.312 | 1.029 | 5 |
| CLBT | 0.025 | 0.083 | 6 | IBU | 0.774 | 2.554 | 6 |
| COCA | 0.034 | 0.113 | 9 | E1 | 0.093 | 0.306 | 14 |
| CARB | 0.02 | 0.067 | 7 | TCS | 0.168 | 0.554 | 6 |
| TETRA | 0.081 | 0.267 | 2 | GEM | 0.009 | 0.031 | 3 |
| BP-3 | 0.016 | 0.052 | 8 | ||||
| EHMC | 0.022 | 0.072 | 7 | ||||
| OC | 0.008 | 0.027 | 9 | ||||
| OD-PABA | 0.002 | 0.008 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bona, D.; Di Carro, M.; Magi, E.; Benedetti, B. Development of an Efficient HPLC-MS/MS Method for the Detection of a Broad Spectrum of Hydrophilic and Lipophilic Contaminants in Marine Waters: An Experimental Design Approach. Separations 2025, 12, 257. https://doi.org/10.3390/separations12100257
Bona D, Di Carro M, Magi E, Benedetti B. Development of an Efficient HPLC-MS/MS Method for the Detection of a Broad Spectrum of Hydrophilic and Lipophilic Contaminants in Marine Waters: An Experimental Design Approach. Separations. 2025; 12(10):257. https://doi.org/10.3390/separations12100257
Chicago/Turabian StyleBona, Daniel, Marina Di Carro, Emanuele Magi, and Barbara Benedetti. 2025. "Development of an Efficient HPLC-MS/MS Method for the Detection of a Broad Spectrum of Hydrophilic and Lipophilic Contaminants in Marine Waters: An Experimental Design Approach" Separations 12, no. 10: 257. https://doi.org/10.3390/separations12100257
APA StyleBona, D., Di Carro, M., Magi, E., & Benedetti, B. (2025). Development of an Efficient HPLC-MS/MS Method for the Detection of a Broad Spectrum of Hydrophilic and Lipophilic Contaminants in Marine Waters: An Experimental Design Approach. Separations, 12(10), 257. https://doi.org/10.3390/separations12100257






