Economic, Environmental, and Safety Multi-Objective Optimization Design for Separation of Tetrahydrofuran/Methanol/Water Mixture
Abstract
1. Introduction
2. Methodology
2.1. Thermodynamic Analysis
2.2. Conceptual Design
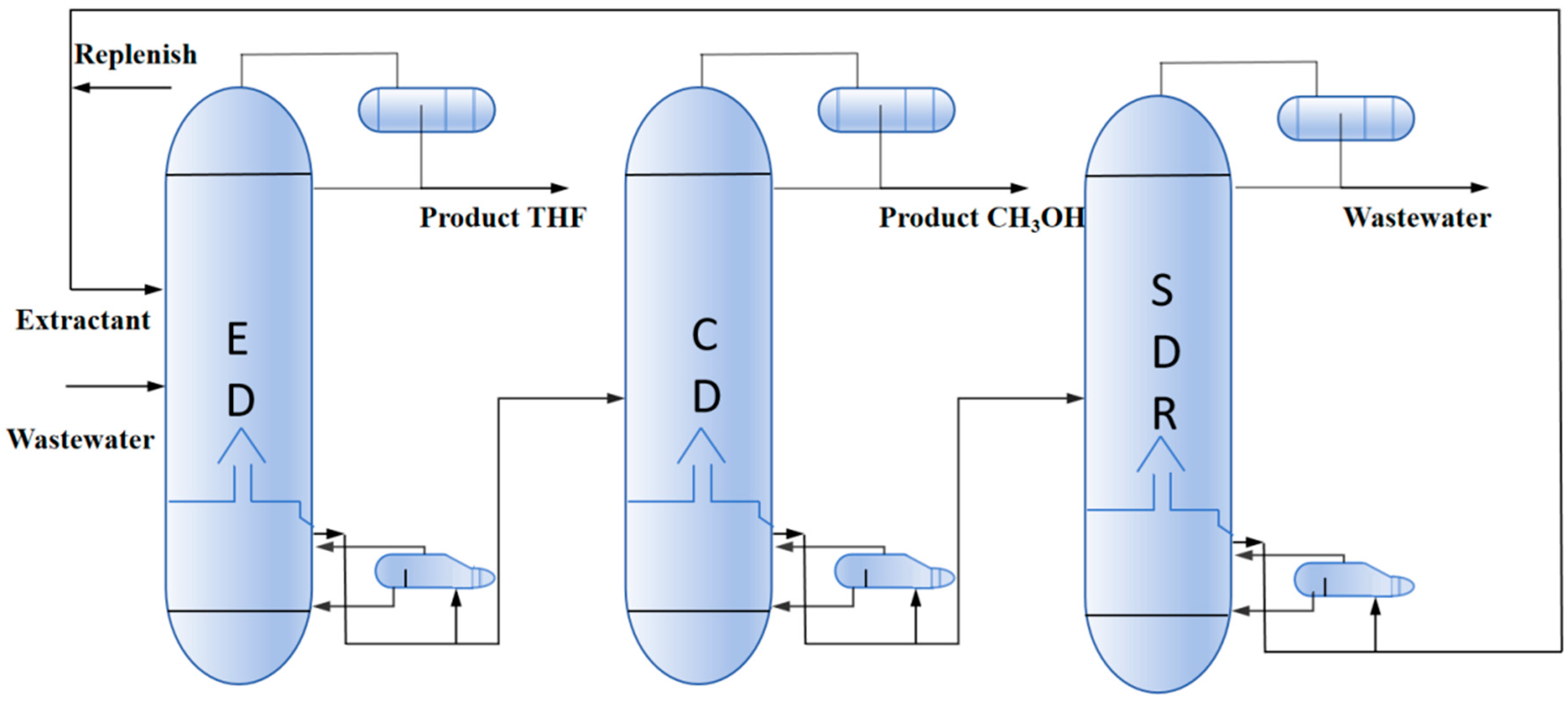
2.2.1. Triple-Column Extractive Distillation (TED) Process
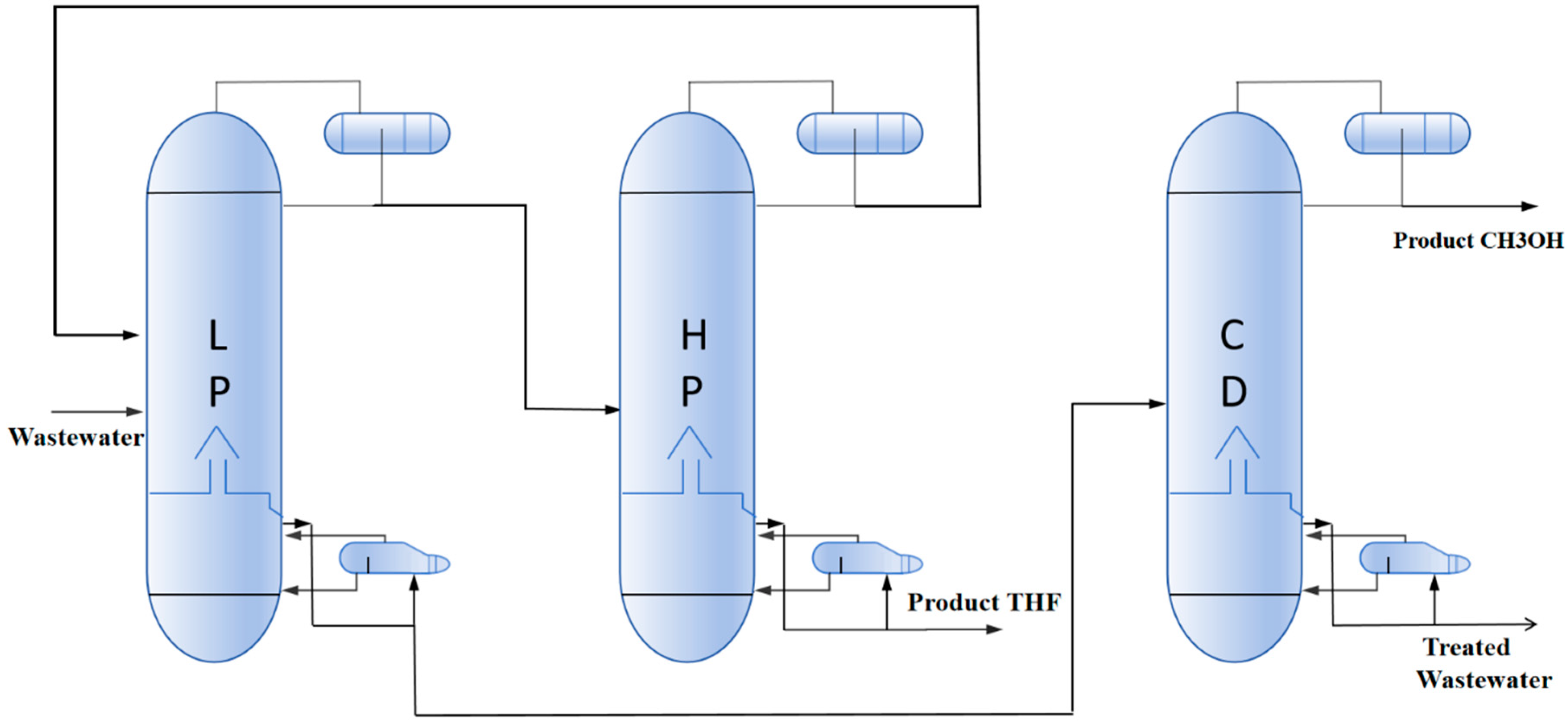
2.2.2. Pressure-Swing Azeotropic Distillation (PSAD) Process
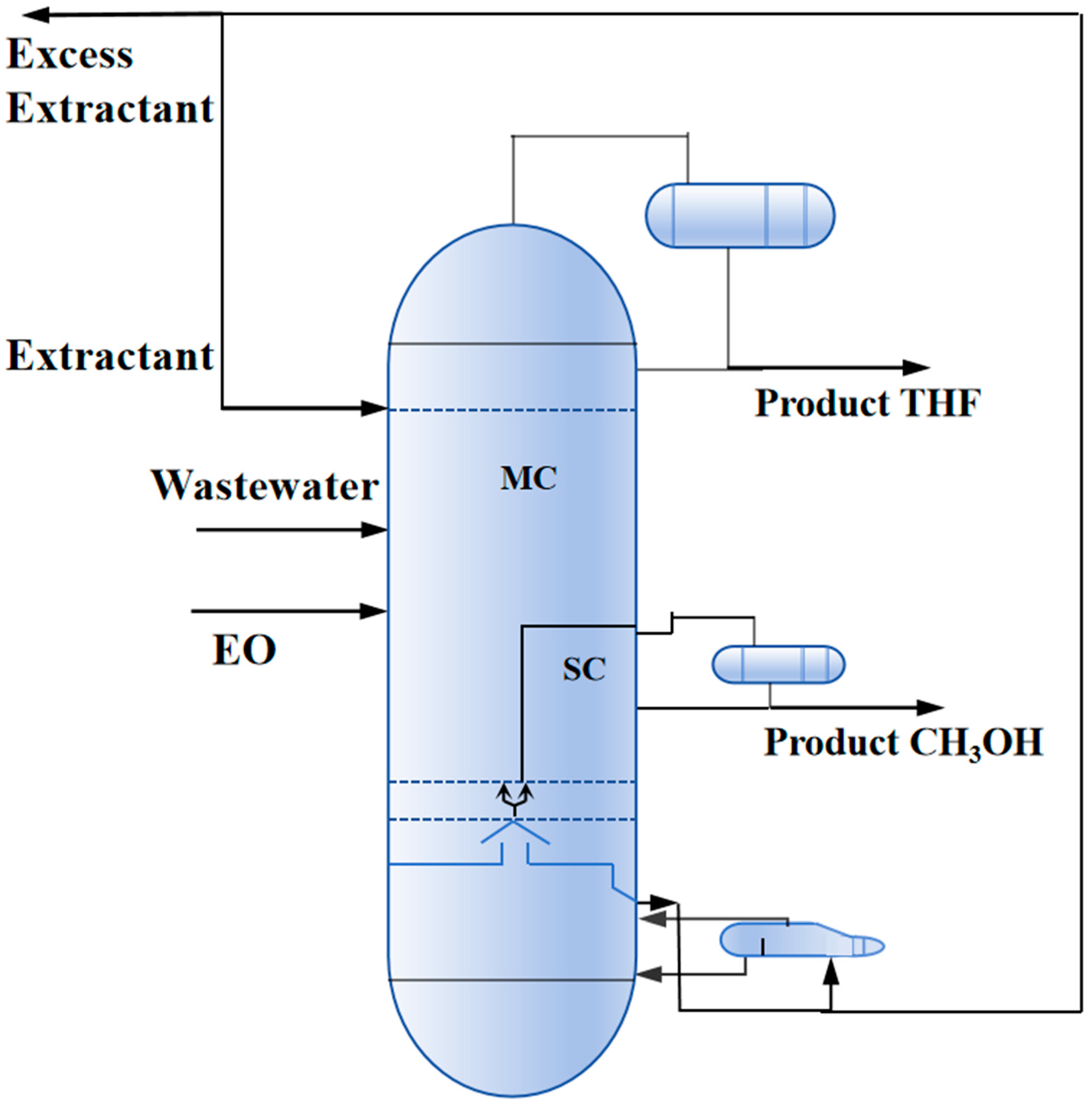
2.2.3. Reactive-Extractive Dividing Wall Column (REDWC)
2.3. Performance Evaluation
2.3.1. Economic Index
2.3.2. Environmental Index
2.3.3. Safety Index
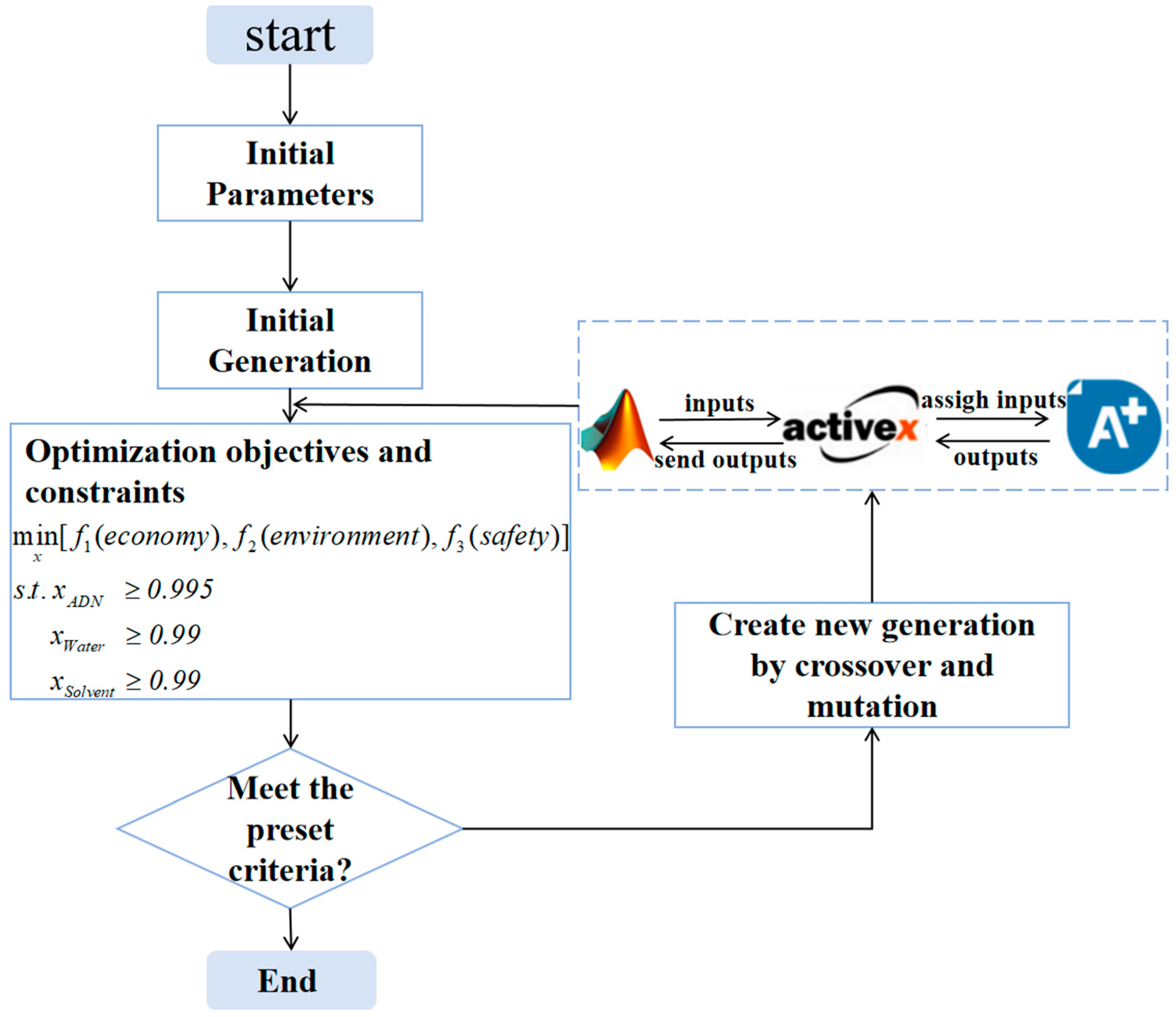
2.4. Optimization Strategy
3. Results and Discussions
3.1. Results of Multi-Objective Optimization
3.2. Results of Optimal Processes
3.3. Economic, Environmental and Safety Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
Abbreviations
| THF | tetrahydrofuran |
| CC | conventional column |
| EG | ethylene glycol |
| MeOH | methanol |
| RD | reactive distillation |
| EtAC | ethyl acetate |
| IPA | isopropanol |
| TED | triple-column extractive distillation |
| DMSO | dimethyl sulfoxide |
| ED | extractive distillation |
| CD | conventional distillation |
| PSD | pressure-swing distillation |
| PSAD | pressure-swing azeotropic distillation |
| SDR | solvent recovery column |
| LP | low-pressure column |
| HP | high-pressure column |
| DRED | reactive-extractive distillation column |
| DWC | dividing wall column |
| REDWC | reactive-extractive dividing wall column |
| NSGA-II | non-dominated sorting genetic algorithm II |
| NSGA-III | non-dominated sorting genetic algorithm III |
| MC | main column |
| SC | side column |
| TAC | total annual cost |
| PRI | The process route index |
| N | stage |
| NF | feed stage |
| RR | reflux ratio |
| DF | distillate flow rate |
| FEG | entrainer content |
References
- Zhao, N.; Yang, J.; Ai, T.; Wang, J. From Methanol to Power: Energy, Economic and Life-Cycle Assessments of Several Pathways. J. Clean. Prod. 2025, 486, 144501. [Google Scholar] [CrossRef]
- Chen, Z.; Du, Z.; Wang, T.; Chen, L. The Impact of THF in Ether-Based Electrolytes for Lithium-Sulfur Battery. Ionics 2025, 31, 1377–1388. [Google Scholar] [CrossRef]
- Wang, Z.; Luo, C.; Wang, B.; Lai, T.; Hong, Y.; Gao, G. Synergistic Catalysis of Sulfate Ionic Liquids and FeCl3 for the Dehydration of 1, 4-Butanediol to Tetrahydrofuran. Fuel 2024, 363, 130806. [Google Scholar] [CrossRef]
- Liu, C.; Karagoz, A.; Alqusair, O.; Ma, Y.; Xiao, X.; Li, J. Design of Hybrid Reactive-Extractive Distillation for Separation of Azeotropic Ternary Mixture Using a Systematic Optimization Framework. Sep. Purif. Technol. 2025, 369, 132805. [Google Scholar] [CrossRef]
- Serpas, C.G.; Samborskaya, M.; Mityanina, O. Parametric Optimization of a Reactive Distillation Column for the Ethyl Tert-Butyl Ether Production. Period. Polytech. Chem. Eng. 2024, 68, 116–123. [Google Scholar] [CrossRef]
- Dai, T.; Li, J.; Wang, H.; Zhang, R.; Ye, Q. Sustainable Recovery of Benzene and Tert-Butanol from Wastewater Using Energy-Efficient Heterogeneous Azeotropic Pressure-Swing Distillation with Multi-Objective Optimization. Process Saf. Environ. Prot. 2025, 200, 107317. [Google Scholar] [CrossRef]
- Zhao, L.; Lyu, X.; Wang, W.; Shan, J.; Qiu, T. Comparison of Heterogeneous Azeotropic Distillation and Extractive Distillation Methods for Ternary Azeotrope Ethanol/Toluene/Water Separation. Comput. Chem. Eng. 2017, 100, 27–37. [Google Scholar] [CrossRef]
- Cui, P.; Zhao, F.; Liu, X.; Shen, Y.; Li, S.; Meng, D.; Zhu, Z.; Ma, Y.; Wang, Y. Sustainable Wastewater Treatment via PV-Distillation Hybrid Process for the Separation of Ethyl Acetate/Isopropanol/Water. Sep. Purif. Technol. 2021, 257, 117919. [Google Scholar] [CrossRef]
- Jin, Z.; Liu, H.; Dai, Z.; Dai, Y. Investigation on Energy-Saving Distillation for Separating Tetrahydrofuran/Methanol/Water Ternary Azeotropic System. J. Clean. Prod. 2024, 441, 140899. [Google Scholar] [CrossRef]
- Modla, G.; Lang, P. Separation of an Acetone−Methanol Mixture by Pressure-Swing Batch Distillation in a Double-Column System with and without Thermal Integration. Ind. Eng. Chem. Res. 2010, 49, 3785–3793. [Google Scholar] [CrossRef]
- Yan, J.; Liu, J.; Ren, J.; Wu, Y.; Li, X.; Sun, T.; Sun, L. Design and Multi-Objective Optimization of Hybrid Reactive-Extractive Distillation Process for Separating Wastewater Containing Benzene and Isopropanol. Sep. Purif. Technol. 2022, 290, 120915. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, J.; Zhou, C.; Cao, Q.; Gao, Y.; Li, X.; Hou, Y.; Zhao, W.; Xiang, S.; Wang, L. Designing of Dividing Wall Columns and Triple-Effect Evaporators for Energy-Saving Separation of Glycol (EG+ DEG+ TEG) and Water: An Integrated Approach Considering Energy, Exergy, Economic, and Environmental Analyses. Sep. Purif. Technol. 2025, 373, 133578. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, Q.; Liu, C.; Yin, T.; Xiang, W. Optimal Design of the Ternary Azeotrope Separation Process Assisted by Reactive-Extractive Distillation for Ethyl Acetate/Isopropanol/Water. Sep. Purif. Technol. 2023, 306, 122708. [Google Scholar] [CrossRef]
- Yin, T.; Zhang, Q.; Chen, Y.; Liu, C.; Xiang, W. Process Design and Optimization of the Reactive-Extractive Distillation Process Assisted with Reaction Heat Recovery via Side Vapor Recompression for the Separation of Water-Containing Ternary Azeotropic Mixture. Process Saf. Environ. Prot. 2024, 184, 1041–1056. [Google Scholar] [CrossRef]
- Yang, A.; Kong, Z.Y.; Sun, S.; Sunarso, J.; Ren, J.; Shen, W. Design and Multiobjective Optimization of a Novel Double Extractive Dividing Wall Column with a Side Reboiler Scheme for the Recovery of Ethyl Acetate and Methanol from Wastewater. Ind. Eng. Chem. Res. 2023, 62, 18591–18602. [Google Scholar] [CrossRef]
- Leong, C.T.; Shariff, A.M. Process Route Index (PRI) to Assess Level of Explosiveness for Inherent Safety Quantification. J. Loss Prev. Process Ind. 2009, 22, 216–221. [Google Scholar] [CrossRef]
- Sharma, D.; Vasanthi, D.R.; Reddy, S.G.K.; Rao, M. NSGA-RM: NSGA-II Evolved Performance Optimized Non-Homogeneous Recursive Polynomial Multiplier Architectures. In Proceedings of the 2025 38th International Conference on VLSI Design and 2024 23rd International Conference on Embedded Systems (VLSID), Bangalore, India, 4–8 January 2025; IEEE: New York, NY, USA, 2025; pp. 121–126. [Google Scholar]
- Wietheger, S.; Doerr, B. A Mathematical Runtime Analysis of the Non-Dominated Sorting Genetic Algorithm III (NSGA-III). In Proceedings of the Genetic and Evolutionary Computation Conference Companion, Melbourne, Australia, 14–18 July 2024; ACM: New York, NY, USA, 2024; pp. 63–64. [Google Scholar]
- Bala, A.M.; Liu, R.; Peereboom, L.; Lira, C.T. Applications of an Association Activity Coefficient Model, NRTL-PA, to Alcohol-Containing Mixtures. Ind. Eng. Chem. Res. 2022, 61, 15603–15619. [Google Scholar] [CrossRef]
- Zhang, Y.-R.; Wu, T.-W.; Chien, I.-L. Intensified Hybrid Reactive-Extractive Distillation Process for the Separation of Water-Containing Ternary Mixtures. Sep. Purif. Technol. 2021, 279, 119712. [Google Scholar] [CrossRef]
- Frolkova, A.V. Structural Analysis of Phase Diagram and Assessment of Possibility for Distillation of Multicomponent Mixtures. Theor. Found. Chem. Eng. 2024, 58, 1017–1026. [Google Scholar] [CrossRef]
- Tavan, Y.; Hosseini, S.H. A Novel Integrated Process to Break the Ethanol/Water Azeotrope Using Reactive Distillation–Part I: Parametric Study. Sep. Purif. Technol. 2013, 118, 455–462. [Google Scholar] [CrossRef]
- Tavan, Y.; Behbahani, R.M.; Hosseini, S.H. A Novel Intensified Reactive Distillation Process to Produce Pure Ethyl Acetate in One Column—Part I: Parametric Study. Chem. Eng. Process. Process Intensif. 2013, 73, 81–86. [Google Scholar] [CrossRef]
- Pandit, S.R.; Jana, A.K. Transforming Conventional Distillation Sequence to Dividing Wall Column: Minimizing Cost, Energy Usage and Environmental Impact through Genetic Algorithm. Sep. Purif. Technol. 2022, 297, 121437. [Google Scholar] [CrossRef]
- Li, E.; Dai, X.; Jia, B. Multi-Dimensional Classification via NSGA-III Algorithm and Sparse Label Encoding. In Proceedings of the 2024 8th International Conference on Electronic Information Technology and Computer Engineering, Haikou, China, 18–20 October 2024; ACM: New York, NY, USA, 2024; pp. 120–128. [Google Scholar]
- Parhi, S.S.; Rangaiah, G.P.; Jana, A.K. Multi-Objective Optimization of Vapor Recompressed Distillation Column in Batch Processing: Improving Energy and Cost Savings. Appl. Therm. Eng. 2019, 150, 1273–1296. [Google Scholar] [CrossRef]
- Luyben, W.L. Comparison of Extractive Distillation and Pressure-Swing Distillation for Acetone/Chloroform Separation. Comput. Chem. Eng. 2013, 50, 1–7. [Google Scholar] [CrossRef]
- Shariff, A.M.; Leong, C.T.; Zaini, D. Using Process Stream Index (PSI) to Assess Inherent Safety Level during Preliminary Design Stage. Saf. Sci. 2012, 50, 1098–1103. [Google Scholar] [CrossRef]
- Aurangzeb, M.; Jana, A.K. Double-Partitioned Dividing Wall Column for a Multicomponent Azeotropic System. Sep. Purif. Technol. 2019, 219, 33–46. [Google Scholar] [CrossRef]


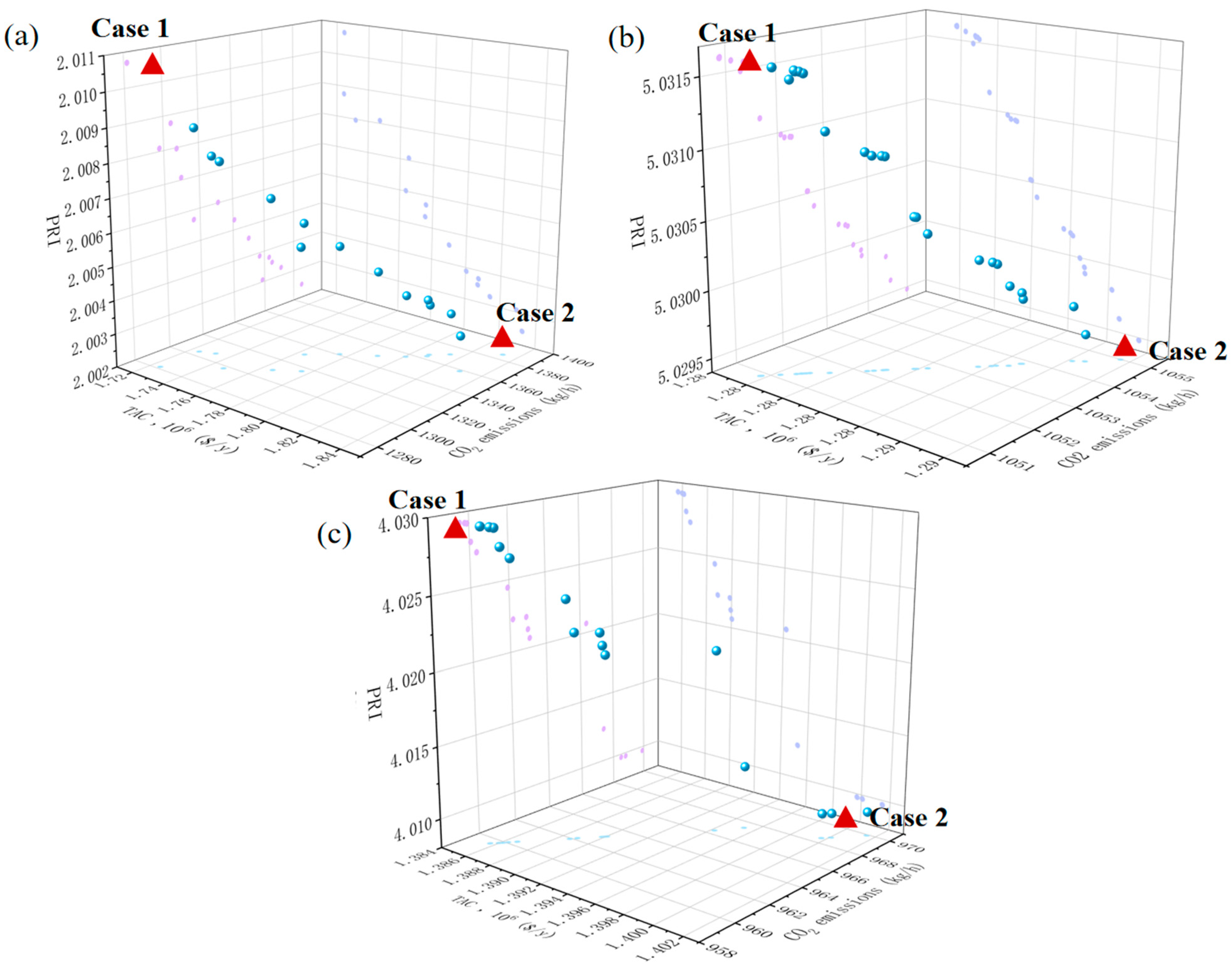
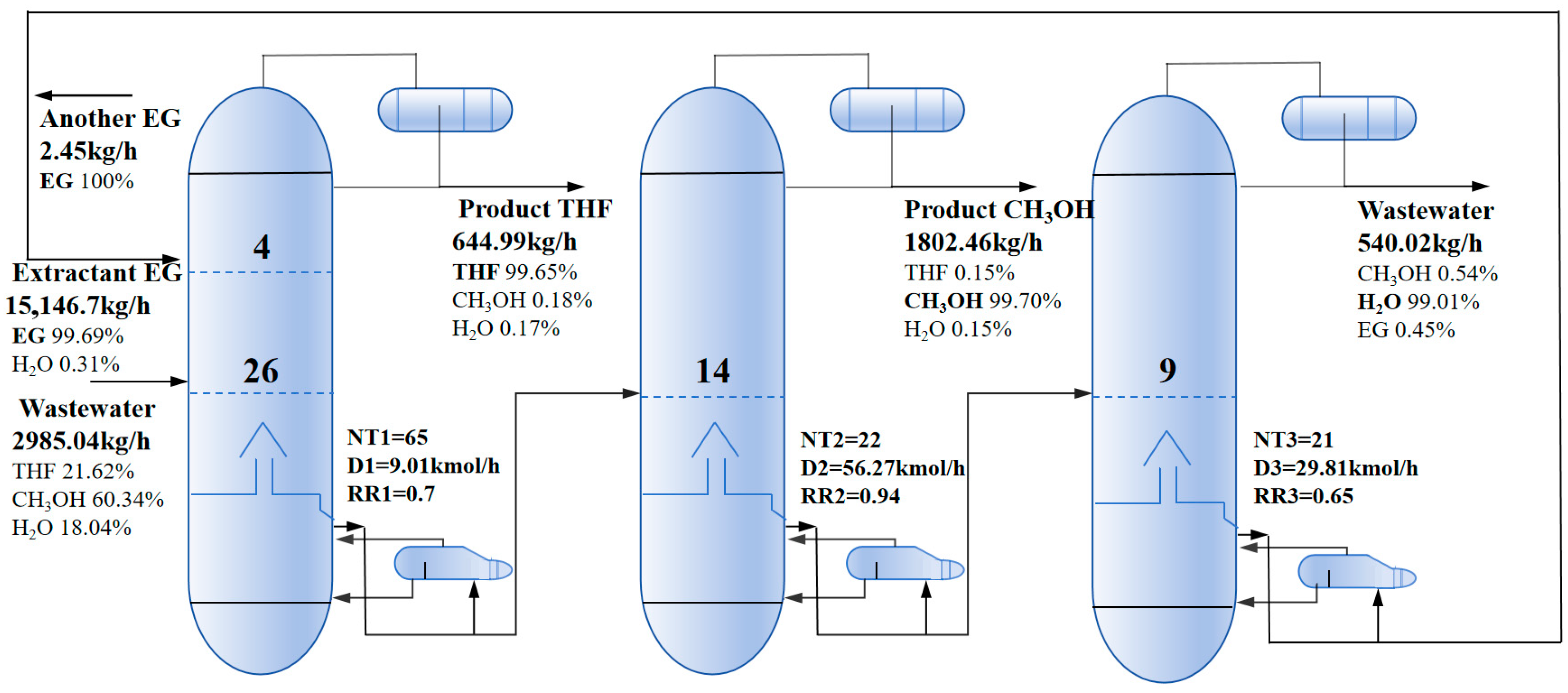

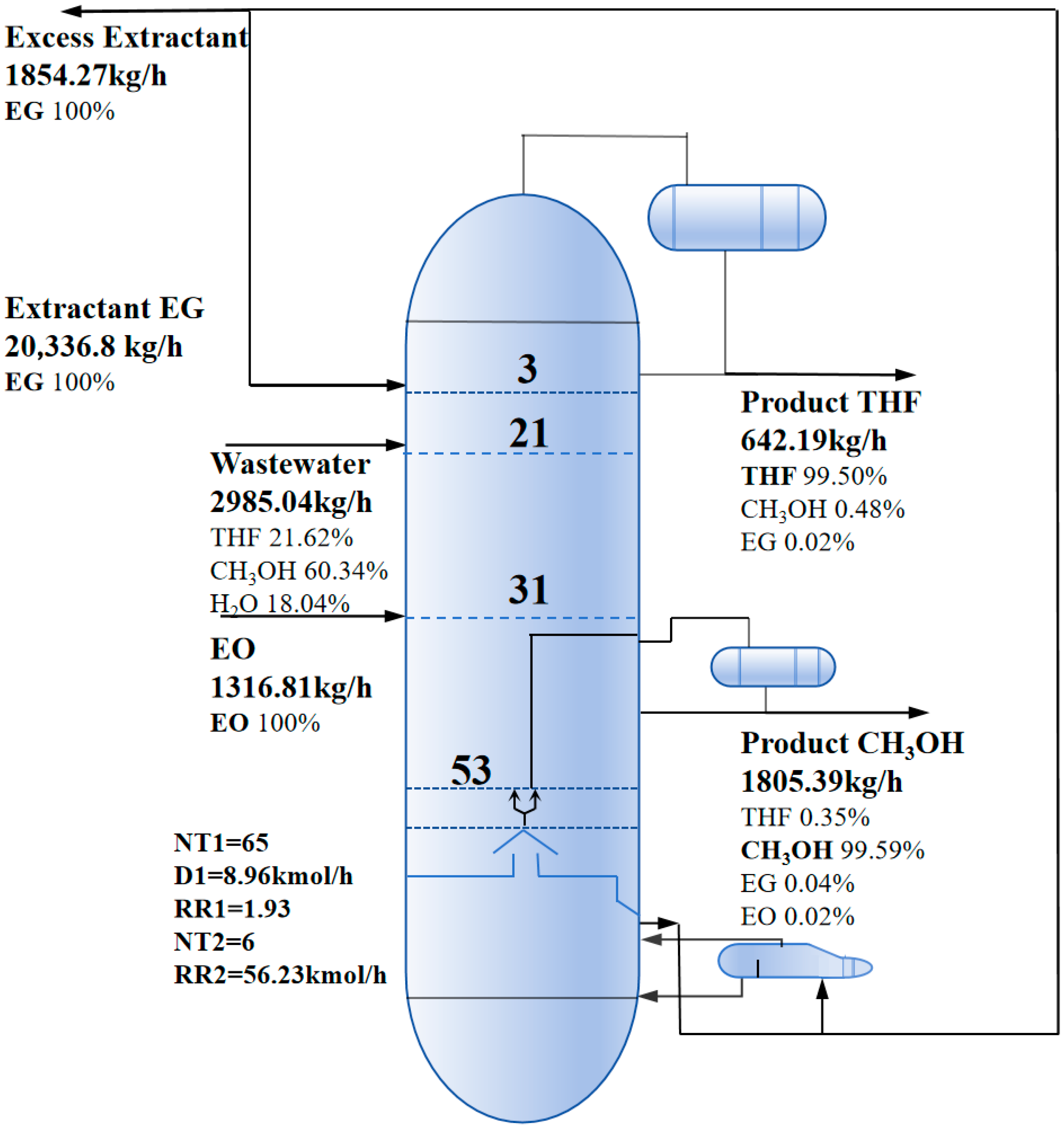
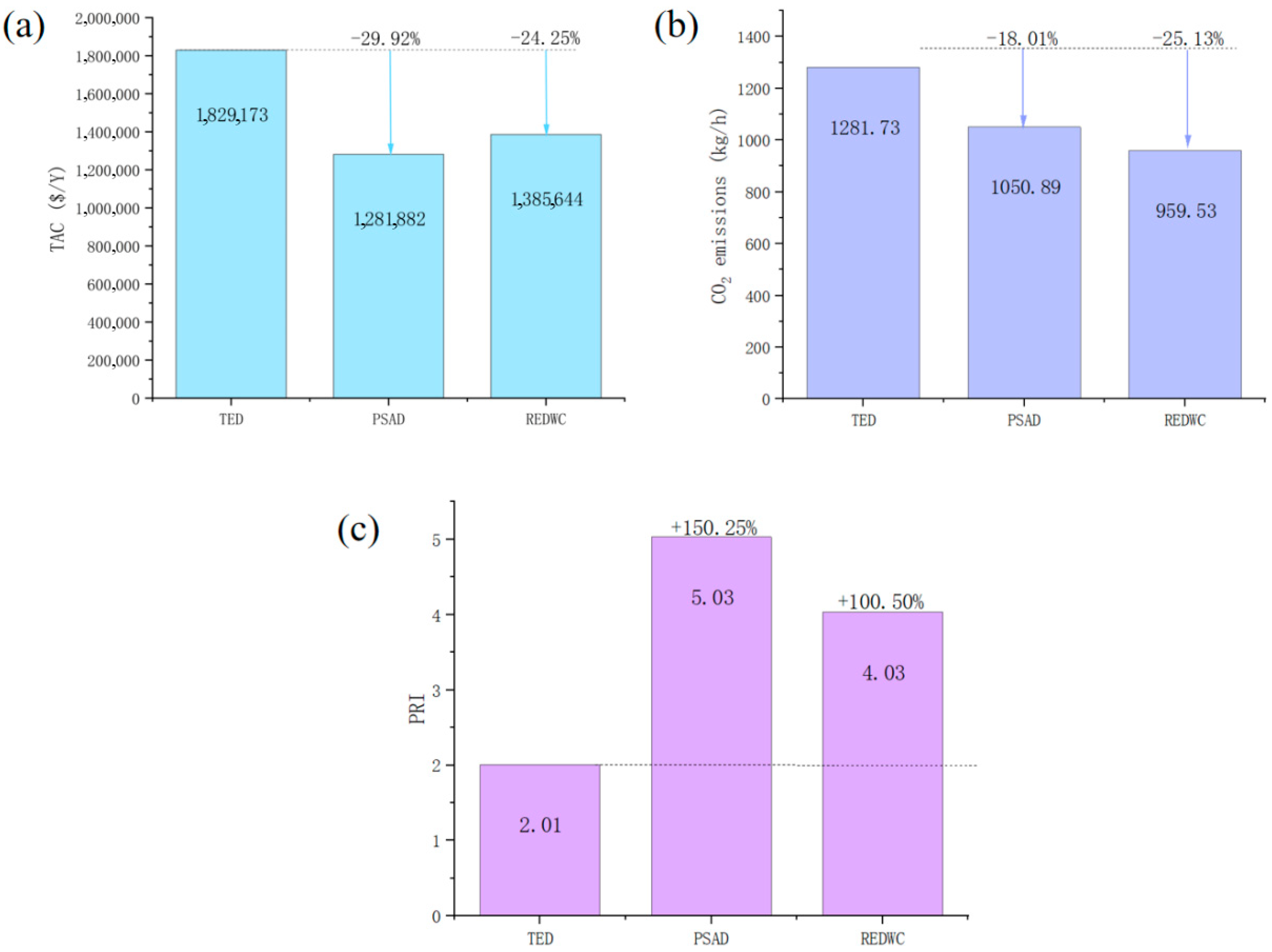
| TED | PSAD | REDWC | ||||
|---|---|---|---|---|---|---|
| Variables | Case 1 | Case 2 | Case 1 | Case 2 | Case 1 | Case 2 |
| N1 | 65 | 62 | 29 | 29 | 65 | 63 |
| NFExtractant | 4 | 3 | 7 | 7 | 3 | 3 |
| NFFeed | 26 | 28 | 11 | 11 | 21 | 22 |
| RR | 0.7 | 0.85 | 1.96 | 1.99 | 1.93 | 2.02 |
| DF1 | 644.99 | 644.75 | 2072.24 | 2079.2 | 642.19 | 642.2 |
| LH | / | / | / | / | 8.41 | 10 |
| V | / | / | / | / | 1848.43 | 1855 |
| VF | / | / | / | / | 53 | 52 |
| N2 | 22 | 20 | 21 | 19 | 7 | 6 |
| NF2 | 14 | 12 | 15 | 13 | / | / |
| RR2 | 0.94 | 0.95 | 1.72 | 1.82 | / | / |
| DF2 | 1802.46 | 1802.01 | 643.09 | 643.68 | 1805.39 | 1805.68 |
| FEG | / | / | / | / | 20,336.75 | 20,488.57 |
| N3 | 21 | 15 | 18 | 18 | / | / |
| NF3 | 9 | 10 | 14 | 14 | / | / |
| RR3 | 0.65 | 0.81 | 1.1 | 1.1 | / | / |
| DF3 | 540.02 | 539.71 | 1802.49 | 1802.42 | / | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, M.; Zhang, Q.; Jin, Z.; Liu, Y.; Dai, Y. Economic, Environmental, and Safety Multi-Objective Optimization Design for Separation of Tetrahydrofuran/Methanol/Water Mixture. Separations 2025, 12, 255. https://doi.org/10.3390/separations12090255
Gao M, Zhang Q, Jin Z, Liu Y, Dai Y. Economic, Environmental, and Safety Multi-Objective Optimization Design for Separation of Tetrahydrofuran/Methanol/Water Mixture. Separations. 2025; 12(9):255. https://doi.org/10.3390/separations12090255
Chicago/Turabian StyleGao, Mengdie, Qiyu Zhang, Zhehao Jin, Yishan Liu, and Yiyang Dai. 2025. "Economic, Environmental, and Safety Multi-Objective Optimization Design for Separation of Tetrahydrofuran/Methanol/Water Mixture" Separations 12, no. 9: 255. https://doi.org/10.3390/separations12090255
APA StyleGao, M., Zhang, Q., Jin, Z., Liu, Y., & Dai, Y. (2025). Economic, Environmental, and Safety Multi-Objective Optimization Design for Separation of Tetrahydrofuran/Methanol/Water Mixture. Separations, 12(9), 255. https://doi.org/10.3390/separations12090255







