Optimization of Organic Micropollutant Adsorption onto Granular Activated Carbon Using Response Surface Methodology
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Adsorbent
2.1.2. Adsorbate
2.2. Methods
2.2.1. Adsorption Study
Response Surface Methodology
Adsorption Experimental Protocols
- Adsorptionkinetics
- Adsorption isotherms
- Thermodynamic study
- Optimized granular activated carbon desorption and regeneration
3. Results and Discussion
3.1. Adsorption of Organic Micropollutants on OGAC: Response Surface Methodology
3.1.1. Experimental Matrix
3.1.2. Statistical Results
Variance Analysis
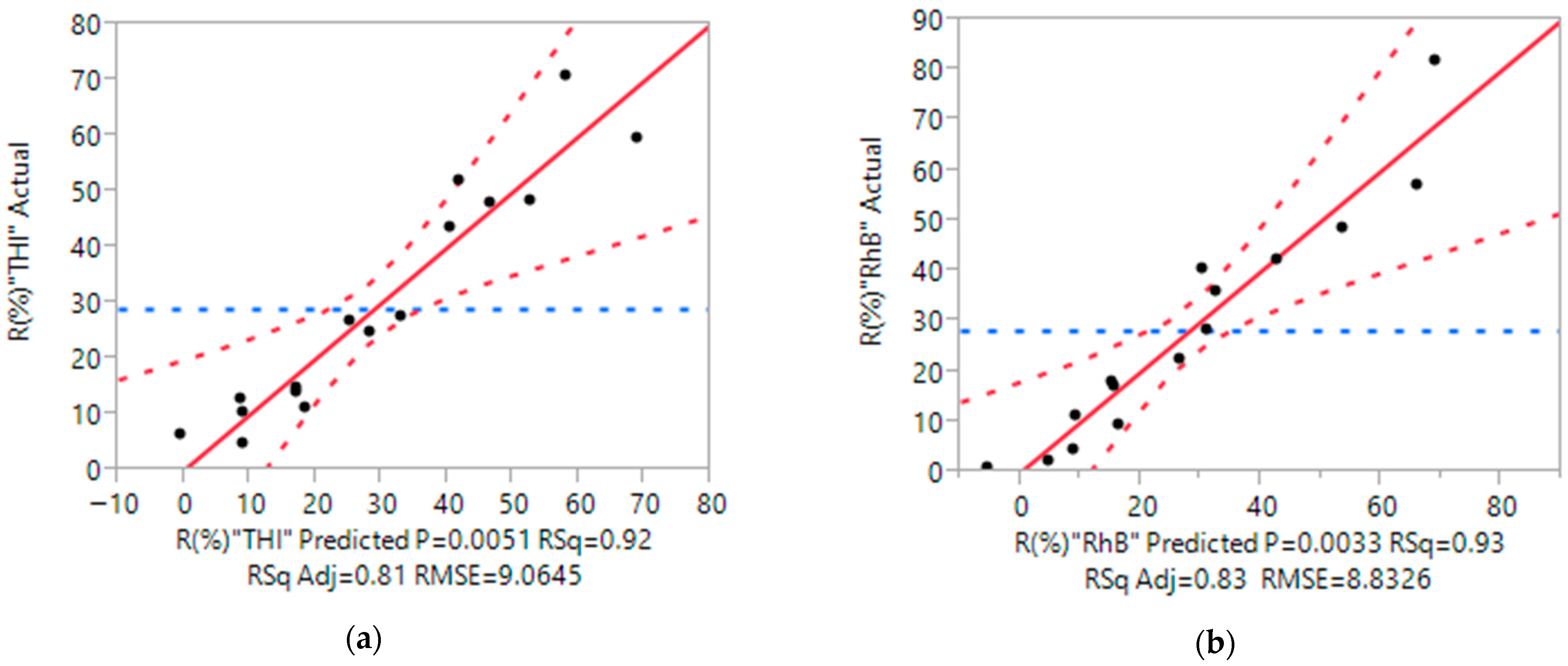
Determination Coefficients
Residual Analysis
Estimated Parameters
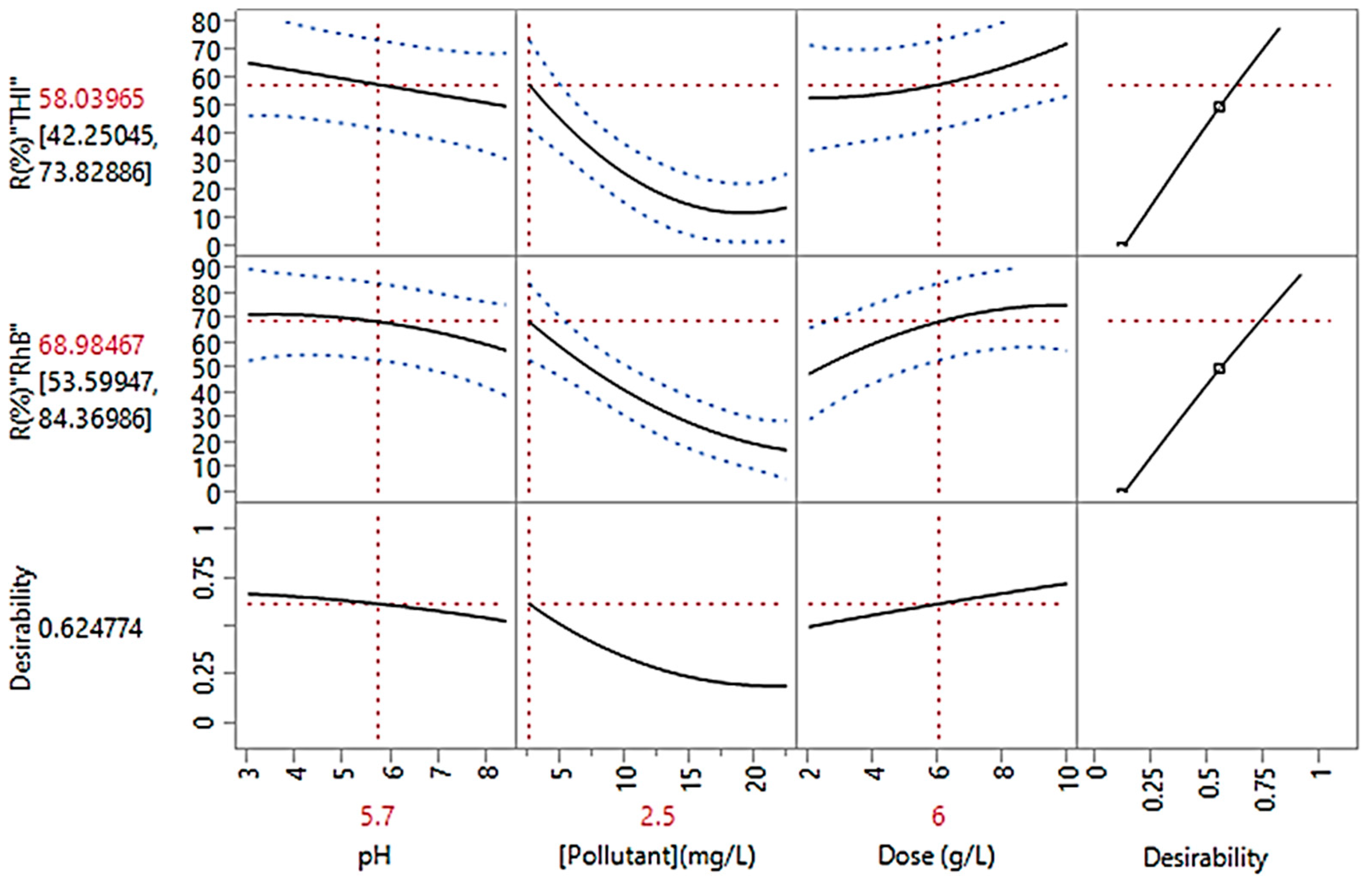
Parameter Optimization of the THI and RhB Adsorption on OGAC
3.2. Adsorption Study
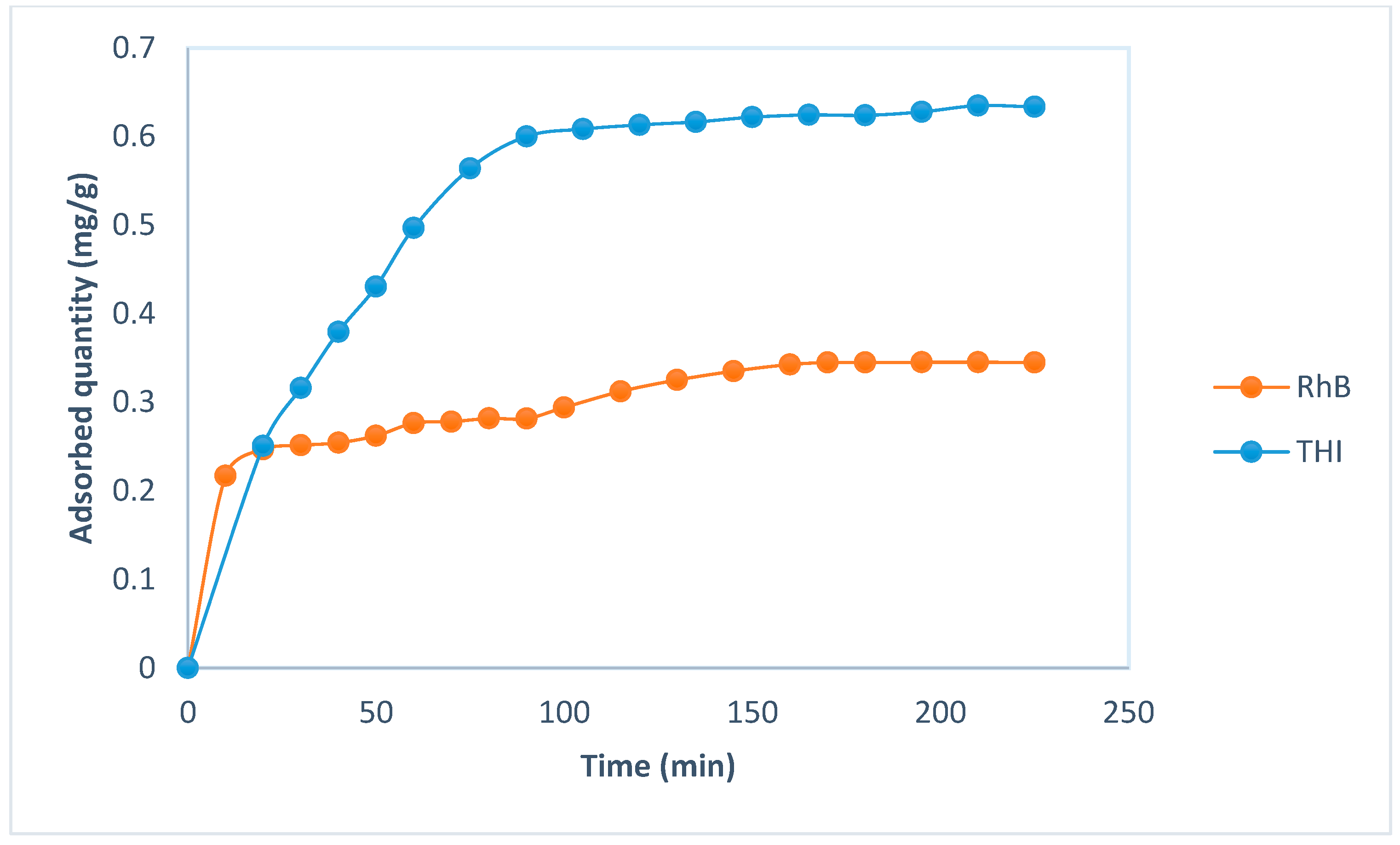
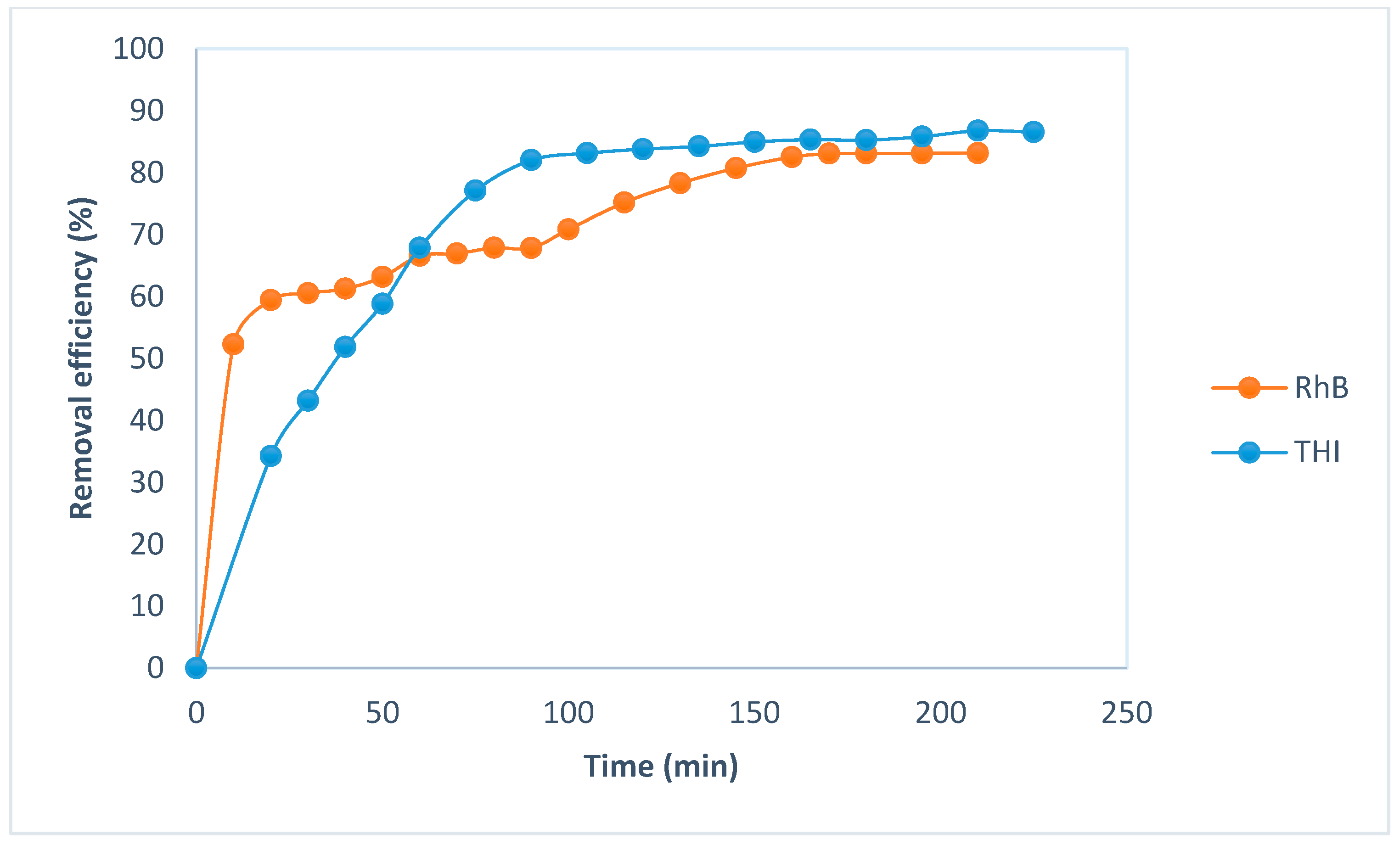
3.2.1. Kinetic Study
Kinetic Models
3.2.2. Study of Adsorption Isotherms
3.2.3. Thermodynamic Study
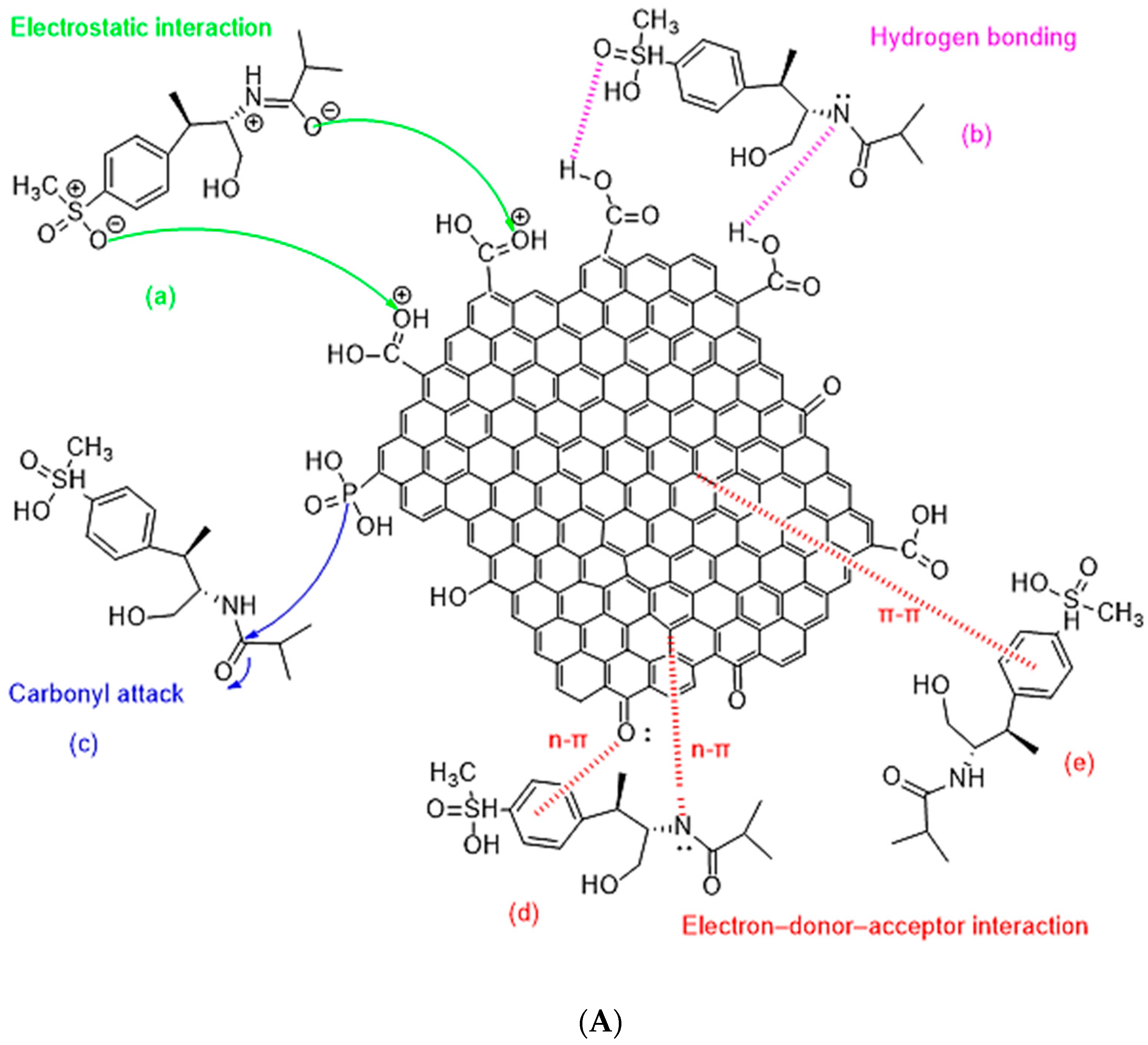
3.3. Adsorption Mechanisms
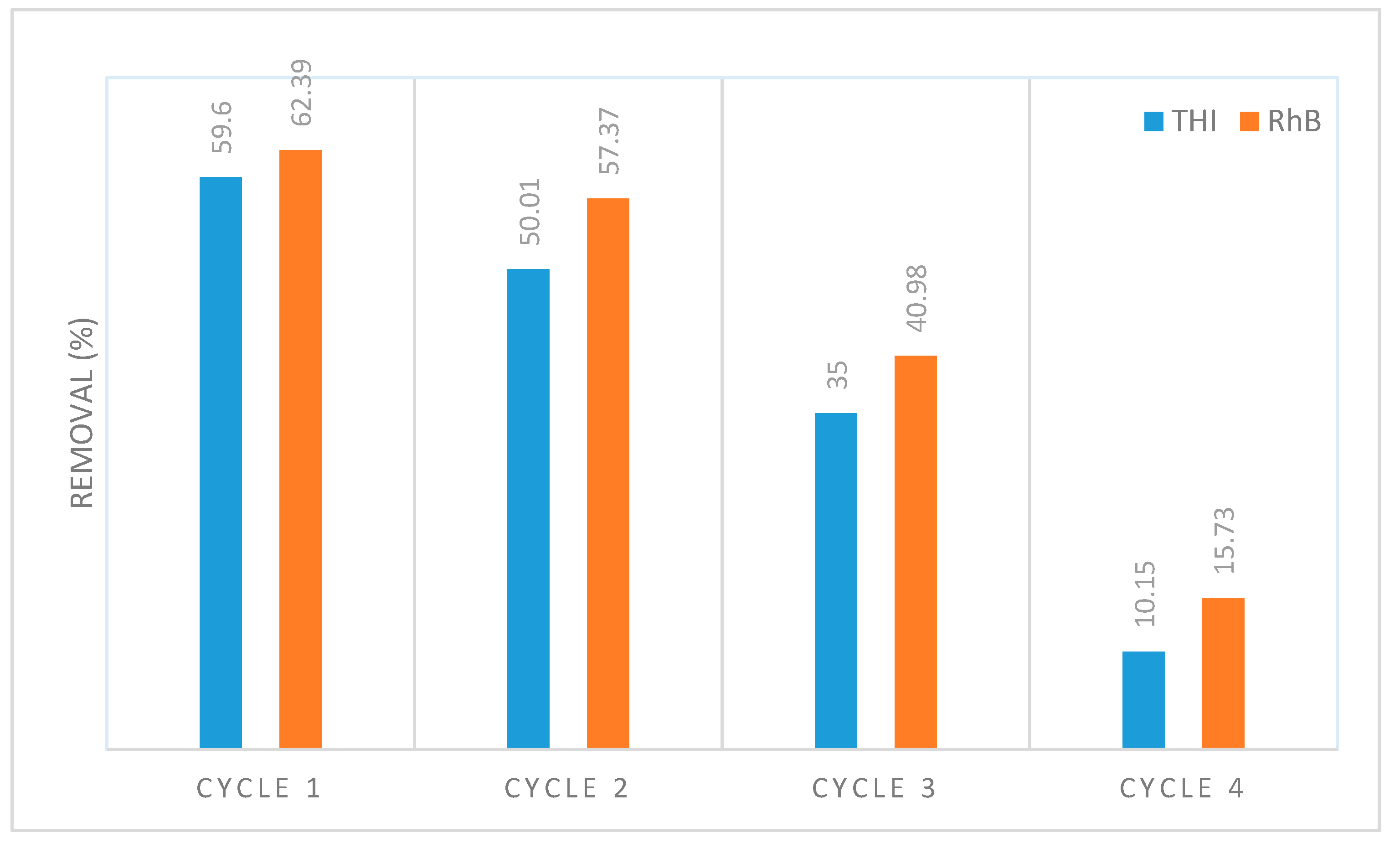
3.4. Regeneration Cycles
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kolpin, D.W.; Furlong, E.T.; Meyer, M.T.; Thurman, E.M.; Zaugg, S.D.; Barber, L.B.; Buxton, H.T. Pharmaceuticals, Hormones, and Other Organic Wastewater Contaminants in U.S. Streams, 1999−2000: A National Reconnaissance. Environ. Sci. Technol. 2002, 36, 1202–1211. [Google Scholar] [CrossRef]
- Snyder, S.A.; Westerhoff, P.; Yoon, Y.; Sedlak, D.L. Pharmaceuticals, Personal Care Products, and Endocrine Disruptors in Water: Implications for the Water Industry. Environ. Eng. Sci. 2003, 20, 449–469. [Google Scholar] [CrossRef]
- Pomati, F.; Castiglioni, S.; Zuccato, E.; Fanelli, R.; Vigetti, D.; Rossetti, C.; Calamari, D. Effects of a Complex Mixture of Therapeutic Drugs at Environmental Levels on Human Embryonic Cells. Environ. Sci. Technol. 2006, 40, 2442–2447. [Google Scholar] [CrossRef]
- Wang, F.; Xiang, L.; Sze-Yin Leung, K.; Elsner, M.; Zhang, Y.; Guo, Y.; Pan, B.; Sun, H.; An, T.; Ying, G.; et al. Emerging Contaminants: A One Health Perspective. Innovation 2024, 5, 100612. [Google Scholar] [CrossRef]
- Shetty, S.S.; D, D.; S, H.; Sonkusare, S.; Naik, P.B.; Kumari N, S.; Madhyastha, H. Environmental Pollutants and Their Effects on Human Health. Heliyon 2023, 9, e19496. [Google Scholar] [CrossRef]
- Geissen, V.; Mol, H.; Klumpp, E.; Umlauf, G.; Nadal, M.; van der Ploeg, M.; van de Zee, S.E.A.T.M.; Ritsema, C.J. Emerging Pollutants in the Environment: A Challenge for Water Resource Management. Int. Soil Water Conserv. Res. 2015, 3, 57–65. [Google Scholar] [CrossRef]
- Karim, M.A.H.; Aziz, K.H.H.; Omer, K.M.; Salih, Y.M.; Mustafa, F.; Rahman, K.O.; Mohammad, Y. Degradation of Aqueous Organic Dye Pollutants by Heterogeneous Photo-Assisted Fenton-like Process Using Natural Mineral Activator: Parameter Optimization and Degradation Kinetics. IOP Conf. Ser. Earth Environ. Sci. 2021, 958, 012011. [Google Scholar] [CrossRef]
- Hama Aziz, K.H.; Mahyar, A.; Miessner, H.; Mueller, S.; Kalass, D.; Moeller, D.; Khorshid, I.; Rashid, M.A.M. Application of a Planar Falling Film Reactor for Decomposition and Mineralization of Methylene Blue in the Aqueous Media via Ozonation, Fenton, Photocatalysis and Non-Thermal Plasma: A Comparative Study. Process Saf. Environ. Prot. 2018, 113, 319–329. [Google Scholar] [CrossRef]
- Lin, S.; Lin, X.; Lou, X.-T.; Yang, F.; Lin, D.-Y.; Lu, Z.-W. Rapid and Sensitive SERS Method for Determination of Rhodamine B in Chili Powder with Paper-Based Substrates. Anal. Methods 2015, 7, 5289–5294. [Google Scholar] [CrossRef]
- Rowiński, P.M.; Chrzanowski, M.M. Influence of Selected Fluorescent Dyes on Small Aquatic Organisms. Acta Geophys. 2011, 59, 91–109. [Google Scholar] [CrossRef]
- Ashfaq, M.H.; Imran, M.; Haider, A.; Shahzadi, A.; Mustajab, M.; Ul-Hamid, A.; Nabgan, W.; Medina, F.; Ikram, M. Antimicrobial Potential and Rhodamine B Dye Degradation Using Graphitic Carbon Nitride and Polyvinylpyrrolidone Doped Bismuth Tungstate Supported with in Silico Molecular Docking Studies. Sci. Rep. 2023, 13, 17847. [Google Scholar] [CrossRef]
- Singh, S.; Parveen, N.; Gupta, H. Adsorptive Decontamination of Rhodamine-B from Water Using Banana Peel Powder: A Biosorbent. Environ. Technol. Innov. 2018, 12, 189–195. [Google Scholar] [CrossRef]
- Lu, Q.; Gao, W.; Du, J.; Zhou, L.; Lian, Y. Discovery of Environmental Rhodamine B Contamination in Paprika during the Vegetation Process. J. Agric. Food Chem. 2012, 60, 4773–4778. [Google Scholar] [CrossRef]
- Priya, P.S.; Pratiksha Nandhini, P.; Vaishnavi, S.; Pavithra, V.; Almutairi, M.H.; Almutairi, B.O.; Arokiyaraj, S.; Pachaiappan, R.; Arockiaraj, J. Rhodamine B, an Organic Environmental Pollutant Induces Reproductive Toxicity in Parental and Teratogenicity in F1 Generation in Vivo. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2024, 280, 109898. [Google Scholar] [CrossRef]
- Umeda, M. Experimental Study of Xanthene Dyes as Carcinogenic Agents. Gann 1956, 47, 51–78_3. [Google Scholar]
- Zhang, X.; Gan, X.; Cao, S.; Shang, J.; Cheng, X. Efficient Removal of Rhodamine B in Wastewater via Activation of Persulfate by MnO2 with Different Morphologies. Water 2023, 15, 735. [Google Scholar] [CrossRef]
- Li, K.; Zhang, P.; Ge, L.; Ren, H.; Yu, C.; Chen, X.; Zhao, Y. Concentration-Dependent Photodegradation Kinetics and Hydroxyl-Radical Oxidation of Phenicol Antibiotics. Chemosphere 2014, 111, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Maita, K.; Kuwahara, M.; Kosaka, T.; Inui, K.; Sugimoto, K.; Takeuchi, Y.; Hatakenaka, N.; Harada, T.; Yasuhara, K.; Mitsumori, K. Testicular Toxicity of Thiamphenicol in Sprague-Dawley Rats. J. Toxicol. Pathol. 1999, 12, 27. [Google Scholar] [CrossRef][Green Version]
- Sim, W.-J.; Lee, J.-W.; Lee, E.-S.; Shin, S.-K.; Hwang, S.-R.; Oh, J.-E. Occurrence and Distribution of Pharmaceuticals in Wastewater from Households, Livestock Farms, Hospitals and Pharmaceutical Manufactures. Chemosphere 2011, 82, 179–186. [Google Scholar] [CrossRef]
- Abdallat, G.A.; Salameh, E.; Shteiwi, M.; Bardaweel, S. Pharmaceuticals as Emerging Pollutants in the Reclaimed Wastewater Used in Irrigation and Their Effects on Plants, Soils, and Groundwater. Water 2022, 14, 1560. [Google Scholar] [CrossRef]
- Tarigan, A.Y.; Effendi, A.J. Kinetic Study of Paracetamol Degradation with Advanced Oxidation Process (AOP) Combination of Ozone, Hydrogen Peroxide and Ultraviolet (O3/H2O2/UV). J. Multidisiplin Madani 2024, 4, 518–527. [Google Scholar] [CrossRef]
- Snyder, S.A.; Adham, S.; Redding, A.M.; Cannon, F.S.; DeCarolis, J.; Oppenheimer, J.; Wert, E.C.; Yoon, Y. Role of Membranes and Activated Carbon in the Removal of Endocrine Disruptors and Pharmaceuticals. Desalination 2007, 202, 156–181. [Google Scholar] [CrossRef]
- Belgiorno, V.; Rizzo, L.; Fatta, D.; Della Rocca, C.; Lofrano, G.; Nikolaou, A.; Naddeo, V.; Meric, S. Review on Endocrine Disrupting-Emerging Compounds in Urban Wastewater: Occurrence and Removal by Photocatalysis and Ultrasonic Irradiation for Wastewater Reuse. Desalination 2007, 215, 166–176. [Google Scholar] [CrossRef]
- Samghouli, N.; Regraguy, B.; Abahdou, F.-Z.; Azoulay, K.; Bencheikh, I.; Mabrouki, J.; Hajjaji, S.E. Removal of a Non-Steroidal Anti-Inflammatory Drug (Piroxicam) in an Aqueous Medium by an Agricultural by-Product. E3S Web Conf. 2022, 337, 05001. [Google Scholar] [CrossRef]
- Kathi, S.; El Din Mahmoud, A. Trends in Effective Removal of Emerging Contaminants from Wastewater: A Comprehensive Review. Desalination Water Treat. 2024, 317, 100258. [Google Scholar] [CrossRef]
- Belahrach, B.; Farah, M.; Samghouli, N.; Gadda, N.; Bensemlali, M.; Labjar, N.; Benabdellah, G.A.; Hajjaji, S.E. Investigation of Equilibrium and Kinetic Aspects of Methylene Blue Dye Adsorption from Aqueous Solutions Using Raw and Extract of Eriobotrya Japonica Seeds. Euro-Mediterr. J. Environ. Integr. 2025, 10, 2197–2211. [Google Scholar] [CrossRef]
- Gadda, N.; Benabdallah, G.A.; Belahrach, B.; Bendany, M.; Labjar, N.; Samghouli, N.; Dahrouch, A.; Hajjaji, S.E. Investigation of Equilibrium and Kinetics in the Removal of Methylene Blue from Aqueous Solutions Using Chamaerops Humilis Fruit. Moroc. J. Chem. 2024, 12, 1446–1461. [Google Scholar] [CrossRef]
- Samghouli, N.; Rghioui, L.; Sebbahi, S.; El Hajji, A.; Guennoun, L.; Khaoulaf, R.; Serghini Idrissi, M.; El Hajjaji, S. Removal of Methylene Blue from Aqueous Solution by Loquats Nuclei. Bulg. Chem. Commun. 2025, 57, 85–93. [Google Scholar]
- Azoulay, K.; Bencheikh, I.; Mabrouki, J.; Samghouli, N.; Moufti, A.; Dahchour, A.; Hajjaji, S.E. Adsorption Mechanisms of Azo Dyes Binary Mixture onto Different Raw Palm Wastes. Int. J. Environ. Anal. Chem. 2021, 0, 1–20. [Google Scholar] [CrossRef]
- Lam, A.; Rivera, A.; Rodrídguez-Fuentes, G. Theoretical Study of Metronidazole Adsorption on Clinoptilolite. Microporous Mesoporous Mater. 2001, 49, 157–162. [Google Scholar] [CrossRef]
- Samghouli, N.; Bencheikh, I.; Azoulay, K.; El Hajjaji, S.; Labjar, N. Optimisation of Olea Europaea Stone—Activated Carbon Preparation Using Response Surface Methodology for Thiamphenicol Removal in Fixed Bed Column. Ecol. Eng. Environ. Technol. 2024, 25, 37–53. [Google Scholar] [CrossRef]
- Masiya, T.T.; Gudyanga, F.P. Investigation of Granular Activated Carbon from Peach Stones for Gold Adsorption in Acidic Thiourea. In Proceedings of the Hydrometallurgy Conference, Muldersdrift, South Africa, 24–26 February 2009; pp. 465–474. [Google Scholar]
- Vohra, M.S. Adsorption-Based Removal of Gas-Phase Benzene Using Granular Activated Carbon (GAC) Produced from Date Palm Pits. Arab. J. Sci. Eng. 2015, 40, 3007–3017. [Google Scholar] [CrossRef]
- Huggins, T.M.; Haeger, A.; Biffinger, J.C.; Ren, Z.J. Granular Biochar Compared with Activated Carbon for Wastewater Treatment and Resource Recovery. Water Res. 2016, 94, 225–232. [Google Scholar] [CrossRef]
- Jjagwe, J.; Olupot, P.W.; Menya, E.; Kalibbala, H.M. Synthesis and Application of Granular Activated Carbon from Biomass Waste Materials for Water Treatment: A Review. J. Bioresour. Bioprod. 2021, 6, 292–322. [Google Scholar] [CrossRef]
- Li, R.; Sun, W.; Xia, L.; U, Z.; Sun, X.; Wang, Z.; Wang, Y.; Deng, X. Adsorption of Toxic Tetracycline, Thiamphenicol and Sulfamethoxazole by a Granular Activated Carbon (GAC) under Different Conditions. Molecules 2022, 27, 7980. [Google Scholar] [CrossRef]
- Bera, A.; Kumar, T.; Ojha, K.; Mandal, A. Adsorption of Surfactants on Sand Surface in Enhanced Oil Recovery: Isotherms, Kinetics and Thermodynamic Studies. Appl. Surf. Sci. 2013, 284, 87–99. [Google Scholar] [CrossRef]
- Sahmoune, M.N.; Louhab, K.; Boukhiar, A. Studies of Chromium Removal from Tannery Effluents by Dead Streptomyces Rimosus. Chem. Product Process Model. 2008, 3. [Google Scholar] [CrossRef]
- Mukherjee, S.; Halder, G. A Review on the Sorptive Elimination of Fluoride from Contaminated Wastewater. J. Environ. Chem. Eng. 2018, 6, 1257–1270. [Google Scholar] [CrossRef]
- Sahmoune, M.N.; Louhab, K.; Boukhiar, A. Biosorption of Cr (III) from Aqueous Solutions Using Bacterium Biomass Streptomyces Rimosus. Int. J. Environ. Res. 2009, 3, 229–238. [Google Scholar]
- Simonin, J.-P. On the Comparison of Pseudo-First Order and Pseudo-Second Order Rate Laws in the Modeling of Adsorption Kinetics. J. Chem. Eng. 2016, 300, 254–263. [Google Scholar] [CrossRef]
- Sahmoune, M.N.; Louhab, K.; Boukhiar, A.; Addad, J.; Barr, S. Kinetic and Equilibrium Models for the Biosorption of Cr(III) on Streptomyces rimosus. Toxicol. Environ. Chem. 2009, 91, 1291–1303. [Google Scholar] [CrossRef]
- Kajjumba, G.W.; Emik, S.; Öngen, A.; Özcan, H.K.; Aydın, S. Modelling of Adsorption Kinetic Processes—Errors, Theory and Application. Adv. Sorpt. Process Appl. 2018, 1–19. [Google Scholar]
- Hossain, M.A.; Ngo, H.H.; Guo, W.S.; Setiadi, T. Adsorption and Desorption of Copper(II) Ions onto Garden Grass. Bioresour. Technol. 2012, 121, 386–395. [Google Scholar] [CrossRef] [PubMed]
- McKay, G.; El-Geundi, M.; Nassar, M.M. Equilibrium Studies for the Adsorption of Dyes on Bagasse Pith. Adsorpt. Sci. Technol. 1997, 15, 251–270. [Google Scholar] [CrossRef]
- Kovalova, L.; Knappe, D.R.U.; Lehnberg, K.; Kazner, C.; Hollender, J. Removal of Highly Polar Micropollutants from Wastewater by Powdered Activated Carbon. Environ. Sci. Pollut. Res. 2013, 20, 3607–3615. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Mayer, B.K.; McNamara, P.J. Adsorption of Organic Micropollutants to Biosolids-Derived Biochar: Estimation of Thermodynamic Parameters. Environ. Sci.: Water Res. Technol. 2019, 5, 1132–1144. [Google Scholar] [CrossRef]
- Dubinin, M.M.; Astakhov, V.A. Development of the Concepts of Volume Filling of Micropores in the Adsorption of Gases and Vapors by Microporous Adsorbents. Russ. Chem. Bull. 1971, 20, 3–7. [Google Scholar] [CrossRef]
- Vadi, M.; Mansoorabad, A.; Mohammadi, M.; Rostami, N. Investigation of Langmuir, Freundlich and Temkin Adsorption Isotherm of Tramadol by Multi-Wall Carbon Nanotube. Asian J. Chem. 2013, 25, 5467. [Google Scholar]
- Guo, J.-Z.; Li, B.; Liu, L.; Lv, K. Removal of Methylene Blue from Aqueous Solutions by Chemically Modified Bamboo. Chemosphere 2014, 111, 225–231. [Google Scholar] [CrossRef]
- Fu, M.; Perlman, M.; Lu, Q.; Varga, C. Pharmaceutical Solid-State Kinetic Stability Investigation by Using Moisture-Modified Arrhenius Equation and JMP Statistical Software. J. Pharm. Biomed. Anal. 2015, 107, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Samghouli, N.; Bencheikh, I.; Azoulay, K.; Abahdou, F.-Z.; Mabrouki, J.; El Hajjaji, S. Study of Piroxicam Removal from Wastewater by Artichoke Waste Using NemrodW® Software: Statistical Analysis. In IoT and Smart Devices for Sustainable Environment; Springer: Cham, Switzerland, 2022; pp. 29–42. ISBN 978-3-030-90082-3. [Google Scholar]
- Bencheikh, I.; Azoulay, K.; Samghouli, N.; Mabrouki, J.; Bouhachlaf, L.; Moufti, A.; Hajjaji, S.E. Mathematical and Statistical Study for the Wastewater Adsorbent Regeneration Using the Central Composite Design. In IoT and Smart Devices for Sustainable Environment; Springer: Cham, Switzerland, 2022; pp. 71–83. [Google Scholar]
- Azoulay, K.; Bencheikh, I.; Samghouli, N.; Mabrouki, J.; Moufti, A.; Hajjaji, S.E. Modeling and Design of Water Treatment Processes by Biosorption Method Using JMP® 11 Software. In IoT and Smart Devices for Sustainable Environment; Springer: Cham, Switzerland, 2022; pp. 53–69. [Google Scholar]
- Ouasif, H.; Yousfi, S.; Bouamrani, M.; Talbi, M.; Tarik, A.; el Kouali, M. Comparative Study of Adsorption of Methylene Blue in Two Natural Materials (Etude Comparative Du Phénomène D’adsorption Du Bleu De Méthylène Sur Deux Matériaux Adsorbants Naturels). Phys. Chem. News 2011, 58, 1–5. [Google Scholar]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the Use and Interpretation of Adsorption Isotherm Models: A Review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef]
- Ghibate, R.; Senhaji, O.; Taouil, R. Kinetic and Thermodynamic Approaches on Rhodamine B Adsorption onto Pomegranate Peel. Case Stud. Chem. Environ. Eng. 2021, 3, 100078. [Google Scholar] [CrossRef]
- Samghouli, N.; Bencheikh, I.; Azoulay, K.; Jansson, S.; El Hajjaji, S. Mechanistic and Reactional Activation Study of Carbons Destined for Emerging Pharmaceutical Pollutant Adsorption. Environ. Monit. Assess. 2025, 197, 259. [Google Scholar] [CrossRef] [PubMed]
- Mohamed Idris, Z.; Hameed, B.; Ye, L.; Hajizadeh, S.; Mattiasson, B.; Mohd Din, A.T. Amino-Functionalised Silica-Grafted Molecularly Imprinted Polymers for Chloramphenicol Adsorption. J. Environ. Chem. Eng. 2020, 8, 103981. [Google Scholar] [CrossRef]
- Yi, L.; Zuo, L.; Wei, C.; Fu, H.; Qu, X.; Zheng, S.; Xu, Z.; Guo, Y.; Li, H.; Zhu, D. Enhanced Adsorption of Bisphenol A, Tylosin, and Tetracycline from Aqueous Solution to Nitrogen-Doped Multiwall Carbon Nanotubes via Cation-π and π-π Electron-Donor-Acceptor (EDA) Interactions. Sci. Total Environ. 2020, 719, 137389. [Google Scholar] [CrossRef]
- Chen, A.; Pang, J.; Wei, X.; Chen, B.; Xie, Y. Fast One-Step Preparation of Porous Carbon with Hierarchical Oxygen-Enriched Structure from Waste Lignin for Chloramphenicol Removal. Environ. Sci. Pollut. Res. 2021, 28, 27398–27410. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Yang, X.; Xu, Z.; Hu, W.; Zhou, Y.; Wan, Z.; Yang, Y.; Wei, Y.; Yang, J.; Tsang, D.C.W. Fabrication of Sustainable Manganese Ferrite Modified Biochar from Vinasse for Enhanced Adsorption of Fluoroquinolone Antibiotics: Effects and Mechanisms. Sci. Total Environ. 2020, 709, 136079. [Google Scholar] [CrossRef]




| Generic Name | Thiamphenicol | Rhodamine B |
|---|---|---|
| Family | Phenicol | xanthene |
| Therapeutic category | Antibiotic | Dye |
| Molecular formula | C12H15Cl2NO5S | C28H31ClN2O3 |
| Molar mass (g/mol) | 356.21 | 479.02 |
| λmax (nm) | 225 | 553 |
| Solubility in water (mg/L) | 2270 | 15,000 |
| Chemical structure | 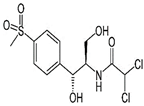 | 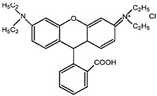 |
| Variables | Units | Coded Symbol | Levels | ||||
|---|---|---|---|---|---|---|---|
| −α | −1 | 0 | +1 | +α | |||
| pH | - | X1 | 2.22 | 3 | 5.7 | 8.4 | 9.18 |
| Initial pollutant concentration | mg/L | X2 | 2.49 | 5 | 13.75 | 22.5 | 25 |
| Dose | g/L | X3 | 0.85 | 2 | 6 | 10 | 11.14 |
| Run | Actual Parameters | ||
|---|---|---|---|
| X1 | X2 | X3 | |
| pH | Initial Pollutant Concentration (mg/L) | Dose (g/L) | |
| 1 | 2.22 | 13.75 | 6 |
| 2 | 3 | 5 | 2 |
| 3 | 3 | 5 | 10 |
| 4 | 3 | 22.49 | 2 |
| 5 | 3 | 22.49 | 10 |
| 6 | 5.7 | 2.49 | 6 |
| 7 | 5.7 | 13.75 | 0.85 |
| 8 | 5.7 | 13.75 | 6 |
| 9 | 5.7 | 13.75 | 6 |
| 10 | 5.7 | 13.75 | 6 |
| 11 | 5.7 | 13.75 | 11.15 |
| 12 | 5.7 | 25 | 6 |
| 13 | 8.4 | 5 | 2 |
| 14 | 8.4 | 5 | 10 |
| 15 | 8.4 | 22.49 | 2 |
| 16 | 8.4 | 22.49 | 10 |
| 17 | 9.18 | 13.75 | 6 |
| Run | Coded Level | Actual Parameters | Responses | |||||
|---|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | ||||||
| X1 | X2 | X3 | pH | [Pollutant] (mg/L) | Dose (g/L) | RTHI (%) | RRhB (%) | |
| 1 | −1.29 | 0 | 0 | 2.23 | 13.75 | 6 | 26.9 | 35.79 |
| 2 | −1 | −1 | −1 | 3 | 5 | 2 | 47.87 | 42.23 |
| 3 | −1 | −1 | 1 | 3 | 5 | 10 | 59.53 | 56.97 |
| 4 | −1 | 1 | −1 | 3 | 22.49 | 2 | 10.49 | 11.39 |
| 5 | −1 | 1 | 1 | 3 | 22.49 | 10 | 43.52 | 16.88 |
| 6 | 0 | −1.29 | 0 | 5.7 | 2.49 | 6 | 70.83 | 81.9 |
| 7 | 0 | 0 | −1.29 | 5.7 | 13.75 | 0.85 | 4.74 | 4.49 |
| 8 | 0 | 0 | 0 | 5.7 | 13.75 | 6 | 14.09 | 28.28 |
| 9 | 0 | 0 | 0 | 5.7 | 13.75 | 6 | 14.74 | 28.32 |
| 10 | 0 | 0 | 0 | 5.7 | 13.75 | 6 | 14.54 | 28.39 |
| 11 | 0 | 0 | 1.29 | 5.7 | 13.75 | 11.15 | 51.81 | 40.62 |
| 12 | 0 | 1.29 | 0 | 5.7 | 25 | 6 | 11.39 | 9.54 |
| 13 | 1 | −1 | −1 | 8.4 | 5 | 2 | 27.62 | 22.65 |
| 14 | 1 | −1 | 1 | 8.4 | 5 | 10 | 48.50 | 48.69 |
| 15 | 1 | 1 | −1 | 8.4 | 22.49 | 2 | 6.43 | 0.87 |
| 16 | 1 | 1 | 1 | 8.4 | 22.49 | 10 | 24.61 | 2.29 |
| 17 | 1.29 | 0 | 0 | 9.18 | 13.75 | 6 | 12.89 | 17.83 |
| Source | DF | Sum of Squares | Mean Square | F Ratio | ||||
|---|---|---|---|---|---|---|---|---|
| THI | RhB | THI | RhB | THI | RhB | THI | RhB | |
| Model | 9 | 9 | 6244.97 | 6871.36 | 693.89 | 763.48 | 8.45 | 9.79 |
| Error | 7 | 7 | 575.16 | 546.10 | 82.17 | 78.02 | p-value | |
| C. Total | 16 | 16 | 6820.13 | 7417.46 | - | 0.0051 * | 0.0033 * | |
| Term | THI | RhB | ||||
|---|---|---|---|---|---|---|
| Estimate | t Ratio | p-Value | Estimate | t Ratio | p-Value | |
| Intercept | 17.06 | 3.75 | 0.0071 * | 31.11 | 7.03 | 0.0002 * |
| pH (3; 8.4) | −6.39 | −2.37 | 0.0496 * | −6.73 | −2.56 | 0.0375 * |
| [Pollutant]i (5; 22.49) (mg/L) | −15.47 | −5.74 | 0.0007 * | −20.53 | −7.82 | 0.0001 * |
| Dose (2; 10) (g/L) | 12.76 | 4.73 | 0.0021 * | 8.33 | 3.17 | 0.0157 * |
| pH * × [Pollutant]i (mg/L) | 1.04 | 0.32 | 0.76 | 0.34 | 0.11 | 0.92 |
| pH × Dose | −0.71 | −0.22 | 0.83 | 0.91 | 0.29 | 0.78 |
| [Pollutant]i × Dose | 2.33 | 0.73 | 0.49 | −4.23 | −1.36 | 0.22 |
| pH × pH | −0.05 | −0.01 | 0.99 | −4.47 | −1.23 | 0.26 |
| [Pollutant]i × [Pollutant]i | 12.76 | 3.42 | 0.0112 * | 6.94 | 1.91 | 0.09 |
| Dose × Dose | 5.01 | 1.34 | 0.22 | −7.04 | −1.94 | 0.09 |
| Kinetic Models | Parameters | Adsorbate | ||
|---|---|---|---|---|
| THI | RhB | |||
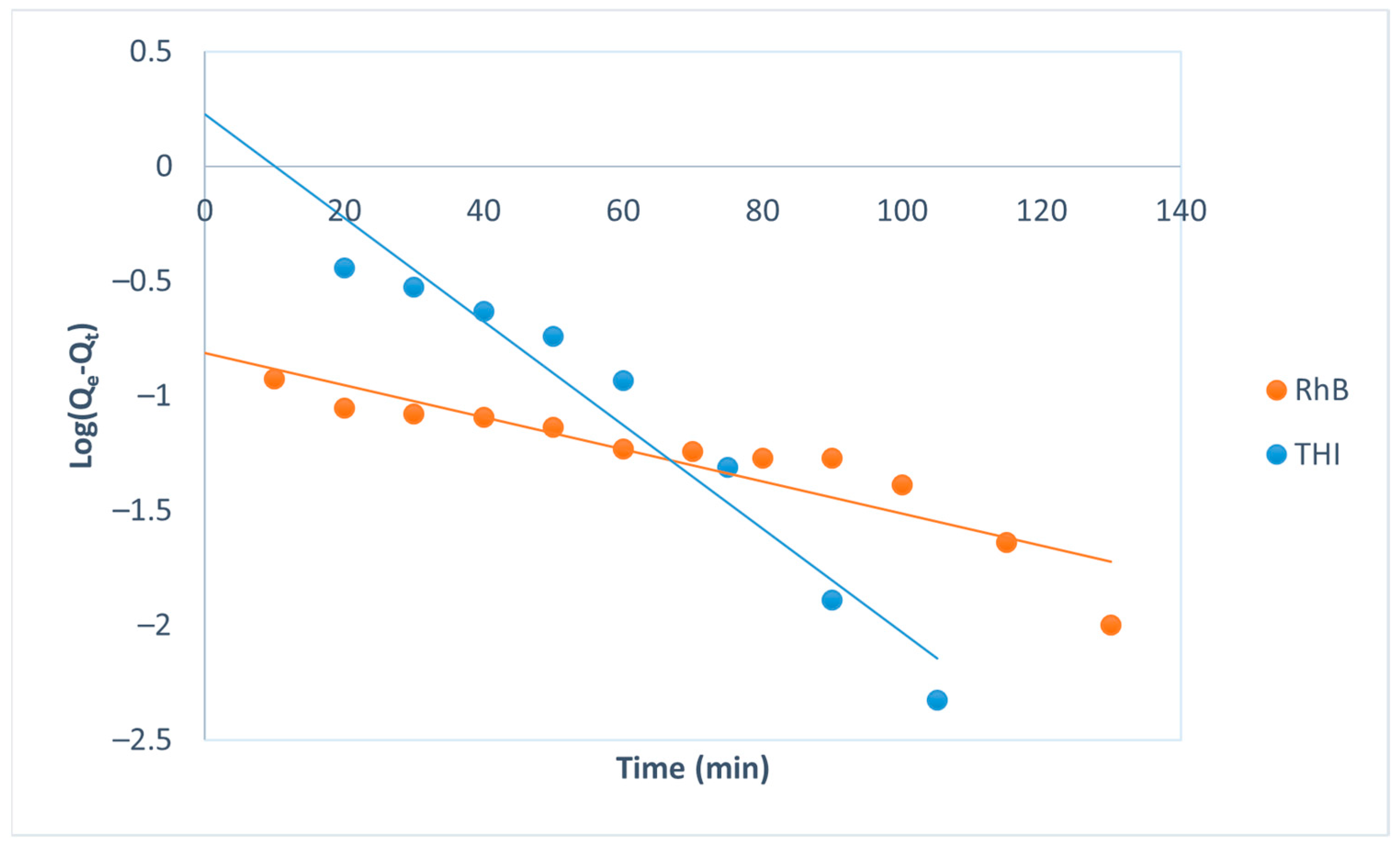
| Pseudo-first-order Log(Qe − Qt) = log(Qe) − t | R2 | 0.95 | 0.84 | |
| k1 (min−1) | 0.052 | 0.016 | ||
| Qth (mg/g) | 1.68 | 0.15 | ||
| Qexp (mg/g) | 0.6 | 0.34 | ||
| Δq = qe theo − qe exp (mg/g) | 1.08 | 0.19 | ||
| ARE (%) | 207.5 | 68.4 | ||
| SSE | 17.74 | 0.41 | ||
| NSD (%) | 101.8 | 67.6 | ||
| χ2 | 11.17 | 1.52 | ||
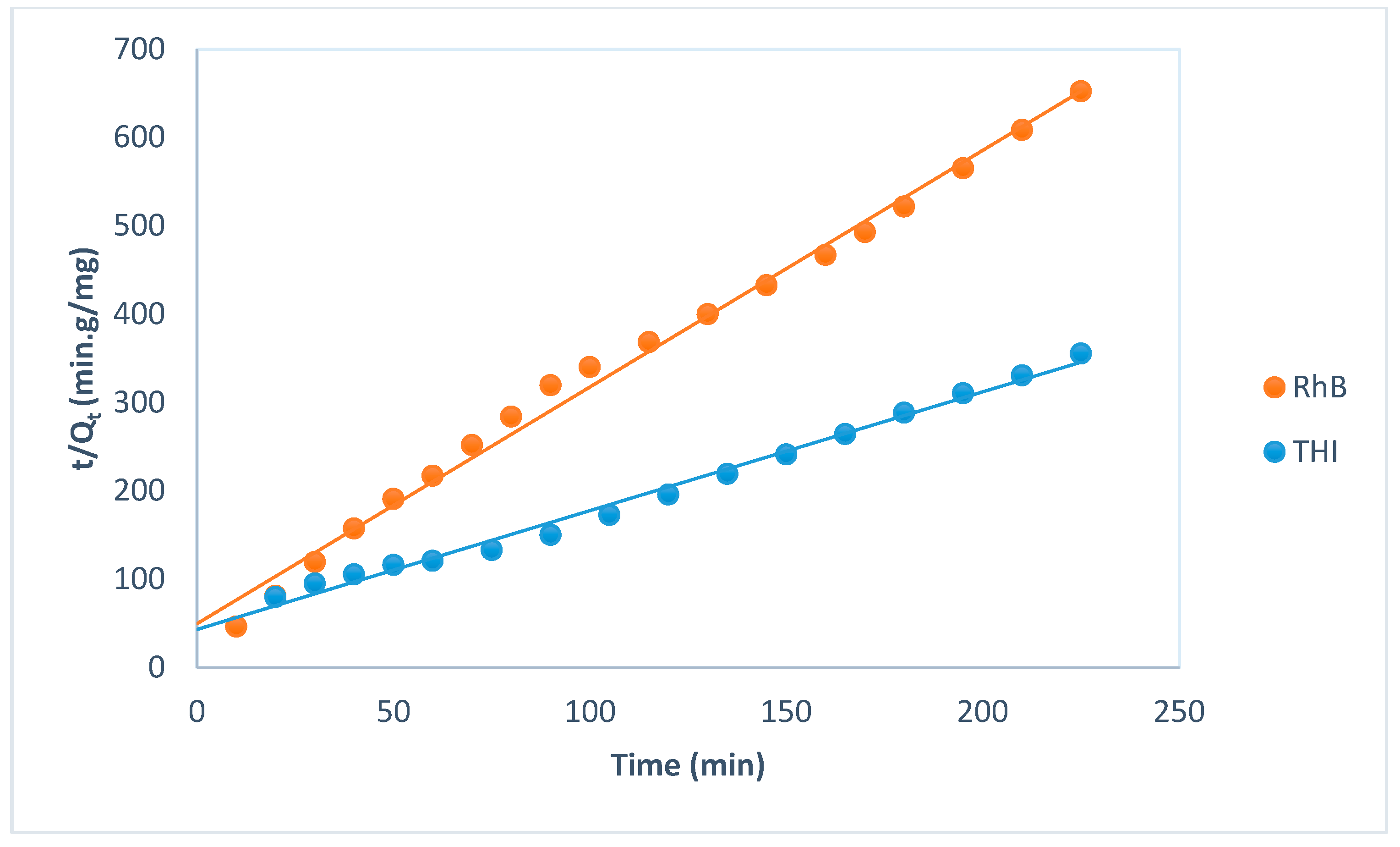
| Pseudo-second-order | R2 | 0.9912 | 0.993 | |
| k2(g·mg−1·min−1) | 0.042 | 0.145 | ||
| Qth (mg/g) | 0.74 | 0.37 | ||
| Qexp (mg/g) | 0.6 | 0.34 | ||
| Δq = qe theo − qe exp (mg/g) | 0.14 | 0.03 | ||
| ARE (%) | 5.1 | 8.9 | ||
| SSE | 0.013 | 0.013 | ||
| NSD (%) | 5.5 | 12.0 | ||
| χ2 | 0.028 | 0.056 | ||
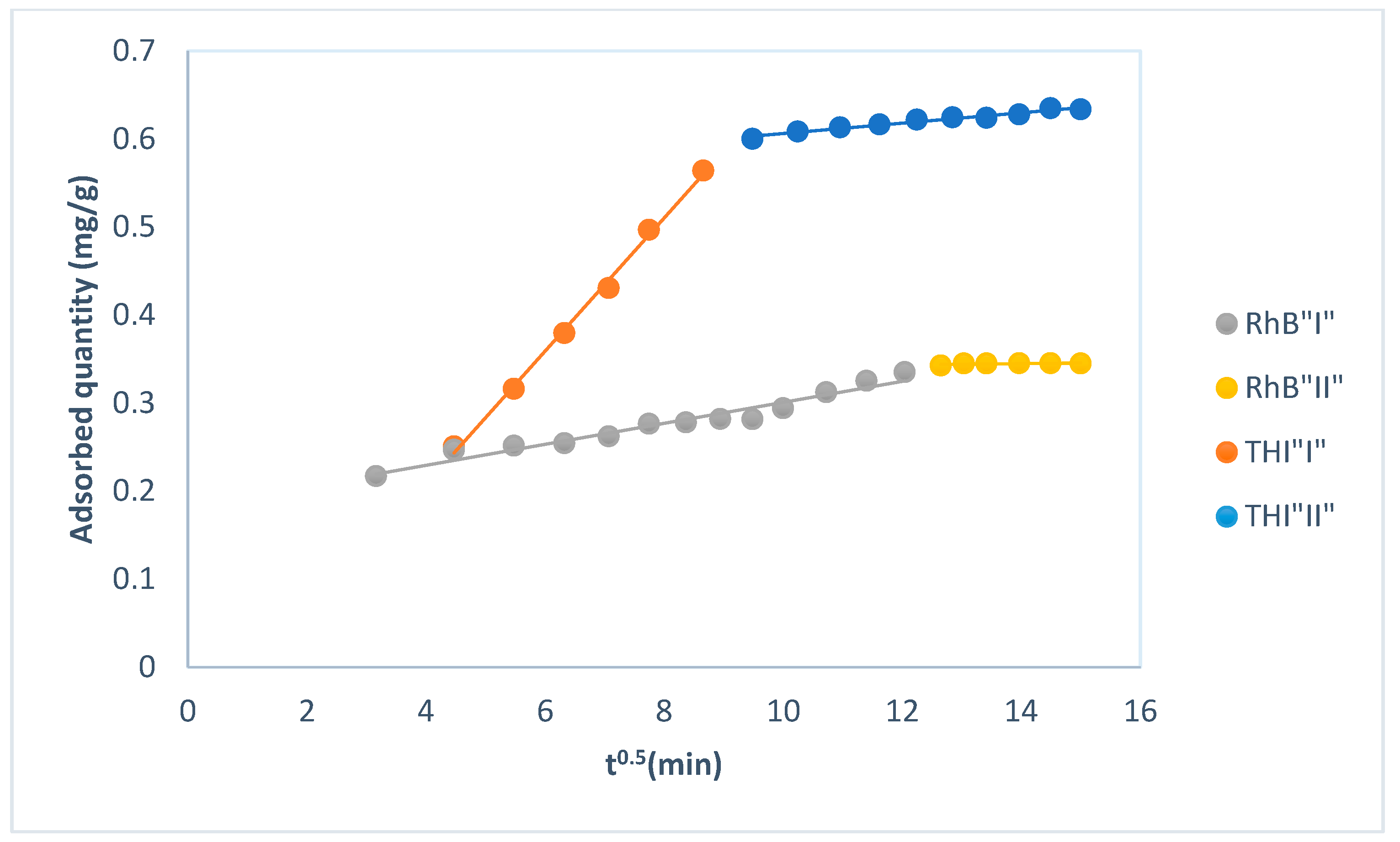
| Intraparticle diffusion Q = Kintt1/2 + C | Type I | R2 | 0.9967 | 0.9527 |
| Kint(mg·g−1·min−1/2) | 0.08 | 0.01 | ||
| CI | −0.09 | 0.18 | ||
| ARE (%) | 31.5 | 5.7 | ||
| SSE | 0.99 | 0.0036 | ||
| NSD (%) | 40.2 | 6.3 | ||
| χ2 | 0.99 | 0.013 | ||
| Type II | R2 | 0.9657 | 0.4373 | |
| Kint(mg·g−1·min−1/2) | 0.0059 | 0.0007 | ||
| CII | 0.55 | 0.33 | ||
| ARE (%) | 21.1 | 24.0 | ||
| SSE | 0.56 | 0.055 | ||
| NSD (%) | 32.5 | 24.7 | ||
| χ2 | 0.99 | 0.219 | ||
| Isotherms | Parameters | Adsorbate | |
|---|---|---|---|
| THI | RhB | ||
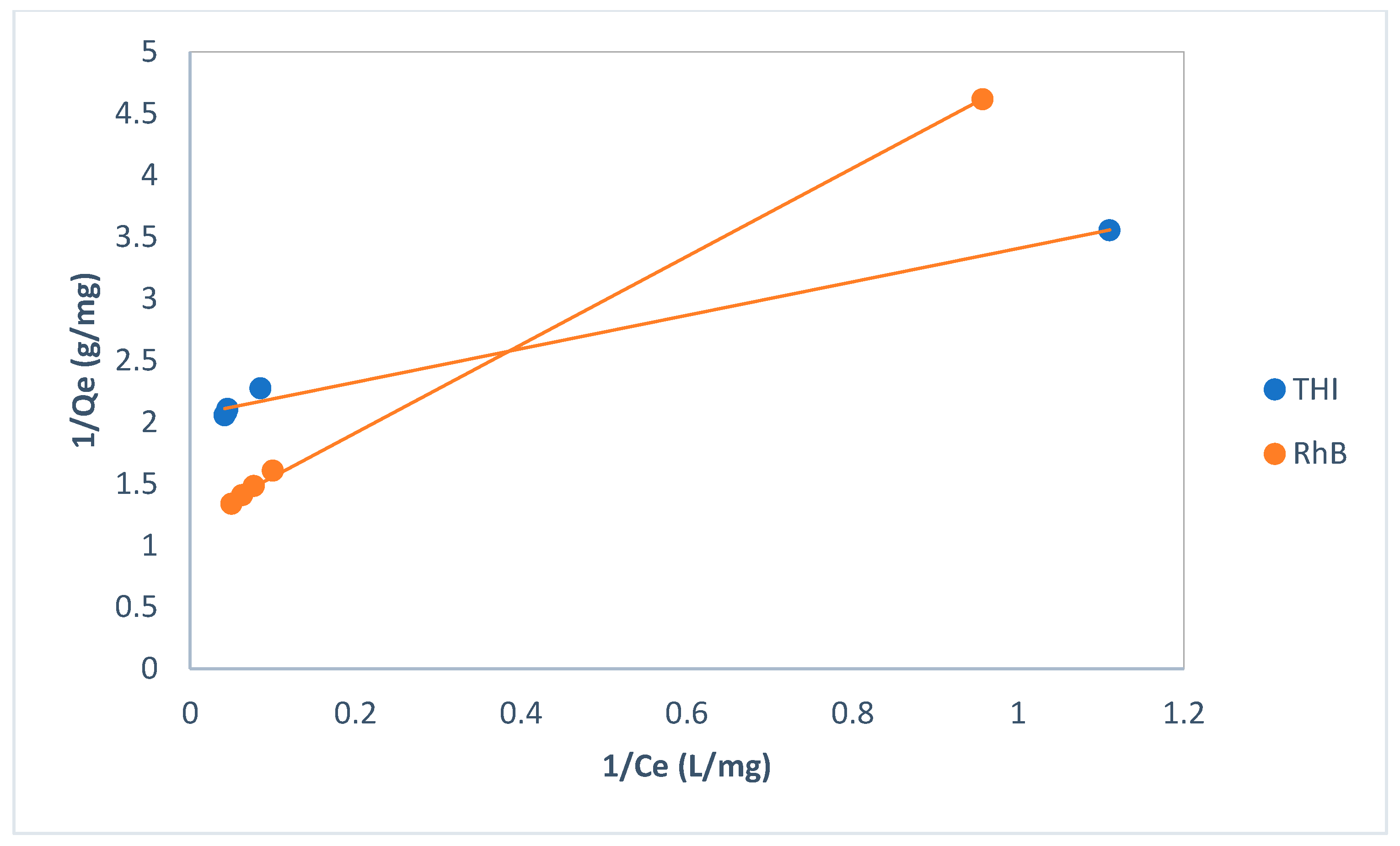
| Langmuir | Qm,cal (mgg−1) | 0.49 | 0.83 |
| KL | 1.51 | 0.34 | |
| RL | 0.12 | 0.37 | |
| R2 | 0.9909 | 0.9994 | |
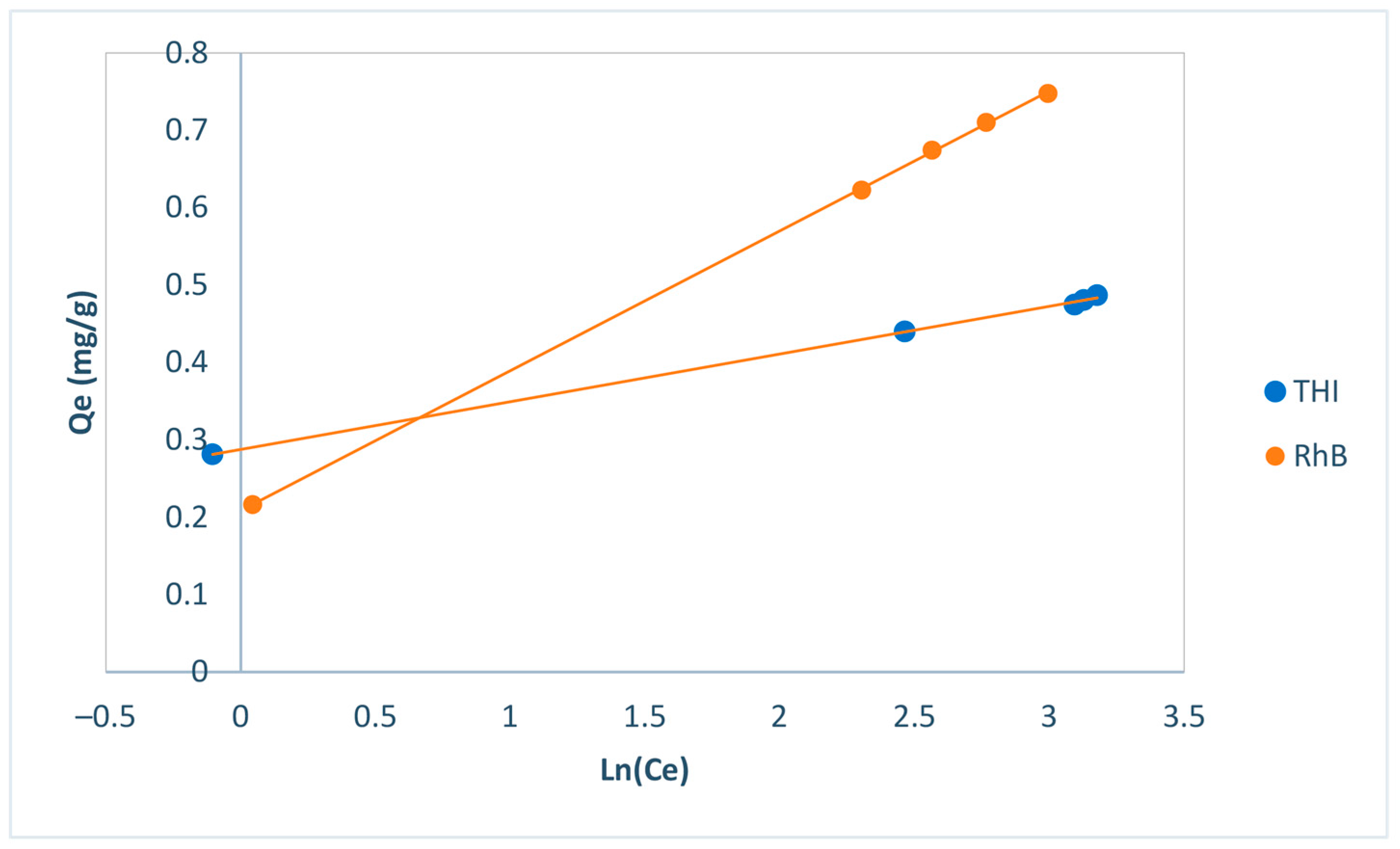
| Freundlich | 1/nf | 0.1658 | 0.43 |
| Kf | 0.29 | 0.21 | |
| R2 | 0.9978 | 0.9927 | |
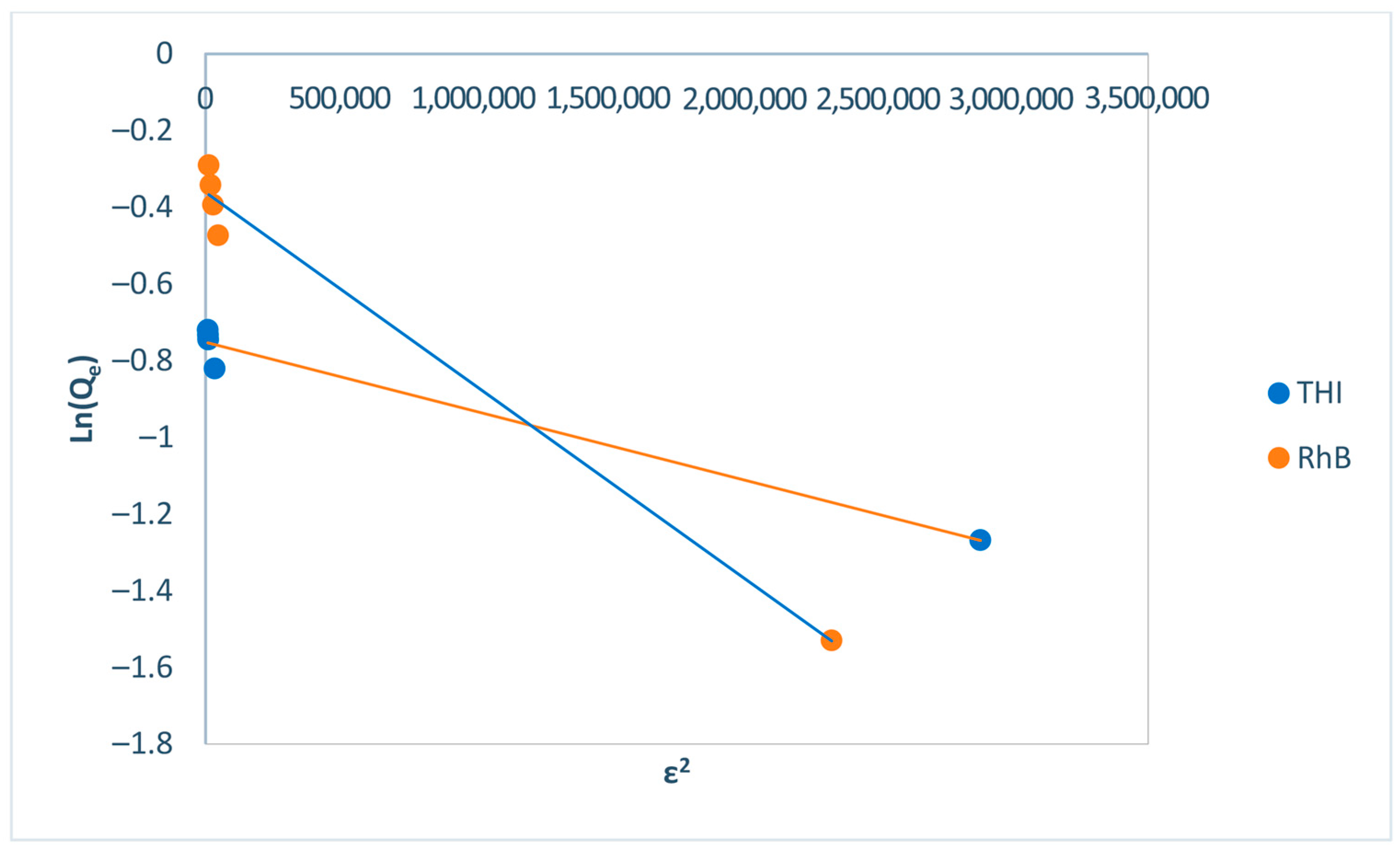
| Dubinin–Radushkevich Ln Qe = Ln Qm − β.ɛ2 | E (KJ/mol) | 1581.14 | 1000 |
| Qm (mg/g) | 0.47 | 0.69 | |
| β (KJ2/mol2) | 2×10−7 | 5×10−7 | |
| R2 | 0.97 | 0.99 | |
| Temkin Qe = BT Ln(KT × Ce) | BT (J/mol) | 0.0616 | 0.1807 |
| KT (L/g) | 106.92 | 3.17 | |
| R2 | 0.9992 | 0.9999 | |
| Adsorbate | ΔH° (KJ·mol−1) | ΔS° (J·mol−1·K−1) | ΔG° (KJ·mol−1) | ||
|---|---|---|---|---|---|
| 303 °K | 313°K | 323°K | |||
| THI | −51.27 | −173.9 | 1.21 | 3.60 | 4.66 |
| RhB | 43.5 | 130.36 | 3.66 | 3.40 | 1.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samghouli, N.; Labjar, N.; Bensemlali, M.; Nasrellah, H.; El Hajjaji, S. Optimization of Organic Micropollutant Adsorption onto Granular Activated Carbon Using Response Surface Methodology. Separations 2025, 12, 254. https://doi.org/10.3390/separations12090254
Samghouli N, Labjar N, Bensemlali M, Nasrellah H, El Hajjaji S. Optimization of Organic Micropollutant Adsorption onto Granular Activated Carbon Using Response Surface Methodology. Separations. 2025; 12(9):254. https://doi.org/10.3390/separations12090254
Chicago/Turabian StyleSamghouli, Nora, Najoua Labjar, Meryem Bensemlali, Hamid Nasrellah, and Souad El Hajjaji. 2025. "Optimization of Organic Micropollutant Adsorption onto Granular Activated Carbon Using Response Surface Methodology" Separations 12, no. 9: 254. https://doi.org/10.3390/separations12090254
APA StyleSamghouli, N., Labjar, N., Bensemlali, M., Nasrellah, H., & El Hajjaji, S. (2025). Optimization of Organic Micropollutant Adsorption onto Granular Activated Carbon Using Response Surface Methodology. Separations, 12(9), 254. https://doi.org/10.3390/separations12090254





