A Focus on the Emission of Volatile Organic Compounds (VOCs) from Raw Materials Potentially Used in Human Odor Sampling
Abstract
1. Introduction
2. Materials and Method
2.1. Materials
2.2. Thermodesorption
2.3. GC×GC/ToFMS
2.4. Data Acquisition and Treatment
3. Results and Discussion
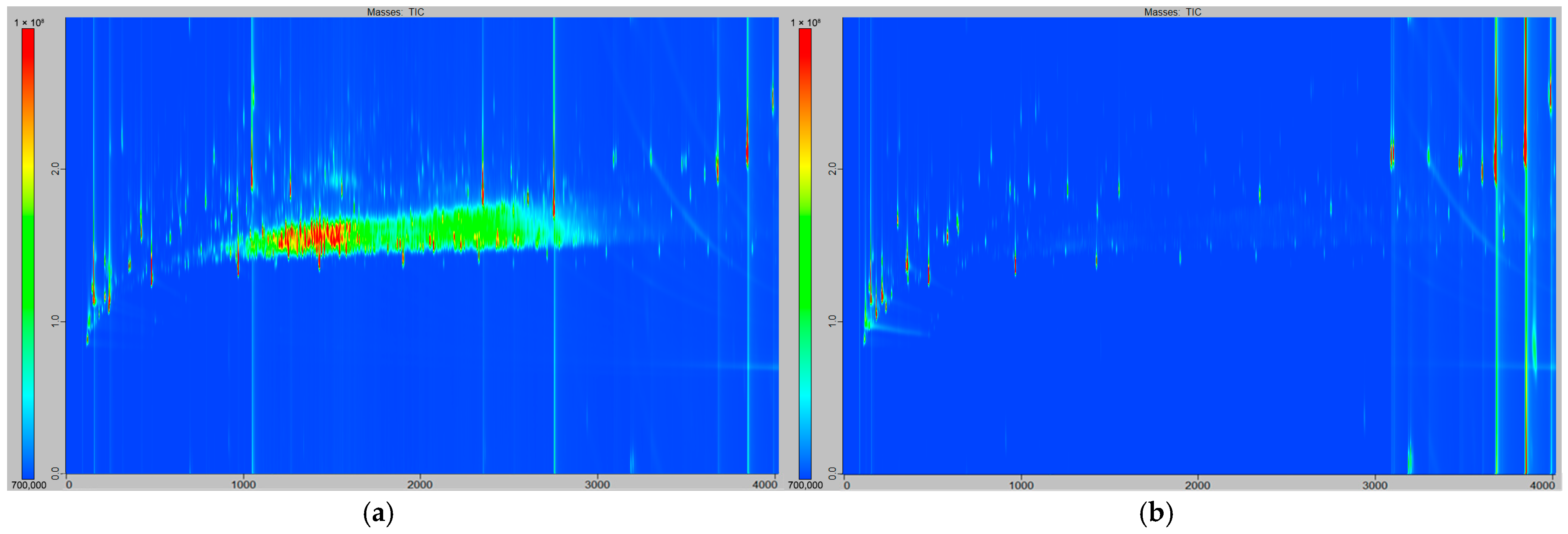
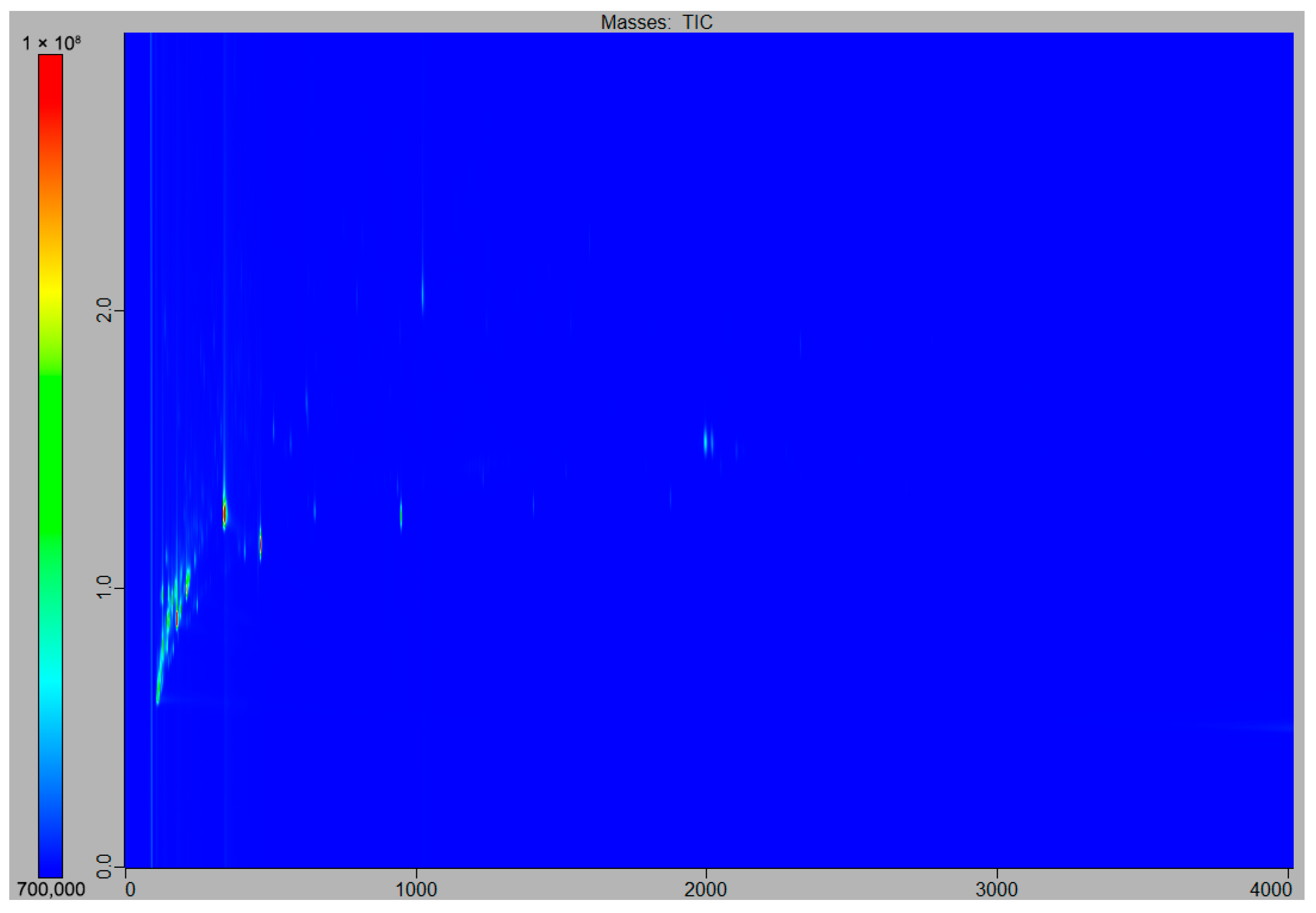
3.1. Preliminary Results: Cleaning Step Evaluation
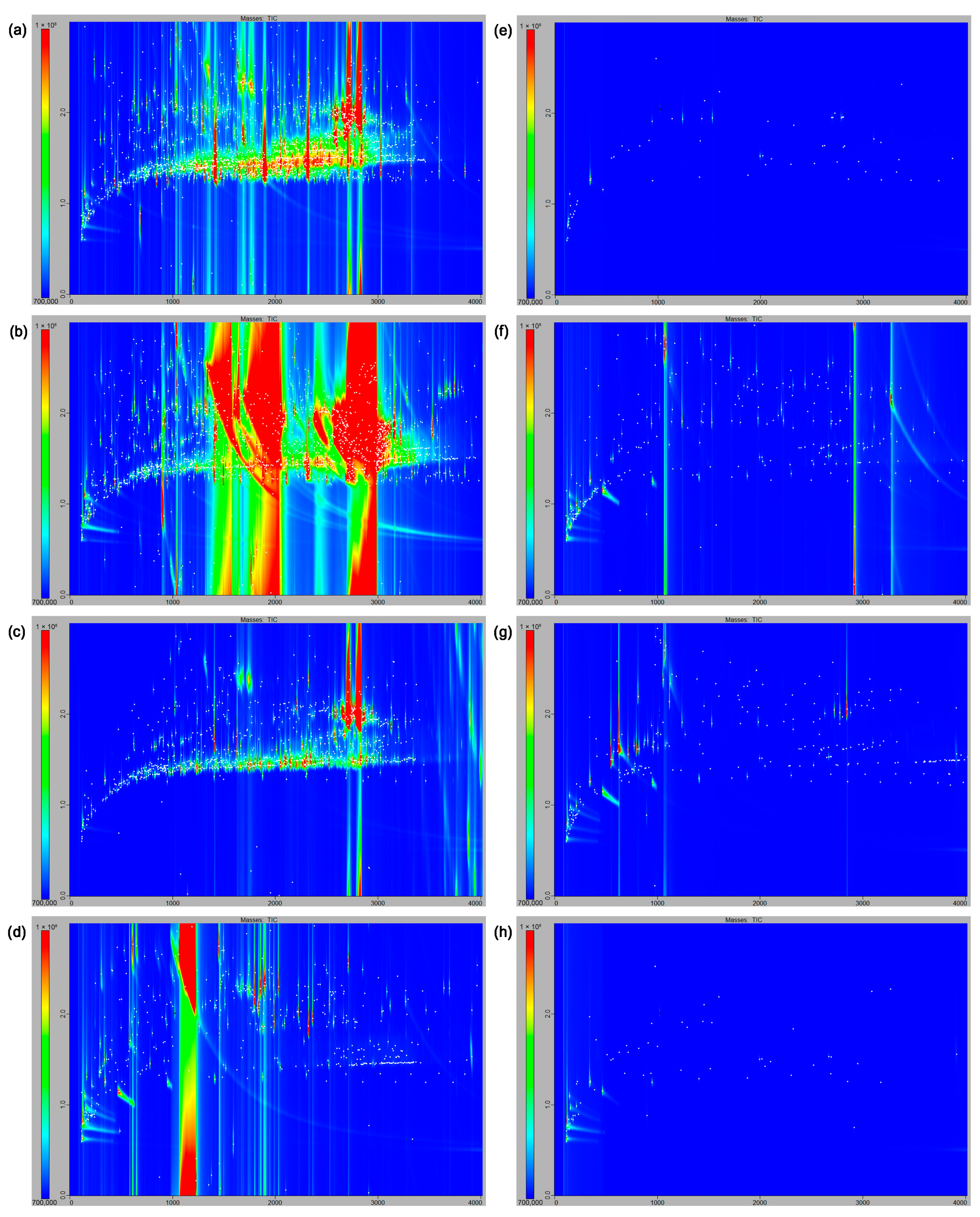
3.2. Qualitative Observations
3.3. Semi-Quantification
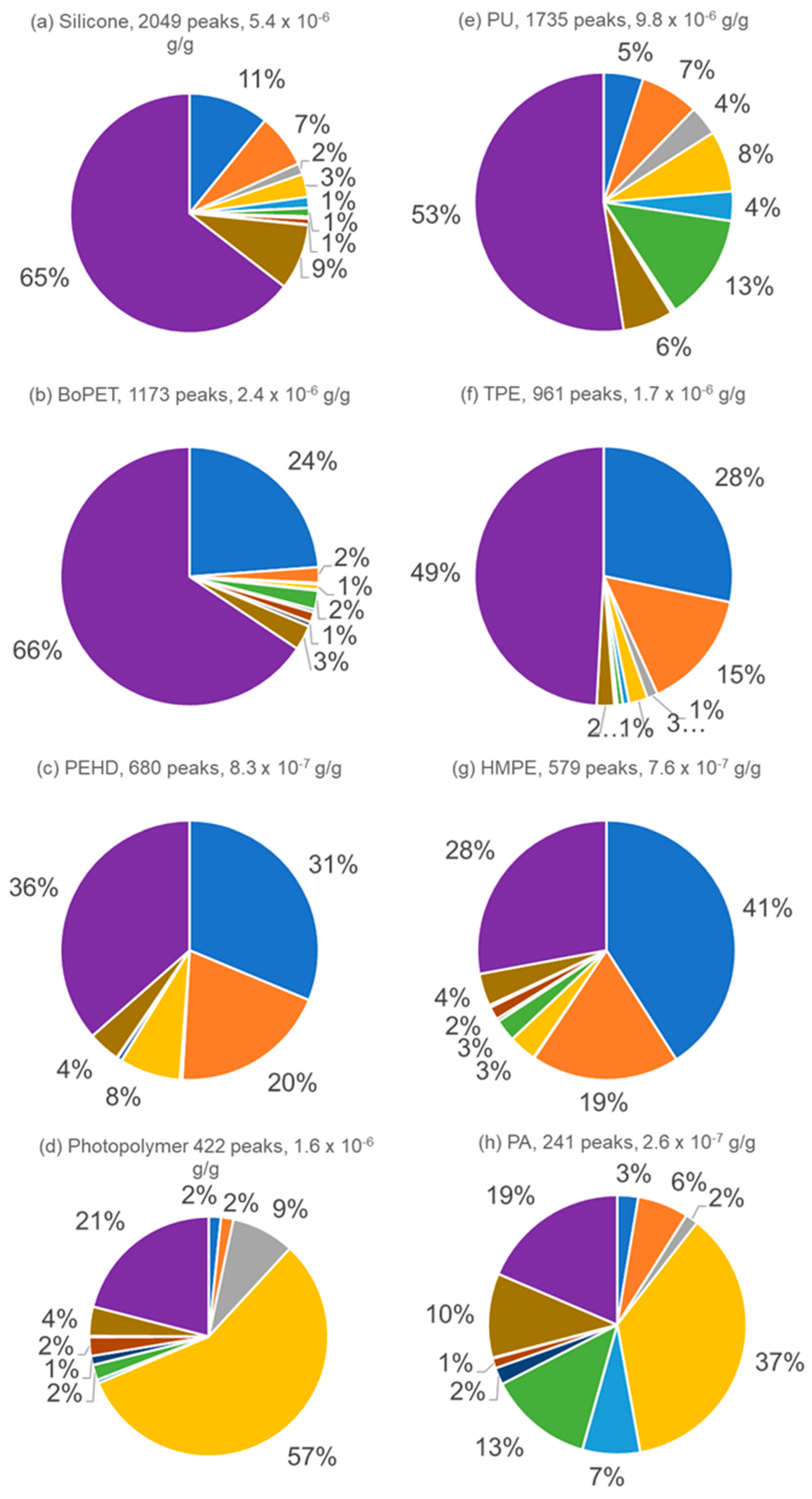
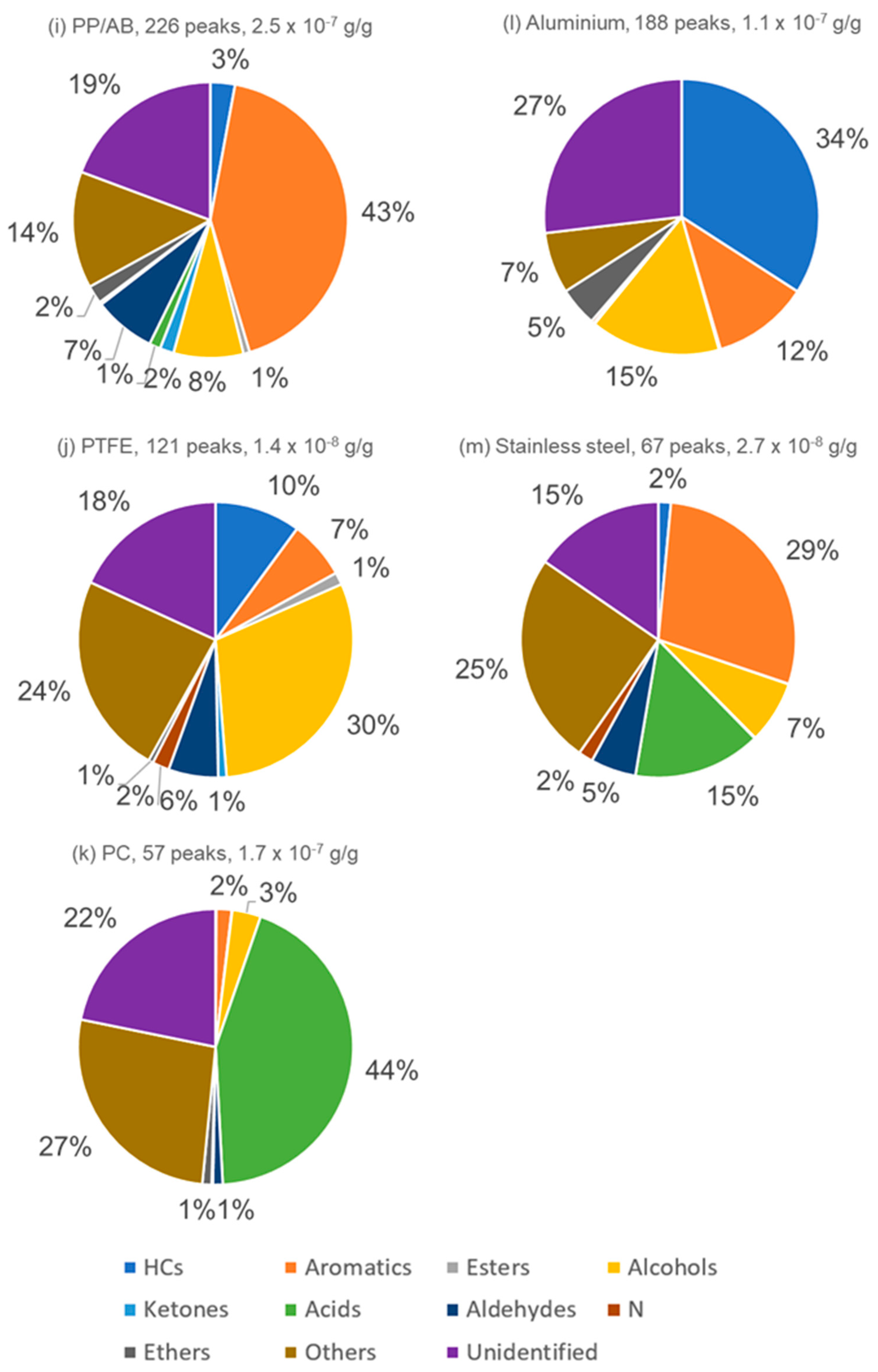
3.4. Major Compound Contributions to TVOC Concentration
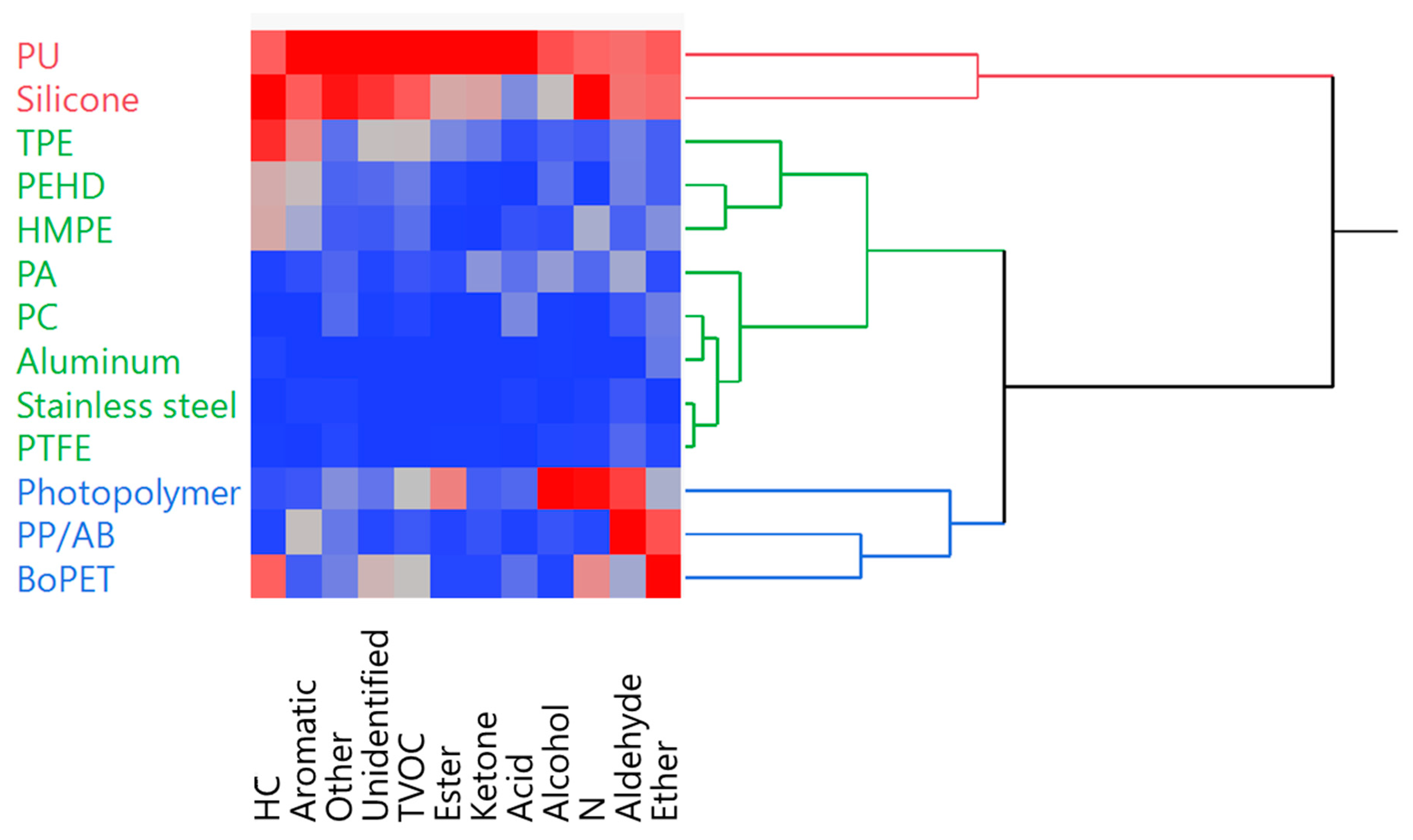
3.5. Spot Assignment in Correlation with Raw Materials
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, P.; Singh, A.B.; Arora, T.; Singh, S.; Singh, R. Critical Review on Emerging Health Effects Associated with the Indoor Air Quality and Its Sustainable Management. Sci. Total Environ. 2023, 872, 162163. [Google Scholar] [CrossRef] [PubMed]
- Caron, F.; Guichard, R.; Robert, L.; Verriele, M.; Thevenet, F. Behaviour of Individual VOCs in Indoor Environments: How Ventilation Affects Emission from Materials. Atmos. Environ. 2020, 243, 117713. [Google Scholar] [CrossRef]
- Yu, C.; Crump, D. A Review of the Emission of VOCs from Polymeric Materials Used in Buildings. Build. Environ. 1998, 33, 357–374. [Google Scholar] [CrossRef]
- Houston, C.T.; Rodrigues, A.D.; Smith, B.B.; Wang, T.; Richardson, M. Principles for Management of Extractables and Leachables in Ophthalmic Drug Products. PDA J. Pharm. Sci. Technol. 2022, 76, 278–294. [Google Scholar] [CrossRef]
- Yoshida, T.; Matsunaga, I. A Case Study on Identification of Airborne Organic Compounds and Time Courses of Their Concentrations in the Cabin of a New Car for Private Use. Environ. Int. 2006, 32, 58–79. [Google Scholar] [CrossRef]
- ISO 16000-6:2021(Fr); Air Intérieur—Partie 6: Dosage Des Composés Organiques (COTV, COV, COSV) Dans l’air Intérieur et l’air de Chambre d’essai Par Prélèvement Actif Sur Tubes à Sorbant, Désorption Thermique et Chromatographie En Phase Gazeuse Avec Détection MS Ou MS-FID. ISO: Geneva, Switzerland, 2021. Available online: https://www.iso.org/obp/ui/fr/#iso:std:iso:16000:-6:ed-3:v1:fr (accessed on 11 July 2023).
- Chien, Y.-C. Variations in Amounts and Potential Sources of Volatile Organic Chemicals in New Cars. Sci. Total Environ. 2007, 382, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Domhagen, F.; Langer, S.; Sasic Kalagasidis, A. Modelling VOC Levels in a New Office Building Using Passive Sampling, Humidity, Temperature, and Ventilation Measurements. Build. Environ. 2023, 238, 110337. [Google Scholar] [CrossRef]
- Dewulf, J.; Van Langenhove, H.; Wittmann, G. Analysis of Volatile Organic Compounds Using Gas Chromatography. TrAC Trends Anal. Chem. 2002, 21, 637–646. [Google Scholar] [CrossRef]
- Cabanes, A.; Valdés, F.J.; Fullana, A. A Review on VOCs from Recycled Plastics. Sustain. Mater. Technol. 2020, 25, e00179. [Google Scholar] [CrossRef]
- de Lacy Costello, B.; Amann, A.; Al-Kateb, H.; Flynn, C.; Filipiak, W.; Khalid, T.; Osborne, D.; Ratcliffe, N.M. A Review of the Volatiles from the Healthy Human Body. J. Breath Res. 2014, 8, 014001. [Google Scholar] [CrossRef]
- Drabińska, N.; Flynn, C.; Ratcliffe, N.; Belluomo, I.; Myridakis, A.; Gould, O.; Fois, M.; Smart, A.; Devine, T.; Costello, B.D.L. A Literature Survey of All Volatiles from Healthy Human Breath and Bodily Fluids: The Human Volatilome. J. Breath Res. 2021, 15, 034001. [Google Scholar] [CrossRef]
- Beauchamp, J.; Herbig, J.; Gutmann, R.; Hansel, A. On the Use of Tedlar® Bags for Breath-Gas Sampling and Analysis. J. Breath Res. 2008, 2, 046001. [Google Scholar] [CrossRef] [PubMed]
- Rankin-Turner, S.; McMeniman, C.J. A Headspace Collection Chamber for Whole Body Volatilomics. Analyst 2022, 147, 5210–5222. [Google Scholar] [CrossRef]
- Roodt, A.P.; Naudé, Y.; Stoltz, A.; Rohwer, E. Human Skin Volatiles: Passive Sampling and GC × GC-ToFMS Analysis as a Tool to Investigate the Skin Microbiome and Interactions with Anthropophilic Mosquito Disease Vectors. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018, 1097–1098, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Prada, P.A.; Curran, A.M.; Furton, K.G. Comparison of Extraction Methods for the Removal of Volatile Organic Compounds (VOCs) Present in Sorbents Used for Human Scent Evidence Collection. Anal. Methods 2010, 2, 470. [Google Scholar] [CrossRef]
- Woolfenden, E. Chapter 10-Thermal Desorption Gas Chromatography. In Gas Chromatography, 2nd ed.; Poole, C.F., Ed.; Handbooks in Separation Science; Elsevier: Amsterdam, The Netherlands, 2021; pp. 267–323. ISBN 978-0-12-820675-1. [Google Scholar]
- Hilaire, F.; Basset, E.; Bayard, R.; Gallardo, M.; Thiebaut, D.; Vial, J. Comprehensive Two-Dimensional Gas Chromatography for Biogas and Biomethane Analysis. J. Chromatogr. A 2017, 1524, 222–232. [Google Scholar] [CrossRef]
- Franchina, F.A.; Zanella, D.; Dubois, L.M.; Focant, J.-F. The Role of Sample Preparation in Multidimensional Gas Chromatographic Separations for Non-Targeted Analysis with the Focus on Recent Biomedical, Food, and Plant Applications. J. Sep. Sci. 2021, 44, 188–210. [Google Scholar] [CrossRef]
- Boudard, E.; Moumane, N.; Dugay, J.; Vial, J.; Thiébaut, D. Body Volatilome Study Strategy for COVID-19 Biomarker Identification Considering Exogenous Parameters. Separations 2024, 11, 336. [Google Scholar] [CrossRef]
- Boudard, E.; Fisson, L.; Moumane, N.; Dugay, J.; Vial, J.; Thiébaut, D. Study of Sampling Phases for Body Odor Sampling Prior to Analysis by TD-GC×GC/ToFMS. Anal. Bioanal. Chem. 2025, 417, 3177–3190. [Google Scholar] [CrossRef]
- Springer Handbook of Odor; Buettner, A., Ed.; Springer Handbooks; Springer International Publishing: Cham, Switzerland, 2017; ISBN 978-3-319-26930-6. [Google Scholar]
- Dorey, S.; Pahl, I.; Uettwiller, I.; Priebe, P.; Hauk, A. Theoretical and Practical Considerations When Selecting Solvents for Use in Extractables Studies of Polymeric Contact Materials in Single-Use Systems Applied in the Production of Biopharmaceuticals. Ind. Eng. Chem. Res. 2018, 57, 7077–7089. [Google Scholar] [CrossRef]
- Venhoven, B.A.M.; De Gee, A.J.; Davidson, C.L. Polymerization Contraction and Conversion of Light-Curing BisGMA-Based Methacrylate Resins. Biomaterials 1993, 14, 871–875. [Google Scholar] [CrossRef] [PubMed]
- Harris, S.H.; Kreter, P.E.; Polley, C.W. Characterization of Polyurethane Foam Odor Bodies. J. Cell. Plast. 1988, 24, 486–494. [Google Scholar] [CrossRef]
- Gauthier, Q.T.; Riley, P.; Simon, A.G. Permeation of Human Scent through Laboratory Examination Gloves. J. Forensic. Sci. 2022, 67, 2308–2320. [Google Scholar] [CrossRef]
- Brodzik, K.; Faber, J.; Łomankiewicz, D.; Gołda-Kopek, A. In-Vehicle VOCs Composition of Unconditioned, Newly Produced Cars. J. Environ. Sci. 2014, 26, 1052–1061. [Google Scholar] [CrossRef]
- Murtagh, F.; Legendre, P. Ward’s Hierarchical Agglomerative Clustering Method: Which Algorithms Implement Ward’s Criterion? J. Classif. 2014, 31, 274–295. [Google Scholar] [CrossRef]
- Li, J.; Sobańtka, A. A Systematic Analysis of the Effect of Extraction Solvents on the Chemical Composition of Extraction Solutions and the Analytical Implications in Extractables and Leachables Studies. J. Pharm. Biomed. Anal. 2023, 222, 115081. [Google Scholar] [CrossRef]
- Arbir, F.W.; Raden, D.S.; Narducy, K.W.; Casati, F.M. Catalyst for Making Polyurethanes 1984. US4456696A, 26 June 1984. [Google Scholar]
- Klein, R. Définition de Critères Moléculaires Simples, Robustes et Industrialisables Pour Prédire la Perception Olfactive et sa Composante Hédonique à Travers Différentes Cultures. Ph.D. Thesis, Université Paris Sciences et Lettres, Paris, France, 2023. [Google Scholar]
- Itoh, T.; Kozaki, M.; Kubo, M.; Iwatsuki, S. Synthesis of Aromatic Polyesters and Polyamides by Alternating Copolymerizations of 1,4-Dicarbonyl-1,4-Dihydronaphthalene with Bezoquinones and Benzoquinone Diimines. J. Polym. Sci. Part A Polym. Chem. 1998, 36, 1929–1936. [Google Scholar] [CrossRef]
- Cotarca, L.; Delogu, P.; Nardelli, A.; Maggioni, P.; Bianchini, R.; Sguassero, S.; Alini, S.; Dario, R.; Clauti, G.; Pitta, G.; et al. Efficient and Scaleable Methods for ω-Functionalized Nonanoic Acids: Development of a Novel Process for Azelaic and 9-Aminononanoic Acids (Nylon-6,9 and Nylon-9 Precursors). Org. Process Res. Dev. 2001, 5, 69–76. [Google Scholar] [CrossRef]
- Kalgutkar, R.S.; Amos, D.T.; Kadoma, I.A.; Severance, J.A.; Nelson, J.M.; Watts, A.; Anderson, J.J.; Schultz, K.C.M. Branched Amorphous Polyamide (Co)Polymers and Methods of Making and Using Same 2021. WO2021209895A1, 21 October 2021. [Google Scholar]
- Cavani, F.; Trifirò, F. Alternative Processes for the Production of Styrene. Appl. Catal. A Gen. 1995, 133, 219–239. [Google Scholar] [CrossRef]

| Material | Abbreviation | Supplier | Additional Information |
|---|---|---|---|
| Polyamide 12 | PA | Silex 3D Print https://www.silex3dprint.fr/ Thizy-Les-Bourgs, France | Nylon |
| Photopolymer (acrylic based polymer) | Xometry Europe https://pages.xometry.eu/ Ottobrunn, Germany | VeroBlackPlus RGD 875 | |
| Polypropylene/acrylonitrile butadiene | PP/AB | ZITFRI https://www.gosupps.com/ Wilmington, Del, USA | |
| Polyurethane | PU | ARRK LCO Protomoule https://fr.arrk.com/ Alby-Sur-Chéran, France | PU GM956 |
| Silicone | ARRK LCO Protomoule https://fr.arrk.com/ Alby-Sur-Chéran, France | ||
| Polycarbonate | PC | Plaqueplastique.fr https://plaqueplastique.fr/ Paris, France | |
| Polyethylene high-density | PEHD | Plaqueplastique.fr https://plaqueplastique.fr/ Paris, France | |
| High-modulus polyethylene | HMPE | Plaqueplastique.fr https://plaqueplastique.fr/ Paris, France | |
| Thermoplastic elastomer | TPE | Avient https://www.avient.com/ Pommerloch, Luxembourg | Versaflex HCMT 224 |
| Polytetrafluoroethylene | PTFE | Action Europe https://www.actioneurope.fr/ Sausheim, France | |
| Aluminum | Fisher Scientific https://www.fishersci.fr Illkirsch, France | Food grade | |
| Stainless steel | SS | HCT group https://hctgroup.com/ Paris, France | |
| Biaxially oriented polyethylene terephtalate | BoPET | LaboModerne https://www.labomoderne.com/ Gennevilliers, France | Mylar |
| Material | Total Number of Peaks | TVOC (µg·g−1) | Minimum Concentration (µg·g−1) | Maximum Concentration (µg·g−1) |
|---|---|---|---|---|
| Blank | 103 (36%) | |||
| PC (LE) | 57 (16%) | 0.170 (49%) | 1.4 × 10−5 | 0.0740 |
| Stainless steel (LE) | 67 (7%) | 0.027 (45%) | 1.4 × 10−5 | 0.0041 |
| PTFE (LE) | 121 (5%) | 0.014 (13%) | 2.4 × 10−6 | 0.0035 |
| Aluminum (LE) | 188 (11%) | 0.110 (17%) | 3.8 × 10−5 | 0.0130 |
| PA (LE) | 241 (20%) | 0.260 (9%) | 8.1 × 10−6 | 0.0440 |
| HMPE (LE) | 579 | 0.760 | 1.0 × 10−5 | 0.0390 |
| PEHD (LE) | 680 | 0.830 | 8.1 × 10−6 | 0.0890 |
| TPE (LE) | 961 | 1.70 | 6.5 × 10−6 | 0.0600 |
| PP/AB (ME) | 226 (14%) | 0.250 (15%) | 5.9 × 10−6 | 0.0310 |
| Photopolymer (ME) | 422 (15%) | 1.60 (10%) | 9.1 × 10−6 | 0.5600 |
| BoPET (ME) | 1173 | 2.40 | 1.7 × 10−5 | 0.0370 |
| PU (HE) | 1735 | 9.80 | 1.3 × 10−5 | 0.5400 |
| Silicone (HE) | 2049 | 5.40 | 1.5 × 10−5 | 0.1000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boudard, E.; Moumane, N.; Dugay, J.; Vial, J.; Sablier, M.; Thiébaut, D. A Focus on the Emission of Volatile Organic Compounds (VOCs) from Raw Materials Potentially Used in Human Odor Sampling. Separations 2025, 12, 250. https://doi.org/10.3390/separations12090250
Boudard E, Moumane N, Dugay J, Vial J, Sablier M, Thiébaut D. A Focus on the Emission of Volatile Organic Compounds (VOCs) from Raw Materials Potentially Used in Human Odor Sampling. Separations. 2025; 12(9):250. https://doi.org/10.3390/separations12090250
Chicago/Turabian StyleBoudard, Elsa, Nabil Moumane, José Dugay, Jérôme Vial, Michel Sablier, and Didier Thiébaut. 2025. "A Focus on the Emission of Volatile Organic Compounds (VOCs) from Raw Materials Potentially Used in Human Odor Sampling" Separations 12, no. 9: 250. https://doi.org/10.3390/separations12090250
APA StyleBoudard, E., Moumane, N., Dugay, J., Vial, J., Sablier, M., & Thiébaut, D. (2025). A Focus on the Emission of Volatile Organic Compounds (VOCs) from Raw Materials Potentially Used in Human Odor Sampling. Separations, 12(9), 250. https://doi.org/10.3390/separations12090250






