Alkali-Resistant Ion-Imprinted Chitosan–Mesoporous Silica Composite for Efficient and Selective Gallium Separation
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of CS/SiO2-Based Mesoporous Silica–Gallium Ion-Imprinted Polymer
2.2. Optimization of Synthesis Conditions for CS/(H-CGCS)-Ga-IIP
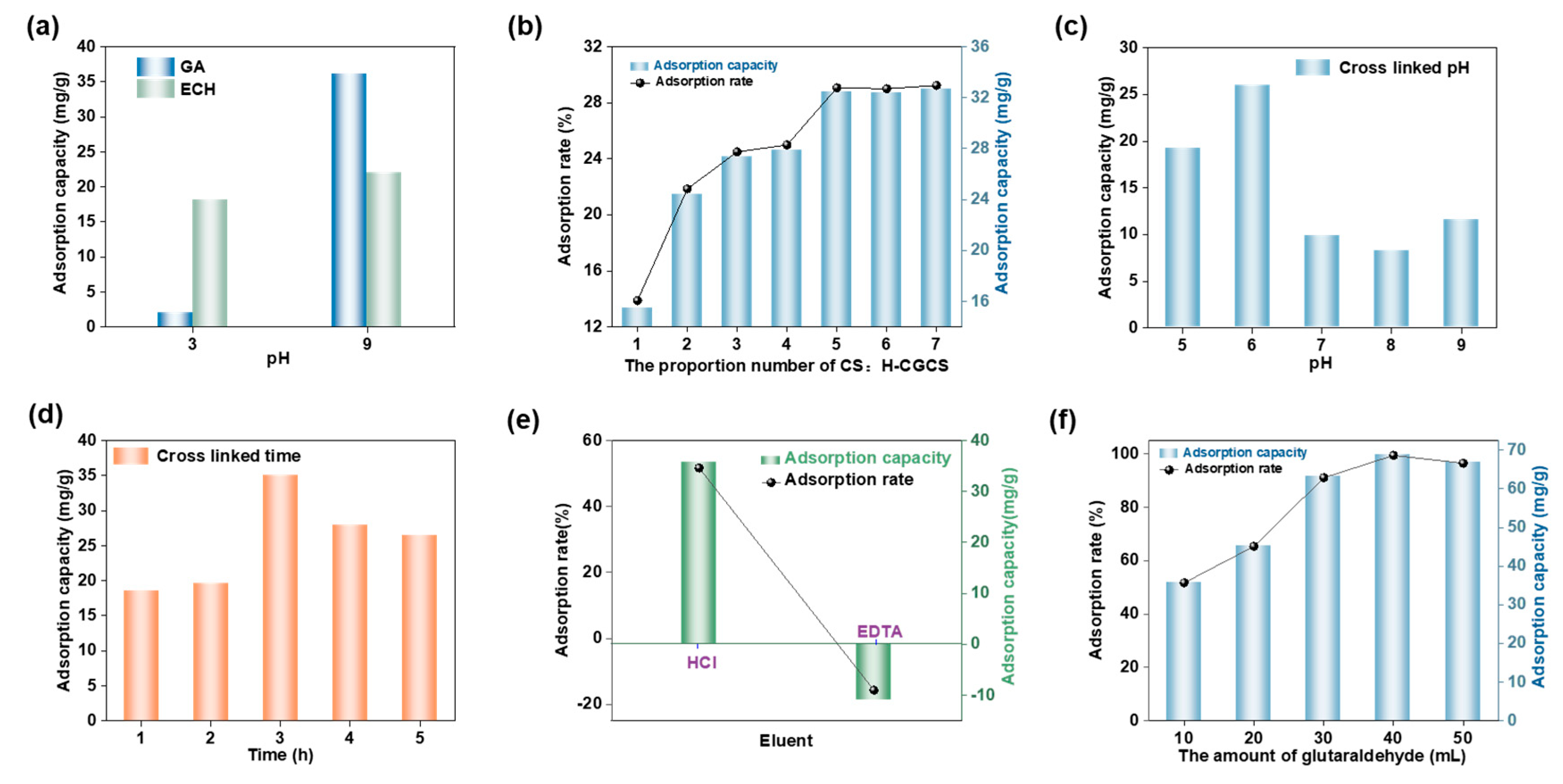
2.2.1. Effect of Cross-Linking Agent Type and Adsorption pH
2.2.2. Effect of CS-to-H-CGCS Mass Ratio
2.2.3. Effect of Cross-Linking pH
2.2.4. Effect of Cross-Linking Time
2.2.5. Selection of Eluent Type
2.2.6. Effect of Cross-Linker Dosage
2.3. Adsorption Experiment
2.4. Adsorption Kinetics Models
2.5. Adsorption Isotherm Models
2.5.1. Langmuir Isotherm Model
2.5.2. Freundlich Isotherm Model
2.6. Adsorption Thermodynamics Model
3. Results and Discussion
3.1. Optimization of Synthesis Conditions for CS/SiO2-Based Mesoporous SiO2-Ga IIPs
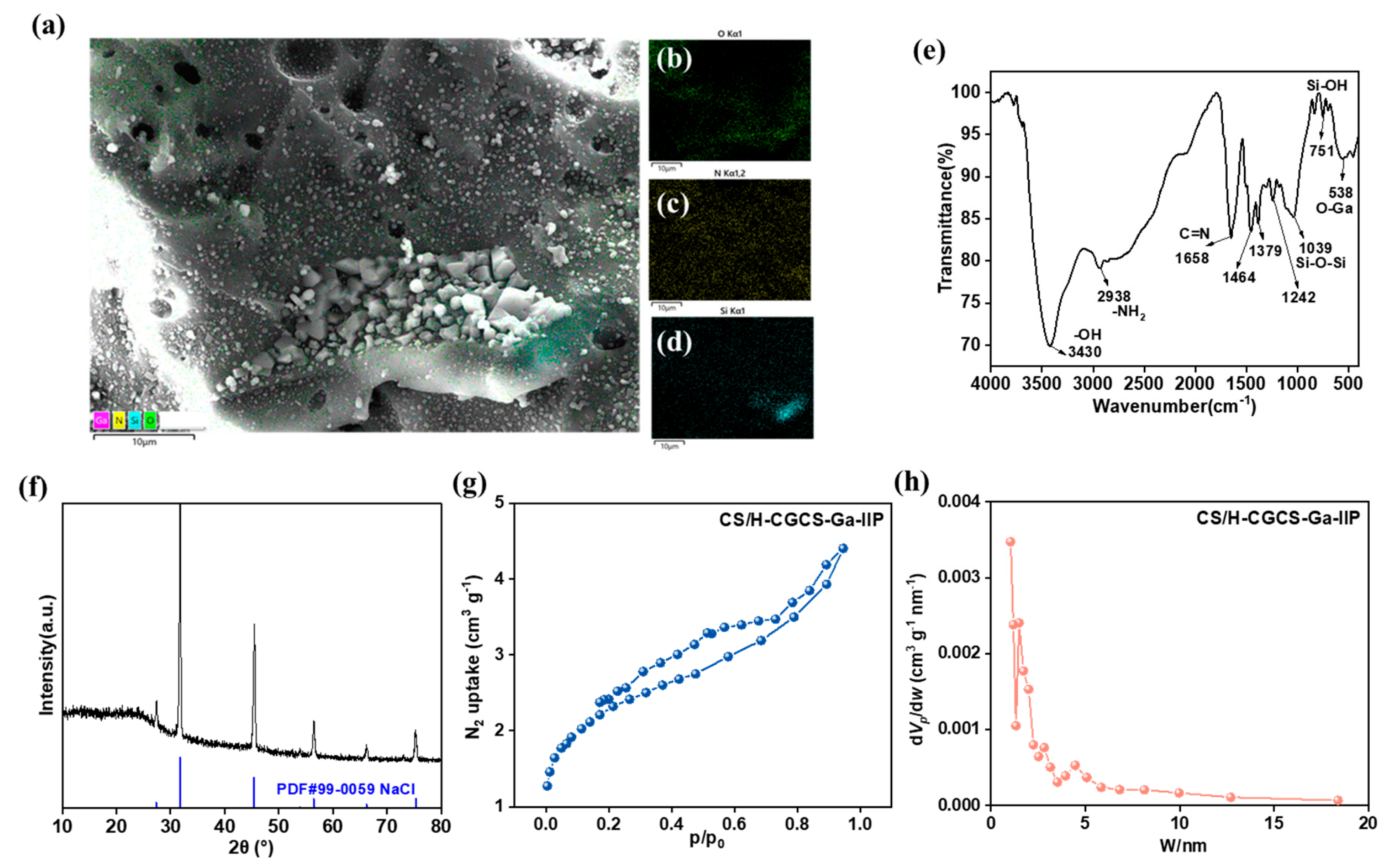
3.2. Characterization and Structural Analysis of CS/(H-CGCS)-Ga-IIP
3.3. Study on the Adsorption Behavior of CS/(H-CGCS)-Ga-IIP
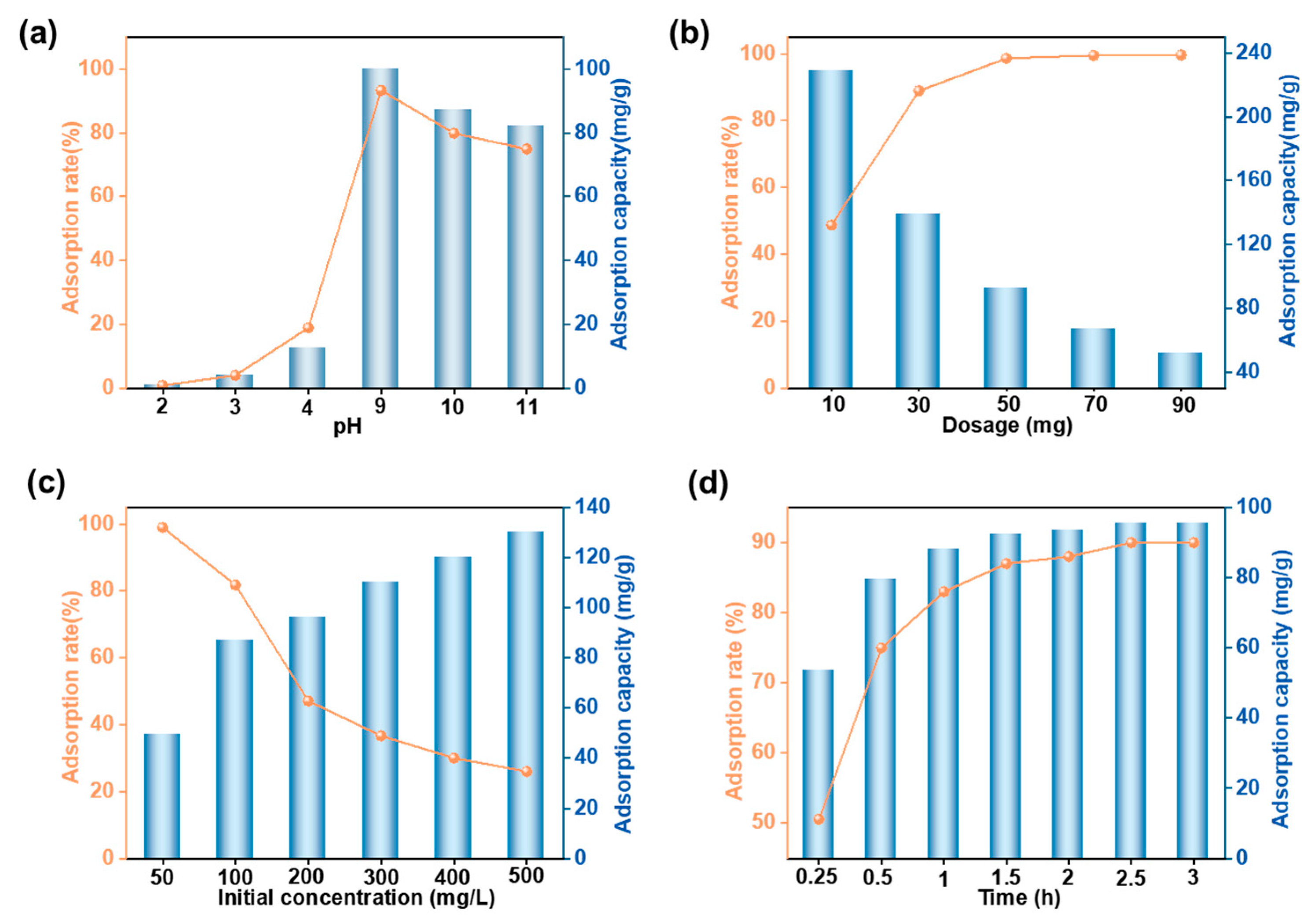
3.3.1. Optimization of Adsorption Conditions
3.3.2. Adsorption Kinetics
3.3.3. Adsorption Isotherms
3.3.4. Adsorption Thermodynamics
- (1)
- ΔG < 0 and decreases with temperature → adsorption is spontaneous and thermodynamically favored at higher temperatures.
- (2)
- ΔH > 0 → the adsorption is endothermic, driven by specific interactions requiring energy input.
- (3)
- ΔS > 0 → increased entropy results from dehydration of Ga(III) ions and structural disordering at the interface.
3.3.5. Simulation of Competitive Adsorption in the Bayer Mother Liquor System
3.3.6. Study on Recycling Performance
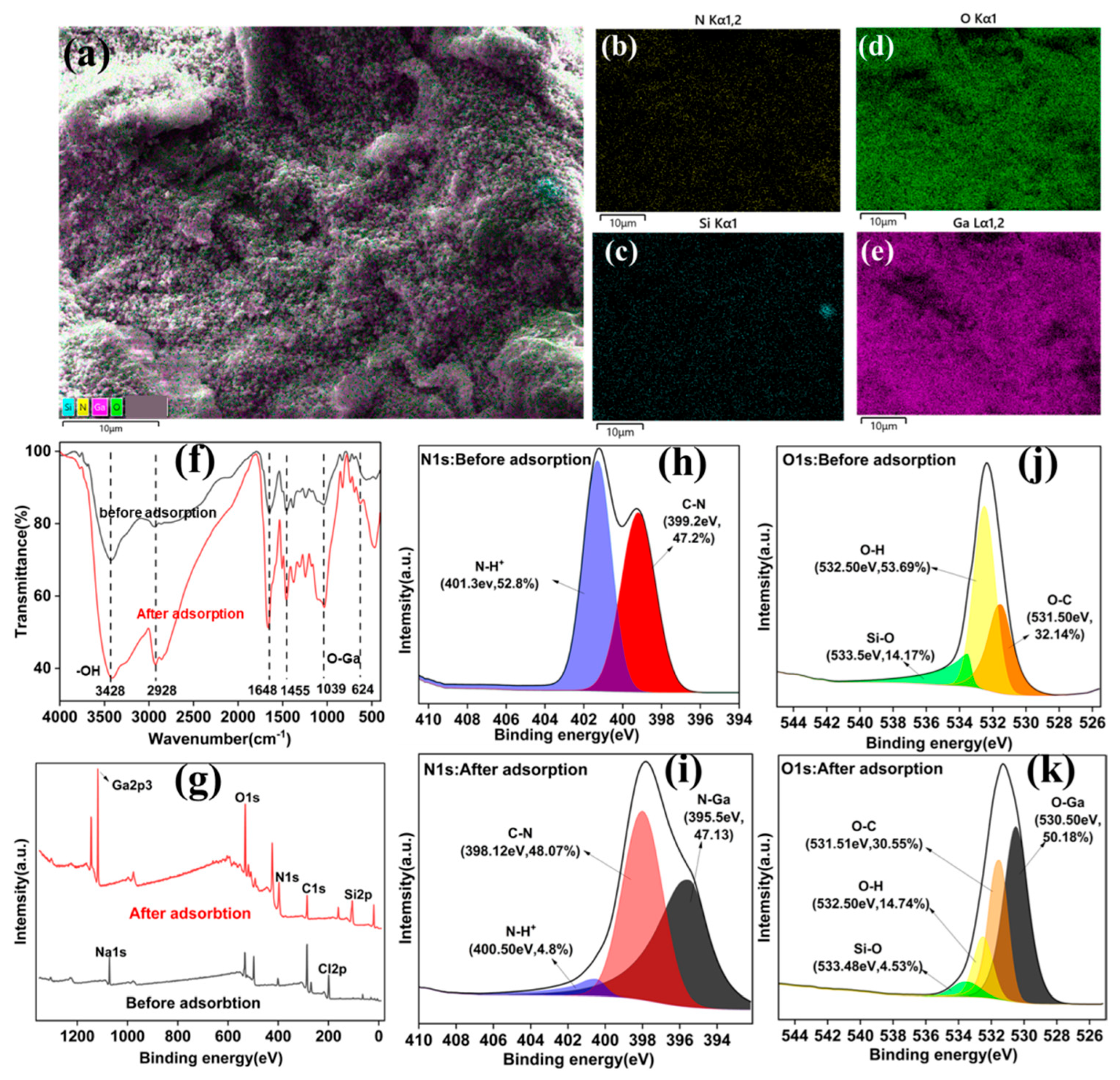
3.4. Adsorption Mechanisms
- (1)
- lectrostatic Interaction: under slightly alkaline conditions (pH 9), positively charged −NH3+ groups on chitosan can electrostatically attract negatively charged Ga(OH)4− species.
- (2)
- Coordination and Dehydration Reaction: Ga(OH)4− undergoes dehydration and forms stable coordination complexes with -OH and -NH2 groups on the polymer matrix, enhancing binding strength and selectivity.
- (3)
- Structural Stability: the siloxane (Si–O–Si) network of H-CGCS remains chemically stable during adsorption, providing mechanical integrity and long-term durability to the adsorbent.

4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lu, S.; Chen, L.; Hamza, M.F.; He, C.; Wang, X.; Wei, Y.; Guibal, E. Amidoxime functionalization of a poly(acrylonitrile)/silica composite for the sorption of Ga(III)—Application to the treatment of Bayer liquor. Chem. Eng. J. 2019, 368, 459–473. [Google Scholar] [CrossRef]
- Nibel, O.; Bon, M.; Agiorgousis, M.L.; Laino, T.; Gubler, L.; Schmidt, T.J. Unraveling the Interaction Mechanism between Amidoxime Groups and Vanadium Ions at Various pH Conditions. J. Phys. Chem. C 2017, 121, 6436–6445. [Google Scholar] [CrossRef]
- Huang, P.; Fang, W.; Yang, L.; Sun, Y.; Yang, H.; Chen, X.-Z.; Zeng, H. Ultralong hydroxyapatite nanowires-based flow-through reactor with high loading of silver nanoparticles for fast continuous catalytic reduction of organic dyes and disinfection of wastewater. Chem. Eng. J. 2023, 475, 146305. [Google Scholar] [CrossRef]
- Gomase, V.; Jugade, R.; Doondani, P.; Saravanan, D.; Pandey, S. Sequential modifications of chitosan biopolymer for enhanced confiscation of Cr(VI). Inorg. Chem. Commun. 2022, 145, 110009. [Google Scholar] [CrossRef]
- Rathi, T.A.; Gomase, V.; Saravanan, D.; Jugade, R. Novel chitosan encapsulated nitrogen-doped multiwalled carbon nanotube composite for enhanced cyanide removal. J. Mol. Liq. 2024, 413, 125922. [Google Scholar] [CrossRef]
- Teng, D.; Liu, C.; Ma, L.; Jin, P.; Fan, G.; Li, P.; Cao, Y.; Wang, W. Selective adsorption of Ga(III) from aqueous solutions using magnetic chitosan-based ion-imprinted polymers: Synthesis optimization and mechanistic insights. Int. J. Biol. Macromol. 2025, 298, 139957. [Google Scholar] [CrossRef]
- Yang, W.; Hu, W.; Zhang, J.; Wang, W.; Cai, R.; Pan, M.; Huang, C.; Chen, X.; Yan, B.; Zeng, H. Tannic acid/Fe3+ functionalized magnetic graphene oxide nanocomposite with high loading of silver nanoparticles as ultra-efficient catalyst and disinfectant for wastewater treatment. Chem. Eng. J. 2021, 405, 126629. [Google Scholar] [CrossRef]
- Lv, Z.; Ma, M.; Huang, Y.; Wang, W.; Li, G.; Si, L.; Fan, G.; Cao, Y.; Li, P.; Teng, D. Hydroxamic acid-functionalized chitosan hydrogel beads for sustainable and continuous gallium recovery. Int. J. Biol. Macromol. 2025, 322, 146869. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Wang, Y.-J.; Liu, D.; Fang, B.; Yan, W.; Zhang, J. Tailoring interfacial interaction in GaN@NG heterojunction via electron/ion bridges for enhanced lithium-ion storage performance. Chem. Eng. J. 2023, 453, 139603. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, F.; Xu, Z.; Hu, W.; Jiang, H.; Liu, L.; Gai, L. Gallium oxynitride@carbon cloth with impressive electrochemical performance for supercapacitors. Chem. Eng. J. 2021, 411, 128481. [Google Scholar] [CrossRef]
- Zhang, M.; Sun, Q.; Wang, Y.; Shan, W.; Lou, Z.; Xiong, Y. Synthesis of porous UiO-66-NH2-based mixed matrix membranes with high stability, flux and separation selectivity for Ga(III). Chem. Eng. J. 2021, 421, 129748. [Google Scholar] [CrossRef]
- Li, W.; Zhou, C.; Li, C.; Zhu, W.; Shi, J.; Liu, G. Synthesis of CNT/UiO-66-NH2 adsorbent and the selective adsorption of gallium in solution. Sep. Purif. Technol. 2023, 323, 124464. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, P.; Guo, Y.; Wang, W.; Fan, G.; Teng, D. Selective Enrichment vs. Dynamic Recovery of Ga(III) Using Functionalized Hydrogels: A Comparative Study of Polypropylene Hydroxamic Acid and Polyacrylic Acid Hydrogels. ACS Sustain. Chem. Eng. 2025, 13, 2141–2153. [Google Scholar] [CrossRef]
- Kou, Z.; Bian, W.; Wang, C. Preparation of novel amidoxime resin and its performance in gallium (III) extraction from Bayer liquor. Chem. Eng. J. Adv. 2022, 11, 100317. [Google Scholar] [CrossRef]
- Gao, L.; Wang, L.; Cao, Y.; Li, S. Persimmon peel-based ion-imprinted adsorbent with enhanced adsorption performance of gallium ions. Miner. Eng. 2022, 176, 107354. [Google Scholar] [CrossRef]
- Chai, X.; Dong, H.; Zhang, Z.; Qi, Z.; Chen, J.; Huang, Z.; Ye, C.; Qiu, T. A novel Zr-MOF modified by 4,6-Diamino-2-mercaptopyrimidine for exceptional Hg (II) removal. J. Water Process Eng. 2022, 46, 102606. [Google Scholar] [CrossRef]
- Bambal, A.; Gaydhane, A.; Chute, A.; Sarvanan, D.; Jugade, R. Novel chitosan-magnetite-silica ternary capsules for highly efficient sequestration of reactive dyes from aqueous media. Environ. Res. 2025, 275, 121359. [Google Scholar] [CrossRef]
- Liu, Q.; He, Y.; Yang, W.; Yan, B.; Lai, F.; Zeng, H. Nanoconfined core-shell heterogeneous fenton reactor: Accelerated degradation of organic pollutants in flow-through systems. Chem. Eng. J. 2025, 505, 158894. [Google Scholar] [CrossRef]
- Dai, X.; Liu, M.; Li, Z.; Jin, A.; Ma, Y.; Huang, X.; Sun, H.; Wang, H.; Zhang, X. Molybdenum Polysulfide Anchored on Porous Zr-Metal Organic Framework To Enhance the Performance of Hydrogen Evolution Reaction. J. Phys. Chem. C 2016, 120, 12539–12548. [Google Scholar] [CrossRef]
- Teng, D.; Lv, Z.; Wang, N.; Wang, W.; Fan, G.; Li, P.; Yang, W. Ligand geometry matters: Comparative study of linear and annular chelating ligands in tailored magnetic chitosan for Cu(II) removal. Int. J. Biol. Macromol. 2025, 303, 140687. [Google Scholar] [CrossRef]
- Saleem, H.; Rafique, U.; Davies, R.P. Investigations on post-synthetically modified UiO-66-NH2 for the adsorptive removal of heavy metal ions from aqueous solution. Microporous Mesoporous Mater. 2016, 221, 238–244. [Google Scholar] [CrossRef]
- Qin, Z.; Liao, Y.; Wang, Z.; Wang, S.; Song, L.; Ma, K.; Luo, D.; Yue, H. Innovative Amidoxime Nanofiber Membranes for Highly Effective Adsorption of Ga(III) from Waste Bayer Solution. Ind. Eng. Chem. Res. 2023, 62, 11140–11150. [Google Scholar] [CrossRef]
- Qin, Z.; Wang, S.; Fan, L.; Zhou, C.-a.; Wang, C.; Song, L.; Ma, K.; Yue, H. A hydrazine amidoxime crosslinked polyacrylonitrile resin for efficient extraction of gallium from vanadium-containing waste solution. Chem. Eng. Sci. 2023, 282, 119240. [Google Scholar] [CrossRef]
- Shi, C.; Wang, K.; Chen, C.; Cao, Y.; Zhou, G.; Wang, J.; Li, C. Highly selective capture of gallium from aqueous solutions using tetradentate amidoxime functionalized MIL-53(Al) nanofiber membranes. Sep. Purif. Technol. 2024, 330, 125303. [Google Scholar] [CrossRef]
- Li, J.; Tuo, K.; Fan, C.; Liu, G.; Pu, S.; Li, Z. Hierarchical Porous Amidoximated Metal–Organic Framework for Highly Efficient Uranium Extraction. Small 2024, 20, 2306545. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; He, C.; Li, Y.; Zhou, J.; Li, J.; Zheng, C.; Zhao, J.; Fujita, T.; Ning, S.; Wei, Y. Enhanced adsorption and separation of gallium using silica-based P507-TBP/SiO2–P adsorbent from sulfuric acid solution. Microporous Mesoporous Mater. 2021, 314, 110859. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, R.; Chen, M.; Liu, Y.; Xie, Z.; Tang, S.; Yuan, Y.; Wang, N. Vertically Aligned Polyamidoxime/Graphene Oxide Hybrid Sheets’ Membrane for Ultrafast and Selective Extraction of Uranium from Seawater. Adv. Funct. Mater. 2022, 32, 2111049. [Google Scholar] [CrossRef]
- Fang, S.; Zhang, B.; Li, W.; Ke, J. Adsorption of Ga(III) by Zn2+, quaternary ammonium and polyamine embellished lignin with the crosslinking agent of epichlorohydrin. Int. J. Biol. Macromol. 2025, 310, 143108. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, X.; Chai, Y.; Hua, Z.; Xiao, Y.; Yang, Y. Adsorption Performances and Mechanisms of Amidoxime Resin toward Gallium(III) and Vanadium(V) from Bayer Liquor. ACS Sustain. Chem. Eng. 2016, 4, 53–59. [Google Scholar] [CrossRef]
- Huang, Z.-W.; Li, X.-B.; Mei, L.; Han, Y.-Z.; Song, Y.-T.; Fu, X.; Zhang, Z.-H.; Guo, Z.-J.; Zeng, J.-R.; Bian, F.-G.; et al. All-in-One: Photo-Responsive Lanthanide-Organic Framework for Simultaneous Sensing, Adsorption, and Photocatalytic Reduction of Uranium. Adv. Funct. Mater. 2024, 34, 2404126. [Google Scholar] [CrossRef]
- Yi, T.; Cen, Z.; Ji, Y.; Huang, J.; Liang, M.; Liu, S. Amidoxime-based Star Polymer Adsorbent with Ultra-High Uranyl Affinity for Extremely Fast Uranium Extraction from Natural Seawater. Adv. Funct. Mater. 2024, 34, 2404220. [Google Scholar] [CrossRef]
- Raj, P.; Patel, M.; Karamalidis, A.K. Chemically modified polymeric resins with catechol derivatives for adsorption, separation and recovery of gallium from acidic solutions. J. Environ. Chem. Eng. 2023, 11, 110790. [Google Scholar] [CrossRef]
- Yang, L.; Wang, Y.; Sun, Y.; Yu, R.; Chu, Y.; Yao, Y.; Liu, C.; Li, N.; Chen, L.; Liu, J.; et al. CO2-Responsive Smart Wood Scaffold for Natural Organic Matter Removal without Secondary Pollution. Adv. Mater. 2025, 37, 2505008. [Google Scholar] [CrossRef]
- Zhou, M.; Yu, N.; Sun, Q.; Gao, H.; Fan, J.; Liang, Z. Combined experimental and computational study on elucidating steric effects in amine-based CO2 capture. Chem. Eng. Sci. 2025, 317, 122015. [Google Scholar] [CrossRef]
- Yang, B.; Han, F.; Xie, Z.; Yang, Z.; Jiang, F.; Yang, S.; Li, Y. Study on adsorption of phosphate from aqueous solution by zirconium modified coal gasification coarse slag. RSC Adv. 2022, 12, 17147–17157. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Ding, L.; Zhang, W. Enhancing unburned carbon recovery from coal gasification coarse slag using waste cooking oil: A grinding-modification synergistic approach. J. Environ. Chem. Eng. 2025, 13, 118282. [Google Scholar] [CrossRef]
- Cao, D.; Cui, F.; Zhang, C.; Yang, Y.; Cao, J.; Song, Y.; Zheng, Y.; Wen, L.; Kong, X.; Ma, H.; et al. Constructing Biomimetic Nanochannels for High-Capacity Capture of Uranyl Tricarbonate Complex Ions. Adv. Mater. 2025, 37, 2500567. [Google Scholar] [CrossRef] [PubMed]
- Hu, E.; Chen, Q.; Gao, Q.; Fan, X.; Luo, X.; Wei, Y.; Wu, G.; Deng, H.; Xu, S.; Wang, P.; et al. Cyano-Functionalized Graphitic Carbon Nitride with Adsorption and Photoreduction Isosite Achieving Efficient Uranium Extraction from Seawater. Adv. Funct. Mater. 2024, 34, 2312215. [Google Scholar] [CrossRef]
- Li, M.; Zhang, L.; Xu, D.; Cheng, N.; Wang, M.; Shao, P.; Yang, L.; Yao, Z.; Zhao, C.; Feng, J.; et al. Deep Separation Between In(III) and Fe(III) Ions by Regulating the Lewis Basicity of Adsorption Sites on Electrospun Fibers. Adv. Funct. Mater. 2024, 34, 2313443. [Google Scholar] [CrossRef]
- Xiang, Y.; Cheng, C.-Y.; Liu, M.-H.; Bai, W.-C.; Zang, Z.-X.; Xu, L.; Yu, Y.; Liu, G.-J. Efficient recovery of gold using Macroporous Metal-Organic framework prepared by the ‘MOF in MOF’ method. Sep. Purif. Technol. 2024, 335, 126131. [Google Scholar] [CrossRef]
- Song, A.-M.; Yang, M.-J.; Wu, Z.; Yang, Q.; Lin, B.; Liang, R.-P.; Qiu, J.-D. Efficient Separation of Adjacent Rare Earths in One Step by Using Ion-microporous Metal–Organic Frameworks. Adv. Funct. Mater. 2025, 35, 2419093. [Google Scholar] [CrossRef]
- Xiong, C.; Wang, S.; Hu, P.; Huang, L.; Xue, C.; Yang, Z.; Zhou, X.; Wang, Y.; Ji, H. Efficient Selective Removal of Pb(II) by Using 6-Aminothiouracil-Modified Zr-Based Organic Frameworks: From Experiments to Mechanisms. ACS Appl. Mater. Interfaces 2020, 12, 7162–7178. [Google Scholar] [CrossRef]
- Qin, Z.; Wang, S.; Zhang, S.; Xie, J.; Zhou, C.-a.; Wang, C.; Song, L.; Ma, K.; Luo, D.; Yue, H. Cross-linked amidoxime porous resin for selective gallium separation in Bayer solutions: Reaction mechanism and kinetic study. Chem. Eng. J. 2024, 481, 148340. [Google Scholar] [CrossRef]
- Hu, S.-z.; Huang, T.; Zhang, N.; Lei, Y.-z.; Wang, Y. Chitosan-assisted MOFs dispersion via covalent bonding interaction toward highly efficient removal of heavy metal ions from wastewater. Carbohydr. Polym. 2022, 277, 118809. [Google Scholar] [CrossRef]
- Song, H.; Wang, C.; Sen, B.; Liu, G. China Factor: Exploring the Byproduct and Host Metal Dynamics for Gallium–Aluminum in a Global Green Transition. Environ. Sci. Technol. 2022, 56, 2699–2708. [Google Scholar] [CrossRef]
- Ma, W.; Lu, T.; Cao, W.; Xiong, R.; Huang, C. Bioinspired Nanofibrous Aerogel with Vertically Aligned Channels for Efficient Water Purification and Salt-Rejecting Solar Desalination. Adv. Funct. Mater. 2023, 33, 2214157. [Google Scholar] [CrossRef]
- Huang, P.; Huang, C.; Sun, Y.; Zhao, Z.; Yang, L.; Yang, H.; Gong, L.; Yang, W.; Zeng, H. Bio-inspired magnetically induced self-assembling Janus solar evaporator with antifouling and antiscaling properties. Chem. Eng. J. 2024, 501, 157824. [Google Scholar] [CrossRef]
- Long, H.; Zhao, Z.; Chai, Y.; Li, X.; Hua, Z.; Xiao, Y.; Yang, Y. Binding Mechanism of the Amidoxime Functional Group on Chelating Resins toward Gallium(III) in Bayer Liquor. Ind. Eng. Chem. Res. 2015, 54, 8025–8030. [Google Scholar] [CrossRef]
- He, W.; Qiu, Z.; Sun, Q.; Li, Z.; Chen, Z.; Hu, L.; Wang, H. Amidoxime-functionalized hollow 2D-COFs for uranium separation. Sep. Purif. Technol. 2024, 331, 125620. [Google Scholar] [CrossRef]
- Lyu, Y.; Xu, H.; Yuan, H.; Cui, Y.; Zhang, C.; Meng, Q. Intermolecular interaction mechanisms between dinitrophenol and water: A molecular dynamics and DFT study. Eur. Phys. J. Plus 2025, 140, 531. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.-H.; Meng, L.-H.; Li, Y.; Sun, M.-Y.; Wang, C.-C.; Wang, P.; Li, H.-Y. Mining Ag+ ions from wastewater with Bio-MOF-1: From adsorption to high value-added application. Sep. Purif. Technol. 2024, 341, 126928. [Google Scholar] [CrossRef]
- Lim, C.; Huang, J.; Kim, S.; Lee, H.; Zeng, H.; Hwang, D.S. Nanomechanics of Poly(catecholamine) Coatings in Aqueous Solutions. Angew. Chem. Int. Ed. 2016, 55, 3342–3346. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Tu, H.; Liu, J.; Zhao, C.; Liao, J.; Yang, Y.; Yang, J.; Liu, N. A novel ion-imprinted polymer induced by the glycylglycine modified metal-organic framework for the selective removal of Co(II) from aqueous solutions. Chem. Eng. J. 2018, 333, 280–288. [Google Scholar] [CrossRef]



| Metal | Ga | Al | V |
|---|---|---|---|
| Contene (g/L) | 2.5 | 30.6 | 0.15 |
| Element | N | O | Si | Ga |
|---|---|---|---|---|
| wt% | 16.33 | 72.7 | 10.97 | 0.00 |
| System | Kd-Ga (L/g) | Kd-M (L/g) | Kd-M (L/g) |
|---|---|---|---|
| Ga/V | 0.4853 | 0.0051 | 1.98 |
| Ga/Al | 0.0033 | 2.17 |
| Element | N | O | Si | Ga |
|---|---|---|---|---|
| wt% | 3.39 | 38.14 | 0.22 | 58.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, Z.; Yang, S.; Wu, J.; Fan, G.; Li, G.; Cao, Y.; Li, P.; Teng, D. Alkali-Resistant Ion-Imprinted Chitosan–Mesoporous Silica Composite for Efficient and Selective Gallium Separation. Separations 2025, 12, 226. https://doi.org/10.3390/separations12090226
Lv Z, Yang S, Wu J, Fan G, Li G, Cao Y, Li P, Teng D. Alkali-Resistant Ion-Imprinted Chitosan–Mesoporous Silica Composite for Efficient and Selective Gallium Separation. Separations. 2025; 12(9):226. https://doi.org/10.3390/separations12090226
Chicago/Turabian StyleLv, Zhifang, Shiqiao Yang, Jiangyan Wu, Guixia Fan, Guosheng Li, Yijun Cao, Peng Li, and Daoguang Teng. 2025. "Alkali-Resistant Ion-Imprinted Chitosan–Mesoporous Silica Composite for Efficient and Selective Gallium Separation" Separations 12, no. 9: 226. https://doi.org/10.3390/separations12090226
APA StyleLv, Z., Yang, S., Wu, J., Fan, G., Li, G., Cao, Y., Li, P., & Teng, D. (2025). Alkali-Resistant Ion-Imprinted Chitosan–Mesoporous Silica Composite for Efficient and Selective Gallium Separation. Separations, 12(9), 226. https://doi.org/10.3390/separations12090226











