Chemistry and Diversity of Nitrogen-Containing Metabolites in Heliotropium procumbens: A Genus-Wide Comparative Profile
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Methanolic Extract Preparation
2.2. UHPLC–HRMS Analysis
2.3. HPLC-ESI-DAD-IT MS
2.3.1. Sample Preparation for HPLC-ESI-DAD-IT MS Analysis
2.3.2. HPLC-ESI-DAD-IT MS Analysis
2.4. GC-MS
2.4.1. Sample Preparation for GC-MS Analysis
2.4.2. GC-MS Analysis
2.5. Isolation and Identification of Helifoline-N-oxide
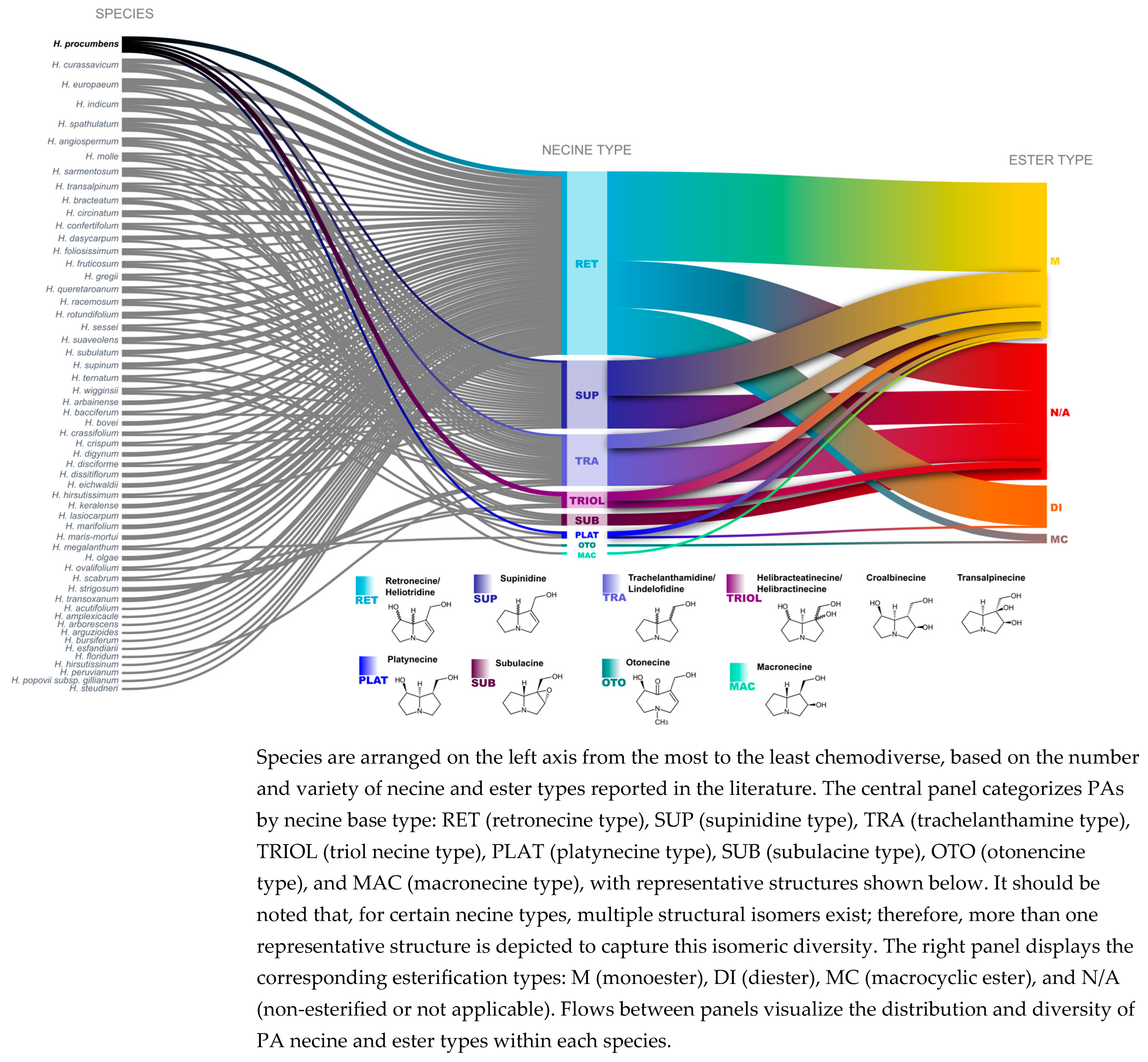
2.6. Data Collection and Visualization of PAs Chemodiversity in the Heliotropium Genus
3. Results
3.1. Phytochemical Profiling of H. procumbens Extracts
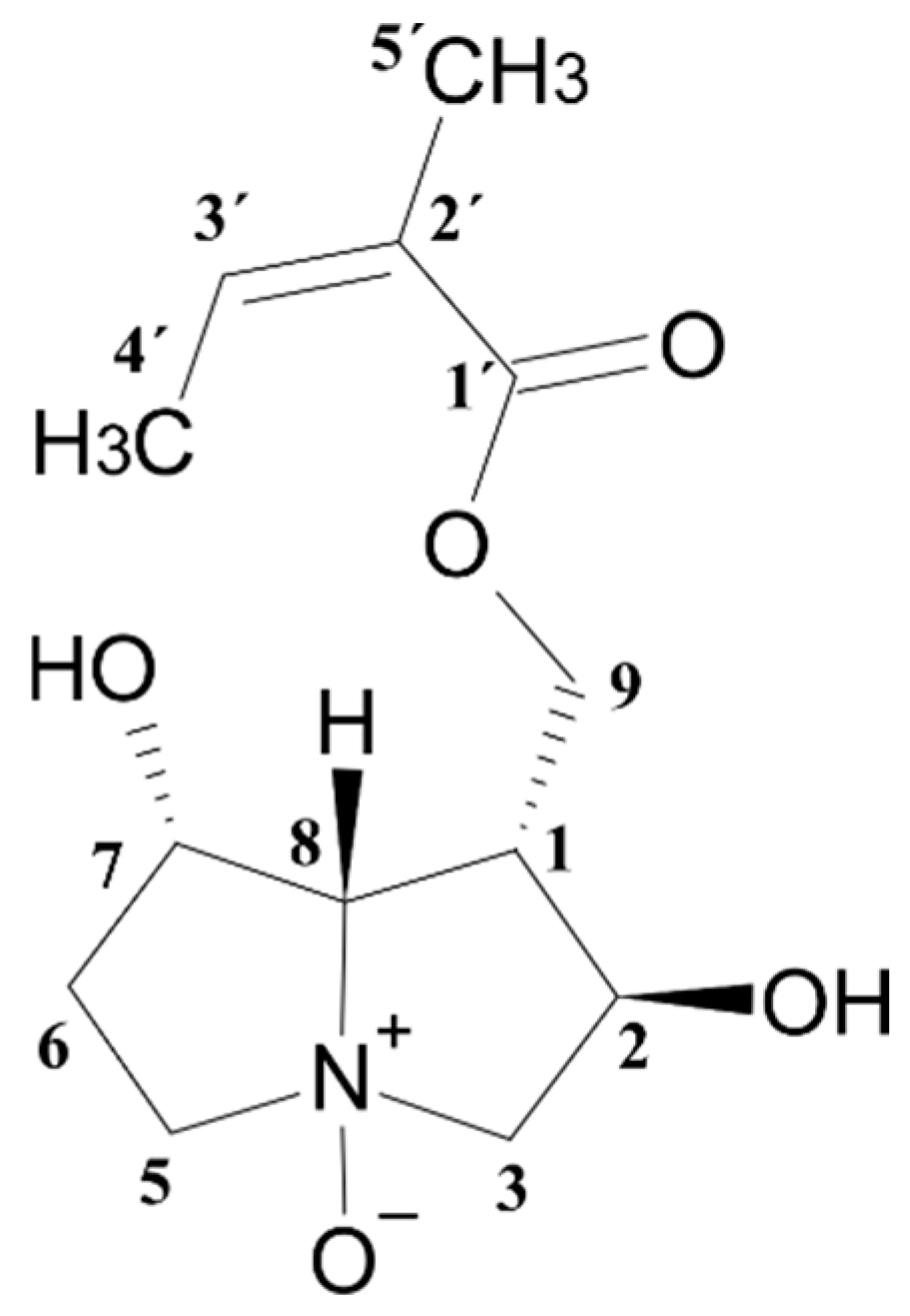
3.2. Structure Elucidation of Helifoline-N-oxide
4. Discussion
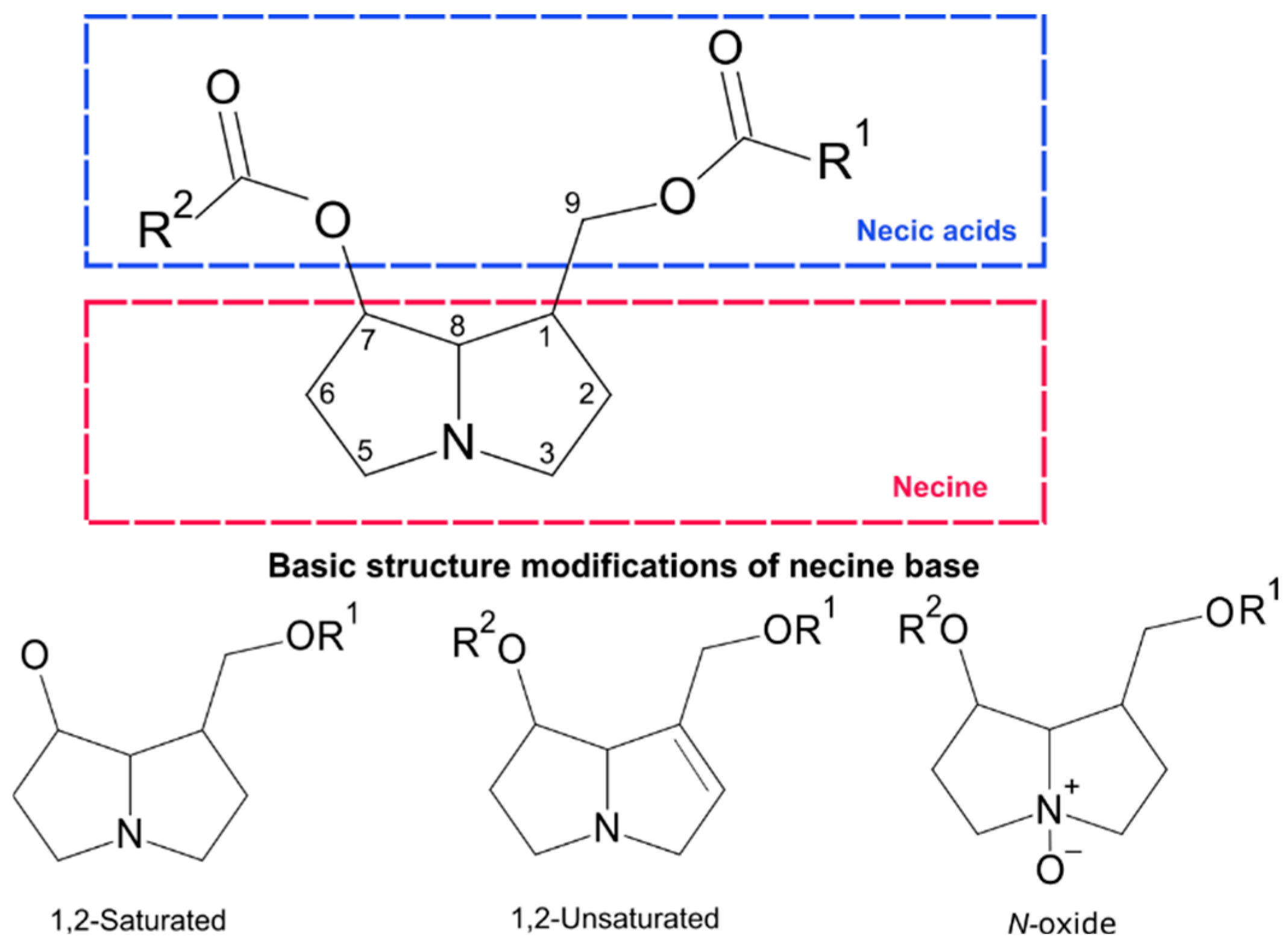
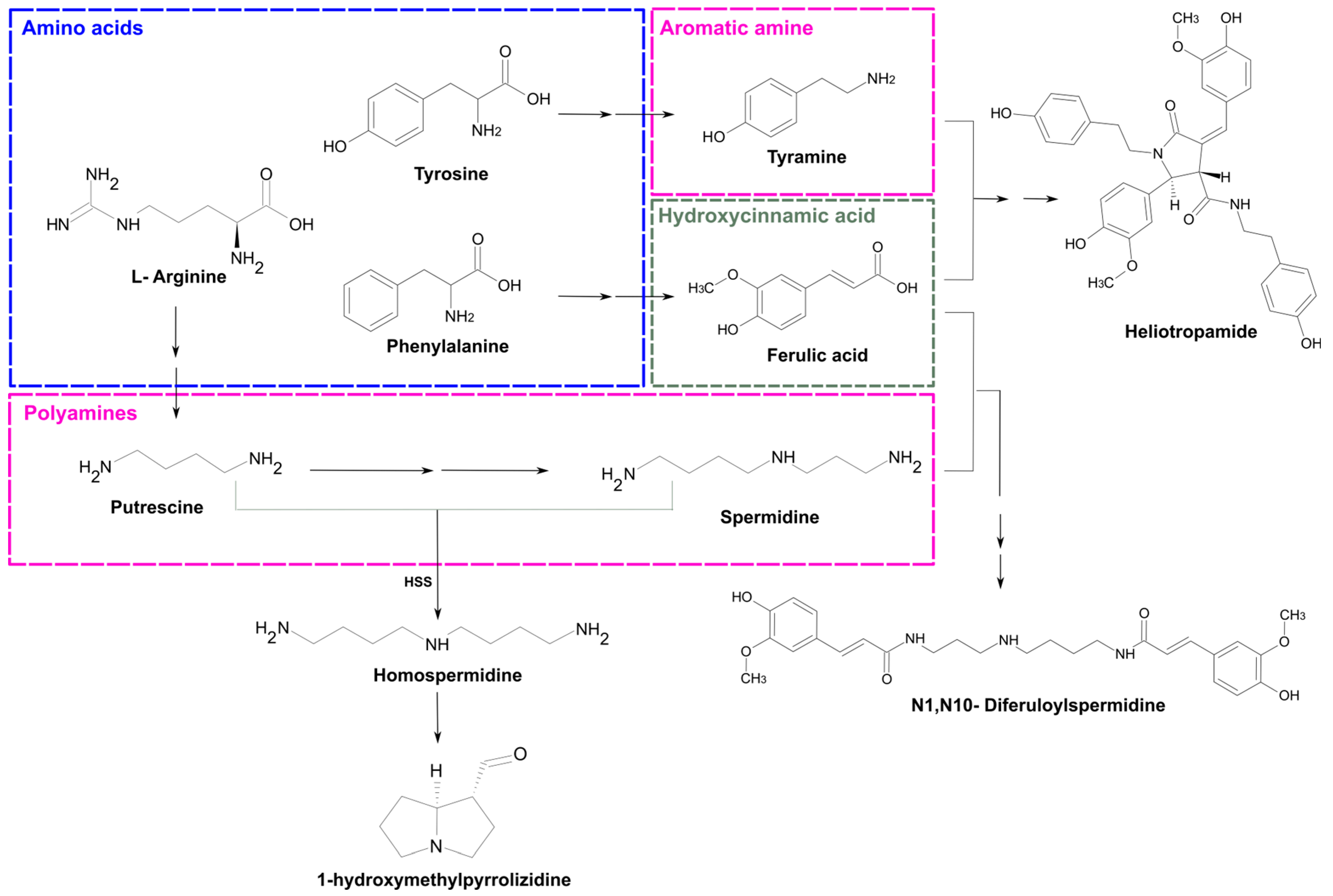
4.1. Profiling of PAs and Phenolamides
4.2. Isolation of Helifoline-N-oxide
4.3. PA Diversity in Heliotropium and H. procumbens
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| HPLC-DAD-IT-MS | High-Performance Liquid Chromatography-Diode-Array Detection-Ion Trap Mass Spectrometry |
| UHPLC–HRMS | Ultra-High Performance Liquid Chromatography—High Resolution Mass Spectrometry |
| GC-MS | Gas Chromatography-Mass Spectrometry |
| PAs | Pyrrolizidine Alkaloids |
| PANOs | Pyrrolizidine Alkaloid N-oxides |
| NMR | Nuclear Magnetic Resonance |
| MeOH | Methanol |
| ACN | Acetonitrile |
| FA | Formic Acid |
| DCM | Dichloromethane |
| SPE | Solid Phase Extraction |
| S | Saturated |
| US | Unsaturated |
| RET | Retronecine type |
| SUP | Supinidine type |
| PLAT | Platynecine type |
| TRIOL | Triol necine type |
| TRA | Trachelanthamine type |
| SUB | Subulacine type |
| OTO | Otonencine type |
| MAC | Macronecine type |
| M | Monoester |
| DI | Diester |
| MC | Macrocyclic ester |
| N/A | Non-esterified or not applicable |
References
- WFO Plant List: Taxon Wfo-4000017267-2025-06. Available online: https://wfoplantlist.org/taxon/wfo-4000017267-2025-06?page=1 (accessed on 16 June 2025).
- Akhani, H. Diversity, Biogeography, and Photosynthetic Pathways of Argusia and Heliotropium (Boraginaceae) in South-West Asia with an Analysis of Phytogeographical Units. Bot. J. Linn. Soc. 2007, 155, 401–425. [Google Scholar] [CrossRef]
- Fayed, M.A.A. Heliotropium; a Genus Rich in Pyrrolizidine Alkaloids: A Systematic Review Following Its Phytochemistry and Pharmacology. Phytomed. Plus 2021, 1, 100036. [Google Scholar] [CrossRef]
- Jain, S.C.; Singh, B.; Jain, R. Antimicrobial Activity of Triterpenoids from Heliotropium ellipticum. Fitoterapia 2001, 72, 666–668. [Google Scholar] [CrossRef]
- Khan, H.; Khan, M.A.; Gul, F.; Hussain, S.; Ashraf, N. Anti-Inflammatory Activity of Heliotropium strigosum in Animal Models. Toxicol. Ind. Health 2015, 31, 1281–1287. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.A.; Singh, B.; Singh, D.; Chandrawat, P. Ethnomedicinal, Pharmacological Properties and Chemistry of Some Medicinal Plants of Boraginaceae in India. J. Med. Plant Res. 2009, 3, 1153–1175. [Google Scholar]
- Wiedenfeld, H. Toxicity of Pyrrolizidine Alkaloids—A Serious Health Problem. MÜSBED 2011, 1, 79–87. [Google Scholar]
- Dreger, M.; Stanisławska, M.; Krajewska-Patan, A.; Mielcarek, S.; Mikołajczak, P.Ł.; Buchwald, W. Pyrrolizidine Alkaloids—Chemistry, Biosynthesis, Pathway, Toxicity, Safety and Perspectives of Medicinal Usage. Herba Pol. 2009, 55, 127–147. [Google Scholar]
- Schramm, S.; Köhler, N.; Rozhon, W. Pyrrolizidine Alkaloids: Biosynthesis, Biological Activities and Occurrence in Crop Plants. Molecules 2019, 24, 498. [Google Scholar] [CrossRef]
- Wiedenfeld, H. (Ed.) Pyrrolizidine Alkaloids: Structure and Toxicity; V&R Unipress Bonn University Press: Göttingen, Germany, 2008; ISBN 978-3-89971-426-5. [Google Scholar]
- Moreira, R.; Pereira, D.M.; Valentão, P.; Andrade, P.B. Pyrrolizidine Alkaloids: Chemistry, Pharmacology, Toxicology and Food Safety. Int. J. Mol. Sci. 2018, 19, 1668. [Google Scholar] [CrossRef]
- Al-Subaie, S.F.; Alowaifeer, A.M.; Mohamed, M.E. Pyrrolizidine Alkaloid Extraction and Analysis: Recent Updates. Foods 2022, 11, 3873. [Google Scholar] [CrossRef]
- Tábuas, B.; Cruz Barros, S.; Diogo, C.; Cavaleiro, C.; Sanches Silva, A. Pyrrolizidine Alkaloids in Foods, Herbal Drugs, and Food Supplements: Chemistry, Metabolism, Toxicological Significance, Analytical Methods, Occurrence, and Challenges for Future. Toxins 2024, 16, 79. [Google Scholar] [CrossRef]
- Zhu, L.; Ruan, J.-Q.; Li, N.; Fu, P.P.; Ye, Y.; Lin, G. A Novel Ultra-Performance Liquid Chromatography Hyphenated with Quadrupole Time of Flight Mass Spectrometry Method for Rapid Estimation of Total Toxic Retronecine-Type of Pyrrolizidine Alkaloids in Herbs without Requiring Corresponding Standards. Food Chem. 2016, 194, 1320–1328. [Google Scholar] [CrossRef]
- Kopp, T.; Abdel-Tawab, M.; Mizaikoff, B. Extracting and Analyzing Pyrrolizidine Alkaloids in Medicinal Plants: A Review. Toxins 2020, 12, 320. [Google Scholar] [CrossRef]
- El-Shazly, A.; Wink, M. Diversity of Pyrrolizidine Alkaloids in the Boraginaceae Structures, Distribution, and Biological Properties. Diversity 2014, 6, 188–282. [Google Scholar] [CrossRef]
- Nunes, E.N.; Ribeiro, J.E.D.S.; Souza, R.S.; De Queiroz, R.T.; Da Cruz, D.D.; De Lucena, R.F.P. Euploca procumbens (Mill.) Diane & Hilger Boraginaceae. In Ethnobotany of the Mountain Regions of Brazil; Farias Paiva De Lucena, R., Dias Da Cruz, D., Eds.; Ethnobotany of Mountain Regions; Springer International Publishing: Cham, Switzerland, 2023; pp. 393–398. ISBN 978-3-030-87250-2. [Google Scholar]
- Nunes, A.T.; Cabral, D.L.V.; Amorim, E.L.C.; Santos, M.V.F.D.; Albuquerque, U.P. Plants Used to Feed Ruminants in Semi-Arid Brazil: A Study of Nutritional Composition Guided by Local Ecological Knowledge. J. Arid Environ. 2016, 135, 96–103. [Google Scholar] [CrossRef]
- Birecka, H.; Frohlich, M.W.; Glickman, L.M. Free and Esterified Necines in Heliotropium Species from Mexico and Texas. Phytochemistry 1983, 22, 1167–1171. [Google Scholar] [CrossRef]
- Damianakos, H.; Jeziorek, M.; Pietrosiuk, A.; Chinou, I. The Chemical Profile of Pyrrolizidine Alkaloids from Selected Greek Endemic Boraginaceae Plants Determined by Gas Chromatography/Mass Spectrometry. J. AOAC Int. 2014, 97, 1244–1249. [Google Scholar] [CrossRef] [PubMed]
- Ganos, C.; Zengin, G.; Chinou, I.; Aligiannis, N.; Graikou, K. Phytochemical Profiling and Biological Assessment of the Aerial Parts from Three Mediterranean Alkanna Species (A. orientalis, A. tinctoria, A. kotschyana) in the Boraginaceae Family. Plants 2024, 13, 278. [Google Scholar] [CrossRef] [PubMed]
- Panou, E.; Zengin, G.; Milic, N.; Ganos, C.; Graikou, K.; Chinou, I. A Comparative UPLC/HRMS Molecular Networking-Enhanced Study on the Phenolic Profiles and Bioactivities of Three Medicinally Significant Species of Onosma (Boraginaceae). Plants 2024, 13, 3468. [Google Scholar] [CrossRef]
- Varvouni, E.-F.; Zengin, G.; Graikou, K.; Ganos, C.; Mroczek, T.; Chinou, I. Chemical Profile and Biological Properties of the Endemic Turkish Species Phyllocara aucheri. S. Afr. J. Bot. 2021, 137, 340–344. [Google Scholar] [CrossRef]
- Ozntamar-Pouloglou, K.-M.; Cheilari, A.; Zengin, G.; Graikou, K.; Ganos, C.; Karikas, G.-A.; Chinou, I. Heliotropium procumbens Mill: Taxonomic Significance and Characterization of Phenolic Compounds via UHPLC–HRMS- In Vitro Antioxidant and Enzyme Inhibitory Activities. Molecules 2023, 28, 1008. [Google Scholar] [CrossRef]
- Federal Institute for Risk Assessment (BfR). Determination of Pyrrolizidine Alkaloids (PA) in Plant Material by SPE-LC-MS/MS; Federal Institute for Risk Assessment (BfR): Berlin, Germany, 2014. [Google Scholar]
- Łuczkiewicz, M.; Migas, P.; Kokotkiewicz, A.; Walijewska, M.; Cisowski, W. Two-Dimensional TLC with Adsorbent Gradient for Separation of Quinolizidine Alkaloids in the Herb and in-Vitro Cultures of Several Genista Species. J. Planar Chromatogr.-Mod. TLC 2004, 17, 89–94. [Google Scholar] [CrossRef]
- Molyneux, R.J.; Roitman, J.N. Specific Detection of Pyrrolizidine Alkaloids on Thin-Layer Chromatograms. J. Chromatogr. A 1980, 195, 412–415. [Google Scholar] [CrossRef]
- Aboelmagd, M.; Elokely, K.; Zaki, M.A.; Said, A.; Haggag, E.G.; Ross, S.A. Anti-Inflammatory of Pyrrolizidine Alkaloids from Heliotropium digynum. Med. Chem. Res. 2018, 27, 1066–1073. [Google Scholar] [CrossRef]
- Arshad, A.; Ahemad, S.; Saleem, H.; Saleem, M.; Zengin, G.; Abdallah, H.H.; Tousif, M.I.; Ahemad, N.; Fawzi Mahomoodally, M. RP-UHPLC-MS Chemical Profiling, Biological and in Silico Docking Studies to Unravel the Therapeutic Potential of Heliotropium crispum Desf. as a Novel Source of Neuroprotective Bioactive Compounds. Biomolecules 2021, 11, 53. [Google Scholar] [CrossRef]
- Carpinelli De Jesus, M.; Hungerford, N.L.; Carter, S.J.; Anuj, S.R.; Blanchfield, J.T.; De Voss, J.J.; Fletcher, M.T. Pyrrolizidine Alkaloids of Blue Heliotrope (Heliotropium amplexicaule) and Their Presence in Australian Honey. J. Agric. Food Chem. 2019, 67, 7995–8006. [Google Scholar] [CrossRef] [PubMed]
- Delnavazi, M.R.; Banihashem, M.; Farsam, H.; Shafiee, A.; Yassa, N. Pyrrolizidine Alkaloids from Heliotropium transoxanum Bunge. Res. J. Pharmacogn. 2016, 3, 1–5. [Google Scholar]
- Feng, C.-L.; Chen, C.-K.; Wei, P.-C.; Yang, Y.-C. Seasonal and Structural Variability of Pyrrolizidine Alkaloids in Heliotropium sarmentosum (Lam.) Craven. Biochem. Syst. Ecol. 2025, 122, 105032. [Google Scholar] [CrossRef]
- Mukhtar, M.; Saleem, M.; Nazir, M.; Riaz, N.; Shafiq, N.; Saleem, H.; Tauseef, S.; Khan, S.; Ehsan Mazhar, M.; Bakhsh Tareen, R.; et al. Identification of Pyrrolizidine Alkaloids and Flavonoid Glycosides through HR-LCMS/MS Analysis, Biological Screening, DFT and Molecular Docking Studies on Heliotropium dasycarpum Ledeb. Arab. J. Chem. 2023, 16, 104655. [Google Scholar] [CrossRef]
- Mustafa, S.; Tousif, M.I.; Raiz, N.; Saleem, M.; Tauseef, S.; Zengin, G.; Hassan, L.; Hassan, A.; Nazir, M.; Muhammad, S. Mapping the Chemodiversity, Antioxidant and Enzyme Inhibitory Potential and in Silico Studies of Heliotropium europaeum. Food Sci. Nutr. 2025, 13, e70119. [Google Scholar] [CrossRef]
- Shimshoni, J.A.; Barel, S.; Mulder, P.P.J. Comparative Risk Assessment of Three Native Heliotropium Species in Israel. Molecules 2021, 26, 689. [Google Scholar] [CrossRef] [PubMed]
- Mauri, M.; Elli, T.; Caviglia, G.; Uboldi, G.; Azzi, M. RAWGraphs: A Visualisation Platform to Create Open Outputs. In Proceedings of the 12th Biannual Conference on Italian SIGCHI Chapter, Cagliari, Italy, 18–20 September 2017; ACM: New York, NY, USA, 2017; pp. 1–5. [Google Scholar]
- Mädge, I.; Gehling, M.; Schöne, C.; Winterhalter, P.; These, A. Pyrrolizidine Alkaloid Profiling of Four Boraginaceae Species from Northern Germany and Implications for the Analytical Scope Proposed for Monitoring of Maximum Levels. Food Addit. Contam.-Chem. Anal. Control Expo. Risk Assess. 2020, 37, 1339–1358. [Google Scholar] [CrossRef] [PubMed]
- Colegate, S.M.; Gardner, D.R.; Davis, T.Z.; Betz, J.M.; Panter, K.E. Dehydropyrrolizidine Alkaloids in Two Cryptantha Species: Including Two New Open Chain Diesters One of Which Is Amphoteric. Phytochem. Anal. 2013, 24, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Grue, M.R.; Liddell, J.R. Pyrrolizidine Alkaloids from Senecio chrysocoma. Phytochemistry 1993, 33, 1517–1519. [Google Scholar] [CrossRef]
- Tundis, R.; Loizzo, M.R.; Bonesi, M.; Menichini, F.; Dodaro, D.; Passalacqua, N.G.; Statti, G.; Menichini, F. In Vitro Cytotoxic Effects of Senecio stabianus Lacaita (Asteraceae) on Human Cancer Cell Lines. Nat. Prod. Res. 2009, 23, 1707–1718. [Google Scholar] [CrossRef]
- Witte, L.; Rubiolo, P.; Bicchi, C.; Hartmannt, T. Comparative Analysis of Pyrrolizidine Alkaloids from Natural Sources by Gas Chromatography-Mass Spectrometry. Phytochemistry 1992, 32, 187–196. [Google Scholar] [CrossRef]
- Mohanraj, S.; Kulanthaive, P.; Subramania, P.S.; Herz, W. Helifoline, a Pyrrolizidine Alkaloid from Heliotropium ovalifolium. Phytochemistry 1981, 20, 1991–1995. [Google Scholar] [CrossRef]
- Qiao, J.; Cai, W.; Wang, K.; Haubruge, E.; Dong, J.; El-Seedi, H.R.; Xu, X.; Zhang, H. New Insights into Identification, Distribution, and Health Benefits of Polyamines and Their Derivatives. J. Agric. Food Chem. 2024, 72, 5089–5106. [Google Scholar] [CrossRef]
- Lo Piparo, E.; Christinat, N.; Badoud, F. From Structural Alerts to Signature Fragment Alerts: A Case Study on Pyrrolizidine Alkaloids. Chem. Res. Toxicol. 2023, 36, 213–229. [Google Scholar] [CrossRef]
- Lakshmanan, A.J.; Shanmugasundaram, S. Helibractinecine, a Pyrrolizidine Alkaloid from Heliotropium bracteatum. Phytochemistry 1994, 36, 245–248. [Google Scholar] [CrossRef]
- Lakshmanan, A.J.; Shanmugasundaram, S. Ester Alkaloids of Heliotropium bracteatum. Phytochemistry 1995, 40, 291–294. [Google Scholar] [CrossRef]
- Reina, M.; Gonzalez-Coloma, A.; Gutierrez, C.; Cabrera, R.; Henriquez, J.; Villarroel, L. Pyrrolizidine Alkaloids from Heliotropium megalanthum. J. Nat. Prod. 1998, 61, 1418–1420. [Google Scholar] [CrossRef] [PubMed]
- Mohanraj, S.; Subramanian, P.S.; Culvenor, C.C.J.; Edgar, J.A.; Frahn, J.L.; Smith, L.W.; Cockrum, P.A. Curassavine, an Alkaloid from Heliotropium curassavicum Linn. with a C8 Necic Acid Skeleton. J. Chem. Soc. Chem. Commun. 1978, 10, 423–424. [Google Scholar] [CrossRef]
- Crowley, H.C.; Culvenor, C.C.J. The Alkaloids of Heliotrpium supinum, with Observations on Viridifloric Acid. Aust. J. Chem. 1959, 12, 694–705. [Google Scholar] [CrossRef]
- Birecka, H.; Dinolfo, T.; William, M.; Frohlich, M.W. Polyamines and Leaf Senescence in Pyrrolizodine Alkaloid Bearing Heliotropium Plants. Phytochemistry 1984, 23, 991–997. [Google Scholar] [CrossRef]
- Guntern, A.; Ioset, J.-R.; Queiroz, E.F.; Sándor, P.; Foggin, C.M.; Hostettmann, K. Heliotropamide, a Novel Oxopyrrolidine-3-Carboxamide from Heliotropium ovalifolium. J. Nat. Prod. 2003, 66, 1550–1553. [Google Scholar] [CrossRef]
- Di Maso, M.J.; Nepomuceno, G.M.; St. Peter, M.A.; Gitre, H.H.; Martin, K.S.; Shaw, J.T. Synthesis of (±)-Bisavenanthramide B-6 by an Anionic Anhydride Mannich Reaction. Org. Lett. 2016, 18, 1740–1743. [Google Scholar] [CrossRef]
- Wang, W.; Snooks, H.D.; Sang, S. The Chemistry and Health Benefits of Dietary Phenolamides. J. Agric. Food Chem. 2020, 68, 6248–6267. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, W.-J.; Kong, J.-B.; Liu, Y.; Zhi, Y.-L.; Cao, Y.-G.; Du, K.; Xue, G.-M.; Li, M.; Zhao, Z.-Z.; et al. Structurally Diverse Phenolic Amides from the Fruits of Lycium barbarum with Potent α-Glucosidase, Dipeptidyl Peptidase-4 Inhibitory, and PPAR-γ Agonistic Activities. J. Agric. Food Chem. 2023, 71, 11080–11093. [Google Scholar] [CrossRef]
- Were, O.; Benn, M.; Munavu, R.M. The Pyrrolizidine Alkaloids of Senecio syringifolius and S. hadiensis from Kenya. Phytochemistry 1993, 32, 1595–1602. [Google Scholar] [CrossRef]
- Boppré, M.; Colegate, S.M.; Edgar, J.A.; Fischer, O.W. Hepatotoxic Pyrrolizidine Alkaloids in Pollen and Drying-Related Implications for Commercial Processing of Bee Pollen. J. Agric. Food Chem. 2008, 56, 5662–5672. [Google Scholar] [CrossRef] [PubMed]
- Dash, G.K.; Abdullah, M.S. A Review on Heliotropium indicum L. (Boraginaceae). Int. J. Pharm. Sci. Res. 2013, 4, 1253–1258. [Google Scholar]
| No | Rt (min) | [M+H]+ | Molecular Formula | Error Δppm | MS/MS | Annotated Compounds | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | 0.41 | 142.1228 | C8H15NO | 1.12 | 124 | Trachelanthamidine/Lindelofidine (S) | [16,37] |
| 2 | 0.39 | 174.1127 | C8H15NO3 | 1.32 | 156, 138, 112 | Helibracteatinecine/Helibractinecine/Croalbinecine (S) | |
| 3 | 0.41 | 190.1075 | C8H15NO4 | 0.61 | 172, 155, 129, 98 | Helibracteatinecine/Helibractinecine/Helifolinecine-N-oxide (S) | |
| 4 | 2.42 | 238.1437 | C13H19NO3 | −0.29 | 156, 138, 120, 94, 83 | 9- angeloylretronecine * (US) | [16,38] |
| 5 | 2.13 | 238.1436 | C13H19NO3 | −0.71 | 156, 138, 120, 94, 83 | 9- angeloylheliotridine * (US) | [37] |
| 6 | 2.81 | 240.1594 | C13H21NO3 | −0.08 | 208, 178, 158, 140, 122, 83 | 7-angeloylplatynecine * (S) | [39,40,41] |
| 7 | 4.19 | 254.1387 | C13H19NO4 | 0.06 | 247, 172, 154, 136, 112, 83 | 9- angeloylheliotridine * -N-oxide (US) | [37] |
| 8 | 1.95 | 254.1388 | C13H19NO4 | 0.45 | 247, 174, 137, 111, 106, 83 | 7- angeloylheliotridine *-N-oxide (US) | [16] |
| 9 | 1.45 | 256.1544 | C13H21NO4 | 0.26 | 238, 174, 156, 138, 120, 83 | Helifoline * (S) | [42] |
| 10 | 1.84 | 256.1544 | C13H21NO4 | 0.26 | 174, 156, 106, 83 | Heliscabine or isomer * (S) | [16] |
| 11 | 2.10 | 272.1492 | C13H21NO5 | −0.18 | 190, 172, 155, 129, 98, 83 | Heliscabine or isomer *-N-oxide (S) | |
| 12 | 2.98 | 272.1493 | C13H21NO5 | 0.19 | 190, 172, 155, 129, 98, 83 | Helifoline *-N-oxide (S) | |
| 13 | 6.53 | 498.2604 | C27H35N3O6 | 1.08 | 322, 234, 177, 145 | N1, N10-Diferuloyl spermidine | [43] |
| 14 | 8.71 | 625.2552 | C36H36N2O8 | 1.21 | 417, 325, 272, 301, 137, 121 | Heliotropamide |
| Position | δC | δH (J in Hz) | COSY | HMBC |
|---|---|---|---|---|
| 1 | 43.6 | 2.76 m | 2, 8, 9a, 9b | - |
| 2 | 71.8 | 4.64 m | 1, 3a, 3b | C-9 |
| 3a | 71.1 | 3.75 dd (11.07, 6.12) | 2, 3b | C-1, C-8 |
| 3b | 3.39 dd (11.07, 9.15) | 2, 3a | C-2, C-5 | |
| 5a | 68.7 | 3.57 m | 5b, 6a, 6b | |
| 5b | 3.91 m | 5a, 6a, 6b | C-6, C-7 | |
| 6a | 34.3 | 2.61 m | 6b, 5a, 5b, 7 | C-5 |
| 6b | 2.12 m | 6a, 5a, 5b, 7 | - | |
| 7 | 73.7 | 4.50 m | 8, 6a, 6b | - |
| 8 | 90.4 | 3.85 dd (8.25, 5.61) | 7, 1 | C-1, C-9 |
| 9a | 62.9 | 4.44 dd (11.23, 4.69) | 9b, 1 | C-1, C-2, C-1′, C-8 |
| 9b | 4.29 dd (11.23, 6.98) | 9a, 1 | C-1, C-2, C-1′, C-8 | |
| 1′ | 167.8 | - | - | - |
| 2′ | 127.5 | - | - | - |
| 3′ | 138.5 | 6.15 dq (7.20/1.40) | 4′ | - |
| 4′ | 15.8 | 1.99 dd (7.20/1.53) | 3′ | C-3′, C-2′ |
| 5′ | 20.9 | 1.92 s | - | C-3′, C-2′, C-1′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozntamar-Pouloglou, K.-M.; Panou, E.; Mroczek, T.; Milic, N.; Graikou, K.; Ganos, C.; Fokialakis, N.; Karikas, G.-A.; Chinou, I. Chemistry and Diversity of Nitrogen-Containing Metabolites in Heliotropium procumbens: A Genus-Wide Comparative Profile. Separations 2025, 12, 225. https://doi.org/10.3390/separations12090225
Ozntamar-Pouloglou K-M, Panou E, Mroczek T, Milic N, Graikou K, Ganos C, Fokialakis N, Karikas G-A, Chinou I. Chemistry and Diversity of Nitrogen-Containing Metabolites in Heliotropium procumbens: A Genus-Wide Comparative Profile. Separations. 2025; 12(9):225. https://doi.org/10.3390/separations12090225
Chicago/Turabian StyleOzntamar-Pouloglou, Kalliopi-Maria, Evgenia Panou, Tomasz Mroczek, Nikola Milic, Konstantia Graikou, Christos Ganos, Nikolas Fokialakis, George-Albert Karikas, and Ioanna Chinou. 2025. "Chemistry and Diversity of Nitrogen-Containing Metabolites in Heliotropium procumbens: A Genus-Wide Comparative Profile" Separations 12, no. 9: 225. https://doi.org/10.3390/separations12090225
APA StyleOzntamar-Pouloglou, K.-M., Panou, E., Mroczek, T., Milic, N., Graikou, K., Ganos, C., Fokialakis, N., Karikas, G.-A., & Chinou, I. (2025). Chemistry and Diversity of Nitrogen-Containing Metabolites in Heliotropium procumbens: A Genus-Wide Comparative Profile. Separations, 12(9), 225. https://doi.org/10.3390/separations12090225









