Abstract
This study utilizes aqueous solutions of organic amines as extractants to remove phenolic compounds from coal tar. It elucidates the extraction mechanism between phenolic compounds and organic amines, and conducts a comprehensive investigation into the extraction performance of various monomeric organic amine aqueous solutions and their composite solutions for phenolic compounds in model oils or light coal tar. The experimental results showed that monoethanolamine (MEA), characterized by a higher reaction equilibrium constant, exhibits superior extraction performance for phenolic compounds in light coal tar compared to diethanolamine (DEA), triethanolamine (TEA), methyldiethanolamine (MDEA), and diethylaminoethanol (DEAE) when used at the same mass concentration of extractant. The addition of a specific concentration of DEA or TEA to a 20 wt%–25 wt% MEA solution significantly reduces the entrainment of neutral oil impurities during the extraction process and enhances the precipitation of phenolic compounds during the acidification phase. Under the optimal process parameters, the composite aqueous solution, comprising 25 wt% MEA, 5 wt% DEA, and 5 wt% TEA, demonstrated a 35.1 wt% increase in the acidification yield of phenolic compounds, Ya,P, a 20.8 wt% increase in the total yield of phenolic compounds, YP, and a 46.0% significant decrease in neutral oil entrainment compared to the solution with a 30% MEA aqueous solution.
1. Introduction
Phenolic compounds are one of the important components of coal tar, accounting for approximately 20–30% of its total content [1,2]. These compounds serve as vital raw materials in a variety of industrial applications, including the production of pesticides, pharmaceuticals, fragrances, and as intermediates in organic synthesis [3,4]. In recent years, with the rapid development of low-rank coal upgrading, global coal tar production reached 20 million tons in 2024, thereby providing a reliable source of raw material for the deep processing of coal tar [5,6]. Furthermore, prioritizing the extraction of phenolic compounds from coal tar can not only reduce hydrogen consumption during the hydrogenation process, but can also enhance the economic value of coal tar [7,8].
Currently, the predominant industrial method for extracting phenolic compounds from coal tar still employs the traditional alkali washing process [9]. This method uses a caustic alkali aqueous solution as the extraction agent and a sulfuric acid aqueous solution as the acidifying agent to extract phenolic compounds from coal tar [10]. According to the relevant chemical reaction equations, the alkali washing process consumes approximately 0.4 tons of caustic alkali (99 wt%), 0.5 tons of concentrated sulfuric acid (98 wt%), and 2.3 tons of fresh water to produce 1 ton of crude phenol oil. Simultaneously, it generates 3.2 tons of wastewater containing phenols and salts, posing severe challenges to the surrounding ecological environment of enterprises.
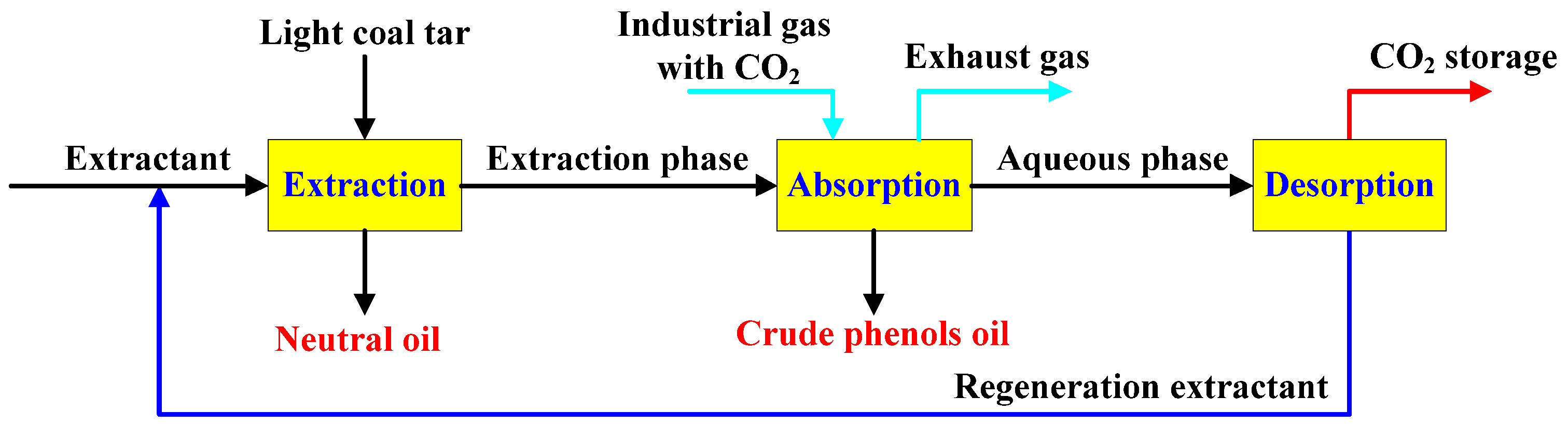
To develop a green, efficient, and cost-effective alternative to the conventional alkali washing process, numerous researchers has been dedicated to the development of extractants and process technologies, accompanied by extensive experimental investigations. For instance, Ge et al. utilized soluble metal salt ions as precipitants, effectively achieving the extraction of phenolic compounds from coal tar [11]. Zhang [12] and Nemati Kharat et al. [13,14] explored the methods of ionic liquids and deep eutectic solvents for the separation of phenolic compounds from coal tar, elucidating the impact of cation and anion structures on the separation processes. Nevertheless, although these methods fundamentally circumvent the use of mineral acids and bases, they are still limited by significant challenges, particularly the trade-off between the extraction performance and separation efficiency of the extractants [15]. The low separation efficiency of the extractants, coupled with the high energy consumption during the separation process, continues to limit their industrial-scale application [16]. This paper conceptually proposed a coupled process technology for the extraction of phenolic compounds and capturing carbon dioxide using a composite organic amine aqueous solution, with the specific flow shown in Figure 1. This process utilized the composite organic amine aqueous solution to efficiently extract phenolic compounds from light coal tar, while applying the phenol-enriched extraction phase to capture CO2 from industrial exhaust gases, thereby displacing the crude phenol oils in the extraction phase. On the one hand, this method achieved the low-energy regeneration and recycling of the extractant by heating the amine-based carbonate aqueous solution to desorb CO2, thus avoiding the consumption of mineral acids and bases, and water resources. On the other hand, it synergistically captures CO2 from industrial exhaust gases through process coupling, which significantly enhances the economic efficiency of production. This study focused on investigating the influence of molecular structure and synergistic effects of organic amine compounds on extraction and separation performance, providing a foundation for the industrial application of the designed process.
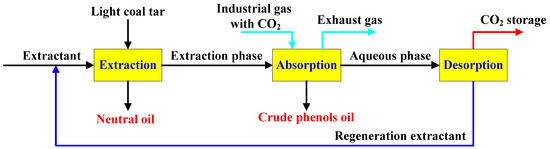
Figure 1.
The experimental flow chart for the coupling process of phenol extraction and CO2 capture by aqueous amine.
2. Materials and Methods
2.1. Reagents and Analytical Method
Monoethanolamine (MEA, AR, ≥99 wt%), Diethanolamine (DEA, AR, ≥99 wt%), Triethanolamine (TEA, AR, ≥99 wt%), Methyldiethanolamine (MDEA, AR, ≥99 wt%), Diethylaminoethanol (DEEA, AR, ≥99 wt%), phenol (AR, ≥99 wt%), o-cresol (AR, ≥99 wt%), 2,6-dimethylphenol (AR, ≥99 wt%), and 2,3,5-trimethylphenol (AR, ≥98 wt%) were purchased from Shanghai Macklin Biochemical Technology Co., Ltd. (Shanghai, China). Toluene (AR, ≥99.5 wt%) and methanol (AR, ≥99.9 wt%) were afforded by Tianjin Kermel Chemical Reagent Co., Ltd. (Tianjin, China), and CO2 (purity ≥99.9 wt%) was provided by the Chengdu Keyuan Gas Co., Ltd. (Chengdu, China). Sodium hydroxide (AR, ≥96.0 wt%) was obtained from Xilong Scientific Co., Ltd. (Shantou, China). The light coal tar was provided by Xinjiang Xuanli Environmental Protection Co., Ltd. (Urumqi, China). The quantitative analysis of the total phenolic content in the model oil and its raffinate was conducted using an Agilent 7890B gas chromatograph, manufactured by Agilent Technologies (Shanghai) Co., Ltd. (Shanghai, China). This instrument was equipped with an HP-5 capillary column (30 m × 0.320 mm × 0.25 µm) and a hydrogen flame ionization detector. Methanol and chlorobenzene were selected as the solvent and internal standard, respectively. The chromatographic conditions for the injector and detector were set at 563.15 K. The temperature program was as follows: the initial oven temperature was 333.15 K, maintained for 1 min, then increased at a rate of 20 K·min−1 to 533.15 K. The phenolic compounds content in the light coal tar and its raffinate phase were determined by the method described in GB/T 24200-2009. Specifically, a 10 wt% aqueous sodium hydroxide solution was used as the solvent to remove phenolic compounds from the test samples in a double-ball measuring tube, thereby enabling quantitative analysis of the phenolic compound content in the test oil phase. The amine in aqueous solution was quantified by acid–base titration with 0.1 mol·L−1 of sulfuric acid solution and 0.1 wt% bromocresol green methanol solution as the standard solution and indicator, respectively.
2.2. Procedure
The experiment utilized light coal tar with a total phenolic content of 22.63 wt% and a model oil consisting of toluene (70.31 wt%), phenol (9.76 wt%), o-cresol (9.78 wt%), 2,6-dimethylphenol (5.10 wt%), and 2,3,5-trimethylphenol (5.05 wt%) as raw materials, along with an aqueous organic amine solution as the extractant. Taking the experiment of extracting phenolic compounds from light coal tar with a composite organic amine aqueous solution containing 25 wt% MEA, 5 wt% DEA, and 5 wt% TEA as an example, the specific experimental procedure was described as follows: 50.0 g of the light coal tar and 150.0 g of composite amine aqueous solution were added in a flask and stirred vigorously with a magnetic stirrer for 45 min at 298.15 K. Upon ceasing agitation, the mixture was transferred to a separatory funnel and allowed to stand for 15 min, during which it separated into two phases. Next, the extraction phase obtained from the extraction process was acidified with a certain flow rate of CO2 at 25 °C. Once the acidification reaction reaches equilibrium, the acidified solution was poured into a separatory funnel for static separation to obtain the crude phenol oil phase and aqueous phase. The samples obtained from the extraction process and acidification process were taken for analysis, and the extraction yield of phenols, YE,P, and the extraction yield of neutral oil, Yo, could be determined by Equation (1). The acidification yield of phenols, Ya,P, and the total yield, YP, were calculated according to Equation (2).
where MP and RP were the mass quantities of phenols in the coal tar and the raffinate phase; Mo and Ro were the mass quantities of neutral oil in the coal tar and the raffinate phase; EP and CP were the mass quantities of phenols in the extraction phase and crude phenol oil, respectively.
3. Extraction Mechanism
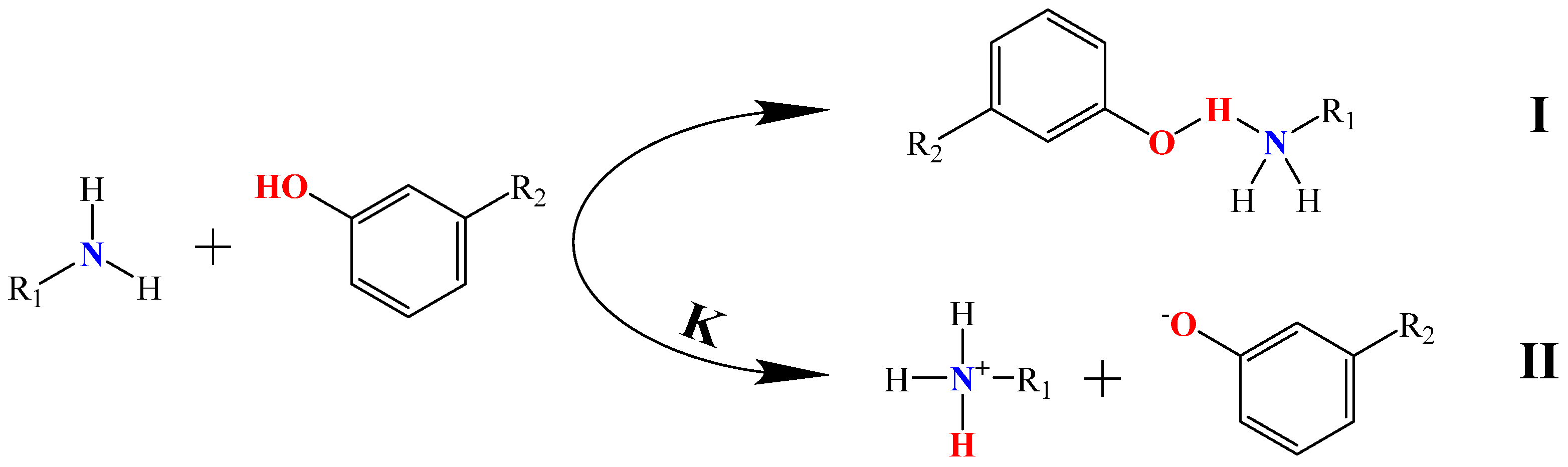
Figure 2 illustrates two coexisting reaction mechanisms between organic amine compounds and phenolic compounds. The first mechanism involves the donation of an electron pair from the lone pair of electrons on the nitrogen atom of the organic amine compound to the hydrogen atom on the hydroxyl group of the phenolic compound, forming a N–H hydrogen bond [17]. The second mechanism occurs in an aqueous solution, where the proton H+ from the hydroxyl group of the phenolic compound is captured by the lone pair of electrons from the organic amine compound, resulting in acid–base proton transfer and generating phenolate anions and protonated amine cations. In general, these two extraction mechanisms coexist during the extraction process of extracting phenolic compounds from coal tar by aqueous organic amine solutions.
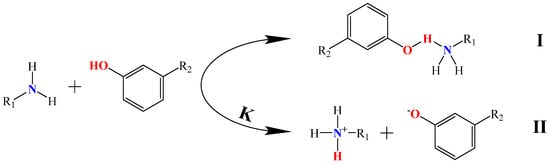
Figure 2.
The extraction mechanisms of organic amine compounds and phenolic compounds.
The equilibrium constants (K) for the reactions between common alkanolamine compounds and phenolic compounds are shown in Table 1. As can be seen from Table 1, it can be observed that the K values for the reactions between alkanolamine compounds and phenolic compounds were all less than 1, indicating that the proton transfer reaction between organic amine compounds and phenolic compounds was limited, and the lone pair of electrons on the N atom of the organic amine compounds was insufficient to completely capture the H protons from the phenolic compounds. Additionally, the equilibrium constants for the reactions between organic amine compounds and individual phenolic compounds follow the order of MEA > DEA > TEA. Furthermore, as the carbon number of phenolic compounds increases from C6 to C8, the K values progressively decrease. This trend suggests that organic amine compounds with higher conjugate acid acidity coefficients predominantly extract lower-carbon-number phenolic compounds from coal tar through ionic interactions, whereas the extraction of organic amine compounds with lower conjugate acid acidity coefficients and higher-carbon-number phenolic compounds primarily depends on hydrogen bonding interactions.

Table 1.
The equilibrium constant of the reaction between organic amine compounds and phenolic compounds.
4. Results and Discussion
4.1. The Effect of Various Alkanolamine Solutions on the Phenolic Compounds Extraction Performance
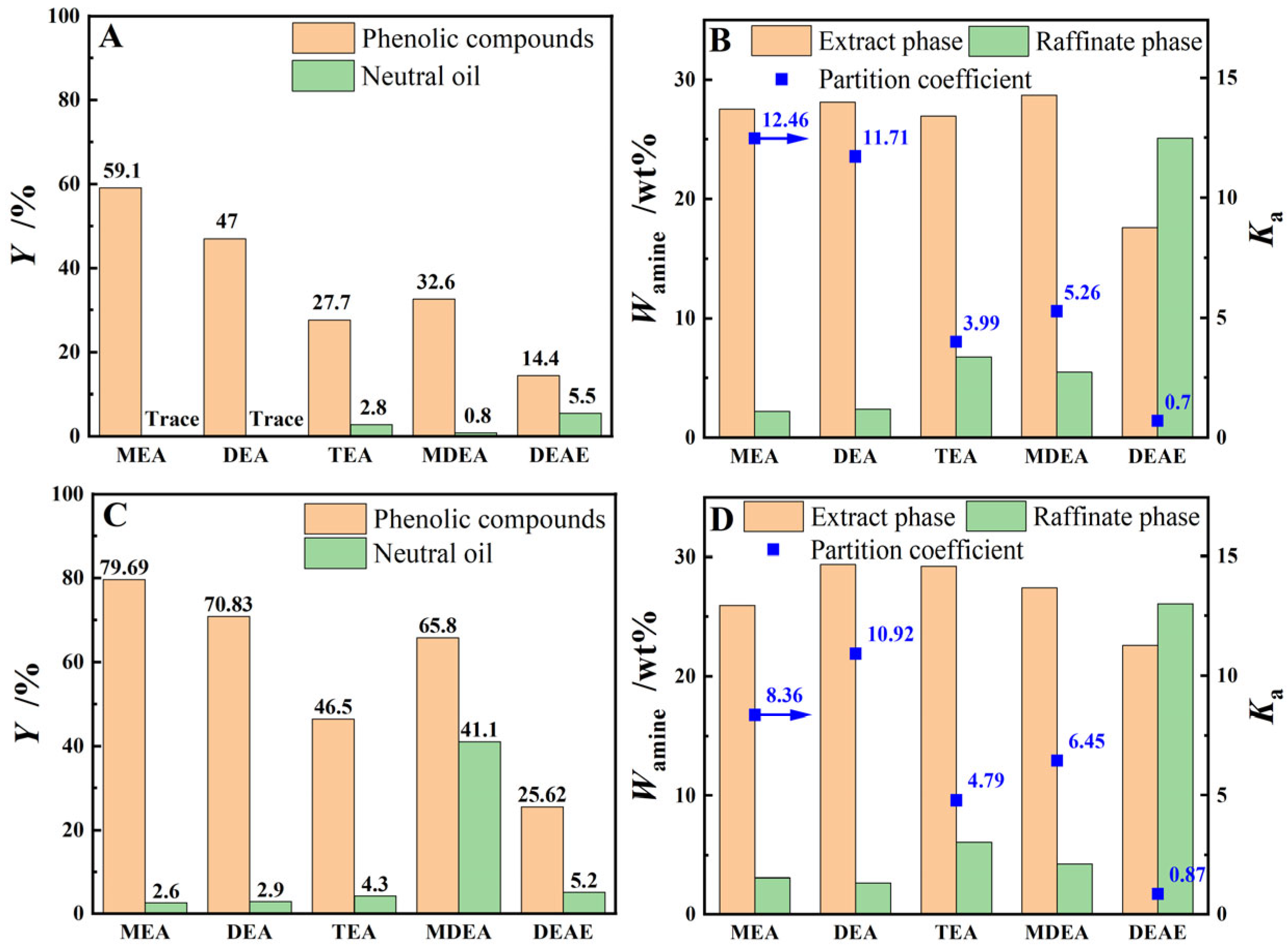
This experiment investigated the extraction performance of various organic amine aqueous solutions, including MEA aqueous solution, DEA aqueous solution, TEA aqueous solution, MDEA aqueous solution, and DEAE aqueous solution, for phenolic compounds in both model oil and light coal tar. The results in Figure 3 shows that the YE,P of the model oil or light coal tar for various extractants follows the order that MEA > DEA > MDEA > TEA > DEAE. As illustrated by Figure 3A,C, although the YE,P for model oil or light coal tar by the MDEA aqueous solution was higher than that of the TEA or even DEA aqueous solution, its YO reaches 41.1%, which had a severe negative effect on the separation and refining of phenolic compounds in the crude phenol oil. Comparing the extraction performance of three secondary amine aqueous solutions, namely DEA, MDEA, and DEAE, in Figure 3, it can be observed that the distribution coefficients () of the organic amine compounds follow the order of DEA > MDEA > DEAE, and the YO of light coal tar by the MDEA and DEAE aqueous solutions were significantly higher than those of the DEA aqueous solution. This was primarily due to the increase in hydrocarbon functional groups such as -CH3 and -CH2-CH3 on the nitrogen atom of the amine group, which weakens the hydrophilicity of the organic amine compounds, leading to an increase in and a decrease in Ka. Simultaneously, affected by the electron-donating effect of the hydrocarbon functional group, the electronegativity of the amino nitrogen atom was also weakened, resulting in a decline in the YE,P for MDEA and DEAE. It can be seen from Figure 3C,D that, even though MEA demonstrated the optimal single-stage extraction yield for real coal tar, DEA with two hydrophilic hydroxyl functional groups exhibits a significantly higher aqueous phase distribution coefficient compared to MEA, which was beneficial for reducing the loss of extractant.
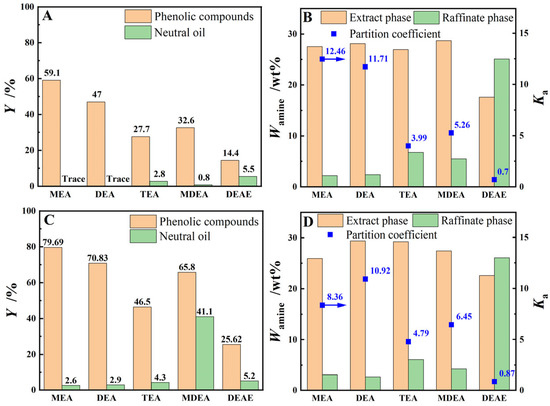
Figure 3.
The extraction performance of various alkanolamine aqueous solutions for phenolic compounds in model oil (Graphs (A,B)) or light coal tar (Graphs (C,D)) at T = 273.15 K, β = 3 and t = 60 min with stirring.
4.2. The Effect of a Composite Alkanolamine Aqueous Solution on the Phenol Extraction Performance
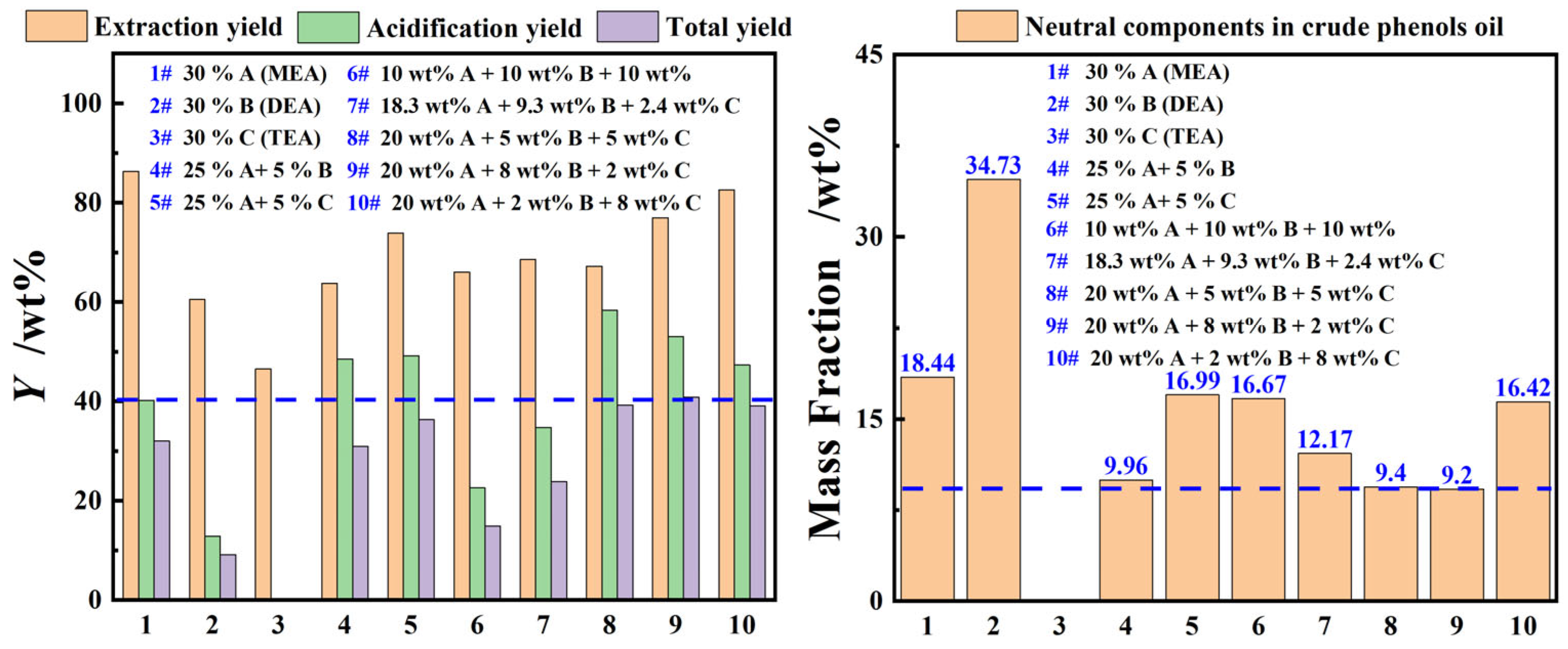
In addition to the extraction performance, the separation efficiency of phenolic compounds and extractant in the extraction phase was also one of the important evaluation indicators. Figure 4 provides a detailed examination of the extraction and separation performance of MEA, DEA, and TEA single-component aqueous organic amine solutions, as well as binary and ternary composite organic amine aqueous solutions, for phenolic compounds in coal tar. The results show that the YE,P, Ya,P, and YP of the single-component organic amine aqueous solutions follow the order of MEA > DEA > TEA. This was mainly because the MEA, which had a primary amine structure, a lower molecular weight, and smaller steric hindrance, could capture and accommodate more phenolic compounds. Thus, the phenolic compounds accommodated in the extraction phase could be released more during the acidification process. More importantly, it can be seen from Figure 4 that the YE,P for coal tar by the composite organic amine aqueous solutions were lower than those of the MEA aqueous solution under identical process parameters, but its Ya,P were significantly higher than those of the single MEA aqueous solution when the mass concentration of MEA in the composite organic amine aqueous solution exceeded 20 wt%. This may be due to the fact that the protonation equilibrium constants of secondary and tertiary amines are greater than those of primary amines, allowing them to absorb more CO2 under equilibrium reaction conditions, thereby displacing a larger number of phenolic compounds [24,25]. Additionally, the experimental results for samples 8#–10# in Figure 4 showed that the neutral oil impurities in the crude phenol oil were merely 9.2% to 9.4%, similar to those of experiment sample 4#, and significantly lower than those of the other experimental groups. This may be attributed to the larger molecular structure, lower viscosity, and the presence of both lipophilic and hydrophilic groups in DEA, which facilitate its directional arrangement at the oil–water interface, forming a dense interfacial film that reduces the entrainment of neutral oils. After comprehensive consideration, an organic amine aqueous solution containing 25 wt% MEA, 5 wt% DEA, and 5 wt% TEA was selected as the extractant.
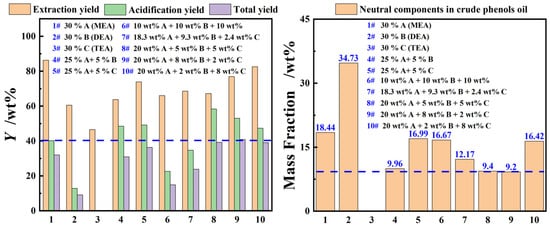
Figure 4.
The yield of single or composite alkanolamine aqueous solutions for phenolic compounds in light coal tar (extraction process: T = 273.15 K, β = 3 and t = 60 min with stirring; acidification process: T = 273.15 K with stirring).
4.3. The Effects of Process Parameters on the Phenol Extraction Performance
4.3.1. The Effect of Extraction Time on the Extraction Performance
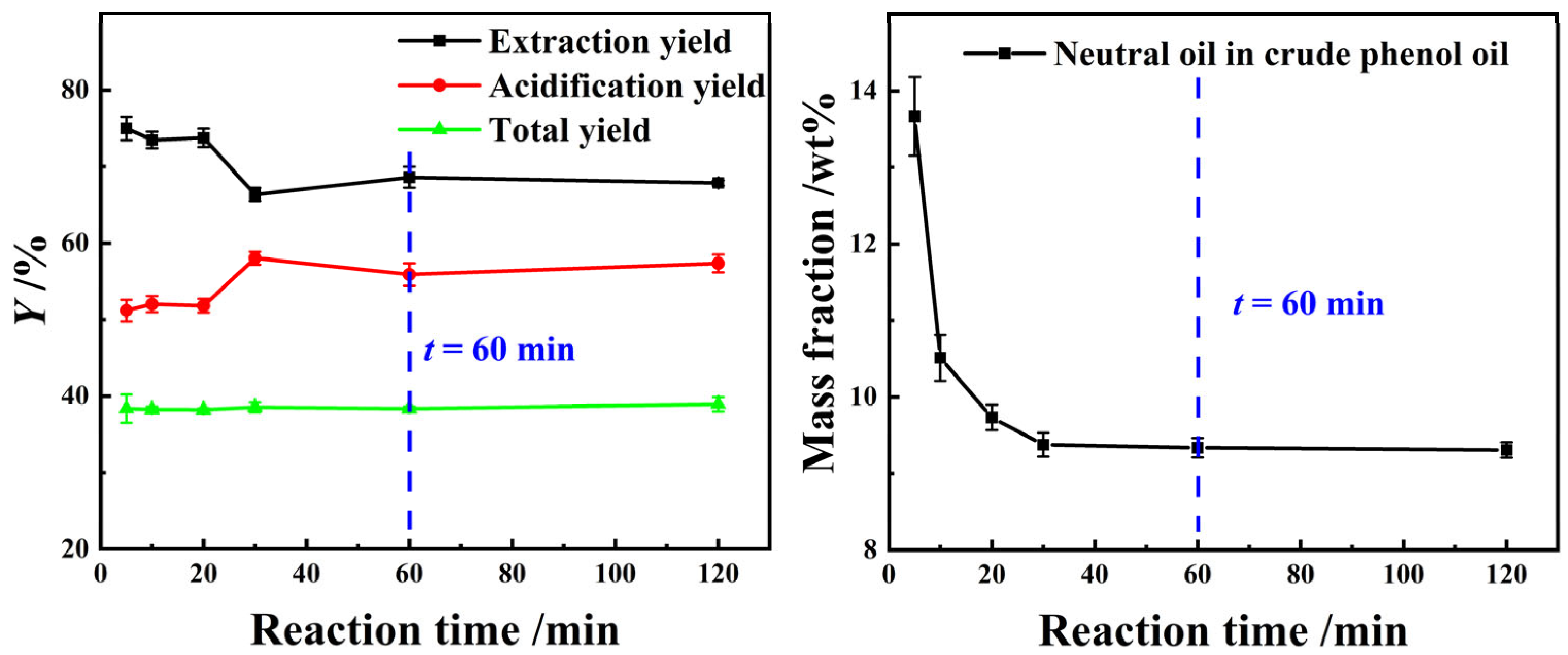
The experimental results in Figure 5 show that as the reaction time increased, the YE,P of light coal tar using a composite alkanolamine aqueous solution and the neutral oil impurity content in crude phenol oil initially decreased, followed by a gradual stabilization. Conversely, the acidification yield displays an opposite trend. This may be due to the fact that in the initial stage of the extraction reaction, the process was dominated by the dissolution equilibrium of organic amines, neutral oil impurities, and phenolic compounds. The intermolecular interactions among these three types of substances promoted the dissolution of neutral oil impurities and phenolic compounds into the alkanolamines aqueous solution. As a result, the YE,P and the concentration of neutral oil impurities in crude phenol oil reached their highest levels when the extraction reaction time was 1 min. Subsequently, as the extraction reaction time was extended from 1 min to 30 min, the reaction equilibrium between the alkanolamines and phenolic compounds became dominant, which resulted in a rapid reduction in neutral oil impurity content in the crude phenol oil, accompanied by an increase in the YE,P. Of course, in addition to the above-mentioned factors, the selective extraction of small molecules and highly hydrophilic phenolic compounds, such as phenol, by the extractant was also one of the major factors contributing to the phenomenon. In conclusion, 60 min was selected as the optimal extraction equilibrium reaction time.
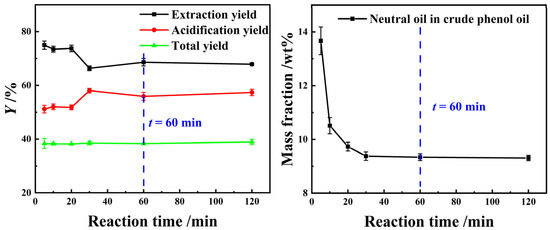
Figure 5.
The effect of t on the extraction process using a 25 wt% MEA–5 wt% DEA–5 wt% TEA aqueous solution at β = 3 and T = 298.15 K with stirring.
4.3.2. The Effect of Extraction Temperature on the Extraction Performance
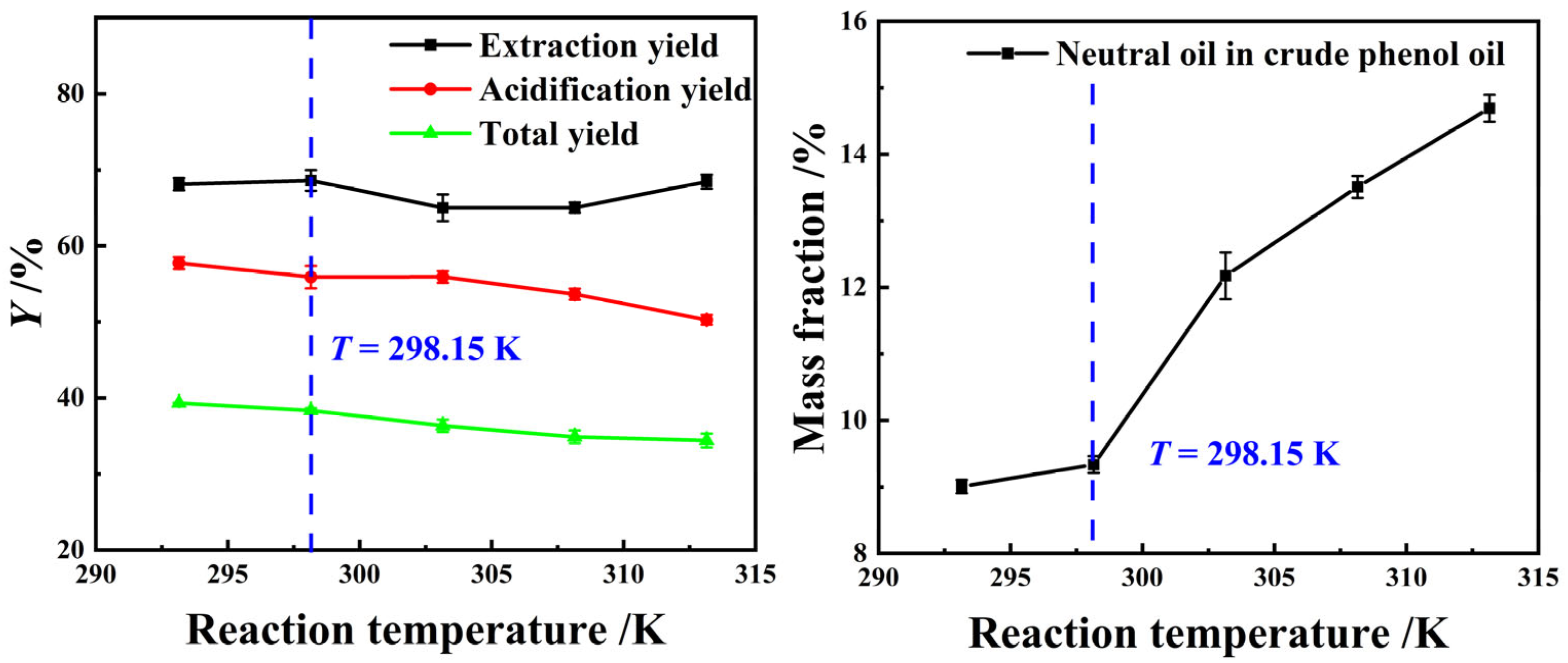
Figure 6 illustrates that as the extraction reaction temperature continuously increases, the YE,P of light coal tar initially decreases and then rises, while the Ya,P and YP exhibit an overall declining trend, and the residue of neutral oil impurities in crude phenol oil show a persistent rise. This may be attributed to the increased solubility of aromatic hydrocarbons or chain hydrocarbons from coal tar as the reaction temperatures during the extraction process elevated, which had negative impacts on the precipitation of phenolic compounds during the acidification process and the residue of neutral oil impurity in crude phenol oil. However, a decrease in the extraction reaction temperature resulted in a prolongation of the extraction reaction equilibrium time. Therefore, room temperature, 298.15 K, was chosen as the optimal extraction reaction temperature.
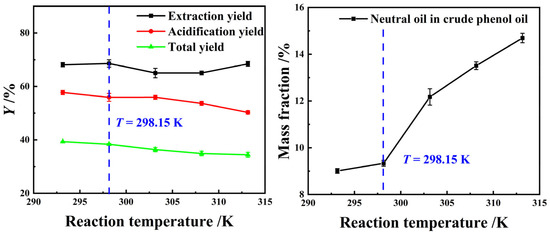
Figure 6.
The effect of T on the extraction process using a 25 wt% MEA–5 wt% DEA–5 wt% TEA aqueous solution at β = 3 and t = 60 min with stirring.
4.3.3. The Effect of Concentration and Phase Ratio on the Extraction Performance
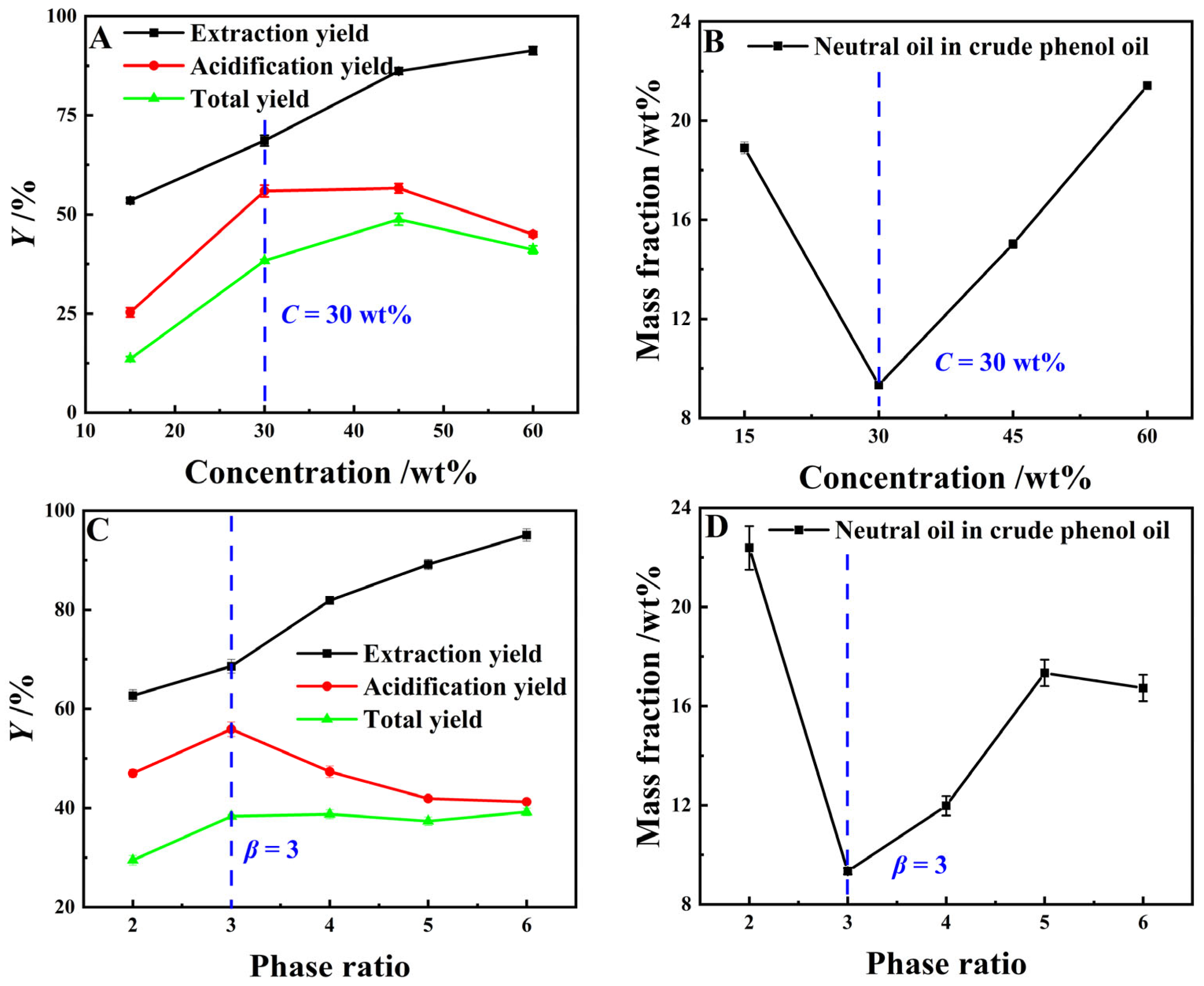
As can be seen from Figure 7A,B, when the extractant concentration increases from 15 wt% to 60 wt%, the YE,P for light coal tar basically shows a linear upward trend, while its Ya,P and YP first increase and then decrease. When the extractant concentration exceeds 30 wt%, the neutral oil content in the crude phenol oil rises linearly. This was because, as the concentration of organic amine compounds increases, the solubility of the extractant for neutral oil in coal tar increases, which leads to a decrease in acidification yield, and an increase in neutral oil residue in crude phenol oil. The results of Figure 7C,D show that as the phase ratio increases from 2 to 6, the YE,P basically shows a linear upward trend, with the highest YE,P reaching 96.16 wt%, while the Ya,P increases first, then decreases subsequently. When the phase ratio exceeds 3, the Ya,P shows a continuous downward trend with the phase ratio expanding further, but the rate of decline gradually slows down. The primary reason for this was that, when the mass ratio of the extractant to the light coal tar feedstock increases from 2 to 6, the concentration of high-carbon-number lipophilic phenolic compounds in the extraction phase rises, while the total phenol concentration in the extraction phase continuously declines. The overlapping influence of these two factors leads to the aforementioned variation pattern in the acidification process. In addition, the neutral oil impurity content in the crude phenol oil reached its lowest level at merely 9.4 wt% when the phase ratio was 3. In summary, the optimal extractant concentration was 30 wt% and the phase ratio was 3.
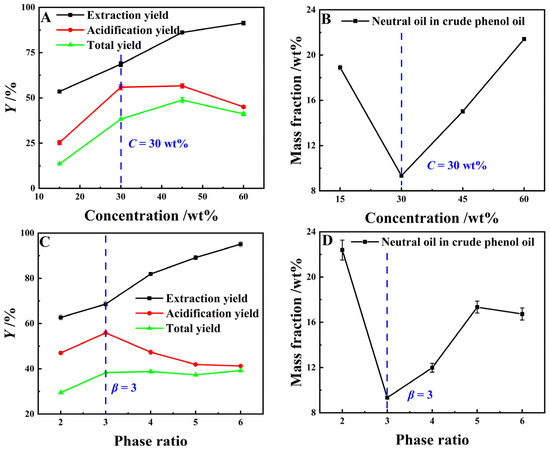
Figure 7.
The effect of C and β on the extraction process (Graphs (A,B) with β = 3, t = 60 min and T = 273.15 K; Graphs (C,D) using a 25 wt% MEA–5 wt% DEA–5 wt% TEA aqueous solution at t = 60 min and T = 273.15 K).
4.4. The Absorption and Desorption Performance Evaluation
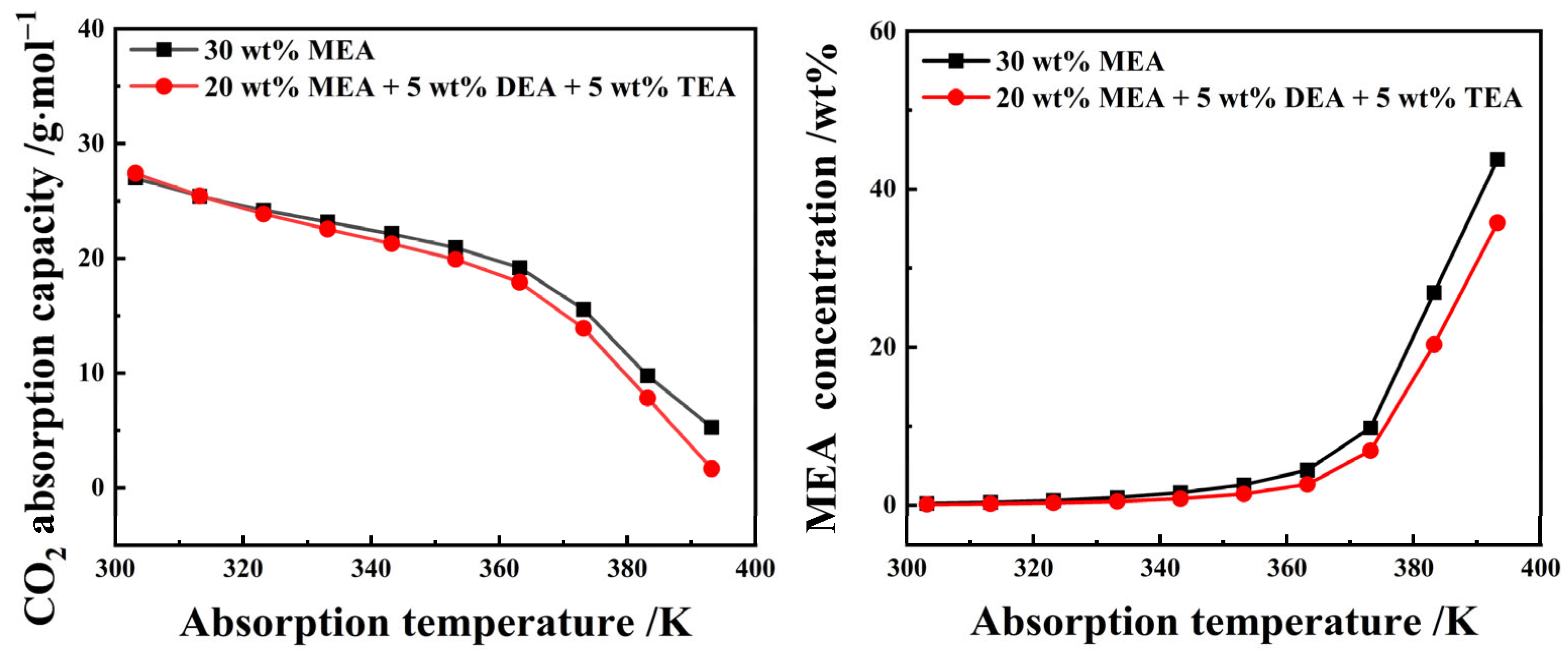
Figure 8 describes the single-stage reaction equilibrium behavior of CO2 absorption by the 20 wt% MEA + 5 wt% DEA + 5 wt% TEA aqueous solution and the 30 wt% MEA aqueous solution based on the equilibrium reaction model of CO2 absorption by MEA, DEA, and TEA aqueous solutions [26,27,28]. The model parameters can be seen in Table S1 of the Supplementary Materials. The simulation results show that the absorption capacity of an organic amine aqueous solution to CO2 decreases with increasing temperature. When the absorption reaction temperature was lower than 40 °C, the CO2 absorption capacity of a 20 wt% MEA + 5 wt% DEA + 5 wt% TEA aqueous solution exceeded that of a 30 wt% MEA aqueous solution. Conversely, after the absorption reaction temperature surpassed 40 °C, the CO2 absorption capacity of the 20 wt% MEA + 5 wt% DEA + 5 wt% TEA aqueous solution was lower than that of the 30 wt% MEA aqueous solution. This phenomenon was beneficial to the precipitation of crude phenol oil in the extraction phase and the regeneration of the extractant. In addition, with the increase in absorption reaction temperature, the equilibrium concentration of MEA in the reaction solution continues to increase, and the equilibrium concentration of MEA in the aqueous solution of 20 wt% MEA + 5 wt% DEA + 5 wt% TEA was lower than the 30 wt% MEA, which might also be one of the reasons why the Ya,P of the aqueous solution of composite organic amine was higher than that of the 30 wt% MEA aqueous solution.
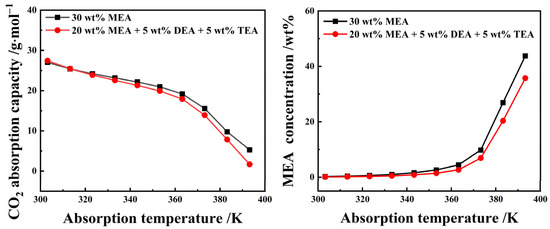
Figure 8.
The CO2 absorption and desorption performance of 25 wt% MEA–5 wt% DEA–5 wt% TEA and 30 wt% aqueous solutions.
5. Conclusions
The extraction mechanisms of organic amines and phenolic compounds via hydrogen bonding and ionic interactions were elucidated, and the extraction performance of phenolic compounds from light coal tar or model oil by mono alkanolamine and composite alkanolamine aqueous solutions were examined. The results showed that MEA, characterized by its primary amine structure and minimal branching, exhibited a higher reaction equilibrium constant with phenolic compounds and superior extraction performance compared to DEA, TEA, MDEA, and DEAE. Specifically, the single-stage YE,P for the light coal tar could reach 79.69% with the 30 wt% MEA aqueous solution. After adding a certain amount of DEA and TEA to the aqueous solution of MEA to prepare an aqueous solution of 20 wt% MEA + 5 wt% DEA + 5 wt% TEA, although the YE,P for light coal tar was only 71.2%, lower than that of the 30 wt% MEA solution. The Ya,P and YP increase by 35.1% and 20.8%, respectively, compared to the 30 wt% MEA solution. Additionally, the neutral oil residue in crude phenol oil was reduced by 46.0%. The observed improvement was attributed to the introduction of DEA and TEA, which possessed large molecular structures and hydrophobic and hydrophilic groups, thereby promoting the formation of an interfacial membrane during extraction and enhancing the absorption capacity of CO2.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/separations12090227/s1, Table S1: The primary chemical reaction equations and model parameters for the aqueous solutions of MEA (monoethanolamine), DEA (diethanolamine), and TEA (triethanolamine) in CO2 absorption.
Author Contributions
Conceptualization, Y.L. and W.Y.; methodology, Y.L., Y.Y. and Y.W.; software, Y.L., W.Y., and Q.A.; validation, B.P. and Y.L.; formal analysis, B.P., J.Z., and Y.Y.; investigation, H.L. and B.P.; resources, Y.L., and Q.A.; data curation, H.L. and B.P.; writing—original draft preparation, Y.Y. and Y.L.; writing—review and editing, Y.L. and Y.W.; visualization, H.L. and B.P.; supervision, J.Z.; project administration, Y.L. and Y.W.; funding acquisition, Y.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Hu Xiang High-Level Talent Aggregation Project, grant number 2024RC4011.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| MDEA | Methyldiethanolamine |
| DEAE | Diethylaminoethanol |
| MEA | Monoethanolamine |
| DEA | Diethanolamine |
| TEA | Triethanolamine |
| t | Reaction time |
| T | Reaction temperature |
| C | Concentration |
| β | Phase ratio |
| K | Reaction equilibrium constant |
| Ka | Distribution coefficient of organic amine |
| YP | The total yield of phenolic compounds |
| YE,P | The extraction yield phenolic compounds |
| Ya,P | The acidification yield of phenolic compounds |
| Yo | The extraction yield of neutral oil |
| CP | The mass quantities of phenols in the crude phenol oil |
| EP | The mass quantities of phenols in the extraction phase |
| RP | The mass quantities of phenols in the raffinate phase |
| MP | The mass quantities of phenols in the light coal tar |
| Ro | The mass quantities of neutral oil in the raffinate phase |
| Mo | The mass quantities of neutral oil in the light coal tar |
References
- Hou, Y.; Kong, J.; Ren, Y.; Ren, S.; Wu, W. Mass transfer dynamics in the separation of phenol from model oil with quaternary ammonium salts via forming deep eutectic solvents. Sep. Purif. Technol. 2017, 174, 554–560. [Google Scholar] [CrossRef]
- Ji, Y.; Hou, Y.; Ren, S.; Yao, C.; Wu, W. Highly efficient extraction of phenolic compounds from oil mixtures by trimethylamine-based dicationic ionic liquids via forming deep eutectic solvents. Fuel Process. Technol. 2018, 171, 183–191. [Google Scholar] [CrossRef]
- Lv, Q.; Ma, Y.; Yao, Q.; Sun, M. A Review on the Extraction of Phenols from Coal Tar: Composition Distribution and Analysis, Methods and Mechanism, Process and Industrialization Prospect. Energy Fuels 2024, 39, 1479–1506. [Google Scholar] [CrossRef]
- Yan, H.; Ma, H.; Li, X.; Dong, S.; Li, Q. Separation of higher-rank phenols from coal tar models: A combination of experiment and mechanism analysis. J. Chem. Thermodyn. 2025, 211, 107550. [Google Scholar] [CrossRef]
- Zhang, X.; Qi, X.; Wang, W.; Li, X.; Zhu, Z. Pyrolysis characteristics of pulverized coal in circulating fluidized bed with a multi-section variable-diameter riser. J. Anal. Appl. Pyrolysis 2025, 191, 107214. [Google Scholar] [CrossRef]
- Tian, Z.; Zhang, B.; Wang, Q.; Deng, Q.; Xu, G.; Ma, D. Effects of different heat carriers on pyrolysis products of high-sodium low-rank coal and optimization of pyrolysis process. Energy 2025, 325, 136095. [Google Scholar] [CrossRef]
- Ma, C.; Zhao, Y.; Guo, X.; Yao, R.; Zou, C.; Miao, Z. Pyrolysis behavior of low-rank coal in an H2-containing atmosphere and combustion properties of the prepared chars. J. Energy Inst. 2024, 114, 101581. [Google Scholar] [CrossRef]
- Li, Y.; Ai, Q.; You, K.; Zhao, F.; Xiao, W.; Luo, H. Modeling and simulating a new process for extracting phenols from model coal tar by low-boiling-point amine aqueous solutions. Fuel 2021, 299, 120921. [Google Scholar] [CrossRef]
- Morgan, J.J.; Meighan, M.H. Extraction of phenols from tar oils by the caustic soda process. Ind. Eng. Chem. 1925, 17, 696–700. [Google Scholar] [CrossRef]
- Chasib, K.F. Extraction of phenolic pollutants (phenol and p-chlorophenol) from industrial wastewater. J. Chem. Eng. Data 2012, 58, 1549–1564. [Google Scholar] [CrossRef]
- Ge, Y.Z.; Jin, H. Recovery process for phenolic compounds from coal-derived oils by ions of soluble metal salts. Fuel 1996, 75, 1681–1683. [Google Scholar] [CrossRef]
- Jiao, T.; Li, C.; Zhuang, X.; Cao, S.; Chen, H.; Zhang, S. The new liquid-liquid extraction method for separation of phenolic compounds from coal tar. Chem. Eng. J. 2015, 266, 148–155. [Google Scholar] [CrossRef]
- Yi, L.; Li, W.; Feng, J. Application of ionic liquid/low eutectic solvent in coal-based liquid separation. Chem. Ind. Eng. Prog. 2020, 39, 2066–2078. [Google Scholar]
- Amirfirouzkouhi, H.; Kharat, A.N. Application of ionic liquids as recyclable green catalysts for selective alkylation of phenol. Sep. Purif. Technol. 2018, 196, 132–139. [Google Scholar] [CrossRef]
- Yan, H.; Zhen, Y.; Zhang, A.; Li, T.; Lu, W.; Li, Q. Research on separation of higher-rank phenols from coal tar: A combination of liquid-liquid extraction experiments and mechanism analysis. J. Mol. Liq. 2024, 415, 126366. [Google Scholar] [CrossRef]
- Gao, D.; Hu, D.; Wang, X.; Abliz, A.; Bi, X.; Musa, B.; Wang, Q. Experimental and simulation studies on extraction and separation of phnolics from coal tar by deep eutectic solvents. Fuel 2025, 399, 135524. [Google Scholar]
- Li, Y.; Luo, H.; Ai, Q.; You, K.; Zhao, F.; Xiao, W. Efficient separation of phenols from coal tar with aqueous solution of amines by liquid-liquid extraction. Chin. J. Chem. Eng. 2021, 35, 180–188. [Google Scholar] [CrossRef]
- Perrin, D. Dissociation Constants of Organic Bases in Aqueous Solution; Butterworths: London, UK, 1972. [Google Scholar]
- Alner, D.; Smeeth, A. The dissociation constants of some substituted benzoic acids in aqueous solution at 25 °C. J. Chem. Soc. 1958, 1, 4128–4132. [Google Scholar]
- Monice, M.; Bart, H.; Wilma, F.; Donald, V.; Ronald, A.; Curtis, D.; Daniel, L.; James, G.; Ronald, C.; Thomas, J.; et al. Safety Assessment of Triethanolamine and Triethanolamine-Containing Ingredients as Used in Cosmetics. Int. J. Toxicol. 2013, 32, 59S–83S. [Google Scholar] [CrossRef]
- Lide, D.R. CRC Handbook of Chemistry and Physics, 83rd ed.; CRC Press Inc.: Boca Raton, FL, USA, 2002; pp. 8–49. [Google Scholar]
- Shiu, W.; Ma, K.; Varhaníčková, D.; Mackay, D. Chlorophenols and alkylphenols: A review and correlation of environmentally relevant properties and fate in an evaluative environment. Chemosphere 1994, 29, 1155–1224. [Google Scholar] [CrossRef]
- Serjeant, E.; Dempsey, B. Ionisation Constants of Prganic Acids in Aqueous Solution; Pergamon Press: Oxford, UK, 1972. [Google Scholar]
- Filburn, T.; Helble, J.; Weiss, R. Development of Supported Ethanolamines and Modified Ethanolamines for CO2 Capture. Ind. Eng. Chem. Res. 2005, 45, 1542–1546. [Google Scholar] [CrossRef]
- Zheng, W.; Luo, Q.; Liu, S.; Wang, L.; Luo, X.; Gao, H.; Liang, Z. New method of kinetic modeling for CO2 absorption into blended amine systems: A case of MEA/EAE/3DEA1P trisolvent blends. AIChE J. 2022, 68, e17628. [Google Scholar] [CrossRef]
- Zhang, Y.; Que, H.; Chen, C. Thermodynamic modeling for CO2 absorption in aqueous MEA solution with electrolyte NRTL model. Fluid Phase Equilibria 2011, 311, 67–75. [Google Scholar] [CrossRef]
- Ahad, G. Mass transfer and thermodynamic modeling of carbon dioxide absorption into MEA aqueous solution. Pol. J. Chem. Technol. 2017, 19, 75–82. [Google Scholar] [CrossRef]
- Shima, K.; Ahad, G.; Kambiz, T. Experimental and Thermodynamic Modeling of CO2 Absorption into Aqueous DEA and DEA+Pz Blended Solutions. Iran. J. Chem. Chem. Eng. 2021, 40, 1162–1178. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).