Stimulus-Responsive Membranes: A Mini Review on Principles, Preparation Methods, and Emerging Applications
Abstract
1. Introduction
2. Stimuli-Responsive Principles
2.1. Thermo-Responsive Stimulus
2.2. pH-Responsive Stimulus
2.3. Photo-Responsive Stimulus
2.4. Electro-Responsive Stimulus
2.5. Magneto-Responsive Stimulus
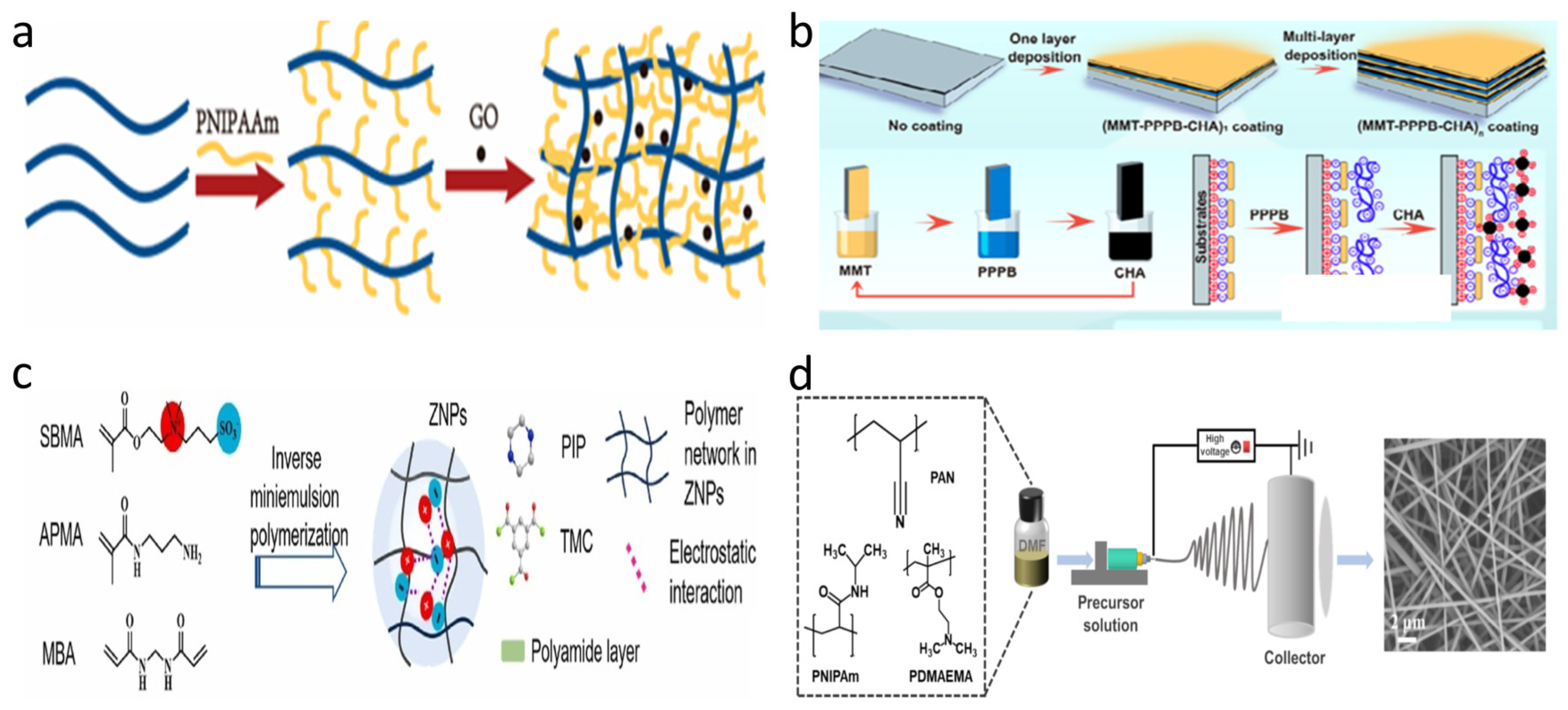
3. Synthesis Strategies of Stimulus-Responsive Membranes
3.1. Blending
3.2. Self-Assembly
3.3. Polymerization
3.4. Electrospinning
4. Applications of Stimulus-Responsive Membranes
4.1. Water Treatment
4.2. Gas Separation
4.3. Biomedical Science
5. Conclusions and Prospects
5.1. Sensitivity and Selectivity
5.2. Stability in Microstructure and Performance
5.3. Multiple Stimuli and Functional Membranes
5.4. Stimuli-Responsive Inorganic Membranes
5.5. Large-Scale Fabrication and Application
Author Contributions
Funding
Conflicts of Interest
References
- Liu, C.; Zhong, J.; Wei, R.; Ruan, J.; Wang, K.; Zhu, Z.; Wang, Y.; Zhong, L. Process design and intensification of multicomponent azeotropes special distillation separation via molecular simulation and system optimization. Chin. J. Chem. Eng. 2024, 71, 24–44. [Google Scholar] [CrossRef]
- Xiang, B.; Liu, Q.; Yan, W.; Wei, Y.; Mu, P.; Li, J. Advances in special wettable materials for adsorption separation of high-viscosity crude oil/water mixtures. Chem. Commun. 2023, 59, 7559–7578. [Google Scholar] [CrossRef]
- Zhang, N.; Yang, X.; Wang, Y.; Qi, Y.; Zhang, Y.; Luo, J.; Cui, P.; Jiang, W. A review on oil/water emulsion separation membrane material. J. Environ. Chem. Eng. 2022, 10, 107257. [Google Scholar] [CrossRef]
- Li, L.; Ye, M.; Gan, X.; Xiao, T.; Zhu, Z. Development of membrane separation technology and membrane-based biore-actor in wastewater treatment: Conventional membrane and dynamic membrane. Desalination Water Treat. 2023, 304, 36–46. [Google Scholar] [CrossRef]
- Kotsuchibashi, Y. Recent advances in multi-temperature-responsive polymeric materials. Polym. J. 2020, 52, 681–689. [Google Scholar] [CrossRef]
- Yue, C.; Zhang, G. Calcium ion crosslinked sodium alginate coated PVDF membrane for improved smart pH-responsive properties. J. Environ. Chem. Eng. 2022, 10, 108684. [Google Scholar] [CrossRef]
- Tripathi, T.; Kamaz, M.; Wickramasinghe, S.R.; Sengupta, A. Designing Electric Field Responsive Ultrafiltration Membranes by Controlled Grafting of Poly (Ionic Liquid) Brush. Int. J. Environ. Res. Public Health 2020, 17, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Su, B.; Tian, Y.; Yu, Z.; Wu, X.; Ding, J.; Wu, C.; Wei, D.; Yin, H.; Sun, J.; et al. Magnetic manipulation of Fe3O4@BaTiO3 nanochains to regulate extracellular topographical and electrical cues. Acta Biomater. 2023, 168, 470–483. [Google Scholar] [CrossRef]
- Rosli, A.; Low, S.C. Molecularly engineered switchable photo-responsive membrane in gas separation for environmental protection. Environ. Eng. Res. 2019, 25, 447–461. [Google Scholar] [CrossRef]
- Huang, T.; Su, Z.; Hou, K.; Zeng, J.; Zhou, H.; Zhang, L.; Nunes, S.P. Advanced stimuli-responsive membranes for smart separation. Chem. Soc. Rev. 2023, 52, 4173–4207. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, Y.; Yang, S.; Zhang, C.; Ullah, Z. Recent research progress on the stimuli-responsive smart membrane: A review. Nanotechnol. Rev. 2023, 12, 20220538. [Google Scholar] [CrossRef]
- Sponchioni, M.; Capasso Palmiero, U.; Moscatelli, D. Thermo-responsive polymers: Applications of smart materials in drug delivery and tissue engineering. Mater. Sci. Eng. C 2019, 102, 589–605. [Google Scholar] [CrossRef] [PubMed]
- Al-Shaeli, M.; Benkhaya, S.; Al-Juboori, R.A.; Koyuncu, I.; Vatanpour, V. pH-responsive membranes: Mechanisms, fabrications, and applications. Sci. Total Environ. 2024, 946, 174865. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Lin, S.; Chen, S.; Shen, X.; Guo, D.; Guo, J. Recent Advances in Smart Windows Based on Photo-Responsive Liquid Crystals Featuring Phase Transition. ChemPlusChem 2024, 89, 202300700. [Google Scholar] [CrossRef] [PubMed]
- Alayande, A.B.; Goh, K.; Son, M.; Kim, C.-M.; Chae, K.-J.; Kang, Y.; Jang, J.; Kim, I.S.; Yang, E. Recent Progress in One- and Two-Dimensional Nanomaterial-Based Electro-Responsive Membranes: Versatile and Smart Applications from Fouling Mitigation to Tuning Mass Transport. Membranes 2020, 11, 5. [Google Scholar] [CrossRef]
- Low, S.C.; Ng, Q.H.; Tan, L.S. Study of magnetic-responsive nanoparticle on the membrane surface as a membrane antifouling surface coating. J. Polym. Res. 2019, 26, 70. [Google Scholar] [CrossRef]
- Musarurwa, H.; Tavengwa, N.T. Thermo-responsive polymers and advances in their applications in separation science. Microchem. J. 2022, 179, 107554. [Google Scholar] [CrossRef]
- Xu, H.-X.; Wang, D.C.; Ho, C.-H.; Chang, M.-C.; Horng, R.-Y.; Liang, T.-M.; Liu, P.-I. LCST-type thermo-responsive ionic liquid used as a recyclable and reusable cleaning agent for fouled membrane. Desalination Water Treat. 2022, 258, 55–63. [Google Scholar] [CrossRef]
- Matsuoka, A.; Motoyama, A.; Kamio, E.; Yoshioka, T.; Nakagawa, K.; Matsuyama, H. Effects of hydrogen-bonding functional groups of ammonium based-ionic liquids with Tf2N anion on the upper critical solution temperature in aqueous solutions. J. Mol. Liq. 2023, 383, 122145. [Google Scholar] [CrossRef]
- Ma, Q.; Zheng, X. Preparation and characterization of thermo-responsive composite for adsorption-based dehumidification and water harvesting. Chem. Eng. J. 2022, 429, 132498. [Google Scholar] [CrossRef]
- Maji, S.; Jerca, V.V.; Hoogenboom, R. Dual pH and thermoresponsive alternating polyampholytes in alcohol/water solvent mixtures. Polym. Chem. 2020, 11, 2205–2211. [Google Scholar] [CrossRef]
- Tian, Y.; Lai, J.; Li, C.; Sun, J.; Liu, K.; Zhao, C.; Zhang, M. Poly(N-acryloyl glycinamide-co-N-acryloxysuccinimide) Nanoparticles: Tunable Thermo-Responsiveness and Improved Bio-Interfacial Adhesion for Cell Function Regulation. ACS Appl. Mater. Interfaces 2023, 15, 7867–7877. [Google Scholar] [CrossRef]
- Wu, J.; Pourdeyhimi, B.; Yarin, A.L. Adaptable Intelligent Filters of Dual Thermo- and pH- Responsive Filter Material. Macromol. Rapid Commun. 2024, 46, 2400861. [Google Scholar] [CrossRef]
- Babazadeh-Mamaqani, M.; Razzaghi, D.; Roghani-Mamaqani, H.; Babaie, A.; Rezaei, M.; Hoogenboom, R.; Salami-Kalajahi, M. Photo-responsive electrospun polymer nanofibers: Mechanisms, properties, and applications. Prog. Mater. Sci. 2024, 146, 101312. [Google Scholar] [CrossRef]
- Wang, B.Y.; Shen, L.G.; Xu, J.J.; Fei, L.Y.; Li, B.S.; Lin, H.J.; Chen, C. Spiropyran molecular aggregates implanted photo-responsive graphene oxide membrane with self-cleaning ability for enhanced water purification. J. Membr. Sci. 2024, 702, 122744. [Google Scholar] [CrossRef]
- Suzuki, T.; Moriya, T.; Endo, R.; Iwasaki, N. A photo-responsive polymeric azopyridine ligand with metal-complexation sensitivity: Application to coordination equilibrium studies on the polymer complexes of a cobalt(ii) Schiff base. Polym. Chem. 2017, 8, 761–768. [Google Scholar] [CrossRef]
- Singh, A.; Kuksenok, O.; Johnson, J.A.; Balazs, A.C. Photo-regeneration of severed gel with iniferter-mediated photo-growth. Soft Matter 2017, 13, 1978–1987. [Google Scholar] [CrossRef]
- Ren, L.; Chen, J.; Han, J.; Liang, J.; Wu, H. Biomimetic construction of smart nanochannels in covalent organic framework membranes for efficient ion separation. Chem. Eng. J. 2024, 482, 148907. [Google Scholar] [CrossRef]
- Song, Y.-P.; Zhang, J.-N.; Wang, J.-R.; Li, K.; Yuan, Y.-X.; Li, B.; Zang, S.-Q. A fast responsive photochromic SCC-MOF for photoswitching and information encryption. Sci. China Mater. 2024, 67, 698–704. [Google Scholar] [CrossRef]
- Zou, L.-B.; Zhou, X.-L.; Zheng, H.; Fan, Z.-W.; Pan, D.-W.; Liu, Z.; Wang, W.; Xie, R.; Ju, X.-J.; Chu, L.-Y. Regulatory effects of cyclodextrins on light-responsive phase transition behaviors of poly(N-isopropylacrylamide-co-N-(4-phenylazophenyl)methylacrylamide). Polymer 2024, 313, 127676. [Google Scholar] [CrossRef]
- Jin, Z.; Wei, X.; He, X.; Wang, Z.; Zhao, Z.; He, H.; Yang, Y.; Chen, N. Research Progress and Emerging Directions in Stimulus Electro-Responsive Polymer Materials. Materials 2024, 17, 4204. [Google Scholar] [CrossRef]
- Xu, L.; Liu, S.; Yu, L.; Li, K.; Zhang, Y.; Wang, J.; Wang, J. Tuneable ion transport by electrically responsive membranes under electrical assistance. J. Membr. Sci. 2022, 663, 121046. [Google Scholar] [CrossRef]
- Puguan, J.M.C.; Rathod, P.V.; More, P.P.; Kim, H. Achieving transmissive-to-black chromism via engineered dual electro-thermoresponsive single-molecule for full modulation of solar transmittance. Chem. Eng. J. 2022, 437, 135157. [Google Scholar] [CrossRef]
- Lee, D.; Kim, H.-I.; Kim, W.-Y.; Cho, S.-K.; Baek, K.; Jeong, K.; Ahn, D.B.; Park, S.; Kang, S.J.; Lee, S.-Y. Water-Repellent Ionic Liquid Skinny Gels Customized for Aqueous Zn-Ion Battery Anodes. Adv. Funct. Mater. 2021, 31, 2104269. [Google Scholar] [CrossRef]
- Song, J.; Lu, Y.; Pan, T.; Wang, J.; Liu, Z.; Xu, L.; Zhang, S.; Li, Y.; Bai, Y.; Heng, B.C.; et al. Manipulation of Surface Electrical Charge on Nanocomposite Membranes Confers Wide. Adv. Funct. Mater. 2024, 34, 2314024. [Google Scholar] [CrossRef]
- Wang, W.; Li, G.; Wang, X.; Huang, F.; Fan, T.; Wang, J. Visible light-driven catalytic degradation of organic pollutants by S-scheme heterojunction Bi4O5I2/NaNbO3 enhanced by piezoelectric effect. Sep. Purif. Technol. 2025, 356, 129830. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, Y.; Zhang, X.; Zhang, W.; Wang, X.; Yue, Y.; Guo, J.; Yu, Y. A CO2-stimulus responsive PVDF/PVDF-g-PDEAEMA blend membrane capable of cleaning protein foulants by alternate aeration of N2/CO2. Sep. Purif. Technol. 2021, 279, 119680. [Google Scholar] [CrossRef]
- Ji, T.; Ji, Y.; Meng, X.; Wang, Q. Temperature-Responsive Separation Membrane with High Antifouling Performance for Efficient Separation. Polymers 2024, 16, 416. [Google Scholar] [CrossRef]
- Li, W.; Hua, G.; Cai, J.; Zhou, Y.; Zhou, X.; Wang, M.; Wang, X.; Fu, B.; Ren, L. Multi-Stimulus Responsive Multilayer Coating for Treatment of Device-Associated Infections. J. Funct. Biomater. 2022, 13, 24. [Google Scholar] [CrossRef]
- Mi, Y.-F.; Xia, W.; Gu, B.-X.; Ma, R.; Ji, Y.-L. Salt-responsive polyamide thin-film nanocomposite membrane based on zwitterionic polymeric nanoparticles for high-efficient dye desalination. J. Membr. Sci. 2024, 709, 123094. [Google Scholar] [CrossRef]
- Dou, Y.-L.; Lv, C.-J.; Yue, X.; Su, Y.; Yasin, A.; Ma, P.-C. Temperature and pH-responsive electrospun membrane with high flux recovery for emulsion separation. Sep. Purif. Technol. 2025, 358, 130247. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, S.; Lyu, Q.; Cheng, M.; Wang, H.; Li, C.; Sha, H.; Faller, R.; Hu, S. Harnessed Dopant Block Copolymers Assist Decorating Membrane Pores: A Dissipative Particle Dynamics Study. Macromol. Rapid Commun. 2019, 41, 1900561. [Google Scholar] [CrossRef]
- Wurm, F.R.; Boyer, C.; Sumerlin, B.S. Progress on Stimuli-Responsive Polymers. Macromol. Rapid Commun. 2021, 42, 2100512. [Google Scholar] [CrossRef]
- Gilmer, C.M.; Bowden, N.B. Reactive Epoxy Nanofiltration Membranes with Disulfide Bonds for the Separation of Multicomponent Chemical Mixtures. ACS Omega 2018, 3, 10216–10224. [Google Scholar] [CrossRef]
- Li, J.; Liu, X.; Sun, H.; Wang, L.; Zhang, J.; Deng, L.; Ma, T. An Optical Fiber Sensor Coated with Electrospinning Polyvinyl Alcohol/Carbon Nanotubes Composite Film. Sensors 2020, 20, 6996. [Google Scholar] [CrossRef] [PubMed]
- Bastidas, J.G.; Maurmann, N.; Oliveira, L.; Alcantara, B.; Pinheiro, C.V.; Leipnitz, G.; Meyer, F.; Oliveira, M.; Rigon, P.; Pranke, P. Bilayer scaffold from PLGA/fibrin electrospun membrane and fibrin hydrogel layer supports wound healing in vivo. Biomed. Mater. 2023, 18, 025020. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Che, H.; Huo, M.; Peng, L.; Fang, T.; Liu, N.; Feng, L.; Wei, Y.; Yuan, J. CO2-Responsive Nanofibrous Membranes with Switchable Oil/Water Wettability. Angew. Chem. Int. Ed. 2015, 54, 8934–8938. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, H.; Wang, R.; Li, Q.; Zhang, L.; Liu, Z.; Jiao, T. Preparation and good antibacterial properties of self-assembled P(AA-AM)/CA/GA fiber membrane by electrospinning. Colloids Surf. A Physicochem. Eng. Asp. 2024, 693, 134077. [Google Scholar] [CrossRef]
- Wang, L.; Gao, N.; Zhang, Y.; Li, B.; Liao, Y. Modified ceramic membrane with temperature responsiveness and self-cleaning property for efficient separation and catalysis. Sep. Purif. Technol. 2024, 349, 127935. [Google Scholar] [CrossRef]
- Li, B.; Gao, N.; Liao, Y.; Zhang, Y.; Mao, Y. Photo-Fenton ceramic composite membrane with Ag-doped FeOOH film for synergistically enhanced dye removal. J. Water Process Eng. 2024, 64, 105728. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, S.; Cao, W.; Zhang, J.; Peng, L.; Lu, H.; Ma, W.; Xiong, R.; Huang, C. Photothermal-Responsive Electrospun Fibrous Membrane for Flux Tunable Oil-In-Water Emulsion Separation. J. Appl. Polym. Sci. 2025, 142, e56635. [Google Scholar] [CrossRef]
- Ahmad, I.; Alayande, A.B.; Jee, H.; Wang, Z.; Park, Y.-J.; Im, K.S.; Nam, S.Y.; Bae, T.-H.; Yang, E. Recent progress of MXene-based membranes for high-performance and efficient gas separation. Diam. Relat. Mater. 2023, 135, 109883. [Google Scholar] [CrossRef]
- Da Conceicao, M.; Nemetz, L.; Rivero, J.; Hornbostel, K.; Lipscomb, G. Gas Separation Membrane Module Modeling: A Comprehensive Review. Membranes 2023, 13, 639. [Google Scholar] [CrossRef] [PubMed]
- Foo, K.; Liang, Y.Y.; Goh, P.S.; Fletcher, D.F. Computational fluid dynamics simulations of membrane gas separation: Overview, challenges and future perspectives. Chem. Eng. Res. Des. 2023, 191, 127–140. [Google Scholar] [CrossRef]
- Ying, Y.; Zhang, Z.; Peh, S.B.; Karmakar, A.; Cheng, Y.; Zhang, J.; Xi, L.; Boothroyd, C.; Lam, Y.M.; Zhong, C.; et al. Pressure-Responsive Two-Dimensional Metal–Organic Framework Composite Membranes for CO2 Separation. Angew. Chem. Int. Ed. 2021, 60, 11318–11325. [Google Scholar] [CrossRef] [PubMed]
- Xin, Q.; Dong, J.; Shao, W.; Ding, X.; Gao, N.; Zhang, L.; Jin, H.; Chen, H.; Zhang, Y. Reversible MOF-Based mixed matrix membranes for SO2/N2 separation: A photo-responsive approach. J. Membr. Sci. 2025, 714, 123431. [Google Scholar] [CrossRef]
- Chen, M.; Li, Y.F.; Besenbacher, F. Electrospun Nanofibers-Mediated On-Demand Drug Release. Adv. Healthc. Mater. 2014, 3, 1721–1732. [Google Scholar] [CrossRef]
- Yang, J.; Tu, J.; Lamers, G.E.M.; Olsthoorn, R.C.L.; Kros, A. Membrane Fusion Mediated Intracellular Delivery of Lipid Bilayer Coated Mesoporous Silica Nanoparticles. Adv. Healthc. Mater. 2017, 6, 1700759. [Google Scholar] [CrossRef]
- Awasthi, N.; Kopec, W.; Wilkosz, N.; Jamróz, D.; Hub, J.S.; Zatorska, M.; Petka, R.; Nowakowska, M.; Kepczynski, M. Molecular Mechanism of Polycation-Induced Pore Formation in Biomembranes. ACS Biomater. Sci. Eng. 2018, 5, 780–794. [Google Scholar] [CrossRef]
- Yi, M.; Nguyen, T.D.; Liu, H.; Liu, Y.; Xiong, S.; Wang, Y. A Boronate Ester Driven Rechargeable Antibacterial Membrane for Fast Molecular Sieving. Adv. Funct. Mater. 2023, 33, 2213471. [Google Scholar] [CrossRef]
- Wang, Z.-L.; Huang, D.; Zhang, M.-Y.; Fu, X.-X.; Luo, Y.; Zou, L.; Gao, S.-J.; Zhao, Z.; Wang, Y.-F.; Zhang, Y.; et al. Responsive Hybrid Poly(vinyl alcohol) Hydrogel Membranes with Embedded Microgels as Valves. Chin. J. Polym. Sci. 2023, 41, 1646–1655. [Google Scholar] [CrossRef]
- Pu, B.; Xiao, G.; Xiao, J.; Yin, H.-B.; Wang, J. A strategy for improving self-healing efficiency: Application of sensitizer in thymine photoresponsive materials. Polymer 2024, 297, 126844. [Google Scholar] [CrossRef]
- Liu, W.; Huang, M.; Liang, J.; Luo, X.; Yang, G.; An, D.; Wei, S.; Xie, Z. pH-responsive hierarchically porous self-assembly bioinspired Al2O3 ceramic membranes. Ceram. Int. 2022, 48, 22246–22253. [Google Scholar] [CrossRef]
- Baig, U.; Al-Kuhaili, M.F.; Dastageer, M.A. Photo-responsive Zinc Oxide-coated alumina ceramic membrane with super-wettable and self-cleaning features fabricated by single step RF magnetron sputtering for oily water treatment. Process Saf. Environ. Prot. 2023, 175, 541–553. [Google Scholar] [CrossRef]


| Response Type | Responsive Substance | Support Layer | Preparation Method | Key Benefits | Reference |
|---|---|---|---|---|---|
| Thermo-responsive | P[P(VMDMO)-co-P(DMAEMA)-co-P (MATE)] | Nano-Ag decorated ceramic ultrafiltration substrate | Blending | Pure water flux increased from 68.8 L‧m−2‧h−1 (25 °C, 0.1 MPa) to 434.4 L‧m−2‧h−1 (85 °C, 0.1 MPa). | [50] |
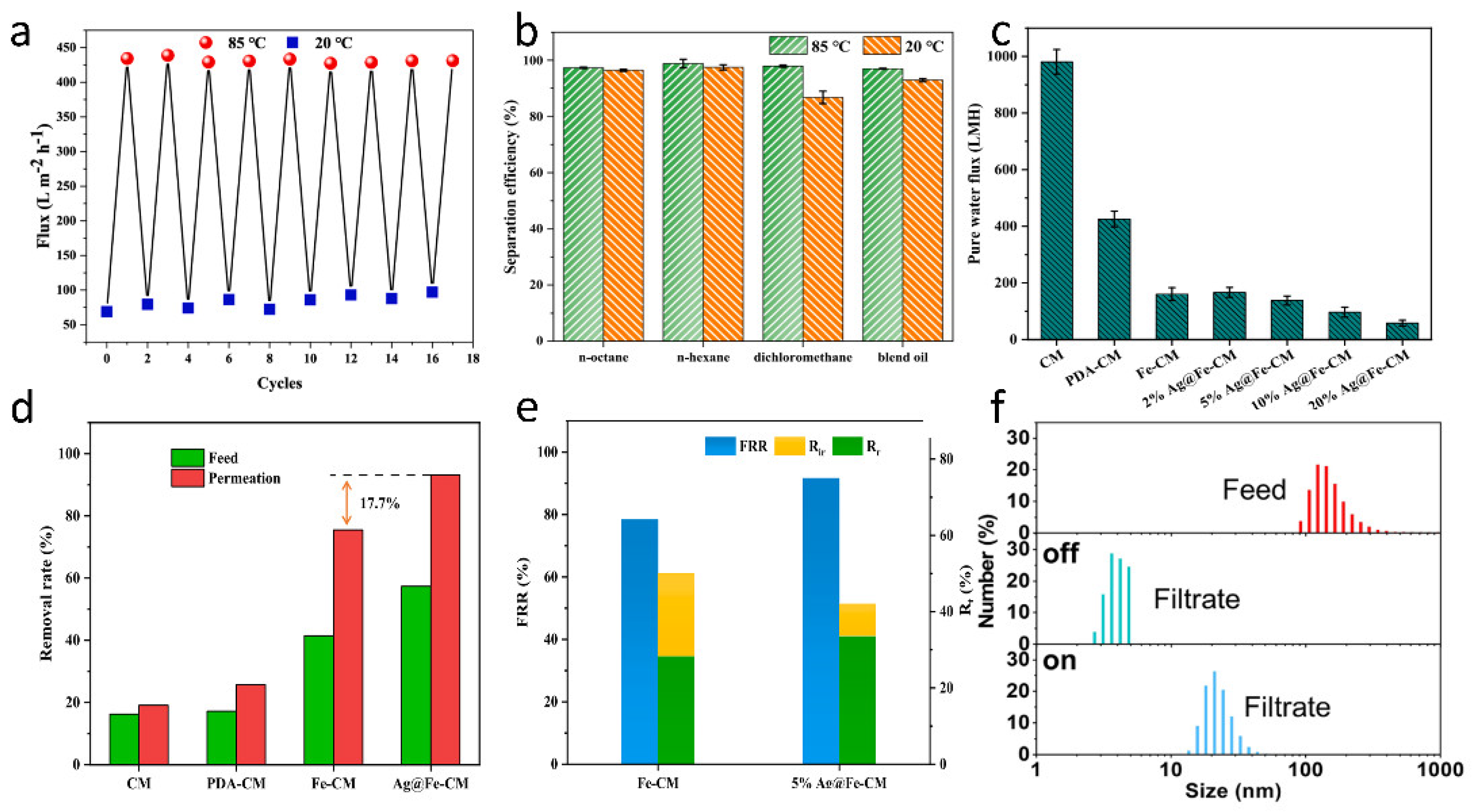
| Photo-responsive | PDA | Ag NPs, FeOOH | Self-Assembly | 5% Ag@Fe-CM showed a higher removal efficiency (93.2%) of MB under visible light compared with pure Fe-CM. | [51] |
| Photo, thermal- responsive | PMMA-b-PNIPAM | PTFMs | Polymerization | Upon laser activation, the filtrate showed droplets between 10 nm and 44 nm. | [52] |
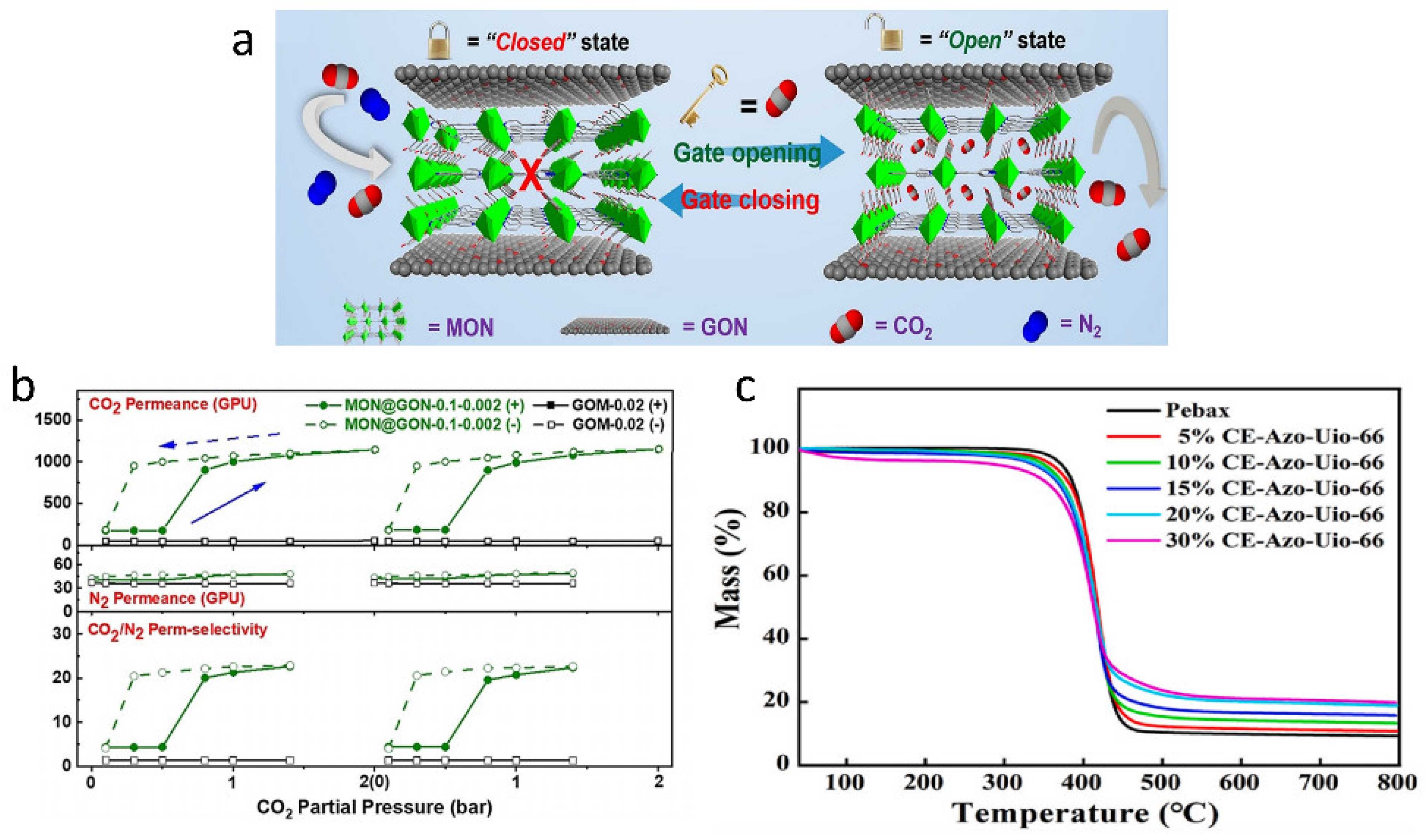
| Photo-responsive | CE-Azo-Uio-66 | Pebax | Blending | The SO2 permeability and selectivity of the Pebax/CE-Azo-UiO-66 membrane increased by 191% and 179% compared to pure Pebax membrane. | [57] |
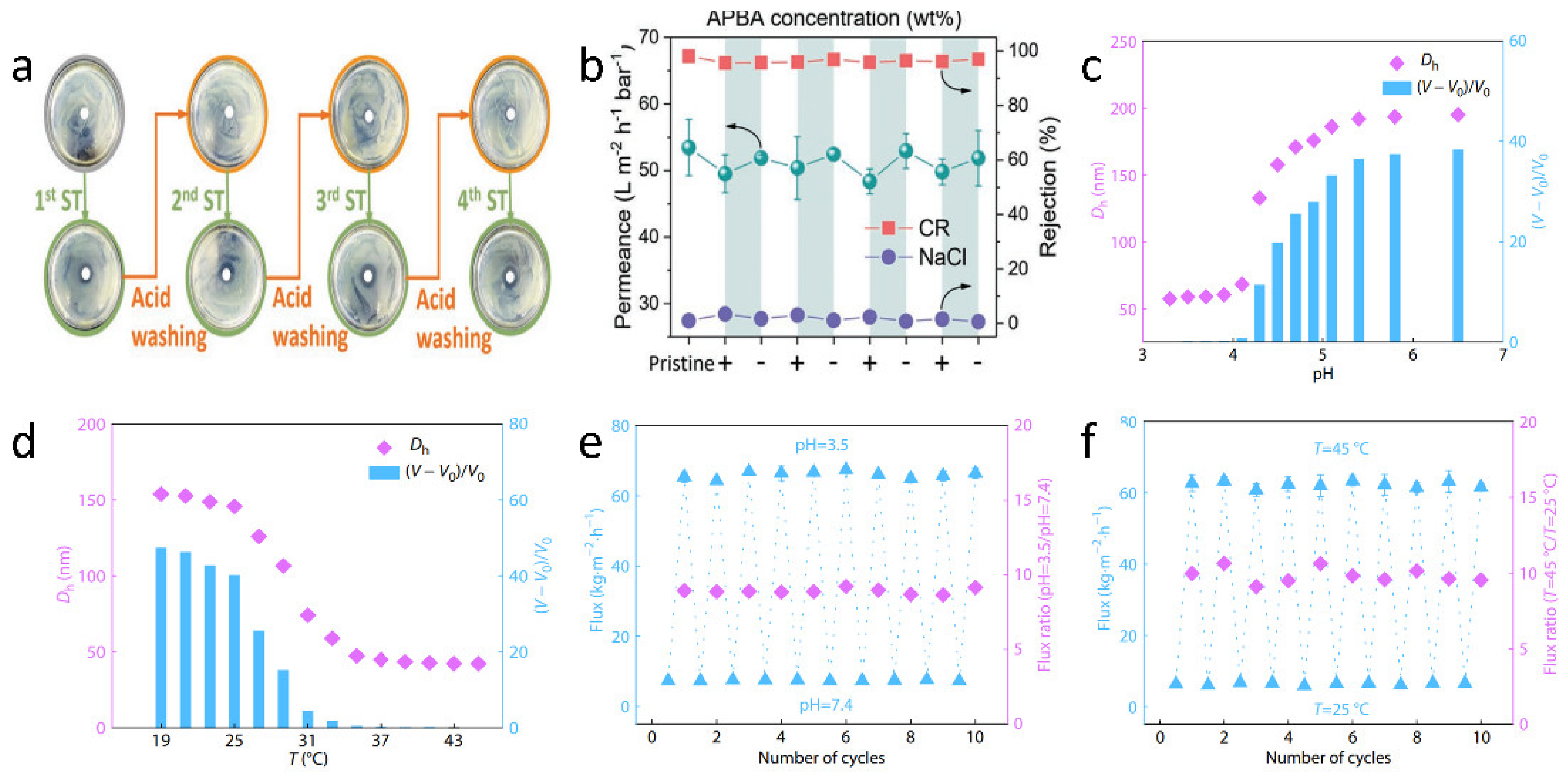
| pH-responsive | boronate ester | Polysulfone | Polymerization | A rejection of 0.93% to NaCl and 98.1% to CR, and fast water permeation of 53.4 L‧m−2‧h−1‧bar−1. | [61] |
| Thermo, pH-responsive | P(NIPAM-AA) | PVA | Blending | Excellent temperature and pH responsiveness, and almost constant flux transition between around 7.5 and 66 kg·m−2·h−1 under certain temperature (37 °C) and constant flux transition between around 6 and 62 kg·m−2·h−1 under certain pH of 3.5. | [62] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Wang, Z.; Zhou, J.; Gu, Q.; Zhong, Z. Stimulus-Responsive Membranes: A Mini Review on Principles, Preparation Methods, and Emerging Applications. Separations 2025, 12, 219. https://doi.org/10.3390/separations12080219
Wu Y, Wang Z, Zhou J, Gu Q, Zhong Z. Stimulus-Responsive Membranes: A Mini Review on Principles, Preparation Methods, and Emerging Applications. Separations. 2025; 12(8):219. https://doi.org/10.3390/separations12080219
Chicago/Turabian StyleWu, Yixin, Ziyu Wang, Jian Zhou, Qilin Gu, and Zhaoxiang Zhong. 2025. "Stimulus-Responsive Membranes: A Mini Review on Principles, Preparation Methods, and Emerging Applications" Separations 12, no. 8: 219. https://doi.org/10.3390/separations12080219
APA StyleWu, Y., Wang, Z., Zhou, J., Gu, Q., & Zhong, Z. (2025). Stimulus-Responsive Membranes: A Mini Review on Principles, Preparation Methods, and Emerging Applications. Separations, 12(8), 219. https://doi.org/10.3390/separations12080219









