Optimization and Component Identification of Ultrasound-Assisted Extraction of Polyphenols from Coriander (Coriandrum sativum L.) and Evaluation of Polyphenol Content Changes and Antioxidant Activity During Storage
Abstract
1. Introduction
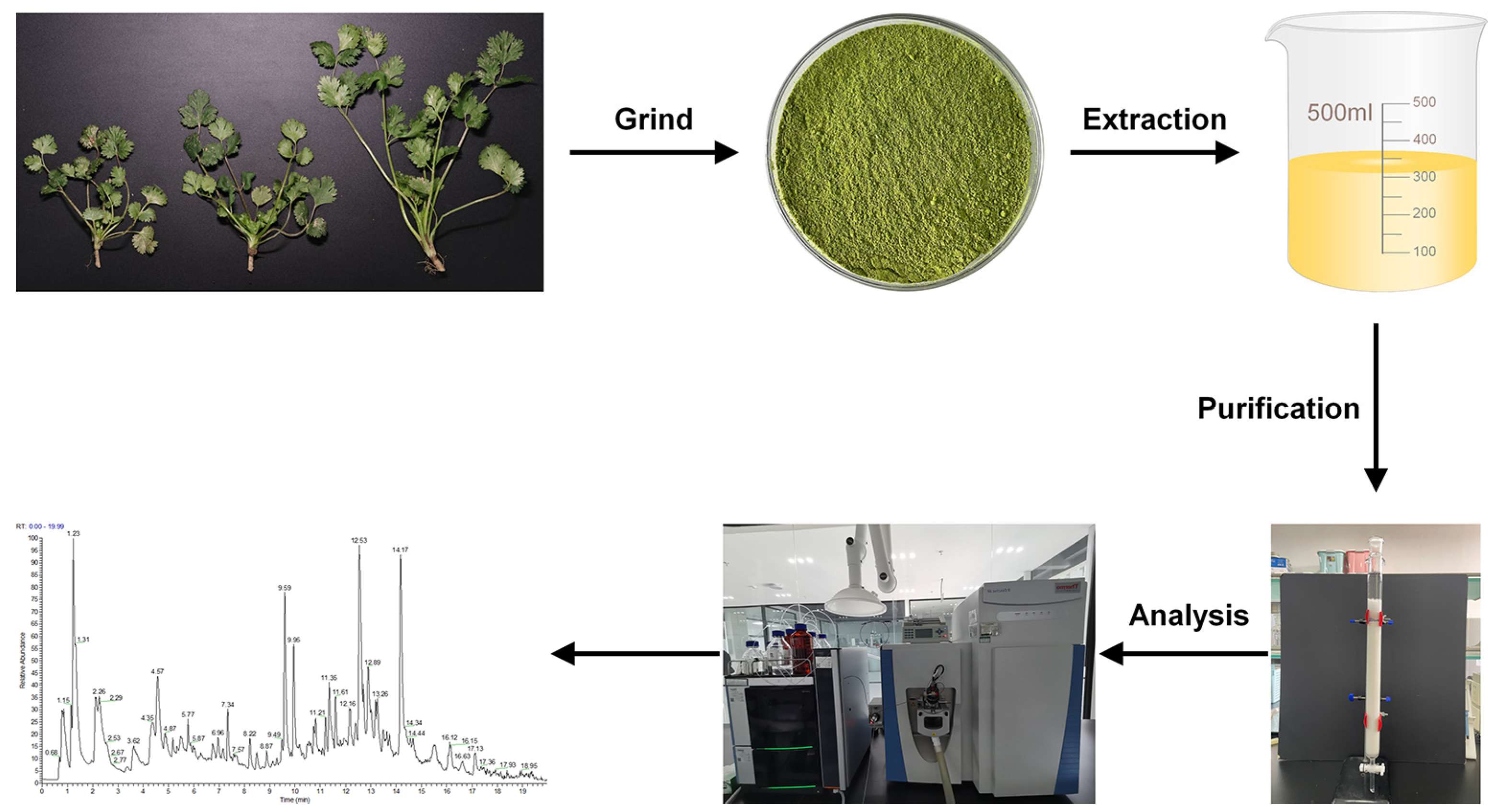
2. Materials and Methods
2.1. Plant Materials
2.2. Chemicals and Reagents
2.3. Optimization of the Extraction of CSPCs
2.3.1. Quantification of Total Phenolic Content
2.3.2. Extraction of CSPCs and Single-Factor Experiment Design
2.3.3. Box–Behnken Experiment
2.4. Identification of Polyphenolic Compounds in CSPCs
2.4.1. Purification of CSPCs
2.4.2. Analysis by UPLC-Q Exactive HF Orbitrap-MS
2.5. Quantitative Determination of the Major Constituents in CSPCs by UPLC-MS/MS
2.5.1. Sample Pretreatment
2.5.2. Chromatographic Conditions
2.5.3. Mass Spectrometry Conditions
2.6. Antioxidant Assays
2.6.1. DPPH Assay
2.6.2. ABTS Radical Scavenging Assay
2.6.3. Ferric Reducing Antioxidant Power Assay
2.7. Coriander Storage Experiment
2.8. Statistical Analysis
3. Results and Discussion
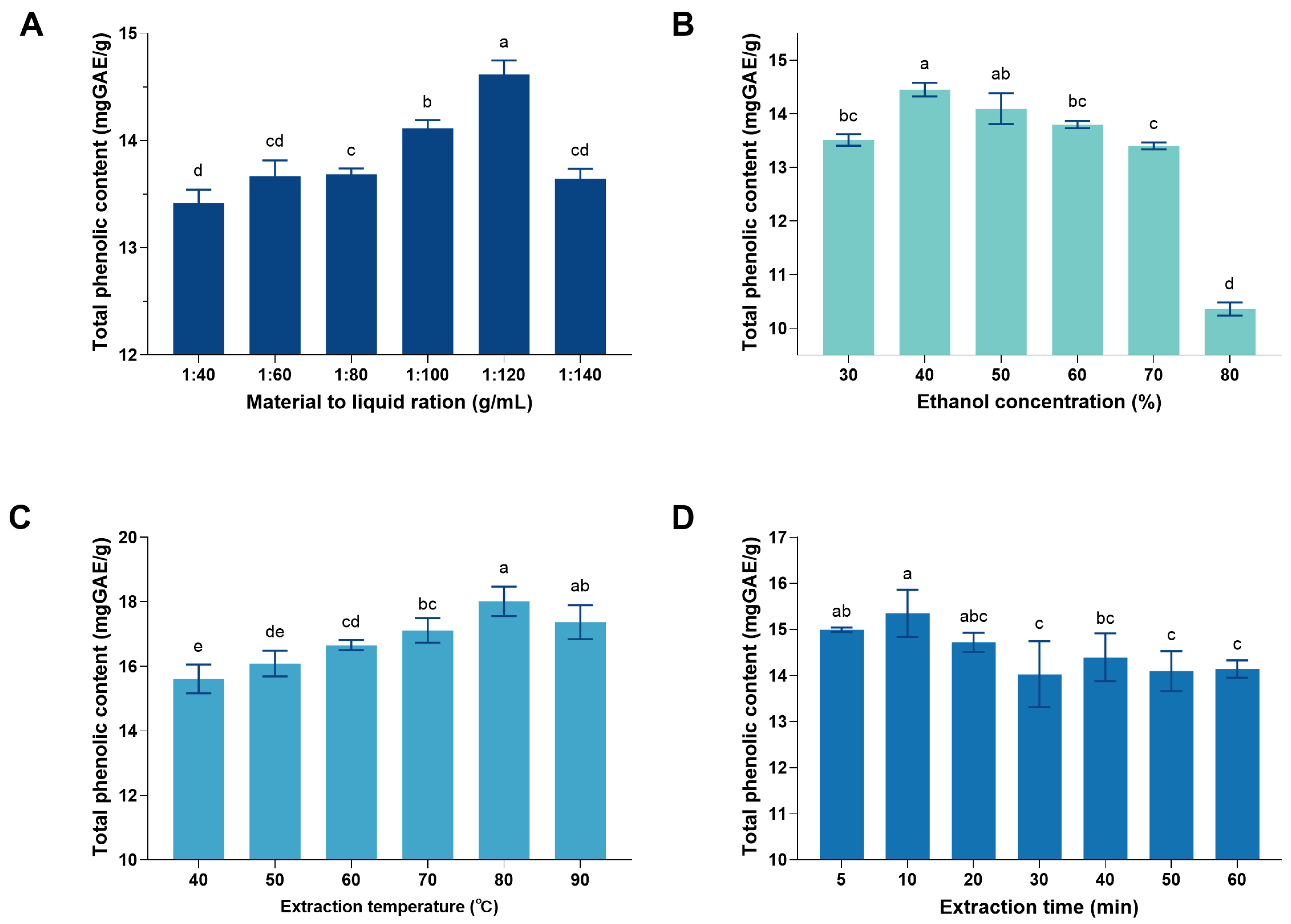
3.1. Single-Factor Experiment
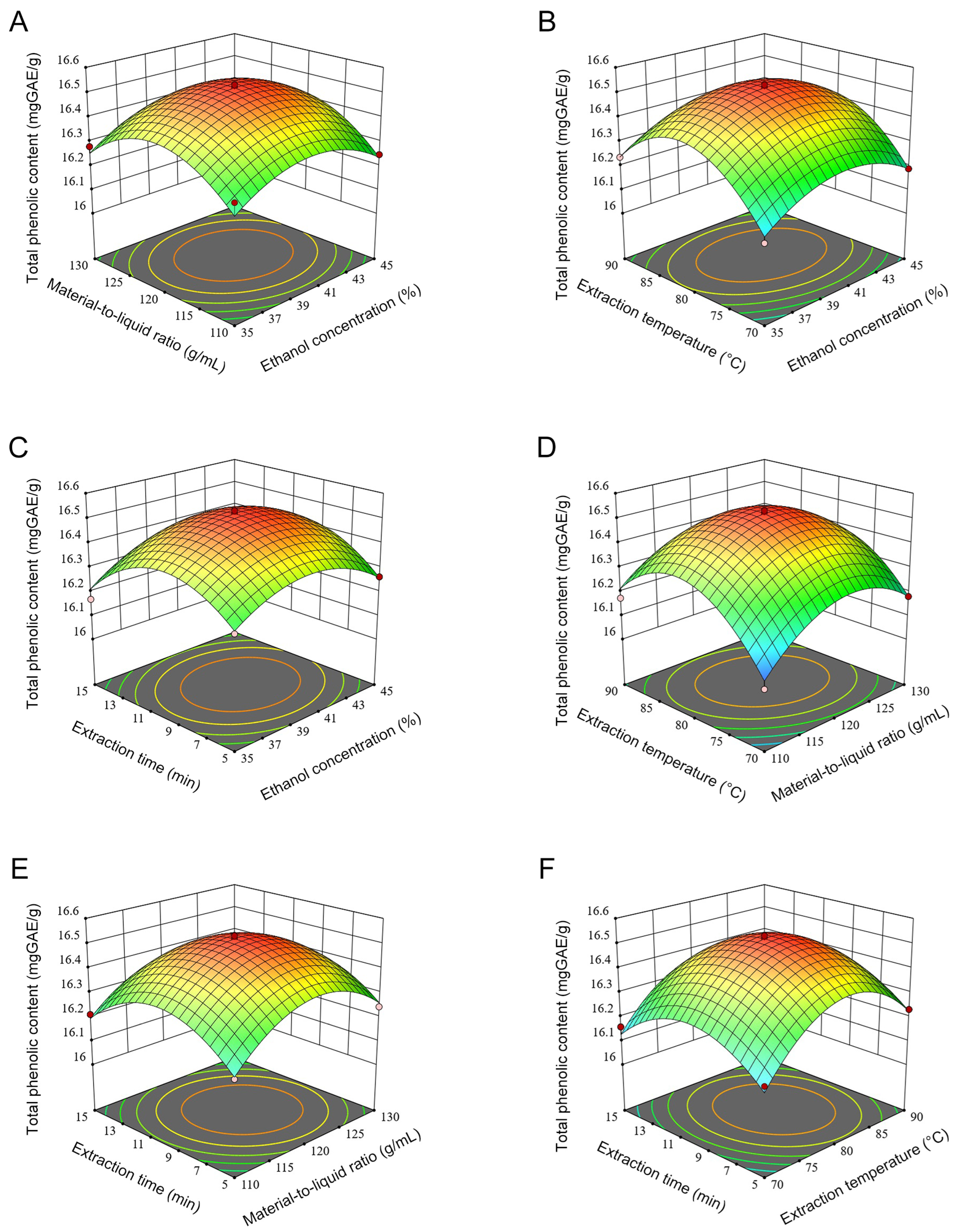
3.2. Box–Behnken Experiment
3.2.1. Establishment of Regression Equation and ANOVA
3.2.2. Verification of the Extraction Conditions Provided by the Predictive Model
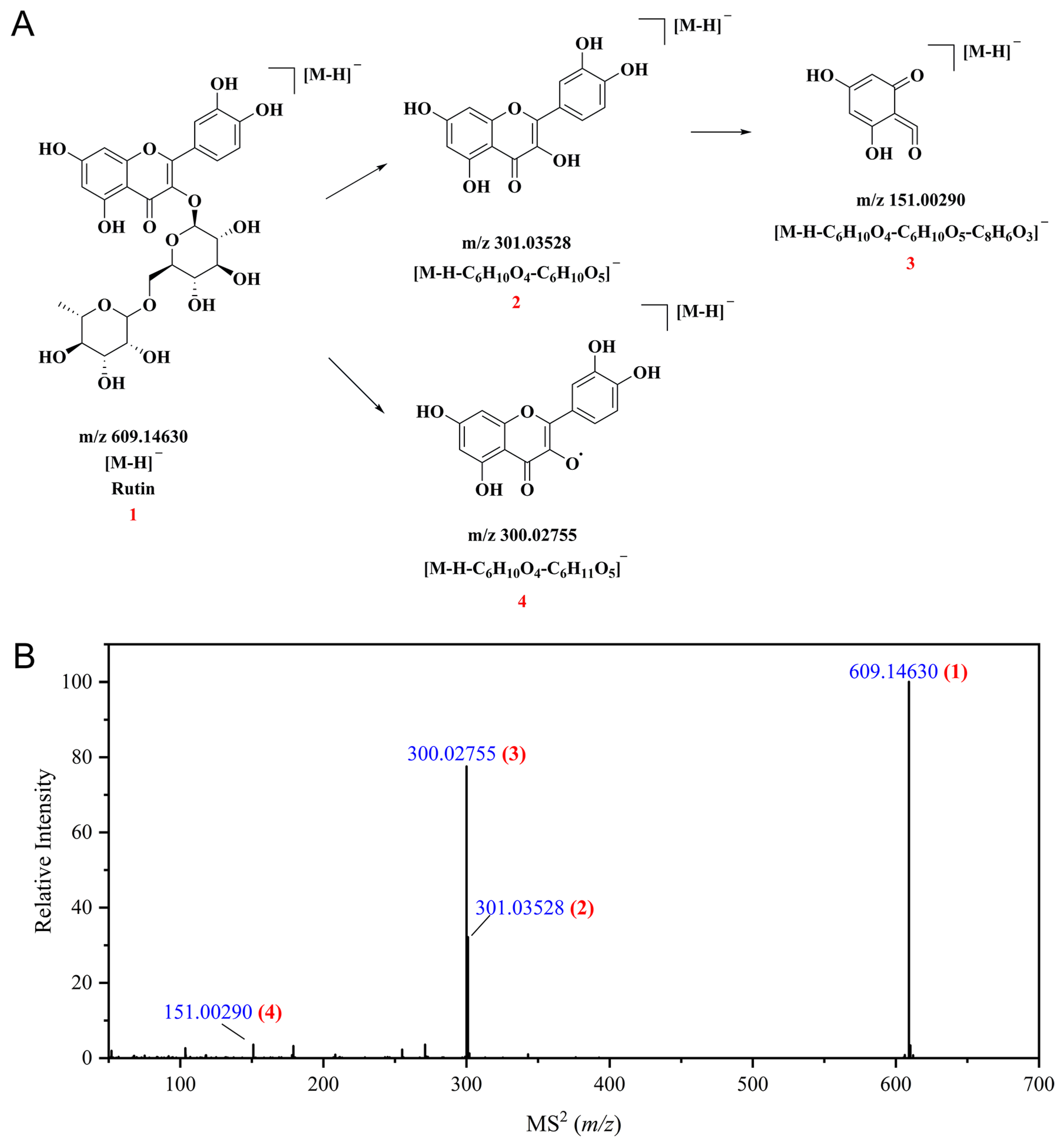
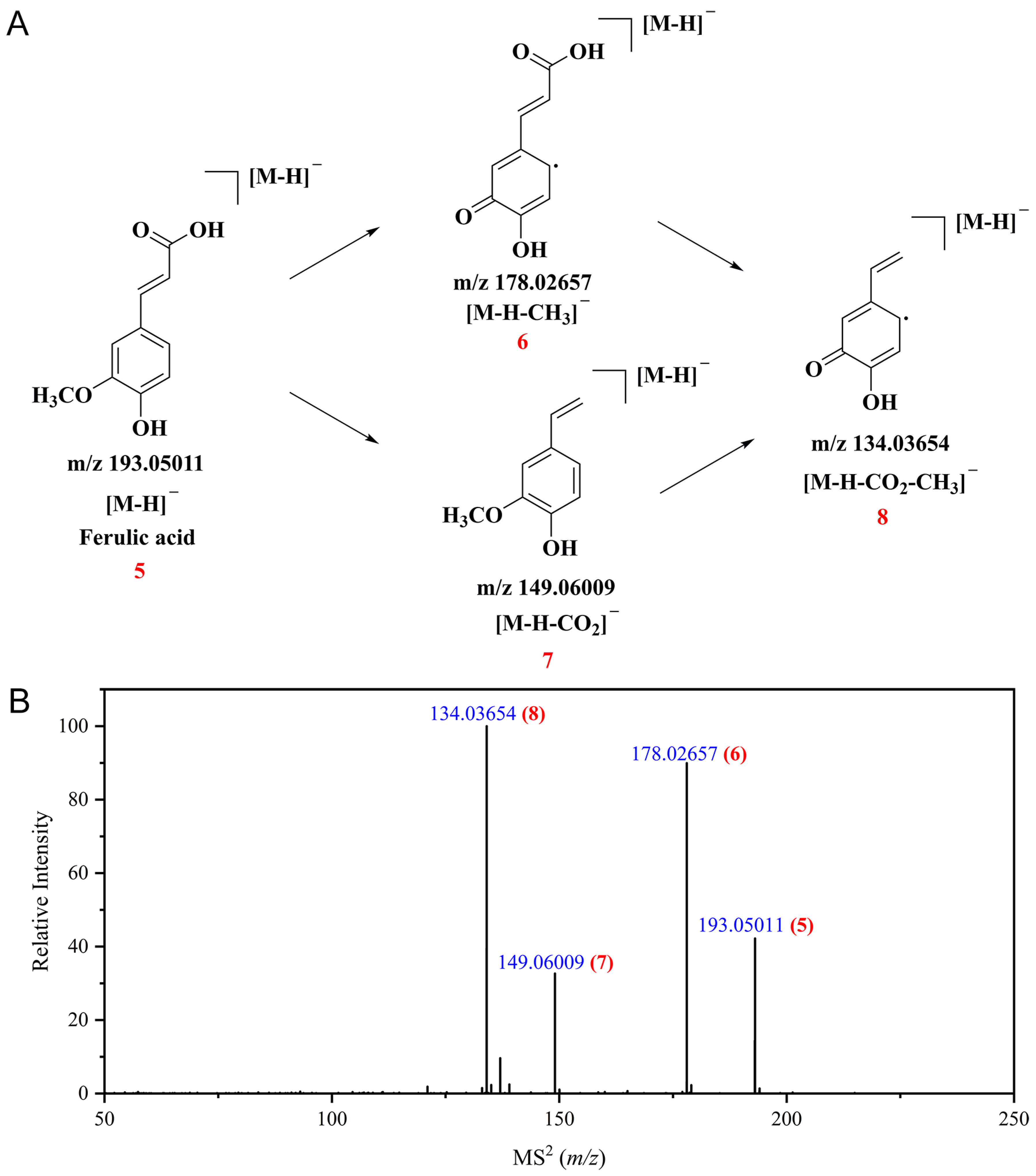
3.3. Identification of Polyphenolic Compounds in CSPCs by UPLC-Q Exactive HF Orbitrap-MS
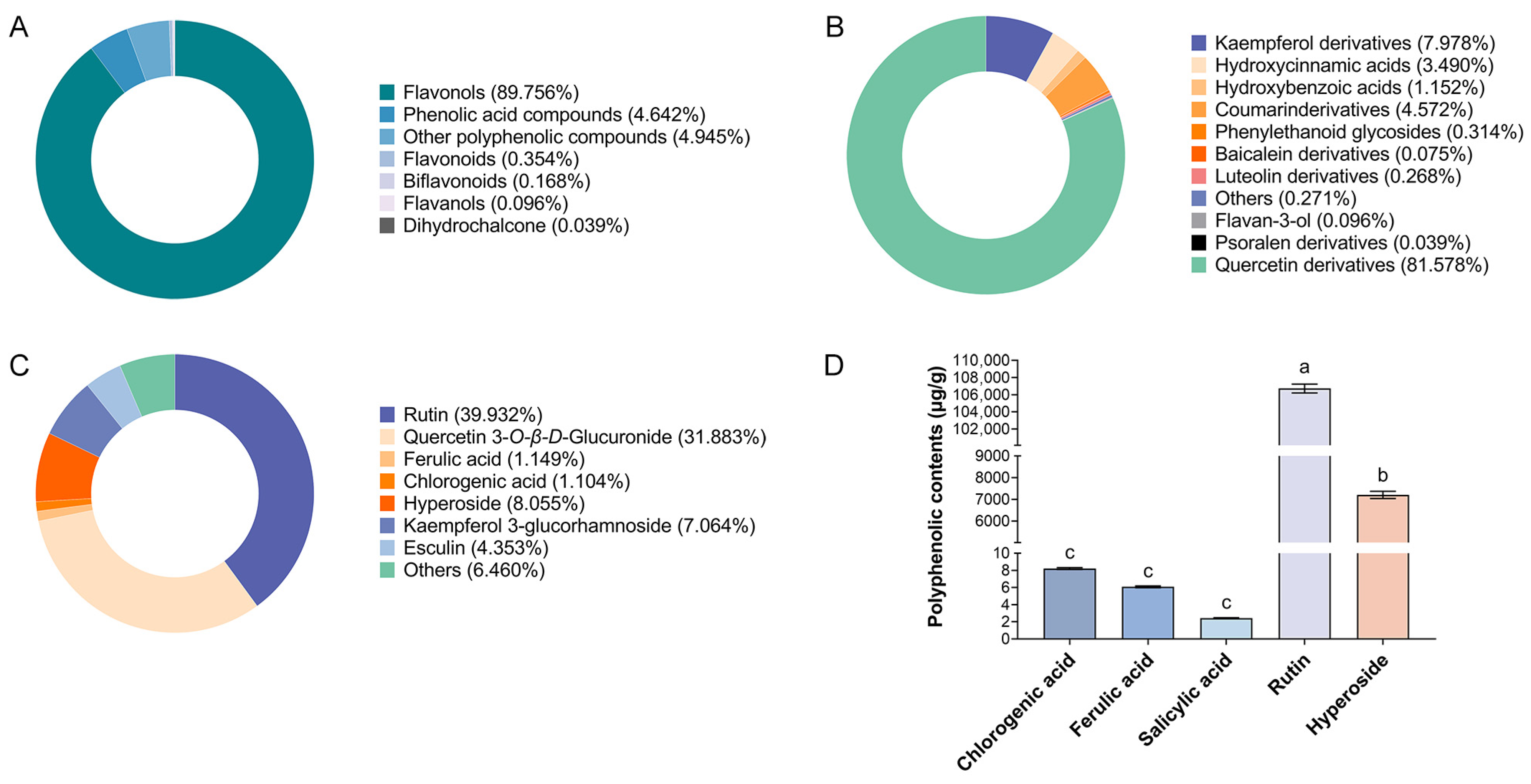
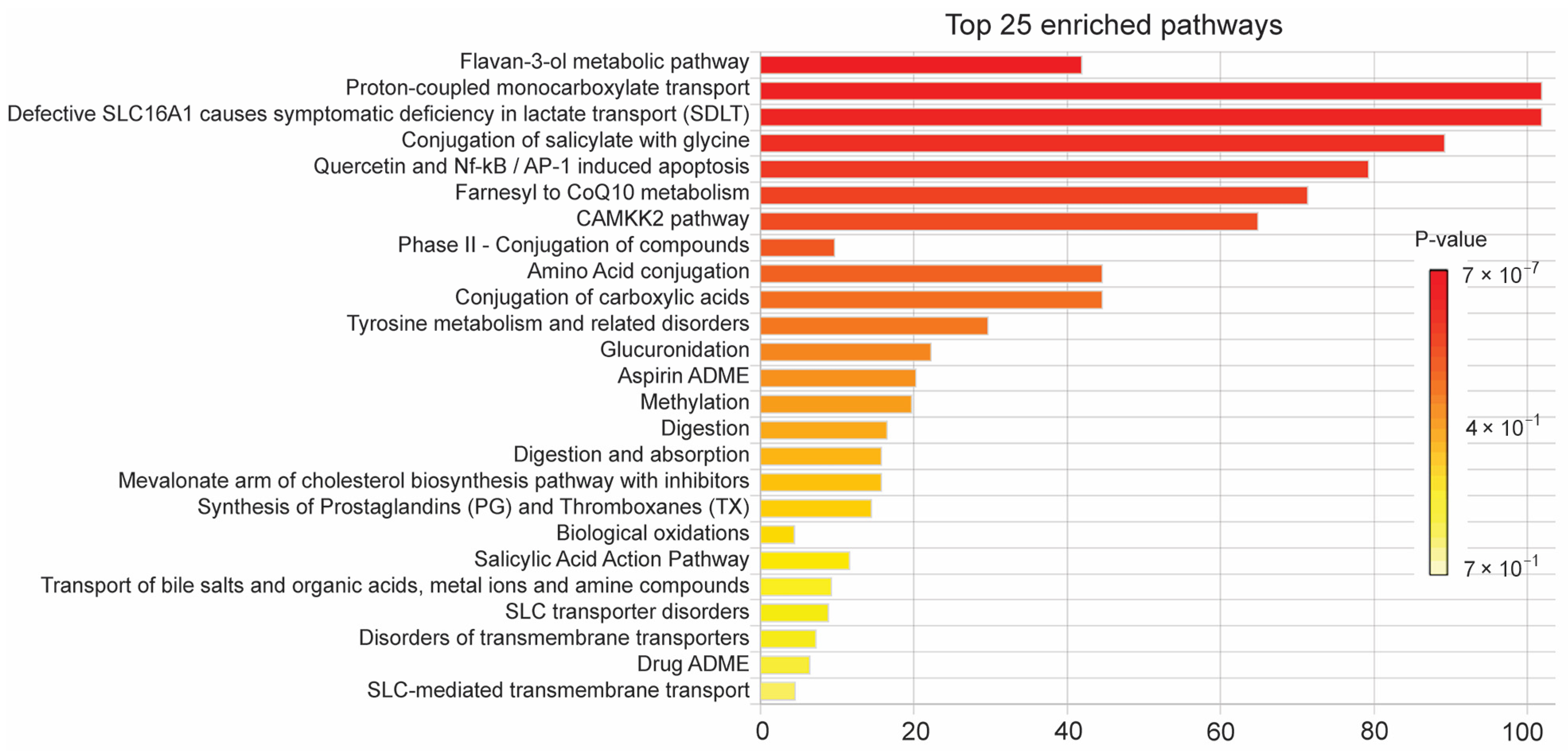
3.4. Polyphenol Profiling and Quantitative Validation
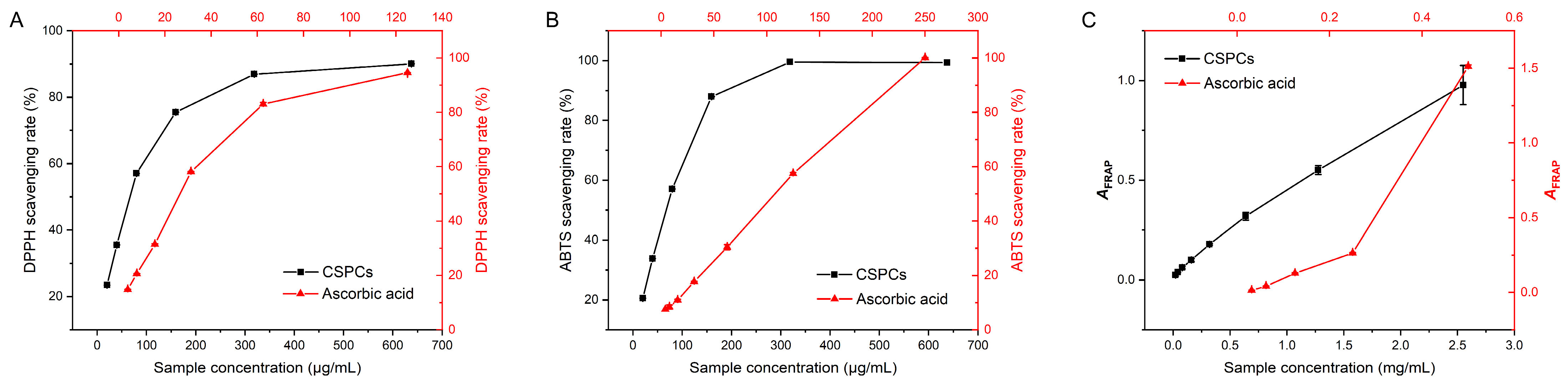
3.5. Antioxidant Activities
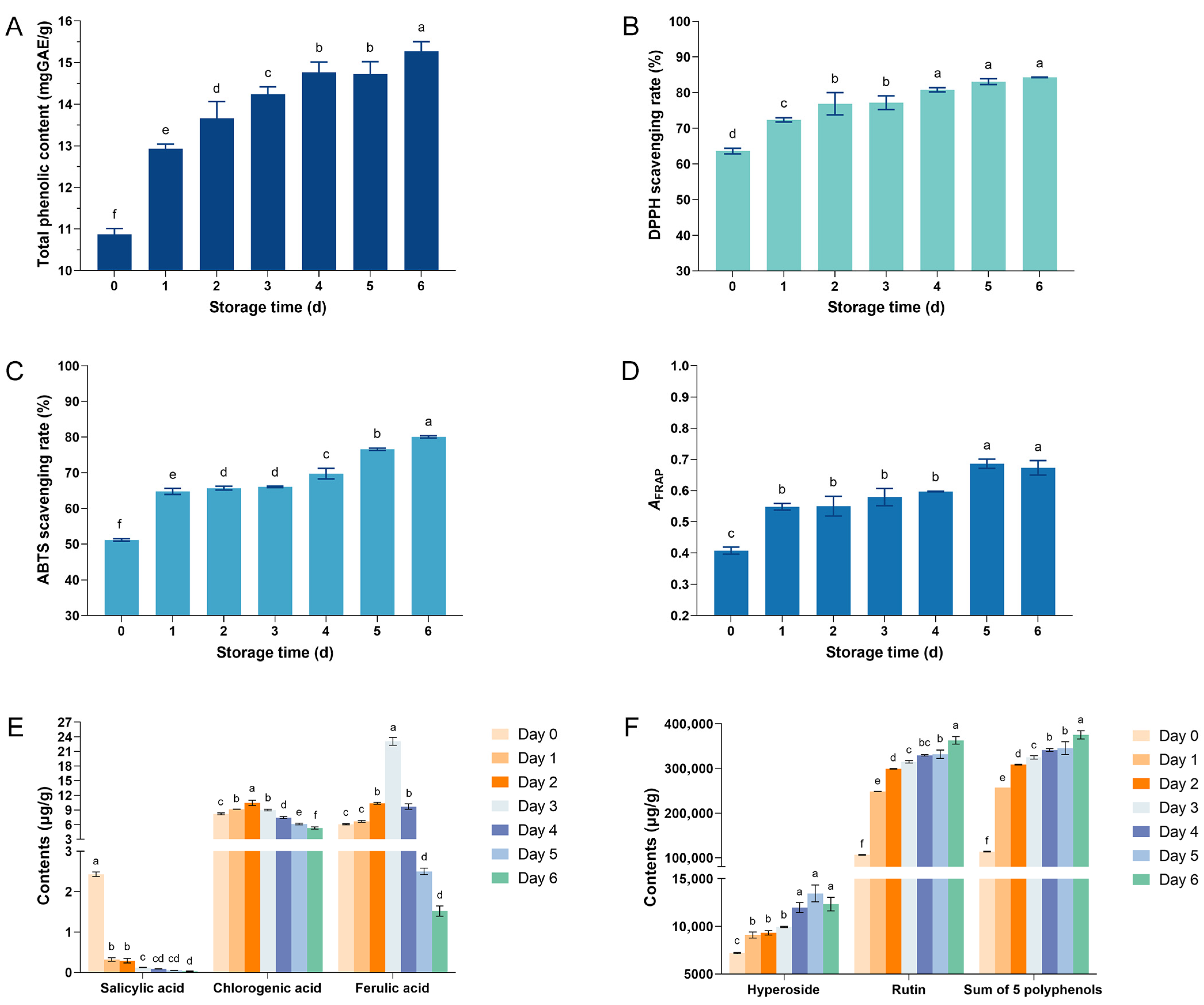
3.6. The Changes in Polyphenol Content and Antioxidant Capacity of Coriander During Storage
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CSPCs | Polyphenolic compounds in coriander |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| ABTS | 2,2′-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) |
| FRAP | Ferric reducing antioxidant power |
| TPC | Total phenolic content |
| ANOVA | Analysis of variance |
| SD | Standard deviation |
| UPLC | Ultra-performance liquid chromatography system |
References
- Mannaa, M.; Seo, Y.; Park, I. Addition of coriander during fermentation of Korean soy sauce (Gangjang) causes significant shift in microbial composition and reduction in biogenic amine levels. Foods 2020, 9, 1346. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.F.; Ali, N.; Mostafa, I.; Hasan, R.A.; Sobeh, M. Coriander oil reverses dexamethasone-induced insulin resistance in rats. Antioxidants 2022, 11, 441. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Xie, Y.; Lyu, J.; Xie, Y.; Zhao, C.; Yu, J. Coriander microgreens and baby greens: Comparison of volatile and non-volatile metabolites and potential therapeutic effects on type 2 diabetes mellitus and obesity. Food Res. Int. 2025, 202, 115759. [Google Scholar] [CrossRef]
- Ishikawa, T.; Kondo, K.; Kitajima, J. Water-soluble constituents of coriander. Chem. Pharm. Bull. 2003, 51, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshi, S.; Harohally, N.V.; Roopavathi, C.; Naidu, M.M. Isolation, identification, structural elucidation and bioactivity of Heneicos-1-ene from Coriandrum sativum L. foliage. Sci. Rep. 2018, 8, 17414. [Google Scholar] [CrossRef]
- Ohara, R.; Sugahara, T.; Sugie, Y.; Onda, H.; Yoshino, N.; Nishi, K.; Ishida, M.; Kikuzaki, H. Identification of components in coriander (Coriandrum sativum L.) inhibiting degranulation of RBL-2H3 cells. Fitoterapia 2022, 163, 105298. [Google Scholar] [CrossRef]
- Scandar, S.; Zadra, C.; Marcotullio, M.C. Coriander (Coriandrum sativum) polyphenols and their nutraceutical value against obesity and metabolic syndrome. Molecules 2023, 28, 4187. [Google Scholar] [CrossRef]
- Chaurasia, P.K.; Bharati, S.L. Coriander: A holistic outlook on its chemistry and pharmacology. Food Chem. 2025, 469, 142444. [Google Scholar] [CrossRef]
- Pawłowska, K.A.; Baracz, T.; Skowrońska, W.; Piwowarski, J.P.; Majdan, M.; Malarz, J.; Stojakowska, A.; Zidorn, C.; Granica, S. The contribution of phenolics to the anti-inflammatory potential of the extract from Bolivian coriander (Porophyllum ruderale subsp. ruderale). Food Chem. 2022, 371, 131116. [Google Scholar] [CrossRef]
- Iqbal, M.J.; Butt, M.S.; Shehzad, A.; Asghar, M. In vitro antioxidant and chromatographic quantification of supercritical fluid extracts obtained from coriander (Coriandrum sativum L.). J. Chem. Soc. Pak. 2018, 40, 733–741. [Google Scholar]
- Šamec, D.; Karalija, E.; Šola, I.; Vujčić Bok, V.; Salopek-Sondi, B. The role of polyphenols in abiotic stress response: The influence of molecular structure. Plants 2021, 10, 118. [Google Scholar] [CrossRef]
- Kaur, G.; Kaur, P.; Kaur, A. Physico-chemical properties, bioactive compounds and color parameters of coriander puree: Effect of pretreatments and freezing. J. Food Sci. Technol. 2018, 55, 3473–3484. [Google Scholar] [CrossRef] [PubMed]
- Thirugnanasambandham, K.; Sivakumar, V. Enhancement of shelf life of Coriandrum sativum leaves using vacuum drying process: Modeling and optimization. J. Saudi Soc. Agric. Sci. 2016, 15, 195–201. [Google Scholar] [CrossRef]
- Gantner, M.; Guzek, D.; Najda, A.; Brodowska, M.; Górska-Horczyczak, E.; Wojtasik-Kalinowska, I.; Godziszewska, J. Oxidative and microbial stability of poultry meatballs added with coriander extracts and packed in cold modified atmosphere. Int. J. Food Prop. 2017, 20, 2527–2537. [Google Scholar] [CrossRef]
- Zamindar, N.; Sadrarhami, M.; Doudi, M. Antifungal activity of coriander (Coriandrum sativum L.) essential oil in tomato sauce. J. Food Meas. Charact. 2016, 10, 589–594. [Google Scholar] [CrossRef]
- Luque-García, J.L.; de Luque Castro, M.D. Ultrasound: A powerful tool for leaching. TrAC Trends Anal. Chem. 2003, 22, 41–47. [Google Scholar] [CrossRef]
- Thakur, B.R.; Singh, R.K.; Nelson, P.E. Quality attributes of processed tomato products: A review. Food Rev. Int. 1996, 12, 375–401. [Google Scholar] [CrossRef]
- Chen, F.; Sun, Y.; Zhao, G.; Liao, X.; Hu, X.; Wu, J.; Wang, Z. Optimization of ultrasound-assisted extraction of anthocyanins in red raspberries and identification of anthocyanins in extract using high-performance liquid chromatography–mass spectrometry. Ultrason. Sonochem. 2007, 14, 767–778. [Google Scholar] [CrossRef]
- Lemmadi, S.; Adoui, F.; Dumas, E.; Karoune, S.; Santerre, C.; Gharsallaoui, A. Optimization of ultrasound-assisted extraction of phenolic compounds from the aerial part of plants in the Chenopodiaceae family using a box–behnken design. Appl. Sci. 2025, 15, 4688. [Google Scholar] [CrossRef]
- Chemat, F.; Zill-e-Huma; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef]
- Gallo, M.; Ferracane, R.; Graziani, G.; Ritieni, A.; Fogliano, V. Microwave assisted extraction of phenolic compounds from four different spices. Molecules 2010, 15, 6365–6374. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Everette, J.D.; Bryant, Q.M.; Green, A.M.; Abbey, Y.A.; Wangila, G.W.; Walker, R.B. Thorough study of reactivity of various compound classes toward the Folin−Ciocalteu reagent. J. Agric. Food Chem. 2010, 58, 8139–8144. [Google Scholar] [CrossRef]
- Tyagi, K.; Lui, A.C.W.; Zhang, S.; Peck, G.M. Folin-Ciocâlteu, RP-HPLC (reverse phase-high performance liquid chromatography), and LC-MS (liquid chromatography-mass spectrometry) provide complementary information for describing cider (Malus spp.) apple juice. J. Food Compos. Anal. 2025, 137, 106844. [Google Scholar] [CrossRef]
- Yang, M.; Wang, S.; Zhou, R.; Zhao, Y.; He, Y.; Zheng, Y.; Gong, H.; Wang, W. Optimization and component identification of ultrasound-assisted extraction of functional compounds from waste blackberry (Rubus fruticosus Pollich) seeds. J. Sci. Food Agric. 2024, 104, 9169–9179. [Google Scholar] [CrossRef]
- Hu, D.; Xue, R.; Zhuang, X.; Zhang, X.; Shi, S. Ultrasound-assisted extraction optimization of polyphenols from Boletus bicolor and evaluation of its antioxidant activity. Front. Nutr. 2023, 10, 1135712. [Google Scholar] [CrossRef]
- Che, H.; Zhang, R.; Wang, X.; Yu, H.; Shi, X.; Yi, J.; Li, J.; Qi, Q.; Dong, R.; Li, Q. Ultrasound-assisted extraction of polyphenols from Phyllanthi Fructus: Comprehensive insights from extraction optimization and antioxidant activity. Ultrason. Sonochem. 2024, 111, 107083. [Google Scholar] [CrossRef]
- Oroian, M.; Ursachi, F.; Dranca, F. Ultrasound-assisted extraction of polyphenols from crude pollen. Antioxidants 2020, 9, 322. [Google Scholar] [CrossRef]
- Al-Dhabi, N.A.; Ponmurugan, K.; Maran, P. Development and validation of ultrasound-assisted solid-liquid extraction of phenolic compounds from waste spent coffee grounds. Ultrason. Sonochem. 2017, 34, 206–213. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Haque, S.K.M. Application of combined Box–Behnken design with response surface methodology and desirability function in optimizing pectin extraction from fruit peels. J. Sci. Food Agric. 2024, 104, 149–173. [Google Scholar] [CrossRef]
- Yang, Y.; Liang, Q.; Zhang, B.; Zhang, J.; Fan, L.; Kang, J.; Lin, Y.; Huang, Y.; Tan, T.-C.; Ho, L.-H. Adsorption and desorption characteristics of flavonoids from white tea using macroporous adsorption resin. J. Chromatogr. A 2024, 1715, 464621. [Google Scholar] [CrossRef]
- Kostikova, V.A.; Chernonosov, A.A.; Kuznetsov, A.A.; Petrova, N.V.; Krivenko, D.A.; Chernysheva, O.A.; Wang, W.; Erst, A.S. Identification of flavonoids in the leaves of Eranthis longistipitata (Ranunculaceae) by liquid chromatography with high-resolution mass spectrometry (LC-HRMS). Plants 2021, 10, 2146. [Google Scholar] [CrossRef]
- Barnaba, C.; Larcher, R.; Nardin, T.; Dellacassa, E.; Nicolini, G. Glycosylated simple phenolic profiling of food tannins using high resolution mass spectrometry (Q-Orbitrap). Food Chem. 2018, 267, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Liu, F.; Sun, M.; Tang, Z.; Wu, Y.; Lyu, J.; Khan, K.S.; Yu, J. Development of a high-performance liquid chromatography method for simultaneous quantification of sixteen polyphenols and application to tomato. J. Chromatogr. A 2024, 1733, 465254. [Google Scholar] [CrossRef] [PubMed]
- Bajkacz, S.; Baranowska, I.; Buszewski, B.; Kowalski, B.; Ligor, M. Determination of flavonoids and phenolic acids in plant materials using SLE-SPE-UHPLC-MS/MS method. Food Anal. Method 2018, 11, 3563–3575. [Google Scholar] [CrossRef]
- Alonso Carrillo, N.; Aguilar Santamaría, M.d.l.Á.; Vernon Carter, E.J.; Jiménez Alvarado, R.; Cruz Sosa, F.; Román Guerrero, A. Extraction of phenolic compounds from Satureja macrostema using microwave-ultrasound assisted and reflux methods and evaluation of their antioxidant activity and cytotoxicity. Ind. Crops Prod. 2017, 103, 213–221. [Google Scholar] [CrossRef]
- Ozgen, M.; Reese, R.N.; Tulio, A.Z., Jr.; Scheerens, J.C.; Miller, A.R. Modified 2,2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) method to measure antioxidant capacity of selected small fruits and comparison to ferric reducing antioxidant power (FRAP) and 2,2′-diphenyl-1-picrylhydrazyl (DPPH) methods. J. Agric. Food Chem. 2006, 54, 1151–1157. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, Y.; Wang, S.; Wang, W.; Yu, N.; Gong, H.; Ni, Z. Optimization of functional compounds extraction from Ginkgo biloba seeds using response surface methodology. Qual. Assur. Saf. Crops Foods 2022, 14, 102–112. [Google Scholar] [CrossRef]
- Li, J.; Chen, Z.; Shi, H.; Yu, J.; Huang, G.; Huang, H. Ultrasound-assisted extraction and properties of polysaccharide from Ginkgo biloba leaves. Ultrason. Sonochem. 2023, 93, 106295. [Google Scholar] [CrossRef]
- Espada-Bellido, E.; Ferreiro-González, M.; Carrera, C.; Palma, M.; Barroso, C.G.; Barbero, G.F. Optimization of the ultrasound-assisted extraction of anthocyanins and total phenolic compounds in mulberry (Morus nigra) pulp. Food Chem. 2017, 219, 23–32. [Google Scholar] [CrossRef]
- Boateng, I.D.; Kumar, R.; Daubert, C.R.; Flint-Garcia, S.; Mustapha, A.; Kuehnel, L.; Agliata, J.; Li, Q.; Wan, C.; Somavat, P. Sonoprocessing improves phenolics profile, antioxidant capacity, structure, and product qualities of purple corn pericarp extract. Ultrason. Sonochem. 2023, 95, 106418. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Qiao, L.; Gao, Q.; Zhang, F.; Zhang, X.; Lei, J.; Ren, M.; Xiao, S.; Kuang, J.; Deng, S.; et al. Total biflavonoids extraction from Selaginella chaetoloma utilizing ultrasound-assisted deep eutectic solvent: Optimization of conditions, extraction mechanism, and biological activity in vitro. Ultrason. Sonochem. 2023, 98, 106491. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Jiang, S.; Liu, M.; Tian, S. Simultaneous process optimization of ultrasound-assisted extraction of polyphenols and ellagic acid from pomegranate (Punica granatum L.) flowers and its biological activities. Ultrason. Sonochem. 2021, 80, 105833. [Google Scholar] [CrossRef]
- Sriti, J.; Wannes, W.A.; Talou, T.; Vilarem, G.; Marzouk, B. Chemical composition and antioxidant activities of Tunisian and Canadian coriander (Coriandrum sativum L.) fruit. J. Essent. Oil Res. 2011, 23, 7–15. [Google Scholar] [CrossRef]
- El-Zaeddi, H.; Calín-Sánchez, Á.; Nowicka, P.; Martínez-Tomé, J.; Noguera-Artiaga, L.; Burló, F.; Wojdyło, A.; Carbonell-Barrachina, Á.A. Preharvest treatments with malic, oxalic, and acetylsalicylic acids affect the phenolic composition and antioxidant capacity of coriander, dill and parsley. Food Chem. 2017, 226, 179–186. [Google Scholar] [CrossRef]
- Li, H.; Zhao, X.; Li, X.; Liang, J.; Qin, S.; Li, J.; Zhang, A.; Xu, L.; Tang, D.; Li, F. Characterization of volatile flavour compounds and characteristic flavour precursors in poultry eggs based on multi-omics and machine learning. Food Chem. 2025, 489, 144840. [Google Scholar] [CrossRef]
- Buszewski, B.; Walczak, J.; Žuvela, P.; Liu, J.J. Non-target analysis of phospholipid and sphingolipid species in egg yolk using liquid chromatography/triple quadrupole tandem mass spectrometry. J. Chromatogr. A 2017, 1487, 179–186. [Google Scholar] [CrossRef]
- Adesegun, S.A.; Alabi, S.O.; Olabanji, P.T.; Coker, H.A.B. Evaluation of antioxidant potential of Melanthera scandens. J. Acupunct. Meridian 2010, 3, 267–271. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Arranz, S.; Tabernero, M.; Díaz- Rubio, M.E.; Serrano, J.; Goñi, I.; Saura-Calixto, F. Updated methodology to determine antioxidant capacity in plant foods, oils and beverages: Extraction, measurement and expression of results. Food Res. Int. 2008, 41, 274–285. [Google Scholar] [CrossRef]
- Li, C.; Wang, E.; Elshikh, M.S.; Alwahibi, M.S.; Wang, W.; Wu, G.; Shen, Y.; Abbasi, A.M.; Shan, S. Extraction and purification of total flavonoids from Gnaphalium affine D. Don and their evaluation for free radicals’ scavenging and oxidative damage inhabitation potential in mice liver. Arab. J. Chem. 2021, 14, 103006. [Google Scholar] [CrossRef]
- Msaada, K.; Jemia, M.B.; Salem, N.; Bachrouch, O.; Sriti, J.; Tammar, S.; Bettaieb, I.; Jabri, I.; Kefi, S.; Limam, F.; et al. Antioxidant activity of methanolic extracts from three coriander (Coriandrum sativum L.) fruit varieties. Arab. J. Chem. 2017, 10, S3176–S3183. [Google Scholar] [CrossRef]
- Zeković, Z.; Pavlić, B.; Cvetanović, A.; Đurović, S. Supercritical fluid extraction of coriander seeds: Process optimization, chemical profile and antioxidant activity of lipid extracts. Ind. Crops Prod. 2016, 94, 353–362. [Google Scholar] [CrossRef]
- Dhakshayani, G.M.; Priya, S.J.A. A comparative study of phytochemical, antioxidant, anticarcinogenic, and antidiabetic potential of coriander (Coriandrum sativum L.): Microgreen and mature plant. Food Raw Mater. 2022, 10, 283–294. [Google Scholar] [CrossRef]
- Kubicova, L.; Bachmann, G.; Weckwerth, W.; Chobot, V. (±)-Catechin—A mass-spectrometry-based exploration coordination complex formation with FeII and FeIII. Cells 2022, 11, 958. [Google Scholar] [CrossRef]
- Boots, A.W.; Haenen, G.R.M.M.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Capanoglu, E.; Beekwilder, J.; Matros, A.; Boyacioglu, D.; Hall, R.D.; Mock, H.P. Correlation of rutin accumulation with 3-O-glucosyl transferase and phenylalanine ammonia-lyase activities during the ripening of tomato fruit. Plant Foods Hum. Nutr. 2012, 67, 371–376. [Google Scholar] [CrossRef]
- Tak, A.M.; Hami, A.; Bhat, B.; Bhat, S.A.; Masoodi, K.Z.; Bhat, M.A.; Shah, M.D.; Khan, M.K.; Zargar, S.M. Unravelling rutin content of tartary buckwheat of north western Himalayas and insights into nucleotide polymorphisms in PAL gene to infer the associations with rutin biosynthesis. 3 Biotech 2022, 12, 156. [Google Scholar] [CrossRef]
- Li, R.; Rosado-Souza, L.; Sampathkumar, A.; Fernie, A.R. The relationship between cell wall and postharvest physiological deterioration of fresh produce. Plant Physiol. Biochem. 2024, 210, 108568. [Google Scholar] [CrossRef]
- Zhong, J.; Ran, Q.; Han, Y.; Gan, L.; Dong, C. Biosynthetic mechanisms of plant chlorogenic acid from a microbiological perspective. Microorganisms 2025, 13, 1114. [Google Scholar] [CrossRef]
- Lallemand, L.A.; Zubieta, C.; Lee, S.G.; Wang, Y.; Acajjaoui, S.; Timmins, J.; McSweeney, S.; Jez, J.M.; McCarthy, J.G.; McCarthy, A.A. A structural basis for the biosynthesis of the major chlorogenic acids found in coffee. Plant Physiol. 2012, 160, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Li, M.; Jin, L.; Xie, X.; Li, M.; Wei, J. Cool temperature enhances growth, ferulic acid and flavonoid biosynthesis while inhibiting polysaccharide biosynthesis in Angelica sinensis. Molecules 2022, 27, 320. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.R.; Poor, P.; Janda, T. Salicylic acid: A versatile signaling molecule in plants. J. Plant Growth Regul. 2022, 41, 1887–1890. [Google Scholar] [CrossRef]

| Independent Variables | Coded Symbols | Level | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| Ethanol concentration (%) | X1 | 35 | 40 | 45 |
| Material-to-liquid ratio (g/mL) | X2 | 1:110 | 1:120 | 1:130 |
| Extraction temperature (°C) | X3 | 70 | 80 | 90 |
| Extraction time (min) | X4 | 5 | 10 | 15 |
| Compounds | Ion Pair (m/z) | Fragmentor Voltage (V) | Collision Energy (V) |
|---|---|---|---|
| Chlorogenic acid | 191.0/353.1 | 107 | 17 |
| Ferulic acid | 134.0/193.0 | 93 | 17 |
| Salicylic acid | 93.1/137.1 | 83 | 17 |
| Rutin | 300.0/609.5 | 241 | 41 |
| Hyperoside | 300.0/463.1 | 180 | 30 |
| Run | X1 (%) | X2 (g/mL) | X3 (°C) | X4 (min) | Total Phenolic Content (mg GAE/g) |
|---|---|---|---|---|---|
| 1 | 45 | 120 | 80 | 15 | 16.255 |
| 2 | 40 | 120 | 80 | 10 | 16.518 |
| 3 | 45 | 130 | 80 | 10 | 16.296 |
| 4 | 40 | 130 | 80 | 15 | 16.231 |
| 5 | 35 | 120 | 80 | 5 | 16.253 |
| 6 | 40 | 130 | 70 | 10 | 16.181 |
| 7 | 40 | 110 | 90 | 10 | 16.174 |
| 8 | 40 | 130 | 90 | 10 | 16.202 |
| 9 | 35 | 120 | 80 | 15 | 16.169 |
| 10 | 40 | 110 | 80 | 15 | 16.211 |
| 11 | 45 | 120 | 70 | 10 | 16.189 |
| 12 | 35 | 130 | 80 | 10 | 16.281 |
| 13 | 40 | 130 | 80 | 5 | 16.243 |
| 14 | 35 | 110 | 80 | 10 | 16.273 |
| 15 | 40 | 120 | 70 | 15 | 16.159 |
| 16 | 40 | 120 | 70 | 5 | 16.152 |
| 17 | 40 | 110 | 70 | 10 | 16.044 |
| 18 | 40 | 120 | 80 | 10 | 16.531 |
| 19 | 40 | 120 | 80 | 10 | 16.522 |
| 20 | 45 | 120 | 90 | 10 | 16.286 |
| 21 | 35 | 120 | 70 | 10 | 16.121 |
| 22 | 40 | 120 | 90 | 15 | 16.212 |
| 23 | 35 | 120 | 90 | 10 | 16.237 |
| 24 | 45 | 110 | 80 | 10 | 16.248 |
| 25 | 40 | 110 | 80 | 5 | 16.179 |
| 26 | 45 | 120 | 80 | 5 | 16.263 |
| 27 | 40 | 120 | 80 | 10 | 16.491 |
| 28 | 40 | 120 | 80 | 10 | 16.533 |
| 29 | 40 | 120 | 90 | 5 | 16.232 |
| Source | Sum of Squares | DFs | Mean Square | F-Value | p-Value | Significance |
|---|---|---|---|---|---|---|
| Model | 0.4568 | 14 | 0.0326 | 33.41 | <0.0001 | ** |
| X1 | 0.0034 | 1 | 0.0034 | 3.52 | 0.0818 | |
| X2 | 0.0078 | 1 | 0.0078 | 7.94 | 0.0137 | * |
| X3 | 0.0206 | 1 | 0.0206 | 21.07 | 0.0004 | ** |
| X4 | 0.0006 | 1 | 0.0006 | 0.6164 | 0.4455 | |
| X1X2 | 0.0004 | 1 | 0.0004 | 0.4095 | 0.5325 | |
| X1X3 | 0.0001 | 1 | 0.0001 | 0.0924 | 0.7656 | |
| X1X4 | 0.0014 | 1 | 0.0014 | 1.48 | 0.2441 | |
| X2X3 | 0.0030 | 1 | 0.0030 | 3.04 | 0.1031 | |
| X2X4 | 0.0005 | 1 | 0.0005 | 0.4955 | 0.4930 | |
| X3X4 | 0.0002 | 1 | 0.0002 | 0.1866 | 0.6723 | |
| X12 | 0.0825 | 1 | 0.0825 | 84.42 | <0.0001 | ** |
| X22 | 0.1484 | 1 | 0.1484 | 151.92 | <0.0001 | ** |
| X32 | 0.2543 | 1 | 0.2543 | 260.36 | <0.0001 | ** |
| X42 | 0.1494 | 1 | 0.1494 | 152.93 | <0.0001 | ** |
| Residual | 0.0137 | 14 | 0.0010 | |||
| Lack of fit | 0.0125 | 10 | 0.0013 | 4.42 | 0.0824 | Not significant |
| Pure error | 0.0011 | 4 | 0.0003 | |||
| Cor total | 0.4705 | 28 | ||||
| R2 | 0.9709 | |||||
| R2adj | 0.9419 | |||||
| C.V.% | 0.1921 |
| No. | Name | Classification | Ionization Mode | RT (min) | Formula | Predicted | Measured | DeltaMass (ppm) | MS/MS (m/z) | Match Score |
|---|---|---|---|---|---|---|---|---|---|---|
| A: Flavonoids | ||||||||||
| 1 | Scutellarin b | Baicalein derivatives | [M-H]− | 6.847 | C21H18O12 | 462.07983 | 462.08011 | 0.6 | 399.07227, 327.05093, 285.04056, 269.04535, 193.49174 | 72.15 |
| 2 | Wogonoside b | [M+H]+ | 9.051 | C22H20O11 | 460.10056 | 460.0998 | −1.65 | 297.07523, 285.07513, 270.05183, 165.05437, 147.04384 | 73.48 | |
| 3 | Luteollin 5-glucoside b | Luteolin derivatives | [M-H]− | 7.193 | C21H20O11 | 448.10056 | 448.10087 | 0.69 | 285.04068, 133.02950, 151.00313 | 80.51 |
| 4 | Cynaroside b | [M-H]− | 7.316 | C21H20O11 | 448.10056 | 448.10062 | 0.14 | 285.04050, 284.03275, 151.00319 | 78.45 | |
| 5 | Luteolin-4′-O-glucoside b | [M-H]− | 7.523 | C21H20O11 | 448.10056 | 448.10078 | 0.5 | 369.05130, 285.04065, 135.04442 | 83.17 | |
| 6 | Nobiletin b | Others | [M+H]+ | 11.209 | C21H22O8 | 402.13147 | 402.13074 | −1.8 | 388.11447, 373.09100, 343.22592 | 75.43 |
| 7 | Sinensetin b | [M+H]+ | 11.735 | C20H20O7 | 372.1209 | 372.12031 | −1.59 | 358.10406, 343.08066, 312.09845 | 70.15 | |
| B: Flavonols | ||||||||||
| 8 | Kaempferol 3-glucorhamnoside a [9] | Kaempferol derivatives | [M-H]− | 6.92 | C27H30O15 | 594.15847 | 594.15868 | 0.35 | 285.04037, 284.0325, 151.00313 | 90.17 |
| 9 | Nicotiflorin b | [M+H]+ | 6.857 | C27H30O15 | 594.15847 | 594.15781 | −1.11 | 449.10699, 287.05435,147.06490 | 88.64 | |
| 10 | Kaempferol-7-O-β-D-glucopyranoside b | [M+H]+ | 6.749 | C21H20O11 | 448.10056 | 448.09991 | −1.45 | 287.05447, 270.05148, 153.01784 | 82.21 | |
| 11 | Ternatumoside II b | [M-H]− | 6.811 | C27H30O15 | 594.15847 | 594.15868 | 0.35 | 285.04053, 284.03259, 255.02975 | 86.34 | |
| 12 | Kaempferol a [7] | [M-H]− | 9.069 | C15H10O6 | 286.04774 | 286.04785 | 0.4 | 267.02969, 151.00298 | 95.16 | |
| 13 | Rutin a [10,46] | Quercetin derivatives | [M-H]− | 6.549 | C27H30O16 | 610.15338 | 610.1535 | 0.19 | 301.03528, 300.02737, 151.00290 | 97.70 |
| 14 | Quercetin 3-O-β-D-Glucuronide a [46] | [M-H]− | 6.784 | C21H18O13 | 478.07474 | 478.07475 | 0.02 | 301.03531, 300.02774, 151.14471 | 95.52 | |
| 15 | Isoquercitrin a [9] | [M+H]+ | 6.738 | C21H20O12 | 464.09548 | 464.09478 | −1.51 | 315.04922, 303.04922, 145.04932 | 84.73 | |
| 16 | Quercetin a [7] | [M+H]+ | 6.736 | C15H10O7 | 302.04265 | 302.04201 | −2.12 | 285.03864, 219.06436, 153.01799 | 98.66 | |
| 17 | Hyperoside b | [M-H]− | 6.766 | C21H20O12 | 464.09548 | 464.09569 | 0.46 | 300.02753, 255.03510, 151.00294 | 92.15 | |
| 18 | Avicularin b | [M+H]+ | 7.007 | C20H18O11 | 434.08491 | 434.08436 | −1.27 | 417.15305, 399.07016, 303.04935, 151.11162, 115.03909 | 96.58 | |
| 19 | Morin b | Others | [M-H]− | 9.168 | C15H10O7 | 302.04265 | 302.04256 | −0.31 | 193.01343, 178.99783, 151.00284 | 85.14 |
| 20 | Fisetin b | [M+H]+ | 6.75 | C15H10O6 | 286.04774 | 286.04717 | −1.97 | 270.05154, 255.10130, 121.06470, | 94.56 | |
| 21 | Narcissoside b | [M-H]− | 6.98 | C28H32O16 | 624.16903 | 624.16896 | −0.12 | 315.05087, 314.04315, 300.02731, 299.01947 | 78.14 | |
| C: Flavanol compounds | ||||||||||
| 22 | Cianidanol b | Flavan-3-ol | [M-H]− | 11.019 | C15H14O6 | 290.07904 | 290.0789 | −0.47 | 251.07666, 245.08165, 243.06599 | 88.59 |
| D: Phenolic acids | ||||||||||
| 23 | Ginkgolic acid C13:0 b | Hydroxybenzoic acids | [M-H]− | 19.138 | C20H32O3 | 320.23514 | 320.23503 | −0.35 | 275.24109, 275.23792, 263.184323, 202.60570 | 71.35 |
| 24 | Ginkgolic Acid C15:1 b | [M-H]− | 19.403 | C22H34O3 | 346.25079 | 346.2503 | −1.44 | 302.25656, 301.25339 | 72.84 | |
| 25 | Protocatechuic acid b | [M-H]− | 3.948 | C7H6O4 | 154.02661 | 154.02587 | −4.79 | 109.02859, 91.01786 | 76.68 | |
| 26 | Salicylic acid b | [M-H]− | 5.061 | C7H6O3 | 138.03169 | 138.03161 | −0.61 | 119.02849, 109.04427, 91.03389 | 90.15 | |
| 27 | 4-Methoxysalicylic acid b | [M-H]− | 3.07 | C8H8O4 | 168.04226 | 168.0417 | −3.33 | 149.02376, 123.04437, 122.02872 | 70.58 | |
| 28 | p-Coumaric acid a [7] | [M+H]+ | 5.681 | C9H8O3 | 164.04734 | 164.04726 | −0.52 | 147.04385, 137.05957, 135.04395 | 86.42 | |
| 29 | Octyl gallate b | [M-H]− | 5.966 | C15H22O5 | 282.14672 | 282.1466 | −0.45 | 263.12863, 237.14928, 189.12787, 123.08064 | 71.52 | |
| 30 | Anacardic acid b | [M+H]+ | 12.516 | C22H36O3 | 348.26644 | 348.2658 | −1.85 | 331.26245, 303.26770, 191.14284, 13107024 | 73.48 | |
| 31 | Diffractic acid b | [M-H]− | 8.453 | C20H22O7 | 374.13655 | 374.13664 | 0.23 | 329.13962, 299.12900, 178.06306 | 71.56 | |
| 32 | Chlorogenic acid a [7,46] | Hydroxycinnamic acids | [M-H]− | 5.463 | C16H18O9 | 354.09508 | 354.09512 | 0.12 | 336.67032, 191.05548, 179.03432, 173.04485 | 95.47 |
| 33 | Cryptochlorogenic acid b | [M-H]− | 5.338 | C16H18O9 | 354.09508 | 354.09521 | 0.35 | 317.62381, 191.05566, 179.03450, 173.04504 | 90.58 | |
| 34 | Sinapic acid b | [M+H]+ | 5.243 | C11H12O5 | 224.06847 | 224.06827 | −0.93 | 207.06480, 175.03867, 147.04376, 119.04914 | 90.45 | |
| 35 | Ferulic acid a [7] | [M-H]− | 7.103 | C10H10O4 | 194.05791 | 194.05735 | −2.9 | 178.02657, 149.06009, 134.03654 | 94.17 | |
| 36 | 1-Caffeoylquinic acid b | [M-H]− | 4.511 | C16H18O9 | 354.09508 | 354.09519 | 0.3 | 191.05565, 179.03445, 135.04442 | 92.78 | |
| 37 | Ethyl caffeate b | [M-H]− | 9.48 | C11H12O4 | 208.07356 | 208.07312 | −2.12 | 189.05530, 179.03447, 161.02382, 135.04442 | 80.42 | |
| 38 | Methyl 4-hydroxycinnamate b | [M+H]+ | 6.837 | C10H10O3 | 178.06299 | 178.06283 | −0.93 | 161.05956, 133.06476, 105.07017 | 79.48 | |
| E: Biflavonoids | ||||||||||
| 39 | Sciadopitysin b | Biflavonoids | [M-H]− | 13.186 | C33H24O10 | 580.13695 | 580.13715 | 0.35 | 565.11438, 547.10358, 415.04605, 403.08221, 388.05878, 165.01862 | 73.89 |
| 40 | Ginkgetin b | [M-H]− | 11.854 | C32H22O10 | 566.1213 | 566.12141 | 0.2 | 533.08832, 403.08249, 389.06705, 117.03377 | 70.71 | |
| 41 | Bilobetin b | [M-H]− | 10.874 | C31H20O10 | 552.10565 | 552.10576 | 0.21 | 519.07202, 389.06671, 269.21216 | 71.48 | |
| F: Dihydrochalcone | ||||||||||
| 42 | Phloridzin b | Psoralen derivatives | [M-H]− | 7.606 | C21H24O10 | 436.13695 | 436.13698 | 0.07 | 391.07040, 273.07675, 167.03427 | 74.02 |
| G: Other polyphenolic compounds | ||||||||||
| 43 | Esculin b | Coumarin derivatives | [M-H]− | 4.875 | C15H16O9 | 340.07943 | 340.07931 | −0.36 | 177.01865, 176.01111, 133.02878 | 98.15 |
| 44 | Esculetin b | [M-H]− | 5.779 | C9H6O4 | 178.02661 | 178.02596 | −3.64 | 158.90924, 133.02864, 105.03358, 89.03854 | 82.33 | |
| 45 | Lithospermic acid b | Others | [M+H]+ | 8.543 | C27H22O12 | 538.11113 | 538.111 | −0.23 | 521.10736, 341.06516, 323.05457, 297.07550, 181.04942 | 75.55 |
| 46 | 1,5-Isoquinolinediol b | [M+H]+ | 4.949 | C9H7NO2 | 161.04768 | 161.04755 | −0.8 | 144.04416, 134.05989, 120.04441 | 83.92 | |
| 47 | Pinoresinol b | [M-H]− | 8.203 | C20H22O6 | 358.14164 | 358.14147 | −0.47 | 339.12360, 324.10019, 309.07672, 177.01862 | 80.50 | |
| 48 | Salidroside b | Phenylethanoid glycosides | [M-H]− | 4.724 | C14H20O7 | 300.1209 | 300.12083 | −0.24 | 179.05545, 176.35088, 161.04488, 119.03410, 89.02330 | 82.70 |
| 49 | Oleuropein b | [M-H]− | 7.626 | C25H32O13 | 540.18429 | 540.18481 | 0.97 | 401.10913, 377.12436, 359.11392, 345.09790 | 70.21 | |
| 50 | Forsythoside E b | [M-H]− | 4.524 | C20H30O12 | 462.17373 | 462.17388 | 0.32 | 317.12265, 309.11725, 293.13855, 179.07007, 147.06500, 129.05455 | 76.16 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, H.; Xie, C.; Ma, Y.; Miao, Y.; Chen, X.; Gong, H.; Wang, J. Optimization and Component Identification of Ultrasound-Assisted Extraction of Polyphenols from Coriander (Coriandrum sativum L.) and Evaluation of Polyphenol Content Changes and Antioxidant Activity During Storage. Separations 2025, 12, 217. https://doi.org/10.3390/separations12080217
Yuan H, Xie C, Ma Y, Miao Y, Chen X, Gong H, Wang J. Optimization and Component Identification of Ultrasound-Assisted Extraction of Polyphenols from Coriander (Coriandrum sativum L.) and Evaluation of Polyphenol Content Changes and Antioxidant Activity During Storage. Separations. 2025; 12(8):217. https://doi.org/10.3390/separations12080217
Chicago/Turabian StyleYuan, Heng, Chunzhi Xie, Yue Ma, Yaqi Miao, Xuehong Chen, Hao Gong, and Jun Wang. 2025. "Optimization and Component Identification of Ultrasound-Assisted Extraction of Polyphenols from Coriander (Coriandrum sativum L.) and Evaluation of Polyphenol Content Changes and Antioxidant Activity During Storage" Separations 12, no. 8: 217. https://doi.org/10.3390/separations12080217
APA StyleYuan, H., Xie, C., Ma, Y., Miao, Y., Chen, X., Gong, H., & Wang, J. (2025). Optimization and Component Identification of Ultrasound-Assisted Extraction of Polyphenols from Coriander (Coriandrum sativum L.) and Evaluation of Polyphenol Content Changes and Antioxidant Activity During Storage. Separations, 12(8), 217. https://doi.org/10.3390/separations12080217






