Directed Message-Passing Neural Networks for Gas Chromatography
Abstract
1. Introduction
2. Materials and Methods
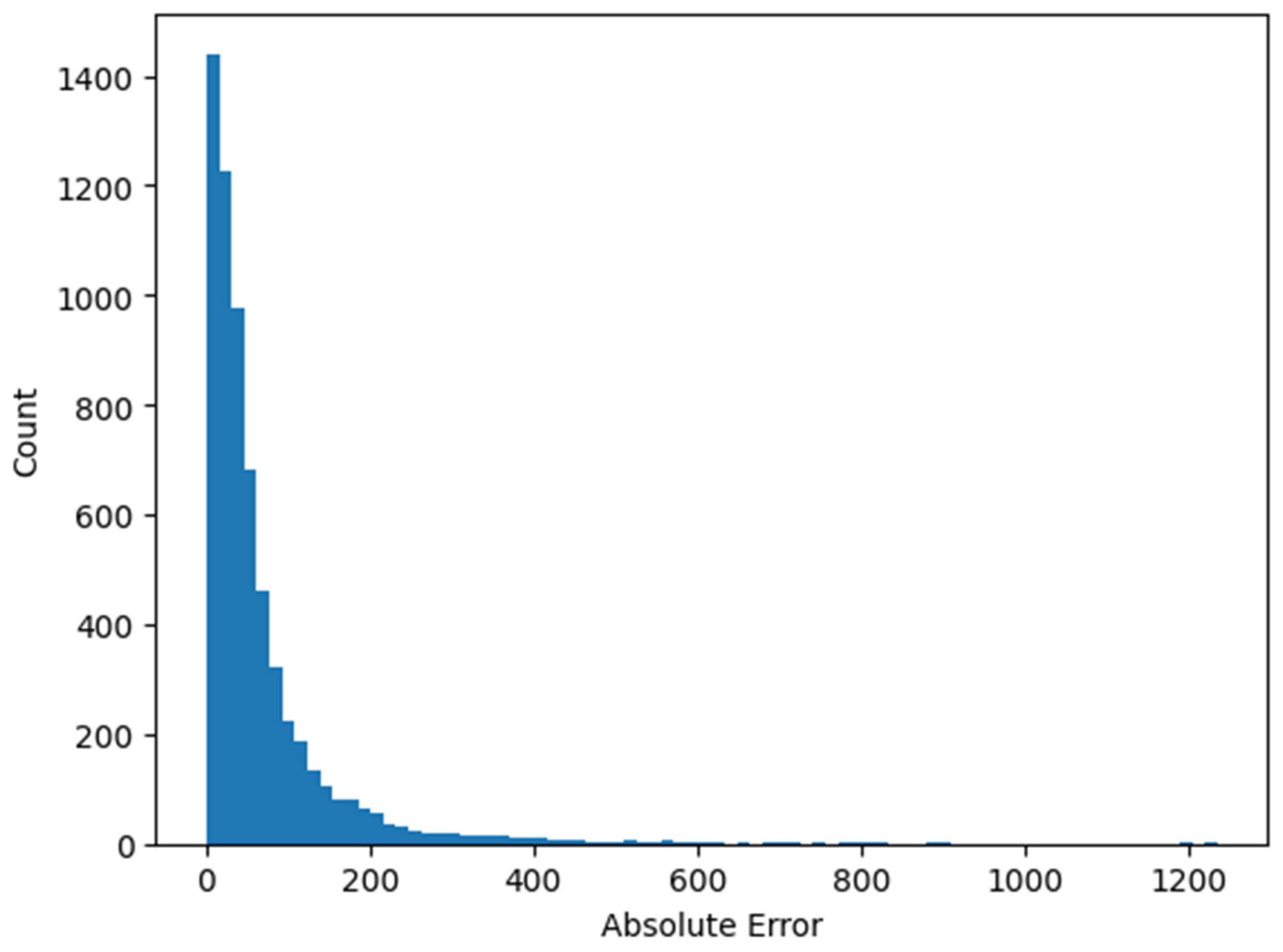
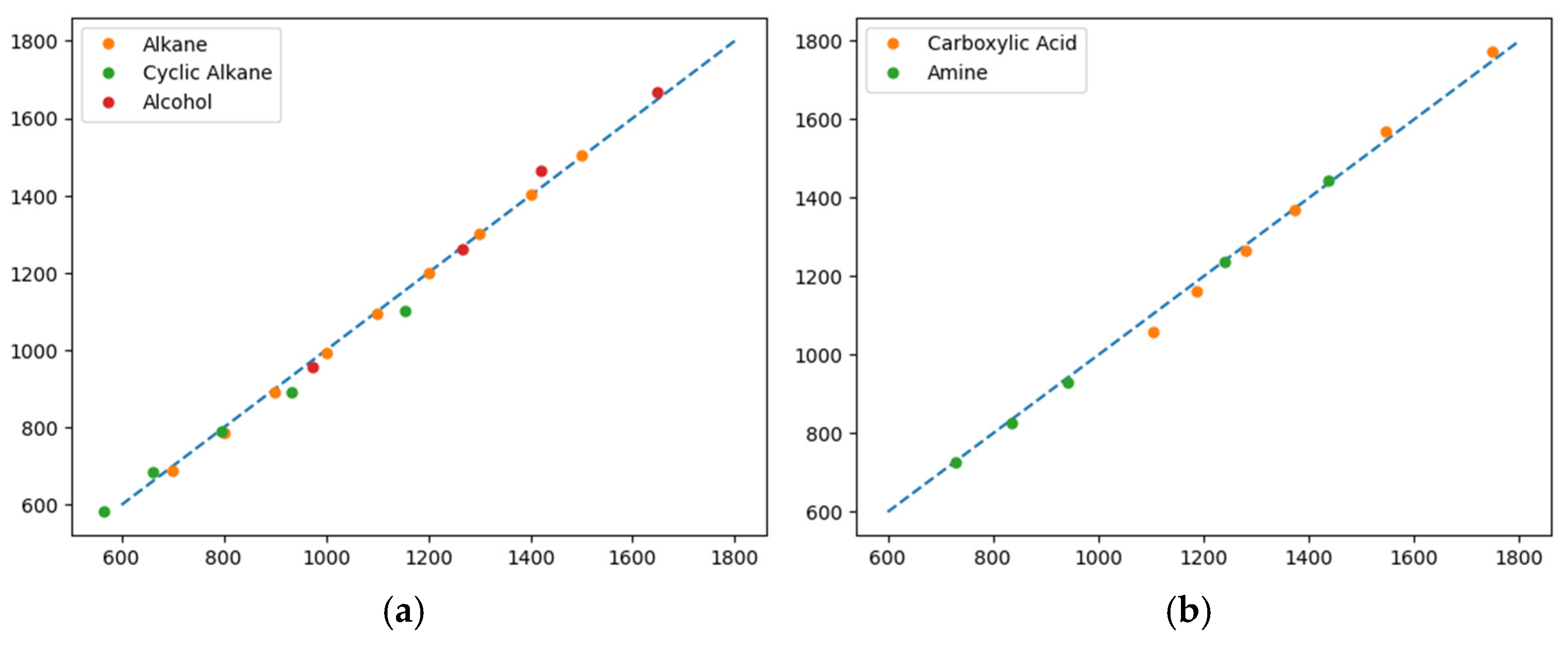
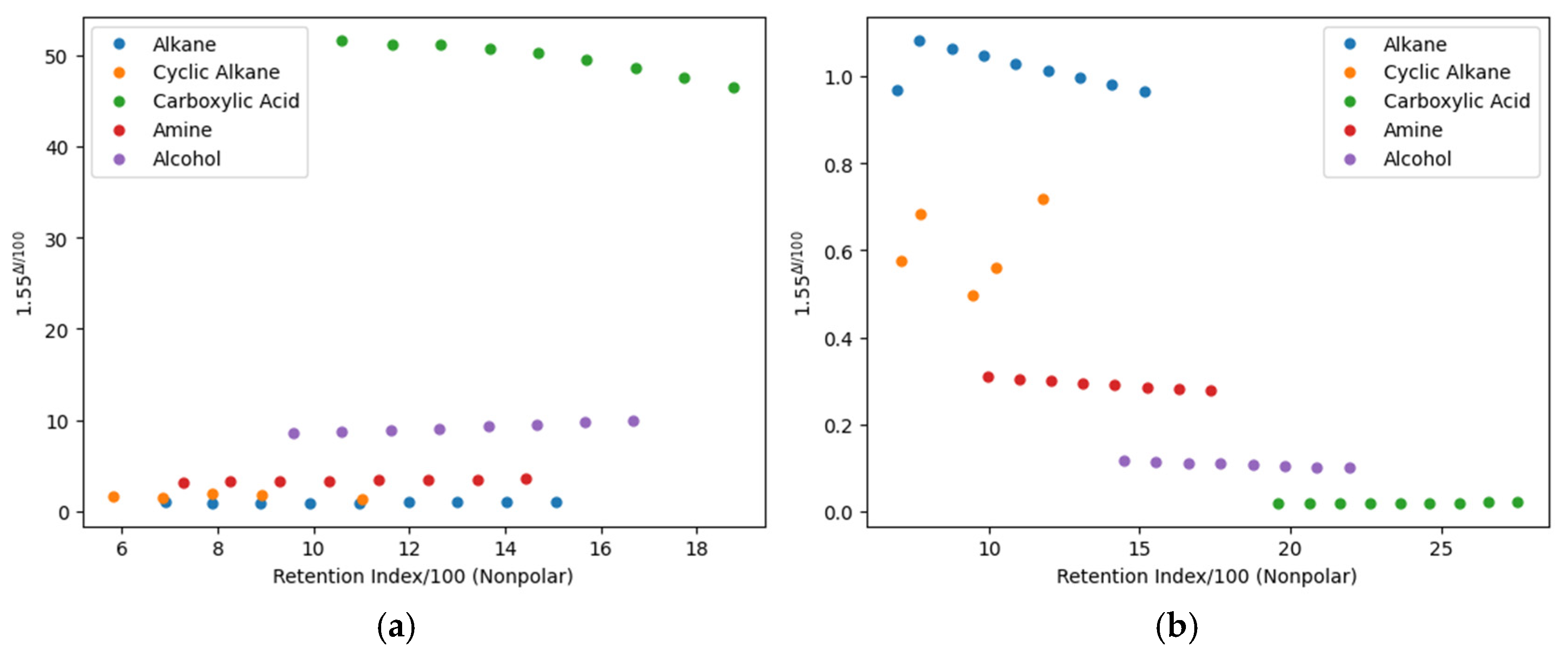
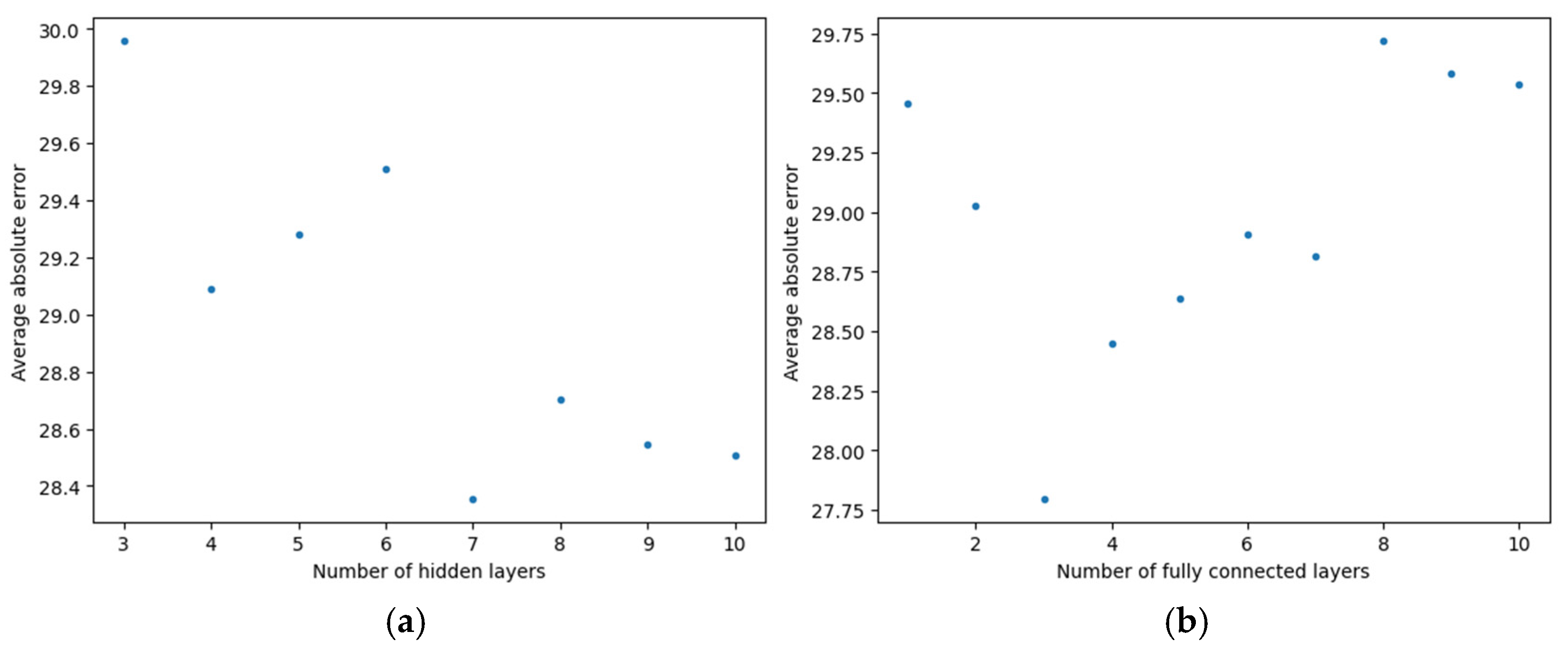
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Artrith, N.; Butler, K.T.; Coudert, F.X.; Han, S.; Isayev, O.; Jain, A.; Walsh, A. Best practices in machine learning for chemistry. Nat. Chem. 2021, 13, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Debus, B.; Parastar, H.; Harrington, P.; Kirsanov, D. Deep learning in analytical chemistry. TrAC Trends Anal. Chem. 2021, 145, 116459. [Google Scholar] [CrossRef]
- Meuwly, M. Machine learning for chemical reactions. Chem. Rev. 2021, 121, 10218–10239. [Google Scholar] [CrossRef] [PubMed]
- Petritis, K.; Kangas, L.J.; Ferguson, P.L.; Anderson, G.A.; Paša-Tolić, L.; Lipton, M.S.; Smith, R.D. Use of artificial neural networks for the accurate prediction of peptide liquid chromatography elution times in proteome analyses. Anal. Chem. 2003, 75, 1039–1048. [Google Scholar] [CrossRef]
- Munro, K.; Miller, T.H.; Martins, C.P.; Edge, A.M.; Cowan, D.A.; Barron, L.P. Artificial neural network modelling of pharmaceutical residue retention times in wastewater extracts using gradient liquid chromatography-high resolution mass spectrometry data. J. Chromatogr. A 2015, 1396, 34–44. [Google Scholar] [CrossRef]
- Yang, J.; Xu, G.; Kong, H.; Zheng, Y.; Pang, T.; Yang, Q. Artificial neural network classification based on high-performance liquid chromatography of urinary and serum nucleosides for the clinical diagnosis of cancer. J. Chromatogr. B 2002, 780, 27–33. [Google Scholar] [CrossRef]
- Srečnik, G.; Debeljak, Ž.; Cerjan-Stefanović, Š.; Novič, M.; Bolanča, T. Optimization of artificial neural networks used for retention modelling in ion chromatography. J. Chromatogr. A 2002, 973, 47–59. [Google Scholar] [CrossRef]
- Madden, J.E.; Avdalovic, N.; Haddad, P.R.; Havel, J. Prediction of retention times for anions in linear gradient elution ion chromatography with hydroxide eluents using artificial neural networks. J. Chromatogr. A 2001, 910, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.D. Derivative formation in the quantitative gas-chromatographic analysis of pharmaceuticals. Part II. A review. Analyst 1978, 103, 193–222. [Google Scholar] [CrossRef]
- König, W.A.; Ernst, K. Application of enantioselective capillary gas chromatography to the analysis of chiral pharmaceuticals. J. Chromatogr. A 1983, 280, 135–141. [Google Scholar] [CrossRef]
- Fang, G.; Min, G.; He, J.; Zhang, C.; Qian, K.; Wang, S. Multiwalled carbon nanotubes as matrix solid-phase dispersion extraction absorbents to determine 31 pesticides in agriculture samples by gas chromatography—Mass spectrometry. J. Agric. Food Chem. 2009, 57, 3040–3045. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Alvarez, M.; Llompart, M.; Lamas, J.P.; Lores, M.; Garcia-Jares, C.; Cela, R.; Dagnac, T. Simultaneous determination of traces of pyrethroids, organochlorines and other main plant protection agents in agricultural soils by headspace solid-phase microextraction—Gas chromatography. J. Chromatogr. A 2008, 1188, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Furton, K.G.; Wang, J.; Hsu, Y.L.; Walton, J.; Almirall, J.R. The use of solid-phase microextraction—Gas chromatography in forensic analysis. J. Chromatogr. Sci. 2000, 38, 297–306. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Frysinger, G.S.; Gaines, R.B. Forensic analysis of ignitable liquids in fire debris by comprehensive two-dimensional gas chromatography. J. Forensic Sci. 2002, 47, 471–482. [Google Scholar] [CrossRef]
- Hou, S.; Stevenson, K.A.; Harynuk, J.J. A simple, fast, and accurate thermodynamic-based approach for transfer and prediction of gas chromatography retention times between columns and instruments Part I: Estimation of reference column geometry and thermodynamic parameters. J. Sep. Sci. 2018, 41, 2544–2552. [Google Scholar] [CrossRef]
- Hou, S.; Stevenson, K.A.; Harynuk, J.J. A simple, fast, and accurate thermodynamic-based approach for transfer and prediction of GC retention times between columns and instruments Part II: Estimation of target column geometry. J. Sep. Sci. 2018, 41, 2553–2558. [Google Scholar] [CrossRef]
- Brehmer, T.; Duong, B.; Marquart, M.; Friedemann, L.; Faust, P.J.; Boeker, P.; Wüst, M.; Leppert, J. Retention database for prediction, simulation, and optimization of GC separations. ACS Omega 2023, 8, 19708–19718. [Google Scholar] [CrossRef]
- Van Den Dool, H.A.N.D.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Kovats, V.E. Gas-chromatographische charakterisierung organischer verbindungen. Teil 1: Retentionsindices aliphatischer halogenide, alkohole, aldehyde und ketone. Helv. Chim. Acta 1958, 41, 1915–1932. [Google Scholar] [CrossRef]
- Lee, M.L.; Vassilaros, D.L.; White, C.M. Retention indices for programmed-temperature capillary-column gas chromatography of polycyclic aromatic hydrocarbons. Anal. Chem. 1979, 51, 768–773. [Google Scholar] [CrossRef]
- NIST Mass Spectrometry Data Center; Wallace, W.E. Retention Indices. In NIST Chemistry WebBook, NIST Standard Reference Database Number 69; Linstrom, P.J., Mallard, W.G., Eds.; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2025; p. 20899. [Google Scholar] [CrossRef]
- Struk, D.R.; Ilhamsyah, R.; Heist, C.A.; Dimandja, J.M.D.; Hesketh, P.J. A Semi-Automated Pipeline for Nontargeted Compound Analysis via GC×GC-MS. J. Chromatogr. Open 2024, 5, 100118. [Google Scholar] [CrossRef]
- Babushok, V.I. Chromatographic retention indices in identification of chemical compounds. TrAC Trends Anal. Chem. 2015, 69, 98–104. [Google Scholar] [CrossRef]
- Koo, I.; Shi, X.; Kim, S.; Zhang, X. iMatch2: Compound identification using retention index for analysis of gas chromatography–mass spectrometry data. J. Chromatogr. A 2014, 1337, 202–210. [Google Scholar] [CrossRef]
- Wei, X.; Koo, I.; Kim, S.; Zhang, X. Compound identification in GC-MS by simultaneously evaluating the mass spectrum and retention index. Analyst 2014, 139, 2507–2514. [Google Scholar] [CrossRef]
- Seeley, J.V.; Seeley, S.K. Model for predicting comprehensive two-dimensional gas chromatography retention times. J. Chromatogr. A 2007, 1172, 72–83. [Google Scholar] [CrossRef]
- Seeley, J.V.; Libby, E.M.; Edwards, K.A.H.; Seeley, S.K. Solvation parameter model of comprehensive two-dimensional gas chromatography separations. J. Chromatogr. A 2009, 1216, 1650–1657. [Google Scholar] [CrossRef]
- Peng, C.T.; Ding, S.F.; Hua, R.L.; Yang, Z.C. Prediction of retention indexes: I. Structure—Retention index relationship on apolar columns. J. Chromatogr. A 1988, 436, 137–172. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.T.; Yang, Z.C.; Ding, S.F. Prediction of retention indexes: II. Structure-retention index relationship on polar columns. J. Chromatogr. A 1991, 586, 85–112. [Google Scholar] [CrossRef]
- Matyushin, D.D.; Buryak, A.K. Gas chromatographic retention index prediction using multimodal machine learning. IEEE Access 2020, 8, 223140–223155. [Google Scholar] [CrossRef]
- Matyushin, D.D.; Sholokhova, A.Y.; Buryak, A.K. Deep learning based prediction of gas chromatographic retention indices for a wide variety of polar and mid-polar liquid stationary phases. Int. J. Mol. Sci. 2021, 22, 9194. [Google Scholar] [CrossRef] [PubMed]
- Larsson, T.; Vermeire, F.; Verhelst, S. Machine Learning for Fuel Property Predictions: A Multi-Task and Transfer Learning Approach (No. 2023-01-0337). SAE Technical Paper, 2023. Available online: https://www.sae.org/publications/technical-papers/content/2023-01-0337/ (accessed on 28 April 2025).
- McNaughton, A.D.; Joshi, R.P.; Knutson, C.R.; Fnu, A.; Luebke, K.J.; Malerich, J.P.; Madrid, P.B.; Kumar, N. Machine learning models for predicting molecular UV–Vis spectra with quantum mechanical properties. J. Chem. Inf. Model. 2023, 63, 1462–1471. [Google Scholar] [CrossRef] [PubMed]
- Koscher, B.A.; Canty, R.B.; McDonald, M.A.; Greenman, K.P.; McGill, C.J.; Bilodeau, C.L.; Jin, W.; Wu, H.; Vermeire, F.H.; Jin, B.; et al. Autonomous, multiproperty-driven molecular discovery: From predictions to measurements and back. Science 2023, 382, eadi1407. [Google Scholar] [CrossRef] [PubMed]
- Heid, E.; Greenman, K.P.; Chung, Y.; Li, S.C.; Graff, D.E.; Vermeire, F.H.; Wu, H.; Green, W.H.; McGill, C.J. Chemprop: A machine learning package for chemical property prediction. J. Chem. Inf. Model. 2023, 64, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2025 update. Nucleic Acids Res. 2025, 53, D1516–D1525. [Google Scholar] [CrossRef]
- Khrisanfov, M.D.; Matyushin, D.D.; Samokhin, A.S. A general procedure for finding potentially erroneous entries in the database of retention indices. Anal. Chim. Acta 2024, 1297, 342375. [Google Scholar] [CrossRef]
- Gholamy, A.; Kreinovich, V.; Kosheleva, O. Why 70/30 or 80/20 relation between training and testing sets: A pedagogical explanation. Int. J. Intell. Technol. Appl. Stat. 2018, 11, 105–111. [Google Scholar]


| Description | Size | Model Output | Source | |
|---|---|---|---|---|
| Thermodynamic Parameters | 287 | A, B, C | [17] | |
| Retention Times | Using the thermodynamic parameters of the prior dataset, the retention times were predicted for temperature ramps of 5, 10, and 15 °C/min | 287 | Raw retention times for 5, 10, 15 °C/min ramps | [15,17] |
| Nonpolar Retention Index Data | Nonpolar retention index data taken from NIST 17 database with different experimental data points averaged together | 60,104 | Nonpolar Retention Index | [21] |
| Polar Retention Index Data | Polar retention index data taken from NIST 17 database with different experimental data points averaged together | 5853 | Polar Retention Index | [21] |
| Filtered Nonpolar Retention Index Data | Nonpolar retention index data filtered according to whether there were at least two distinct sources reporting the information and if the standard deviation was less than 20 unit | 7817 | Nonpolar Retention Index | [21] |
| Filtered Polar Retention Index Data | Polar retention index data taken from NIST 17 database filtered according to whether there were at least two distinct sources reporting the information and if the standard deviation was less than 20 unit | 3192 | Polar Retention Index | [21] |
| Combined Filtered Retention Index Data | Both nonpolar and polar | 2463 | Nonpolar and Polar Retention Indices | [21] |
| 5 °C/min (Test Subset) | 10 °C/min (Test Subset) | 15 °C/min (Test Subset) | 5 °C/min (Full Set) | 10 °C/min (Full Set) | 15 °C/min (Full Set) | |
|---|---|---|---|---|---|---|
| Mean absolute error (s) | 141.75 | 122.13 | 131.96 | 155.38 | 132.84 | 137.35 |
| Mean absolute percent error | 15.78 | 14.60 | 17.81 | 20.10 | 16.59 | 19.02 |
| A (Test Subset) | B (Test Subset) | C (Test Subset) | A (Full Set) | B (Full Set) | C (Full Set) | |
|---|---|---|---|---|---|---|
| Mean absolute error | 16.23 | 1292.73 | 2.25 | 16.49 | 1305.77 | 2.30 |
| Mean absolute percent error | 19.50 | 12.17 | 21.27 | 25.49 | 14.04 | 28.25 |
| Mean Absolute Error, Test Subset | Mean Absolute Error, Full List | Mean Absolute Percent Error, Test Subset | Mean Absolute Percent Error, Full List | |
|---|---|---|---|---|
| Nonpolar | 46.05 ± 1.64 | 39.51 ± 3.61 | 2.50 ± 0.01% | 1.92 ± 0.14% |
| Polar | 78.37 ± 1.15 | 66.96 ± 1.57 | 4.49 ± 0.10% | 3.90 ± 0.06% |
| Nonpolar, filtered | 30.81 ± 3.43 | 24.52 ± 4.86 | 2.24 ± 0.16% | 2.06 ± 0.23% |
| Polar, filtered | 55.21 ± 0.41 | 42.43 ± 0.48 | 3.50 ± 0.03% | 2.58 ± 0.02% |
| Combined, filtered | 34.97 ± 0.62 | 32.05 ± 0.67 | 2.61 ± 0.04% | 2.30 ± 0.06% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Struk, D.; Ilhamsyah, R.; Dimandja, J.-M.D.; Hesketh, P.J. Directed Message-Passing Neural Networks for Gas Chromatography. Separations 2025, 12, 200. https://doi.org/10.3390/separations12080200
Struk D, Ilhamsyah R, Dimandja J-MD, Hesketh PJ. Directed Message-Passing Neural Networks for Gas Chromatography. Separations. 2025; 12(8):200. https://doi.org/10.3390/separations12080200
Chicago/Turabian StyleStruk, Daniel, Rizky Ilhamsyah, Jean-Marie D. Dimandja, and Peter J. Hesketh. 2025. "Directed Message-Passing Neural Networks for Gas Chromatography" Separations 12, no. 8: 200. https://doi.org/10.3390/separations12080200
APA StyleStruk, D., Ilhamsyah, R., Dimandja, J.-M. D., & Hesketh, P. J. (2025). Directed Message-Passing Neural Networks for Gas Chromatography. Separations, 12(8), 200. https://doi.org/10.3390/separations12080200







