Abstract
Magnetic nanoparticles (MNPs), especially iron oxide (Fe3O4), display distinctive superparamagnetic characteristics and elevated surface-area-to-volume ratios, facilitating improved physicochemical interactions with solutes and pollutants. These characteristics make MNPs strong contenders for use in water treatment applications. This research investigates the application of iron oxide MNPs synthesized via co-precipitation as innovative draw solutes in forward osmosis (FO) for treating synthetic produced water (SPW). The FO membrane underwent surface modification with sulfobetaine methacrylate (SBMA), a zwitterionic polymer, to increase hydrophilicity, minimize fouling, and elevate water flux. The SBMA functional groups aid in electrostatic repulsion of organic and inorganic contaminants, simultaneously encouraging robust hydration layers that improve water permeability. This adjustment is vital for sustaining consistent flux performance while functioning with MNP-based draw solutions. Material analysis through thermogravimetric analysis (TGA), scanning electron microscopy (SEM), and Fourier-transform infrared spectroscopy (FTIR) verified the MNPs’ thermal stability, consistent morphology, and modified surface chemistry. The FO experiments showed a distinct relationship between MNP concentration and osmotic efficiency. At an MNP dosage of 10 g/L, the peak real-time flux was observed at around 3.5–4.0 L/m2·h. After magnetic regeneration, 7.8 g of retrieved MNPs generated a steady flow of ~2.8 L/m2·h, whereas a subsequent regeneration (4.06 g) resulted in ~1.5 L/m2·h, demonstrating partial preservation of osmotic driving capability. Post-FO draw solutions, after filtration, exhibited total dissolved solids (TDS) measurements that varied from 2.5 mg/L (0 g/L MNP) to 227.1 mg/L (10 g/L MNP), further validating the effective dispersion and solute contribution of MNPs. The TDS of regenerated MNP solutions stayed similar to that of their fresh versions, indicating minimal loss of solute activity during the recycling process. The combined synergistic application of SBMA-modified FO membranes and regenerable MNP draw solutes showcases an effective and sustainable method for treating produced water, providing excellent water recovery, consistent operational stability, and opportunities for cyclic reuse.
1. Introduction
Produced water is a byproduct of oil and gas, geothermal energy extraction, and petroleum production. This wastewater or brine is generated in high volumes during oil and gas extraction from underground reservoirs [1,2]. This untreated produced water or brine is generated in high volumes during oil and gas extraction from underground reservoirs in drilling [3]. High salt concentrations can harm soil and vegetation by impairing their ability to germinate and absorb water [4,5]. Oil and grease can reduce oxygen levels in the water, and alkylphenols are toxic to aquatic life and can bioaccumulate inside fish [6,7]. Benzene, Toluene, Ethylbenzene, and Xylenes (BTEX) and polycyclic aromatic hydrocarbons (PAHs) are toxic environmental carcinogens, while organic acids with low pH levels can be harmful to microorganisms while harming the water treatment process [8,9]. Decaying organic matter can cause eutrophication or excessive plant life growth, causing high mortality in aquatic animals due to a lack of oxygen in the water. Intemperate inorganic elements and compounds like nitrogen pollution can also significantly weaken aquatic life with their toxic nature [10]. Spent drilling fluid contains a low but harmful number of heavy metals that contain dangerous non-threshold genotoxic carcinogens [11,12,13].
Produced water is treated to minimize effluent contamination through ‘zero liquid discharge,’ which assures no readmittance of untreated water into the environment. Following treatment, the water can be reutilized for industrial or environmental purposes, saving energy, reducing pollution, decreasing discharge to sensitive water bodies, enhancing wetlands and riparian habitats, and decreasing freshwater diversion from sensitive ecosystems [14,15]. Lots of produced water is not treated for reuse or admission into the environment; it is injected into disposal wells [16]. Disposal wells are designated zones underground that are considered safe and efficient for discarding contaminated water. Despite its perceived security, the wells pose many risks to surrounding ecosystems and bodies of water. Leaks, cracks, and abandoned wells can leave surface-level bodies of water vulnerable to increased concentrations of chloride and bromide and other toxin contamination emanating from disposal wells [17,18]. Rather than discarding and neglecting the contaminated water, zero liquid discharge can be attained by various methods, including evaporation, electrodialysis, membrane distillation, reverse osmosis, and forward osmosis [14,19].
Forward osmosis (FO) is an osmotic process that utilizes a semipermeable membrane and the energy of osmotic pressure to remove contaminants and other solutes from water. The membrane comprises two layers: a selective active layer with a high rejection rate and a support layer that is comparatively permeable [20,21]. FO has two modes of operation: FO mode and Pressure-retarded osmosis (PRO) mode. FO mode consists of an active layer positioned towards the feed solution and a support layer placed towards the draw solution, causing a lower flux because of its concentration polarization [21,22]. In PRO mode, the layer placements are inverted, so the active layer is positioned towards the draw solution, and the support layer is towards the feed solution, resulting in the reliable obtention of higher flux [22,23]. The process is progressively becoming more prevalent as a form of wastewater treatment because it requires the least energy compared to other membrane processes, as it works based on the osmotic pressure gradient [24]. The Osmotic pressure gradient is rooted in the variation in concentration levels between phases, serving as an impetus for water flow to the outer phase from the inner phase and to the double emulsion globules from the outer phase. The gradient is crucial to the stability of double emulsions. Despite its increasing use and relevance, the process is considered technologically “immature” due to its use of draw solution regeneration, making procedures expensive to trial and replicate. Draw solutions require periodical reconcentration so that production is sustainable. Regeneration is imperative to the efficiency and net energy generation in a closed-loop pressure-retarded osmosis (CLPRO) system, as its complete reconcentration ensures high-quality permeated water will be produced while disallowing salt leakage [24,25,26].
Magnetic Nanoparticles (MNPs) are a promising sustainable draw solution for the FO membrane process, though development is necessary before the practice can be widely used for wastewater treatment [27,28]. MNPs are the most energy-efficient draw solute, using magnetic force to regenerate their high osmotic pressure easily [27]. According to Yavuz et al. (2006) [29], the low-field magnetic separation of Fe3O4 nanocrystals can be successfully facilitated with magnetic arrangements generating gradients below 100 T/m, demonstrating the technique’s practicality for large-scale, energy-efficient water purification. Osmotic pressure generation varies based on particles’ electrostatic surface charge, size, and hydrophilicity; all variables can be controlled with intentional procedural changes [30]. The surface charge and mean size of the particles are affected by the pH and ionic strength of the precipitation medium. However, in doing so, the uniformity and monodispersed formation of the nanoparticles are detrimentally affected [31]. The size of MNPs is also controllable through the synthesis process, where, once dispersed into water, the colloidal stability property mainly determines the particle size [32]. It maintains the initial local structure and radius of curvature. MNPs generally have low colloidal stability and poor dispersion due to the dipole–dipole reactions between particles. Their stability can be enhanced by increasing the electrostatic repulsion between particles using a thin layer of hydrophilic coating polymer or surfactant overlaying the nanoparticles and protecting them from the effects of dipole–dipole reactions. The coating layer of the MNP surface can intensify osmotic pressure via so or deprotonation, while a constant pH level will further aid colloidal stability [27,28]. This study focused on FO membranes using sulfobetaine methacrylate (SBMA) to improve their antifouling properties, water permeability, and durability in high-salinity environments characteristic of produced water. Modifiers such as SBMA, which are zwitterionic, are becoming more favored because they create thick, electrically neutral hydration layers that effectively prevent organic fouling, oil emulsions, and mineral scaling [33,34]. In contrast to conventional hydrophilic coatings like polyethylene glycol (PEG) or carboxylic acid functionalization, which frequently experience oxidative degradation or limited compatibility with the polyamide selective layer, SBMA offers a strong, covalently attached, and highly hydrated surface [35,36]. Different modification methods, such as silver nanoparticle doping or amine-functionalized brushes, are susceptible to leaching, agglomeration, and instability in complicated wastewater matrices [37]. Additionally, to enhance water flux and osmotic driving force, MNPs were employed as innovative draw solutes [27,38]. These nanoparticles demonstrate significant osmotic pressure, high colloidal stability, and can be magnetically retrieved and reused, minimizing secondary pollution and preventing solute loss. SBMA-modified thin-film composite FO membranes combined with MNP draw solutes generate a selective antifouling interface along with a reusable and adjustable draw system [35]. This combined method is especially beneficial for managing produced water, as the significant organic load and salinity demand a membrane system that can maintain flux while avoiding membrane damage or permanent fouling. Consequently, the FO system based on SBMA-MNP provides a synergistic and sustainable foundation for efficient treatment and reuse of produced water [27,38,39].
2. Materials and Methods
2.1. Materials
Ferrous chloride (FeCl2), ferric chloride (FeCl3), citric acid, ammonium hydroxide (NH4OH), and ethyl acetate were procured from Sigma Aldrich and used without further purification. All solutions were prepared using deionized water. The preparation of Synthetic Produced Water (SPW) was prepared using the following analytical-grade chemicals: sodium chloride (NaCl), sodium bicarbonate (NaHCO3), sodium carbonate (Na2CO3), sodium sulfate (Na2SO4), calcium chloride (CaCl2), magnesium chloride hexahydrate (MgCl2·6H2O), and sodium dodecyl sulfate (SDS). Sterlitech Corporation, FTSH2O flat Sheet CTA, forward osmosis membrane, sulfobetaine methacrylate (SBMA), potassium persulfate (K2S2O8). All reagents were procured from Thermo Fischer Scientific and utilized without further purification. Deionized water was used throughout the experimental procedure.
2.2. Preparation of Magnetic Nanoparticles
Magnetic nanoparticles were synthesized using a co-precipitation approach. Initially, FeCl2 and FeCl3 were mixed in a molar ratio of 2:1 to serve as the iron precursors. The solution was purged with nitrogen gas for 10 min to eliminate dissolved oxygen and prevent premature oxidation. The precipitation process was initiated by the controlled dropwise addition of ammonium hydroxide under continuous magnetic stirring at 80 °C. The pH of the mixture was gradually raised and maintained around 11 to facilitate the complete formation of iron oxide nanoparticles. Citric acid was introduced as a surface-capping agent to enhance the colloidal stability and hydrophilicity of the nanoparticles. The ratio of citric acid to the iron precursors was maintained at 2:2:1 (citric acid: FeCl2:FeCl3). Following the completion of the precipitation reaction, the nanoparticles were repeatedly washed with ethyl acetate to remove residual ions and impurities. The washed precipitate was then dried under ambient conditions to yield a fine powder. The synthesized magnetic nanoparticles were utilized as an advanced draw solute for FO applications [38].
2.3. Surface Modification of Forward Osmosis Membrane
A commercially available flat sheet cellulose triacetate FO membrane (FTSH2O Flat Sheet Membrane, CTA, FO, CF042) was chosen for surface modification with the zwitterionic monomer SBMA according to a previous study [33]. The membrane was initially washed extensively with deionized (DI) water and kept in DI water for 12 h to eliminate leftover preservatives. The membrane was subsequently dried at ambient temperature prior to being stored for future use. A zwitterionic monomer solution was made by dissolving 1 g of SBMA in 100 mL of deionized water. To start the polymerization, 0.1 g of potassium persulfate (K2S2O8) was incorporated as a thermal initiator. The mixture was agitated at room temperature for 15 min to guarantee uniformity. The activated membrane was positioned on a clean glass plate with the active side facing upward and submerged in the SBMA monomer solution. The configuration was kept at 60 °C for 3 h in a convection oven to commence and uphold the polymerization of SBMA on the membrane surface. Following polymerization, the membrane was extracted from the solution and thoroughly washed with deionized water to remove unreacted monomers and weakly attached polymers. Rinsing occurred three times, utilizing 200 mL of DI water for 10 min with gentle agitation. The altered membranes were preserved in fresh deionized water at 4 °C for later utilization.
2.4. Preparation of Synthetic Produced Water
Individual stock solutions were prepared by dissolving specific quantities of each salt in 1 L of deionized water to achieve the following concentrations: NaCl: 150 g/L, NaHCO3: 285 g/L, Na2CO3: 16.5 g/L, Na2SO4: 1 g/L, CaCl2: 5.55 g/L, MgCl2·6H2O: 3.63 g/L. Each chemical was weighed using an analytical balance and dissolved completely under magnetic stirring. The solutions were labeled appropriately with the chemical name, concentration, and date of preparation, and stored in sealed containers at room temperature. To prepare 1 L of the SPW brine matrix, calculated volumes from each stock solution were measured and added to a container with approximately 950 mL of deionized water. The final concentrations targeted in the SPW were as follows: NaCl: 1500 mg/L → 10 mL of 150 g/L stock, NaHCO3: 2850 mg/L → 10 mL of 285 g/L stock, Na2CO3: 165 mg/L → 10 mL of 16.5 g/L stock, Na2SO4: 10 mg/L → 10 mL of 1 g/L stock, CaCl2: 55.5 mg/L → 10 mL of 5.55 g/L stock, MgCl2·6H2O: 10 mL of 3.90 g/L stock. The combined solution was stirred using a magnetic stirrer until it was homogenized. In total, 500 mL of the prepared brine was transferred to a 1 L glass beaker to simulate the organic load often present in real produced water; 30 mg of SDS was added as a surfactant to this solution. The mixture was gently heated to 45 °C and maintained at this temperature for 1 h to ensure dissolution. Subsequently, 5 mL of hydra pump oil was introduced into the solution, followed by high-speed magnetic stirring at 1200 rpm for 45 min to promote emulsification. The emulsion was then subjected to probe sonication for 30 min to enhance dispersion and simulate stable oil-water emulsions typical of produced water. The resulting SPW solution was aliquoted into 1 L amber glass bottles and stored at room temperature until further use in experiments [40].
2.5. Forward Osmosis Experiments
A specially designed laboratory-scale FO system was employed for this study. The experimental setup included a cross-flow membrane module, dual gear pumps for consistent circulation of the solution, separate tanks for feed and draw solutions, a high-accuracy digital scale for tracking mass, and a computer system for real-time data logging. Positioning the FO membrane inside the cell formed two identical flow channels, allowing directional water movement through the membrane as a result of the osmotic pressure difference between the feed and draw compartments. The osmotic driving force, determined by the Van’t Hoff equation (Equation (1)), is influenced by internal concentration polarization (ICP), which diminishes effective transmembrane pressure. The degree of this decrease is represented by the membrane performance ratio (Pm), defined as the ratio of the measured water flux (Jexp) to the theoretical flux (Jthr) calculated from the osmotic pressure difference between bulk solutions, as stated in Equation (2).
where i is the van’t Hoff factor, C is the molar concentration (mol/L), R is the universal gas constant, and T is the absolute temperature (K).
Throughout each trial, the flow rate was held steady at 0.75 L/min to guarantee uniform hydrodynamic conditions. The digital scale captured the mass variation in the draw solution reservoir every minute, allowing for accurate flux tracking. Water flux (Jw) was determined using Equation (3), which includes the observed mass change over time (∆Weight), surface area of the membrane (A), and water density (ρ).
To evaluate the membrane’s separation capability, total solids (TS) rejection was assessed using Equation (4). Here, TSFeed denotes the initial solute concentration in the feed, while TSIncreased refers to the final concentration in the draw solution following the FO run.
2.6. Characterization
The detailed characterization of the produced magnetic nanoparticles was performed utilizing a range of sophisticated analytical methods. A field emission scanning electron microscope (FE-SEM) (JSM-6010LA, JEOL, Tokyo, Japan) was utilized for surface morphological analysis, equipped with an energy-dispersive X-ray spectroscopy (EDS) detector and operated using InTouchScope software (version 1.11), allowing for detailed examination of particle shape and distribution. Thermogravimetric analysis (TGA) was performed with a PerkinElmer-S11 thermal analyzer (PerkinElmer, Waltham, MA, USA) to assess the thermal stability and degradation characteristics of the nanoparticles under a nitrogen atmosphere, the MNP samples were heated from 25 to 800 °C at 10 °C min−1. Differential scanning calorimetry (DSC) was utilized to assess the thermal transition characteristics with a DSC-3 system (Mettler Toledo, Columbus, OH, USA). FTIR spectroscopy was performed using a Thermo Scientific Nicolet device (Thermo Fisher Scientific, Houston, TX, USA) to identify surface functional groups. Crystallographic structure and phase identification were performed through X-ray diffraction (XRD) analysis utilizing a Shimadzu 7000 diffractometer, enabling thorough evaluation of crystalline properties. The pristine membrane, and SBMA surface-modified membrane were characterized by FTIR spectroscopy to confirm the integration and uniformity of surface functionalization achieved via thermal polymerization.
The analysis of Total Dissolved Solids (TDS) was performed to assess the concentration of leftover solutes in MNP draw solutions following the FO process. This measurement offers an understanding of the osmotic strength preserved in the draw solution and the degree of MNP dispersion or dissolution after filtration. After each FO experiment, 25 mL of the draw solution containing MNPs was meticulously collected and passed through a 1.4 µm pore size, Whatman filter paper to eliminate undispersed particles or aggregates. The filtrate was moved to a 50 mL glass beaker, and TDS levels were assessed with a calibrated Fisher Scientific TDS meter.
3. Results and Discussion
3.1. Characterization of Magnetic Nanoparticle
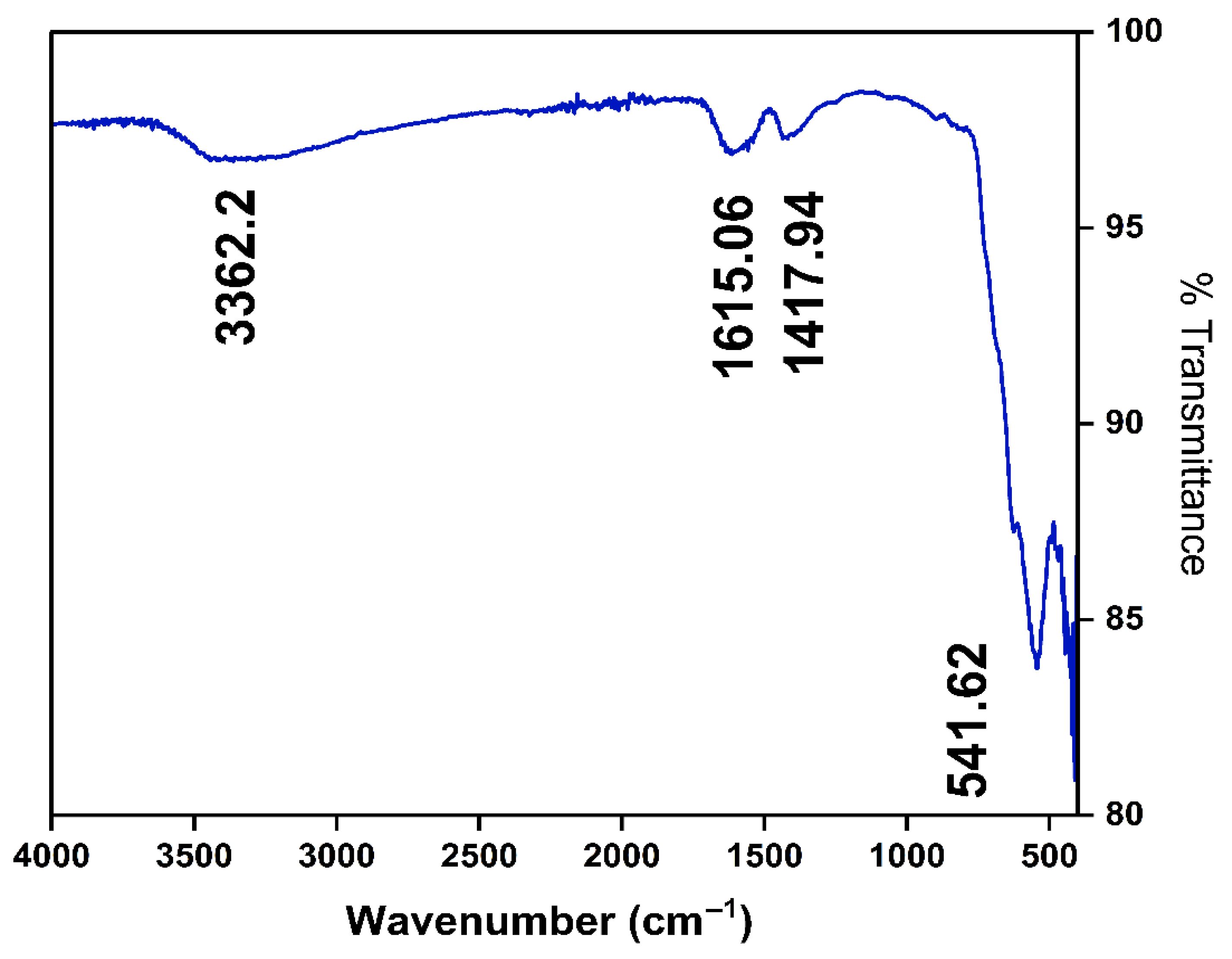
The Fourier-transform infrared spectroscopy (FT-IR) spectrum presented in Figure 1 illustrates the chemical and functional characteristics of the synthesized MNPs. A prominent absorption band is observed at around 3362.2 cm−1, indicative of the presence of hydroxyl (-OH) functional groups, likely arising from adsorbed moisture or citric acid hydroxyl groups associated with nanoparticle surfaces [41,42]. Additionally, characteristic peaks at approximately 1615.06 cm−1 and 1417.94 cm−1 correspond to bending vibrations and stretching frequencies typically assigned to surface-bound water molecules or carboxylate groups, confirming the surface functionalization of the nanoparticles [43]. Notably, a distinct peak observed at approximately 541.62 cm−1 represents the intrinsic stretching vibration of the Fe–O bond, a signature of iron oxide nanoparticles, thus validating their magnetic nature [41]. Collectively, the observed FT-IR bands substantiate the successful synthesis and confirm the anticipated chemical structure and surface chemistry of the MNPs, which are essential for their effectiveness in environmental remediation and FO applications.
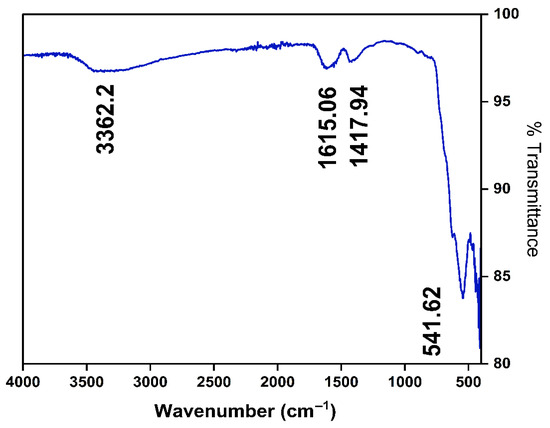
Figure 1.
FTIR spectra of MNP.
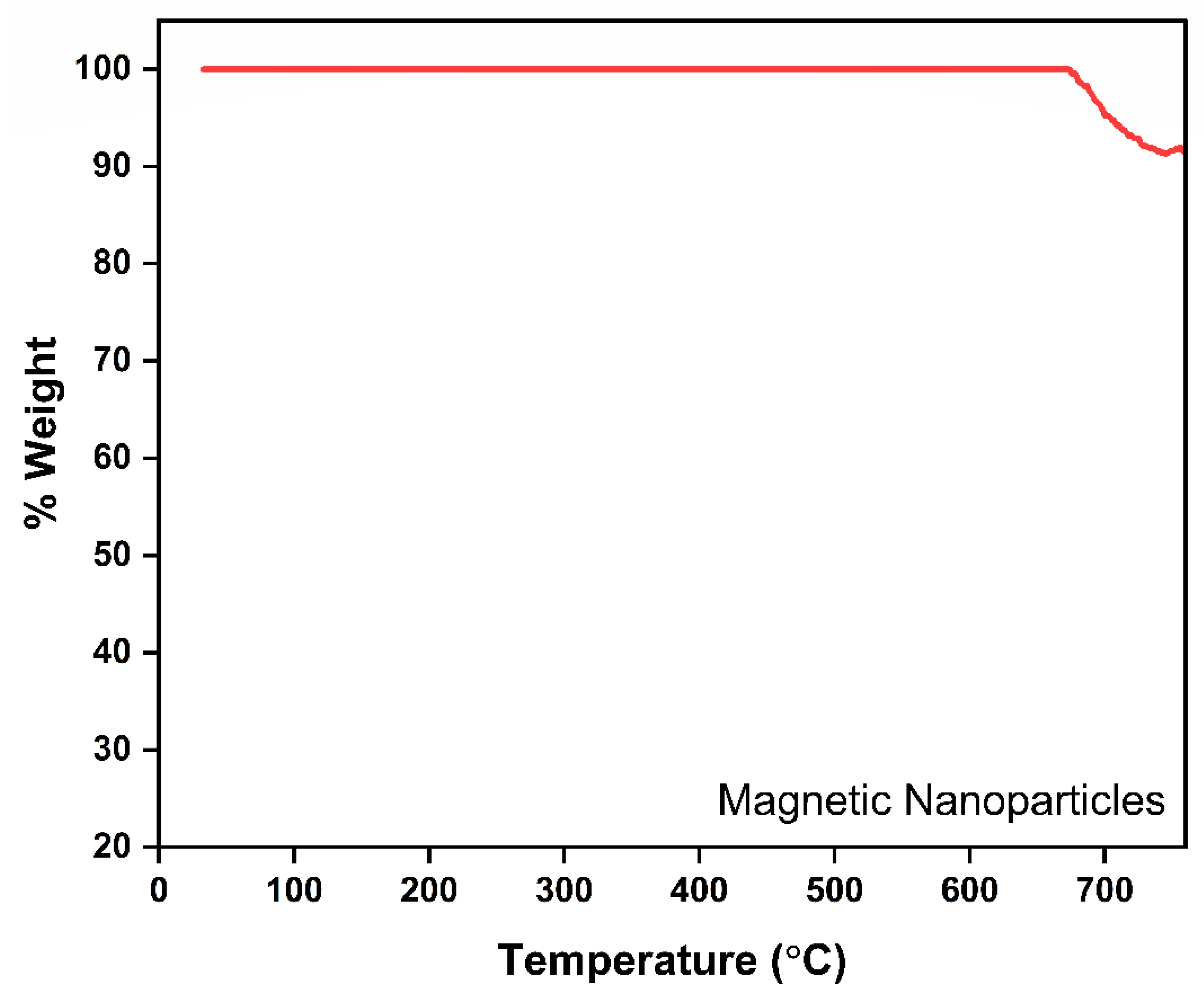
The presented thermogravimetric analysis (TGA) curve effectively demonstrates the thermal stability and compositional integrity of the synthesized MNPs (Figure 2). Initially, the weight loss remains relatively negligible across an extensive temperature range, from ambient conditions to approximately 650 °C, indicating higher thermal stability of MNPs. This negligible weight variation suggests a higher weight fraction of Fe3O4 than citric acid functionalized on the Fe3O4 surface. A distinct (~10%) weight loss observed beyond approximately 650 °C likely corresponds to the decomposition of Fe3O4 to hematic γ-Fe2O3 in the presence of atmospheric oxygen. However, the total weight reduction remains minimal, signifying the high stability and predominantly inorganic composition of the nanoparticles, specifically their iron oxide core. Overall, this TGA profile confirms the magnetic nanoparticles’ excellent thermal resilience, validating their suitability and robustness for high-temperature environmental remediation processes, such as adsorption treatments and FO applications. Differential Scanning Calorimetry (DSC) assessment was performed to examine the thermal characteristics of the MNPs (Figure S1).
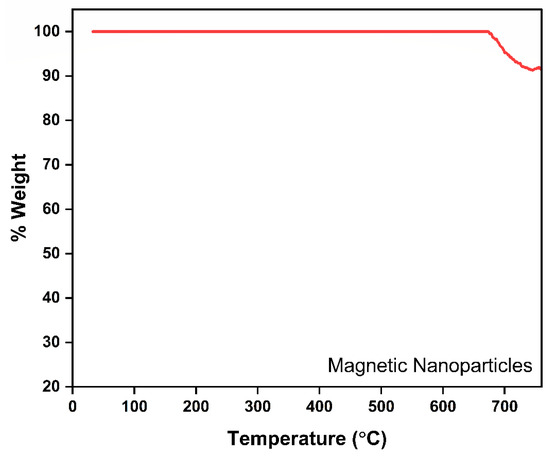
Figure 2.
TGA-DTG thermograph of MNP.
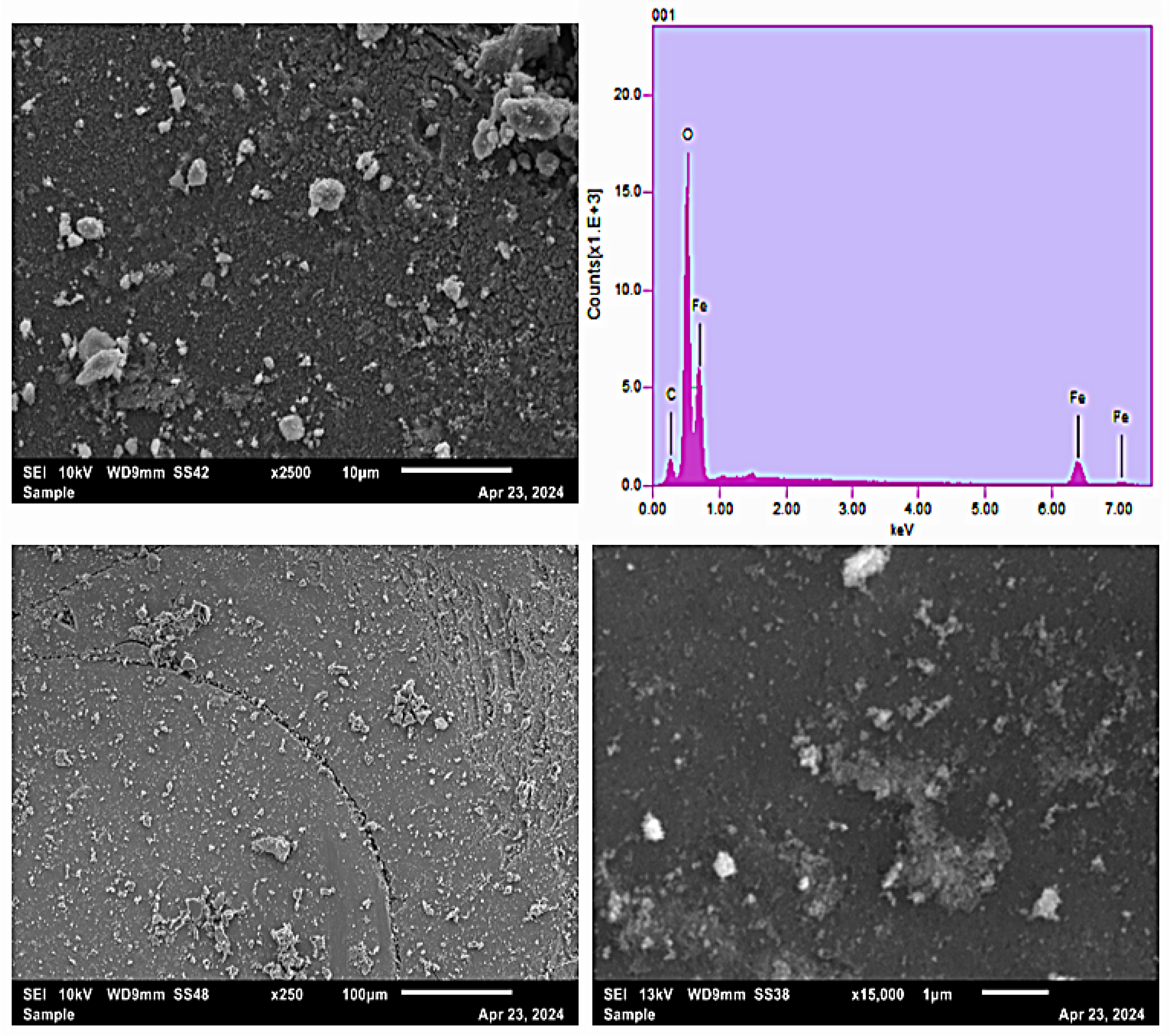
The provided scanning electron microscopy (SEM) images coupled with energy dispersive X-ray spectroscopy (EDS) analysis offer valuable insights into the morphological features and elemental composition of the synthesized MNPs (Figure 3). The SEM micrographs at various magnifications demonstrate that the nanoparticles exhibit irregular yet roughly spherical morphology with a certain degree of aggregation, typical of nanoparticles prepared via the co-precipitation method [44,45]. At higher magnification (15,000x), the particles reveal detailed nanostructures characterized by their sub-micron size, thus confirming their nanoscale dimensions [46]. The accompanying EDS spectrum confirms the primary elemental constituents as iron (Fe) and oxygen (O), indicative of iron oxide-based magnetic nanoparticles. Additionally, the presence of carbon (C) signals likely originates from surface functional groups or contamination associated with sample handling or preparation [47]. The distinct Fe peaks observed at energy levels around 6.4 keV and 7.1 keV strongly suggest the nanoparticles are predominantly composed of iron oxide phases, corroborating their magnetic character. This combined SEM-EDS investigation effectively validates the successful formation of iron oxide nanoparticles, affirming their morphology, nanoscale size distribution, and elemental integrity, making them suitable candidates for environmental applications such as pollutant removal through adsorption and FO processes.
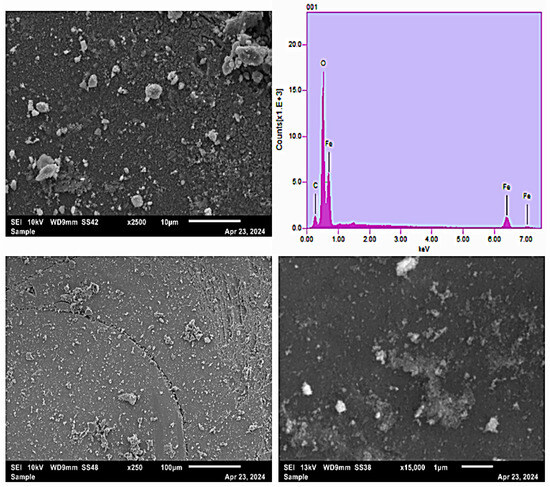
Figure 3.
SEM micrograph of MNP at different resolutions and corresponding EDS.
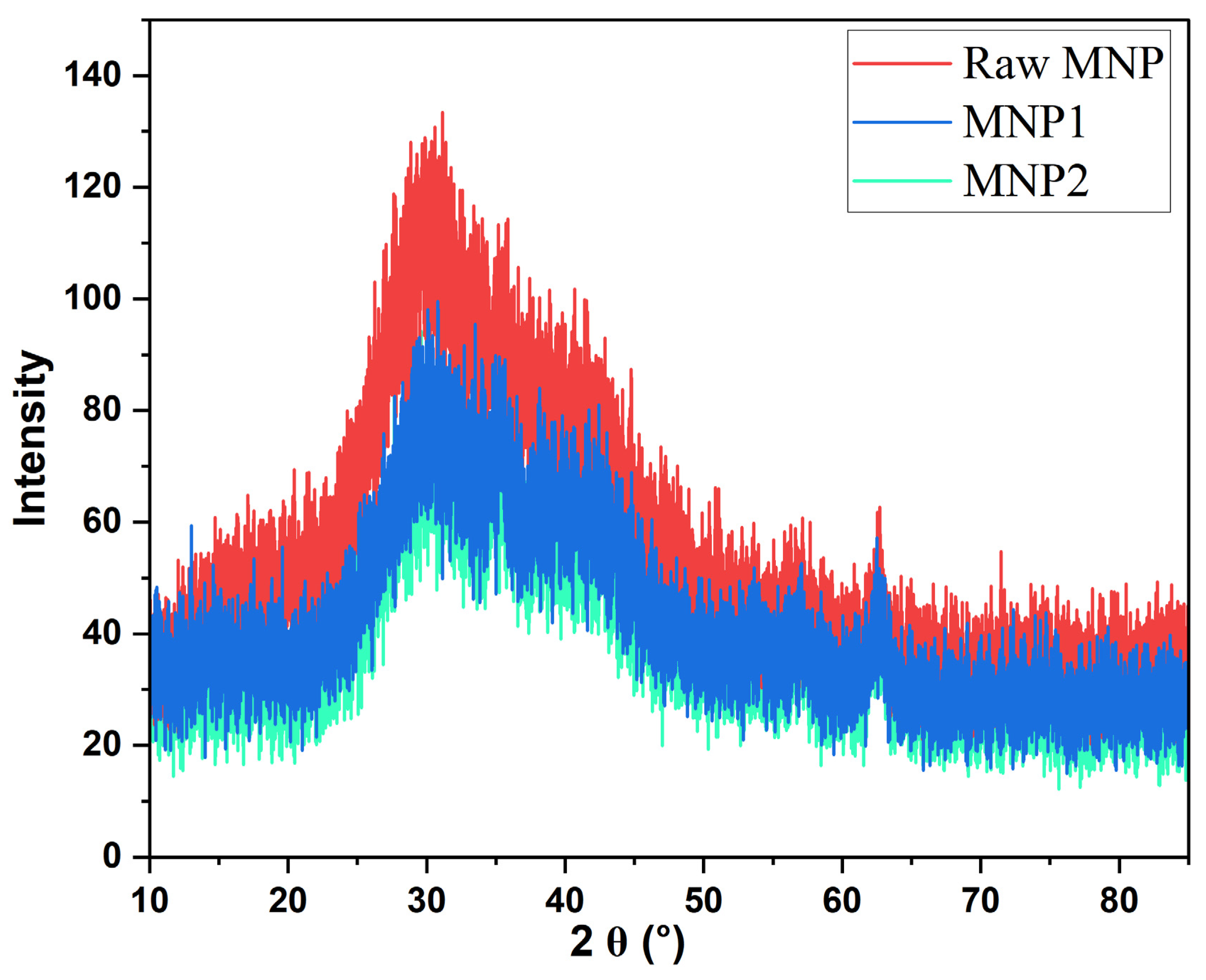
The X-ray diffraction (XRD) patterns of the MNPs showed clear crystalline structures throughout various regeneration cycles (Figure 4). The distinctive diffraction peaks of magnetite generally manifest at 2θ values around 30.1°, 35.5°, 43.1°, 53.4°, 57.0°, and 62.6°, aligning with the (220), (311), (400), (422), (511), and (440) crystallographic planes, respectively, as referenced to the standard JCPDS card No. 19-0629 [41]. The raw MNPs showed pronounced and, intense diffraction peaks aligned with the specific planes of magnetite (Fe3O4), signifying elevated crystallinity. During initial regeneration and reuse as a draw solute (MNP1), the XRD pattern displayed slight broadening and decreased peak intensity, indicating minor structural alterations resulting from the osmotic environment exposure. Following the second regeneration (MNP2), additional peak broadening and lower intensities were noted, suggesting a progressive decline in crystallinity and possible surface alteration or partial aggregation. These subtle alterations indicate that the structural integrity of MNPs is affected by their repeated application in FO cycles using 1 L of deionized water as the draw solution and synthetic produced water as the feed solution.
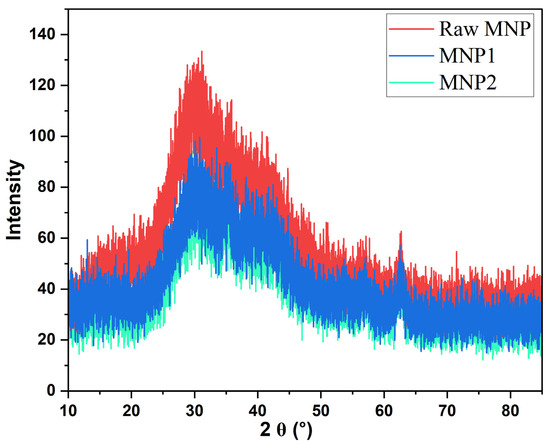
Figure 4.
The XRD patterns of raw MNP, MNP1, and MNP2.
3.2. Characterization of Pristine and SBMA Modified Membrane
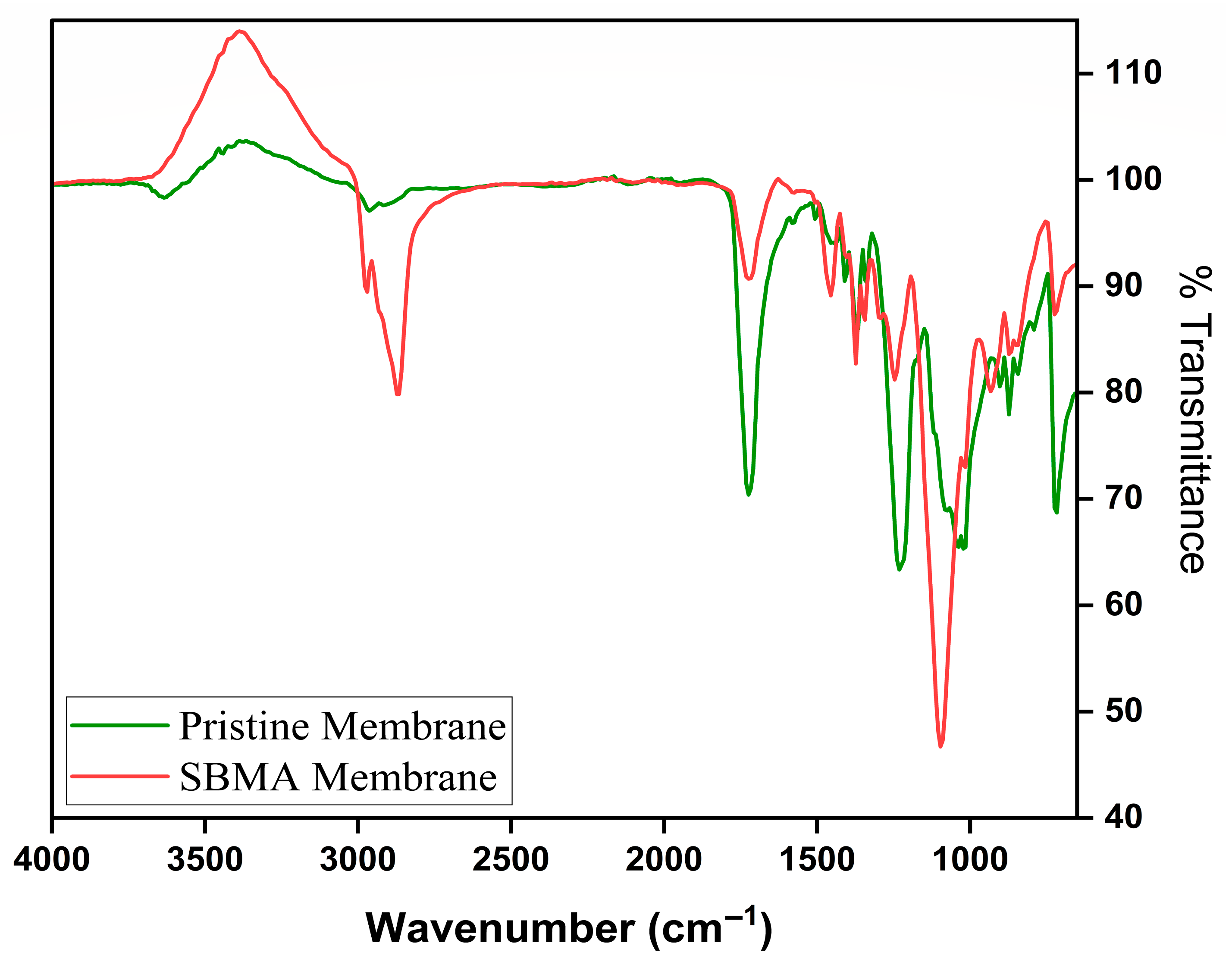
The FTIR spectra for the raw and SBMA-modified FO membranes clearly distinguish between functional group signatures, verifying successful surface modification. The FTIR spectrum of the raw flat sheet CTA FO membrane (Figure 5) shows distinctive absorption bands related to the cellulose triacetate structure. Significantly, intense peaks identified near ~1735 cm−1 align with the C=O stretching vibrations of ester functionalities in the triacetate portion. The widespread absorption from 3200 to 3500 cm−1 signifies O–H stretching, typical of hydroxyl groups, whereas the zone from 1050 to 1250 cm−1 shows C–O stretching associated with acetyl and ether bonds. In contrast, the SBMA-modified membrane (Figure 5) shows notable spectral alterations that suggest successful attachment of the zwitterionic polymer. A widened and more pronounced O–H/N–H stretching band between 3000 and 3400 cm−1 is observed, likely due to the addition of the sulfobetaine group. The appearance of new peaks near ~1180–1200 cm−1 is attributed to the symmetric and asymmetric stretching of SO3− groups, specific to SBMA, thereby verifying the presence of the sulfonate group. Additionally, the C–N+ stretching of quaternary ammonium groups is observed around ~950–1050 cm−1, indicating the development of the zwitterionic layer. These spectral characteristics provide strong evidence for the chemical attachment of SBMA to the membrane surface. The combination of sulfonate and quaternary ammonium groups improves hydrophilicity and resistance to fouling [48].
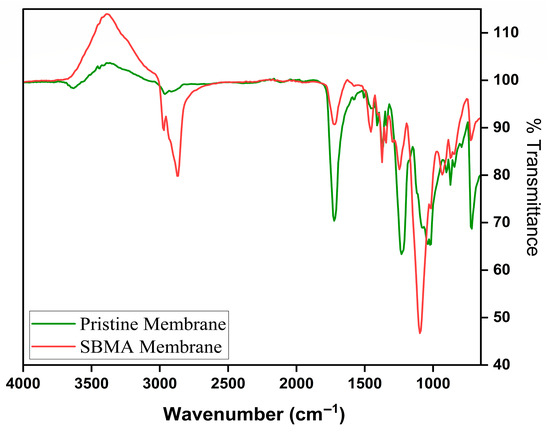
Figure 5.
FTIR spectra of Pristine membrane, and SBMA modified membrane.
The contact angle measurements showed a significant enhancement in the membrane’s surface hydrophilicity following SBMA modification. The unaltered CTA membrane showed a contact angle of 67.4°, signifying moderate hydrophilicity. After grafting with the zwitterionic monomer SBMA, the contact angle was reduced to 51.1°, indicating improved surface wettability. The decreased contact angle is due to the functional groups in SBMA, which interact with water molecules through electrostatic and hydrogen bonding, creating a hydration layer. The enhanced hydrophilicity is anticipated to positively impact water permeability and antifouling efficacy in FO uses [49].
3.3. Performance of Magnetic Particle as Draw Solute in Forward Osmosis
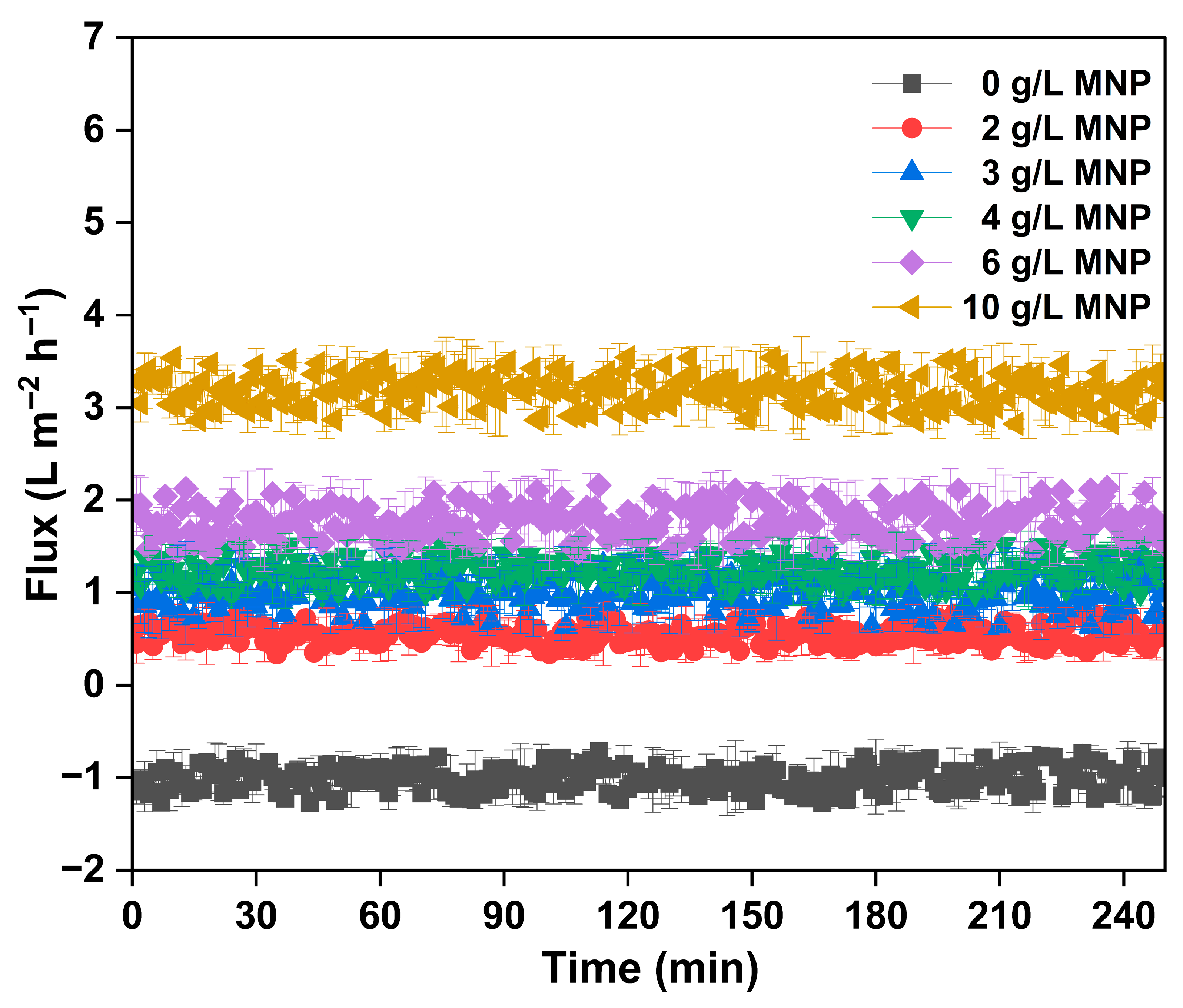
The flux performance of the FO system utilizing SBMA-modified cellulose triacetate (CTA) membranes was assessed with different concentrations of MNPs as draw solutes for the processing of synthetic produced water (Figure 6). Modifying the membrane surface with SBMA, a zwitterionic monomer, improved the hydrophilicity and antifouling traits of the CTA membrane, facilitating better water transport capabilities in high salinity environments. The flux-time profile shows that the control experiment with only deionized water (0 g/L MNP) demonstrated minimal or slightly negative flux, underscoring the lack of a significant osmotic gradient and possible membrane compaction or back-diffusion. In comparison, the addition of MNPs at concentrations between 2 g/L and 10 g/L significantly improved the osmotic driving force, leading to an increase in water flux. A distinct dose-dependent relationship was noted, where water flux consistently enhanced as MNP concentration increased. At 2 g/L MNP, the FO system demonstrated a modest flux (~1.5 L/m2·h), which steadily rose to ~3.5 L/m2·h at 6 g/L MNP. The most remarkable performance was observed at 10 g/L MNP, producing a consistent and elevated flux of around 5.5–6 L/m2·h during a 240 min operating period. This reliable performance highlights the capacity of MNPs to create a robust and lasting osmotic gradient while reducing reverse solute flow, probably because of their comparatively large molecular size and elevated osmotic activity. Additionally, the durability of the flux over time with minimal reduction indicates strong membrane resistance to fouling and deterioration, which is linked to the SBMA modification. These findings collectively reinforce the promise of MNPs as an advanced category of draw solutes and the success of zwitterionic membrane modification techniques in improving FO performance for difficult wastewater processes like produced water recovery. In this study, a concentration of 10 g/L was chosen based on initial flux performance tests and literature-supported ranges where MNPs have shown effective osmotic activity with minimal back-diffusion and aggregation [27,28]. This dosage resulted in a consistent, repeatable, and elevated water flux (~2.9–3.6 L/m2·h) throughout the 240 min testing period, suggesting a strong osmotic driving force and satisfactory colloidal dispersion. Additionally, employing a higher MNP concentration facilitated a clearer assessment of the synergistic effects between SBMA membrane modification and draw solute efficacy. This dosage also enabled the gathering of regeneration performance data throughout various cycles, confirming the practicality of magnetic recovery under realistic FO operating conditions. Subsequent research will focus on building upon these results through comprehensive optimization studies to better fine-tune the operational parameters for MNP dosage in intricate produced water matrices.
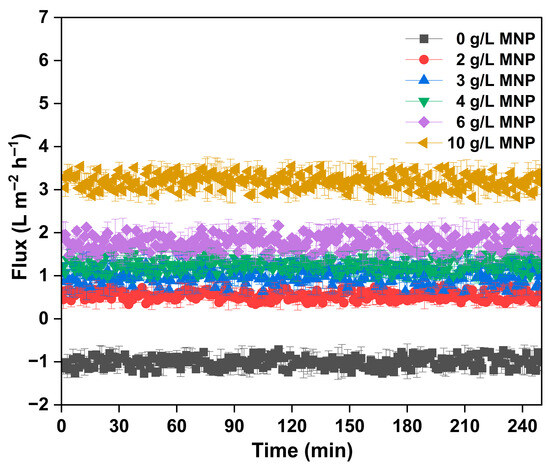
Figure 6.
The real-time flux graph of MNP as draw solute at different doses in treatment of produced water.
3.4. Performance of Regenerated Magnetic Nanoparticle as Draw Solute
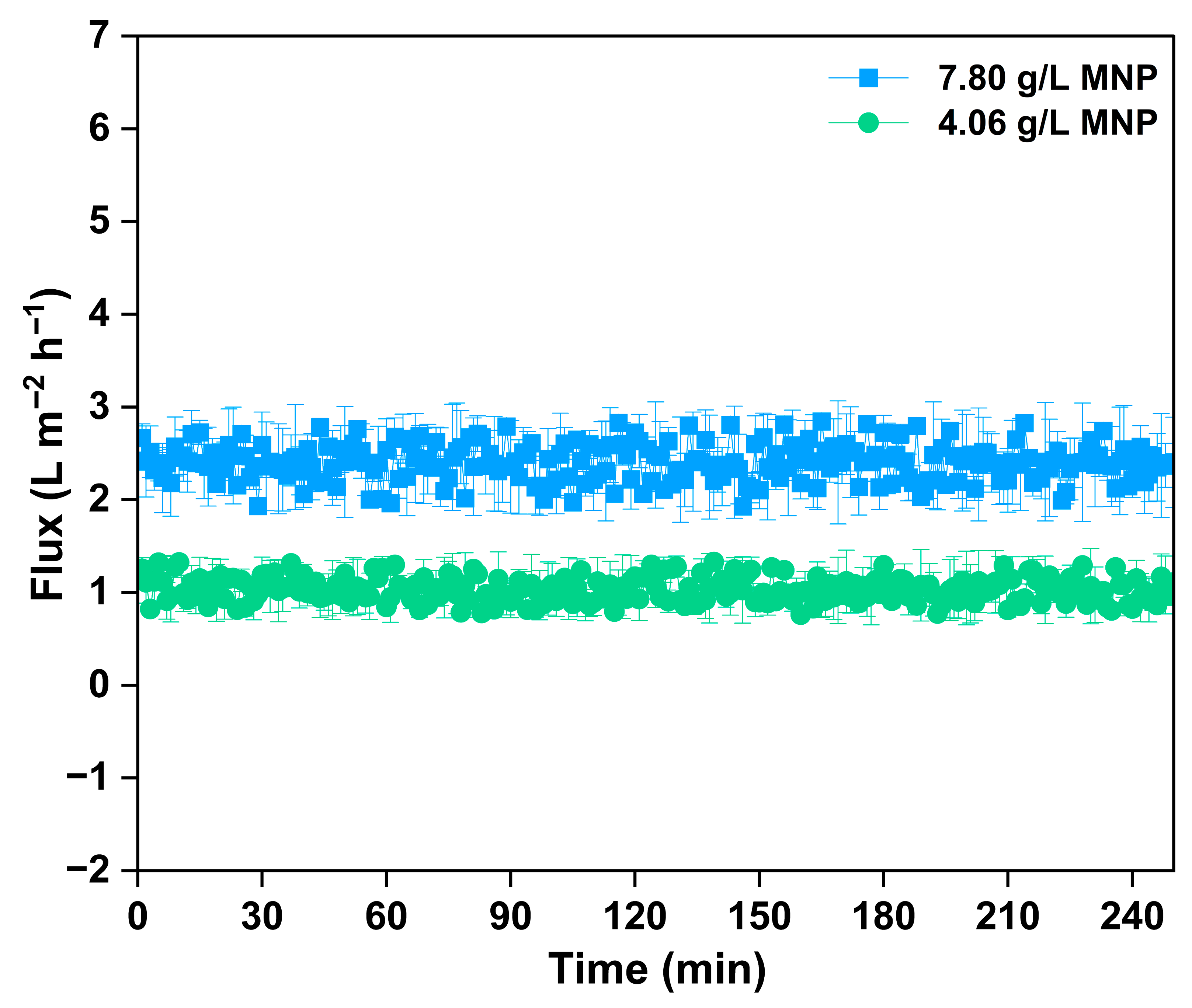
The flux efficiency of regenerated MNPs within an FO system was examined to assess the reusability and functional stability of the nanoparticles in treating produced water (Figure 7). The MNPs were regenerated through a magnetic separation method, which involved retrieving nanoparticles from the draw solution after the FO process by utilizing an external magnetic field. This efficient and non-invasive approach enabled the retrieval of MNPs with little structural change, confirming their suitability for several reuse cycles. During the initial regeneration cycle, 7.80 g/L of MNPs were effectively recovered and reutilized as the draw solute in later FO experiments. The water flux measured for the regenerated MNPs at this concentration aligned with the flux characteristics of newly prepared MNPs, remaining steady at approximately 2.9–3.6 L/m2·h over a period of 240 min. This demonstrates that the osmotic activity and colloidal stability of the MNPs stayed robust following the first separation and reuse, showing no noticeable aggregation or considerable decline in performance. After a second regeneration cycle, a lesser amount of 4.06 g/L of MNPs was obtained and utilized in the next FO run. As anticipated, the flux performance correspondingly diminished because of the reduced draw solute concentration, achieving about 2.0–2.5 L/m2·h. Even with the decrease in flux, the operation stayed stable during the 240 min duration, suggesting the enduring osmotic potential of the recycled MNPs. These results highlight the practical viability of magnetic separation as an efficient approach for recovering and reusing MNPs in FO systems. The capability to regenerate and reuse MNPs over several cycles with little reduction in flux enhances their feasibility as a sustainable and economical draw solute for long-term produced water treatment uses. This also aligns with circular resource use principles, boosting the ecological and financial attractiveness of magnetic nanomaterials in cutting-edge water recovery methods.
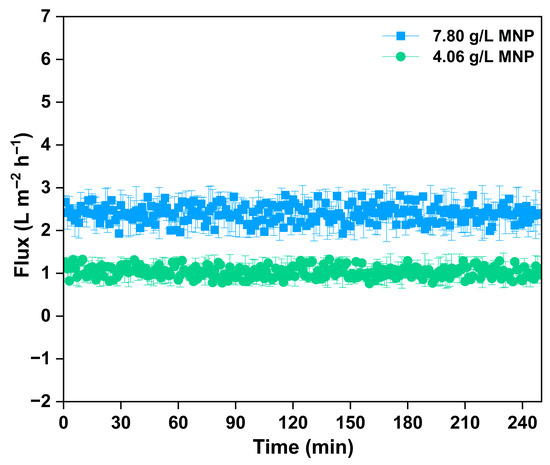
Figure 7.
The real-time flux graph of regenerated MNP as draw solute at two cycles in treatment of produced water.
3.5. Total Dissolved Solids
Table 1 depicts total dissolved solids results. The baseline sample (0 g/L MNP) exhibited a minimal TDS of 2.5 mg/L, validating the lack of background solutes in the system. As MNP concentrations increased, a steady increase in TDS values was noted, showing a direct relationship between MNP dosage and the ionic content, either dissolved or suspended, that remained in the draw solution post-FO. The peak TDS measurement of 227.1 mg/L was noted for the 10 g/L MNP solution, indicating the total influence of the ions linked to the nanoparticles or surfactant coatings that stayed suspended or dissolved post-filtration. Significantly, the TDS value for the regenerated MNP samples (7.8 and 4.06 g/L) was 206.4 and 144.8 mg/L, respectively (Table 2), which aligns closely with the fresh 4 g/L sample (151.6 mg/L), and indicating a negligible loss of osmotic strength during regeneration and reuse. These findings confirm the effectiveness of MNPs as draw solutes in FO and support their stability and partial solubility in aqueous environments, which are essential for ensuring steady osmotic performance across several cycles.

Table 1.
Total dissolved solids of magnetic nanoparticle draw solute after FO.

Table 2.
Total dissolved solids of regenerated magnetic nanoparticle draw solute after FO.
3.6. Mechanism of Magnetic Nanoparticle as a Draw Solute
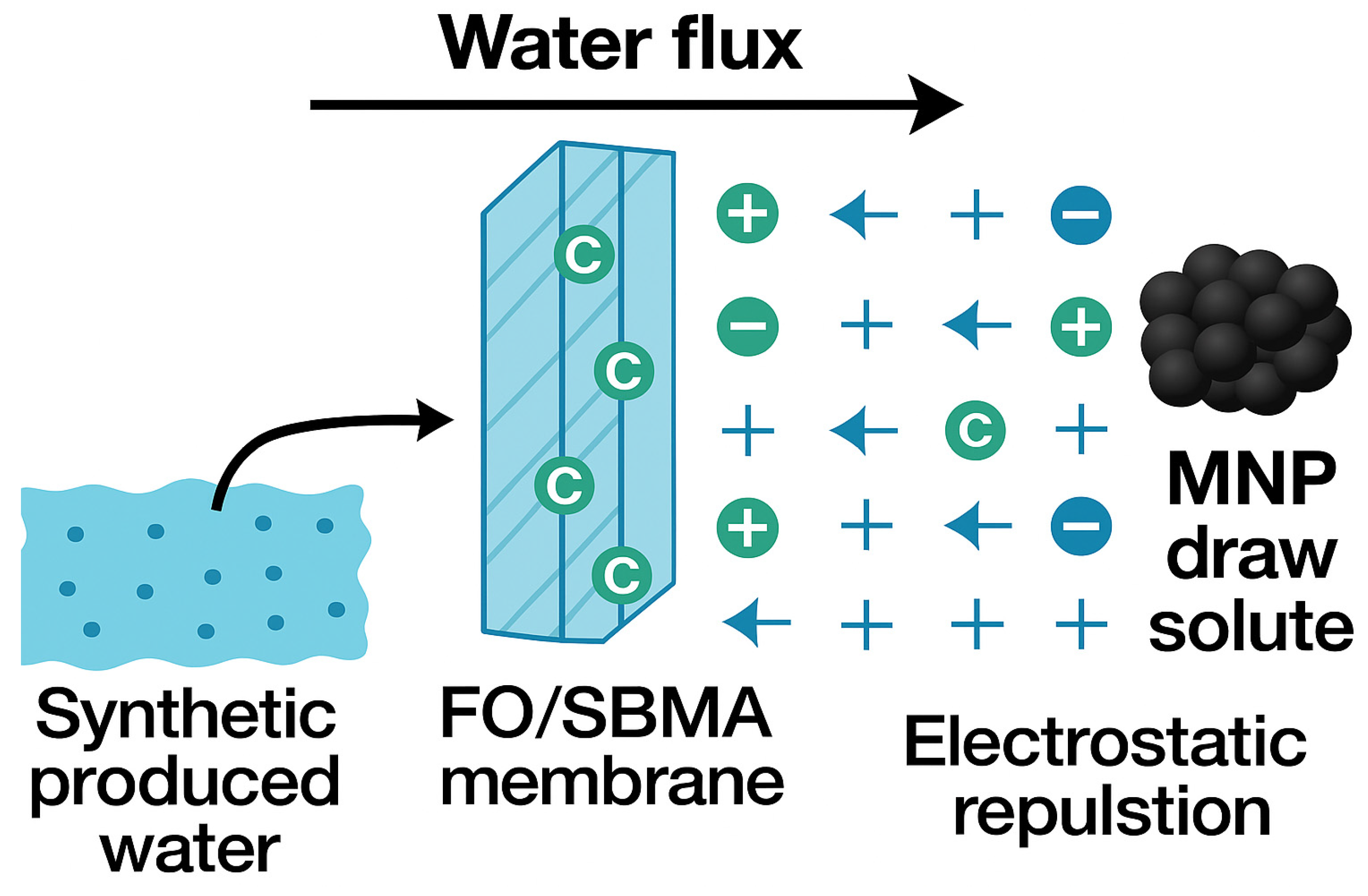
The way MNPs act as draw solutes in FO systems with SBMA-modified membranes is determined by their distinct physicochemical characteristics and interaction behaviors (Figure 8). Iron oxide-based MNPs, owing to their nanoscale dimensions and elevated surface-area-to-volume ratio, demonstrate notable osmotic activity when distributed in water. During the FO process, these particles create an osmotic pressure gradient that facilitates the movement of water from the low-salinity feed solution from synthetic produced water through the semi-permeable FO membrane into the draw solution containing MNPs. The FO membrane modified with SBMA significantly contributes to improving this process. The zwitterionic surface enhances hydrophilicity and resistance to fouling by creating a hydration layer that repels charged organic and inorganic substances found in the feed. This diminishes concentration polarization and enhances water permeability. As water passes through the membrane, MNPs stay trapped on the draw side because of their size and are later retrieved through external magnetic separation. This magnetic recoverability enables easy regeneration and reuse of the MNPs, rendering the process sustainable and energy-efficient. The combination of SBMA membrane modification and MNP-based draw solutes allows efficient and recyclable water extraction from difficult feedwaters such as produced water. Besides their osmotic properties, possible interactions between MNPs and contaminants in the feed solution deserve attention. The iron oxide nanoparticles modified with citric acid possess negatively charged carboxyl groups at neutral to alkaline pH, potentially promoting weak electrostatic interactions with divalent cations like Ca2+ and Mg2+, or with polar organic compounds that may be found in produced water. Nonetheless, as the MNPs remain on the draw side and do not directly interact with the feed solution through the semi-permeable membrane, minimal interactions are anticipated during the FO process. Additionally, the zwitterionic SBMA-altered membrane further reduces contaminant movement by creating a hydration barrier that deters charged and hydrophobic substances. Post-operational analysis (XRD) of MNPs after reuse cycles revealed no significant signs of contaminant accumulation or surface degradation, affirming the chemical stability and selective functionality of the MNPs over numerous FO runs. These results validate the enduring osmotic efficiency and ecological safety of MNP-based draw solutes in practical treatment scenarios.
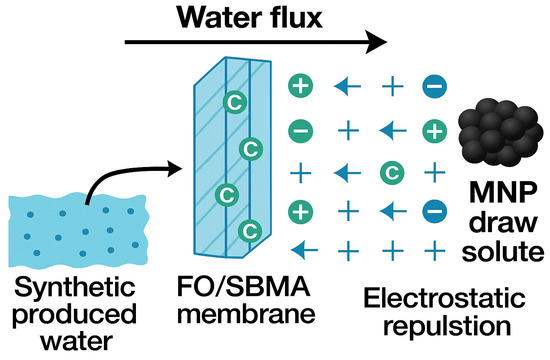
Figure 8.
Mechanism of magnetic nanoparticle as a draw solute for FO/SBMA membrane in synthetic produced water treatment.
4. Conclusions
This research confirms using iron oxide magnetic nanoparticles as innovative and reusable draw solutes in FO systems for treating synthetic produced water. The MNPs, created through co-precipitation, underwent extensive characterization employing X-ray diffraction (XRD), thermogravimetric analysis (TGA), differential scanning calorimetry (DSC), scanning electron microscopy (SEM), and Fourier-transform infrared spectroscopy (FTIR). The XRD analysis verified the crystalline spinel structure typical of Fe3O4, whereas DSC and TGA demonstrated their thermal stability and compositional purity qualities that endorse their strength under FO operating conditions. Applying a FO membrane modified with SBMA improved system efficiency even more. The zwitterionic characteristics of SBMA enhanced membrane hydrophilicity, decreased fouling, and alleviated internal concentration polarization, leading to increased and more consistent water flux when combined with MNP draw solutes. FO experiments in real-time showed a distinct relationship between MNP dosage and water flux, with the peak flux (around 2.9–3.6 L/m2·h) reached at a concentration of 10 g/L MNP. The magnetic regeneration of the MNPs proved effective, with the initial regeneration (7.8 g) sustaining a flux of approximately 2.8 L/m2·h, while the subsequent regeneration (4.06 g) reached around 1.5 L/m2·h showing the usability of MNPs with minor flux reduction. Furthermore, total dissolved solids (TDS) assessments post-FO treatment validated the role of MNPs in generating osmotic driving force, as elevated TDS values aligned with increased concentrations of MNPs. The regenerated MNPs exhibited similar TDS profiles, demonstrating consistent solute activity over cycles. Combining SBMA-modified FO membranes with magnetically recoverable MNP draw solutes provides an eco-friendly, low-energy, and recyclable approach for treating produced water. The thorough assessment and evaluation of performance highlight the practicality of this system for scalable, real-world applications in water reclamation and resource recovery.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/separations12080199/s1, Figure S1: DSC analysis of MNP.
Author Contributions
Conceptualization, R.R.K. and S.B.M.; methodology, S.B.M. and R.R.K.; data curation, S.B.M.; software, S.B.M.; validation, S.B.M.; resources, R.R.K.; writing—original draft preparation, S.B.M.; funding acquisition, R.R.K.; supervision, R.R.K.; reviewing and editing, S.B.M. and R.R.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Science Foundation (NSF) through the NSF Excellence in Research project (Award #1900787) and with partial support from the CREST Center for Energy and Environmental Sustainability-Phase II (CEES) (Award #1914692).
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors would like to acknowledge the support of Hongbo Du, formerly a research scientist in the Center for Energy and Environmental Sustainability at PVAMU, for support with earlier stages of the research project, Prashan M Rodrigo for reviewing the manuscript, and Gabriel M Morales for DSC analysis.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jiménez, S.; Micó, M.M.; Arnaldos, M.; Medina, F.; Contreras, S. State of the art of produced water treatment. Chemosphere 2018, 192, 186–208. [Google Scholar] [CrossRef]
- Amakiri, K.T.; Canon, A.R.; Molinari, M.; Angelis-Dimakis, A. Review of oilfield produced water treatment technologies. Chemosphere 2022, 298, 134064. [Google Scholar] [CrossRef] [PubMed]
- Olajire, A.A. Recent advances on the treatment technology of oil and gas produced water for sustainable energy industry-mechanistic aspects and process chemistry perspectives. Chem. Eng. J. Adv. 2020, 4, 100049. [Google Scholar] [CrossRef]
- Soni, S.; Jha, A.B.; Dubey, R.S.; Sharma, P. Nanowonders in agriculture: Unveiling the potential of nanoparticles to boost crop resilience to salinity stress. Sci. Total Environ. 2024, 925, 171433. [Google Scholar] [CrossRef] [PubMed]
- Green, A.; DeSutter, T.M.; Meehan, M.A.; Daigh, A.L.M.; O’Brien, P.L. Produced water’s impact on soil properties: Remediation challenges and opportunities. Agrosystems Geosci. Environ. 2020, 3, e20042. [Google Scholar] [CrossRef]
- Tornero, V.; Hanke, G. Chemical contaminants entering the marine environment from sea-based sources: A review with a focus on European seas. Mar. Pollut. Bull. 2016, 112, 17–38. [Google Scholar] [CrossRef]
- Honda, M.; Suzuki, N. Toxicities of Polycyclic Aromatic Hydrocarbons for Aquatic Animals. Int. J. Environ. Res. Public Health 2020, 17, 1363. [Google Scholar] [CrossRef]
- Akinsanya, B.; Ayanda, I.O.; Onwuka, B.; Saliu, J.K. Bioaccumulation of BTEX and PAHs in Heterotis niloticus (Actinopterygii) from the Epe Lagoon, Lagos, Nigeria. Heliyon 2020, 6, e03272. [Google Scholar] [CrossRef]
- Eldos, H.I.; Zouari, N.; Saeed, S.; Al-Ghouti, M.A. Recent advances in the treatment of PAHs in the environment: Application of nanomaterial-based technologies. Arab. J. Chem. 2022, 15, 103918. [Google Scholar] [CrossRef]
- Mangotra, A.; Singh, S.K. Volatile organic compounds: A threat to the environment and health hazards to living organisms—A review. J. Biotechnol. 2024, 382, 51–69. [Google Scholar] [CrossRef]
- Antia, M.; Ezejiofor, A.N.; Obasi, C.N.; Orisakwe, O.E. Environmental and public health effects of spent drilling fluid: An updated systematic review. J. Hazard. Mater. Adv. 2022, 7, 100120. [Google Scholar] [CrossRef]
- Madduri, S.B.; Kommalapati, R.R. Harnessing Novel Reduced Graphene Oxide-Based Aerogel for Efficient Organic Contaminant and Heavy Metal Removal in Aqueous Environments. Nanomaterials 2024, 14, 1708. [Google Scholar] [CrossRef]
- Madduri, S.; Elsayed, I.; Hassan, E.B. Novel oxone treated hydrochar for the removal of Pb(II) and methylene blue (MB) dye from aqueous solutions. Chemosphere 2020, 260, 127683. [Google Scholar] [CrossRef] [PubMed]
- Tong, T.; Elimelech, M. The Global Rise of Zero Liquid Discharge for Wastewater Management: Drivers, Technologies, and Future Directions. Environ. Sci. Technol. 2016, 50, 6846–6855. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, Y.; Lee, W. Zero-liquid discharge (ZLD) technology for resource recovery from wastewater: A review. Sci. Total Environ. 2019, 681, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Delanka-Pedige, H.M.K.; Zhang, Y.; Young, R.B.; Wang, H.; Hu, L.; Danforth, C.; Xu, P. Safe reuse of treated produced water outside oil and gas fields? A review of current practices, challenges, opportunities, and a risk-based pathway for produced water treatment and fit-for-purpose reuse. Curr. Opin. Chem. Eng. 2023, 42, 100973. [Google Scholar] [CrossRef]
- Hammond, G.P.; O’Grady, Á. Indicative energy technology assessment of UK shale gas extraction. Appl. Energy 2017, 185, 1907–1918. [Google Scholar] [CrossRef]
- Johnston, J.E.; Werder, E.; Sebastian, D. Wastewater Disposal Wells, Fracking, and Environmental Injustice in Southern Texas. Am. J. Public Health 2016, 106, 550–556. [Google Scholar] [CrossRef]
- Panagopoulos, A.; Michailidis, P. Membrane Technologies for Sustainable Wastewater Treatment: Advances, Challenges, and Applications in Zero Liquid Discharge (ZLD) and Minimal Liquid Discharge (MLD) Systems. Membranes 2025, 15, 64. [Google Scholar] [CrossRef]
- Hu, Y.; Cai, X.; Xue, Y.; Du, R.; Ji, J.; Chen, R.; Sano, D.; Li, Y.-Y. Recent developments of anaerobic membrane bioreactors for municipal wastewater treatment and bioenergy recovery: Focusing on novel configurations and energy balance analysis. J. Clean. Prod. 2022, 356, 131856. [Google Scholar] [CrossRef]
- Mohammadifakhr, M.; de Grooth, J.; Roesink, H.D.W.; Kemperman, A.J.B. Forward Osmosis: A Critical Review. Processes 2020, 8, 404. [Google Scholar] [CrossRef]
- Chung, T.-S.; Luo, L.; Wan, C.F.; Cui, Y.; Amy, G. What is next for forward osmosis (FO) and pressure retarded osmosis (PRO). Sep. Purif. Technol. 2015, 156, 856–860. [Google Scholar] [CrossRef]
- Alsvik, I.L.; Hägg, M.-B. Pressure Retarded Osmosis and Forward Osmosis Membranes: Materials and Methods. Polymers 2013, 5, 303–327. [Google Scholar] [CrossRef]
- Abounahia, N.; Ibrar, I.; Kazwini, T.; Altaee, A.; Samal, A.K.; Zaidi, S.J.; Hawari, A.H. Desalination by the forward osmosis: Advancement and challenges. Sci. Total Environ. 2023, 886, 163901. [Google Scholar] [CrossRef] [PubMed]
- Feria-Díaz, J.J.; Correa-Mahecha, F.; López-Méndez, M.C.; Rodríguez-Miranda, J.P.; Barrera-Rojas, J. Recent Desalination Technologies by Hybridization and Integration with Reverse Osmosis: A Review. Water 2021, 13, 1369. [Google Scholar] [CrossRef]
- Sun, M.; Wang, X.; Winter, L.R.; Zhao, Y.; Ma, W.; Hedtke, T.; Kim, J.-H.; Elimelech, M. Electrified Membranes for Water Treatment Applications. ACS EST Eng. 2021, 1, 725–752. [Google Scholar] [CrossRef]
- Hafiz, M.; Hassanein, A.; Talhami, M.; Al-Ejji, M.; Hassan, M.K.; Hawari, A.H. Magnetic nanoparticles draw solution for forward osmosis: Current status and future challenges in wastewater treatment. J. Environ. Chem. Eng. 2022, 10, 108955. [Google Scholar] [CrossRef]
- Hafiz, M.; Talhami, M.; Ba-Abbad, M.M.; Hawari, A.H. Optimization of Magnetic Nanoparticles Draw Solution for High Water Flux in Forward Osmosis. Water 2021, 13, 3653. [Google Scholar] [CrossRef]
- Yavuz, C.T.; Mayo, J.T.; Yu, W.W.; Prakash, A.; Falkner, J.C.; Yean, S.; Cong, L.; Shipley, H.J.; Kan, A.; Tomson, M.; et al. Low-Field Magnetic Separation of Monodisperse Fe3O4 Nanocrystals. Science 2006, 314, 964–967. [Google Scholar] [CrossRef]
- Medved, I.; Černý, R. Osmosis in porous media: A review of recent studies. Microporous Mesoporous Mater. 2013, 170, 299–317. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Shaterabadi, Z.; Nabiyouni, G.; Soleymani, M. Correlation between effects of the particle size and magnetic field strength on the magnetic hyperthermia efficiency of dextran-coated magnetite nanoparticles. Mater. Sci. Eng. C 2020, 117, 111274. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Dong, Y.; Yu, C.; Liu, Y.; Teng, X. A novel Nafion-g-PSBMA membrane prepared by grafting zwitterionic SBMA onto Nafion via SI-ATRP for vanadium redox flow battery application. J. Membr. Sci. 2018, 554, 324–330. [Google Scholar] [CrossRef]
- Hartanto, Y.; Corvilain, M.; Mariën, H.; Janssen, J.; Vankelecom, I.F.J. Interfacial polymerization of thin-film composite forward osmosis membranes using ionic liquids as organic reagent phase. J. Membr. Sci. 2020, 601, 117869. [Google Scholar] [CrossRef]
- Kim, I.; Kang, S.M. Formation of Amphiphilic Zwitterionic Thin Poly(SBMA-co-TFEMA) Brushes on Solid Surfaces for Marine Antifouling Applications. Langmuir 2024, 40, 3213–3221. [Google Scholar] [CrossRef]
- Farahbakhsh, J.; Golgoli, M.; Khiadani, M.; Najafi, M.; Suwaileh, W.; Razmjou, A.; Zargar, M. Recent advances in surface tailoring of thin film forward osmosis membranes: A review. Chemosphere 2024, 346, 140493. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, R.; Liu, K.; Zhang, Y.; Shi, X.; Sand, W.; Hou, B. Application of nanomaterials in antifouling: A review. Nano Mater. Sci. 2024, 6, 672–700. [Google Scholar] [CrossRef]
- Dey, P.; Izake, E.L. Magnetic nanoparticles boosting the osmotic efficiency of a polymeric FO draw agent: Effect of polymer conformation. Desalination 2015, 373, 79–85. [Google Scholar] [CrossRef]
- Gong, Y.; Fan, M.; Gao, F.; Hong, J.; Liu, S.; Luo, S.; Yu, J.; Huang, J. Preparation and characterization of amino-functionalized magnetic nanogels via photopolymerization for MRI applications. Colloids Surf. B Biointerfaces 2009, 71, 243–247. [Google Scholar] [CrossRef]
- Deng, S.; Bai, R.; Chen, J.P.; Jiang, Z.; Yu, G.; Zhou, F.; Chen, Z. Produced water from polymer flooding process in crude oil extraction: Characterization and treatment by a novel crossflow oil–water separator. Sep. Purif. Technol. 2002, 29, 207–216. [Google Scholar] [CrossRef]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic Iron Oxide Nanoparticles: Synthesis, Stabilization, Vectorization, Physicochemical Characterizations, and Biological Applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Khan, R.; Daverey, A. Synthesis and characterization of magnetic nanoparticles, and their applications in wastewater treatment: A review. Environ. Technol. Innov. 2021, 24, 101924. [Google Scholar] [CrossRef]
- Xu, P.; Zeng, G.M.; Huang, D.L.; Feng, C.L.; Hu, S.; Zhao, M.H.; Lai, C.; Wei, Z.; Huang, C.; Xie, G.X.; et al. Use of iron oxide nanomaterials in wastewater treatment: A review. Sci. Total Environ. 2012, 424, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; He, Q.; Jiang, C. Magnetic Iron Oxide Nanoparticles: Synthesis and Surface Functionalization Strategies. Nanoscale Res. Lett. 2008, 3, 397. [Google Scholar] [CrossRef]
- Laurent, S.; Dutz, S.; Häfeli, U.O.; Mahmoudi, M. Magnetic fluid hyperthermia: Focus on superparamagnetic iron oxide nanoparticles. Adv. Colloid Interface Sci. 2011, 166, 8–23. [Google Scholar] [CrossRef]
- Lu, A.-H.; Salabas, E.L.; Schüth, F. Magnetic Nanoparticles: Synthesis, Protection, Functionalization, and Application. Angew. Chem. Int. Ed. 2007, 46, 1222–1244. [Google Scholar] [CrossRef]
- Reddy, D.H.K.; Yun, Y.-S. Spinel ferrite magnetic adsorbents: Alternative future materials for water purification? Coord. Chem. Rev. 2016, 315, 90–111. [Google Scholar] [CrossRef]
- Chen, S.; Li, L.; Zhao, C.; Zheng, J. Surface hydration: Principles and applications toward low-fouling/nonfouling biomaterials. Polymer 2010, 51, 5283–5293. [Google Scholar] [CrossRef]
- Han, L.; Tan, Y.Z.; Xu, C.; Xiao, T.; Trinh, T.A.; Chew, J.W. Zwitterionic grafting of sulfobetaine methacrylate (SBMA) on hydrophobic PVDF membranes for enhanced anti-fouling and anti-wetting in the membrane distillation of oil emulsions. J. Membr. Sci. 2019, 588, 117196. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).