Functionalized Magnetic Nanoparticles as Recyclable Draw Solutes for Forward Osmosis: A Sustainable Approach to Produced Water Reclamation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Magnetic Nanoparticles
2.3. Surface Modification of Forward Osmosis Membrane
2.4. Preparation of Synthetic Produced Water
2.5. Forward Osmosis Experiments
2.6. Characterization
3. Results and Discussion
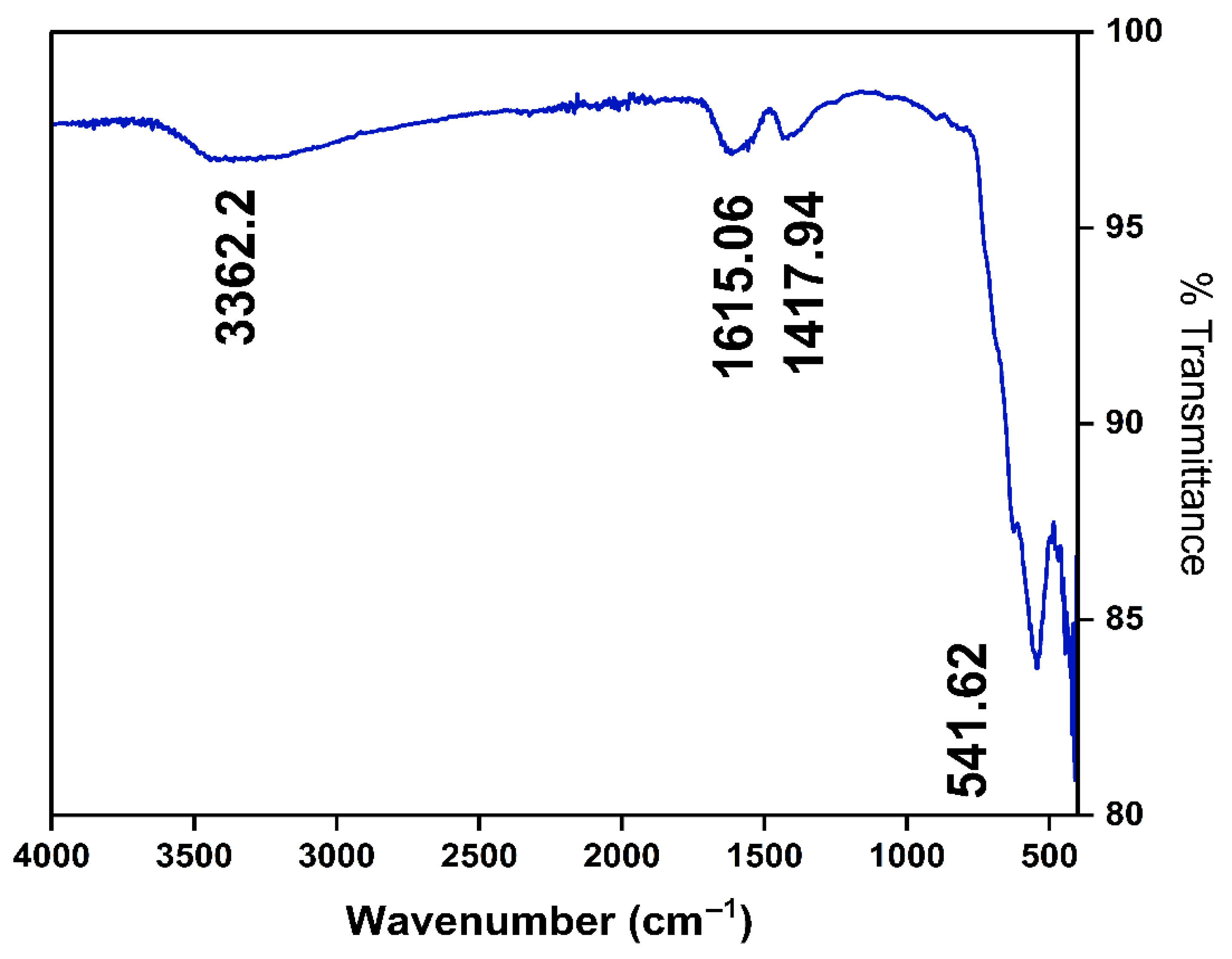
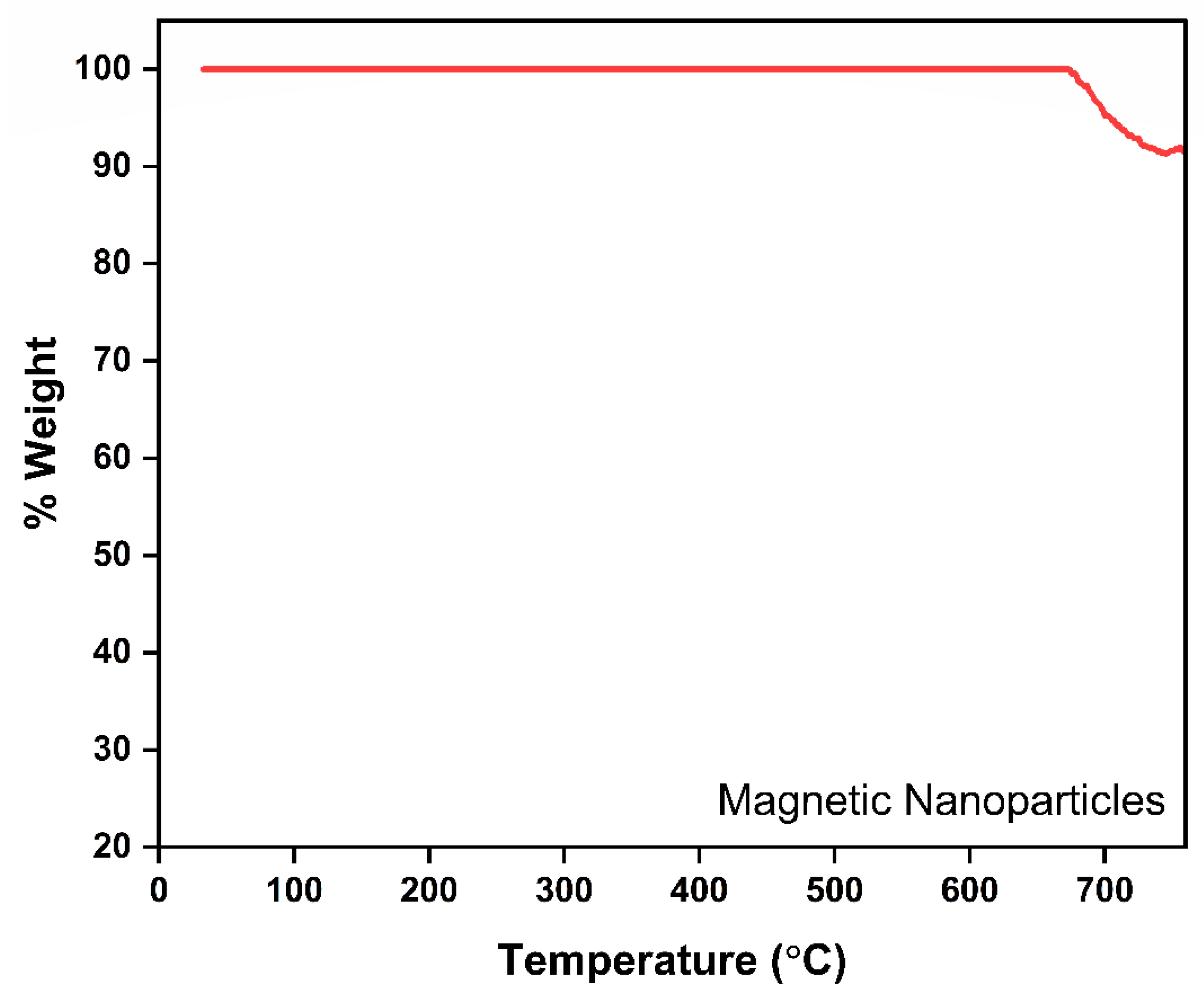
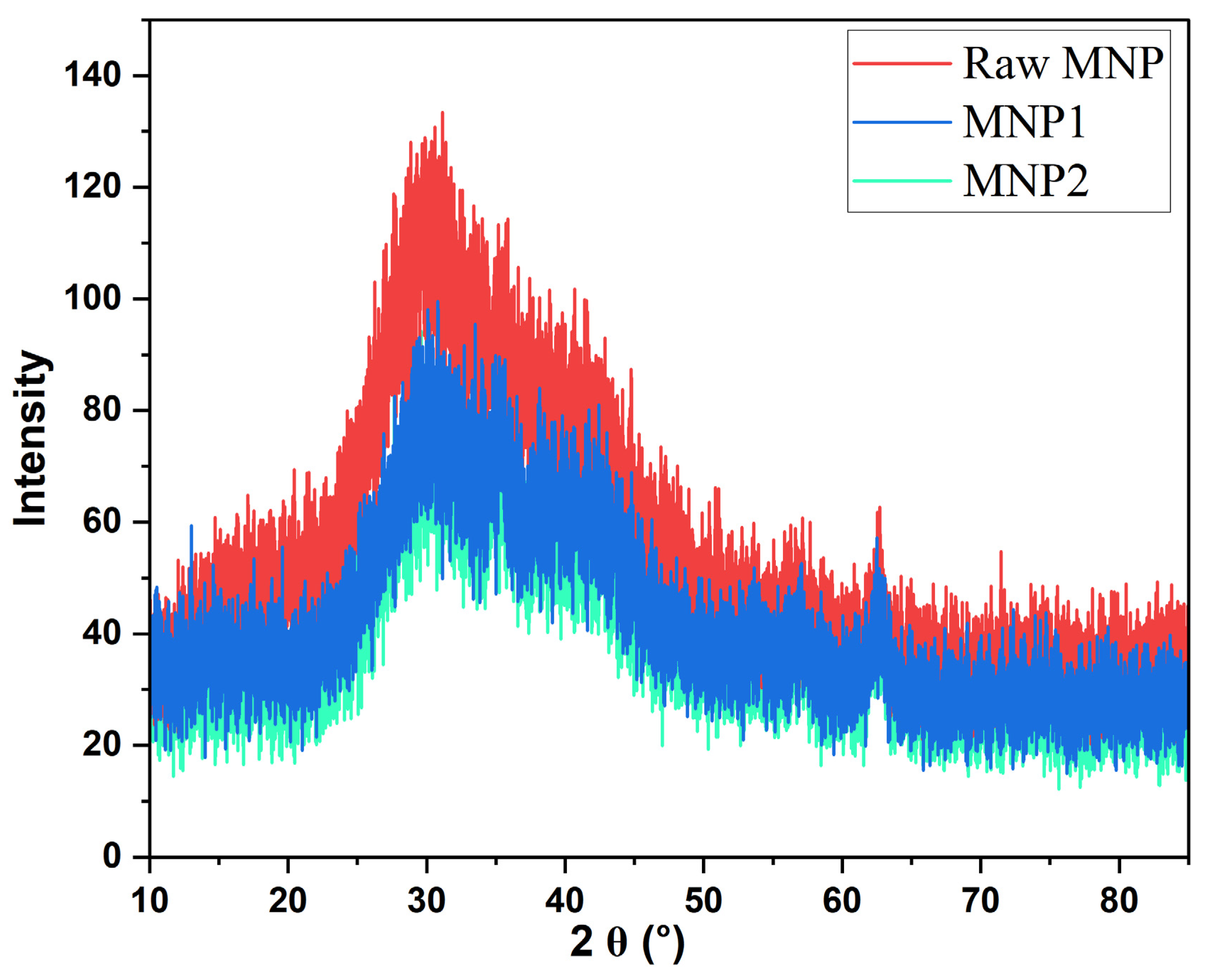
3.1. Characterization of Magnetic Nanoparticle
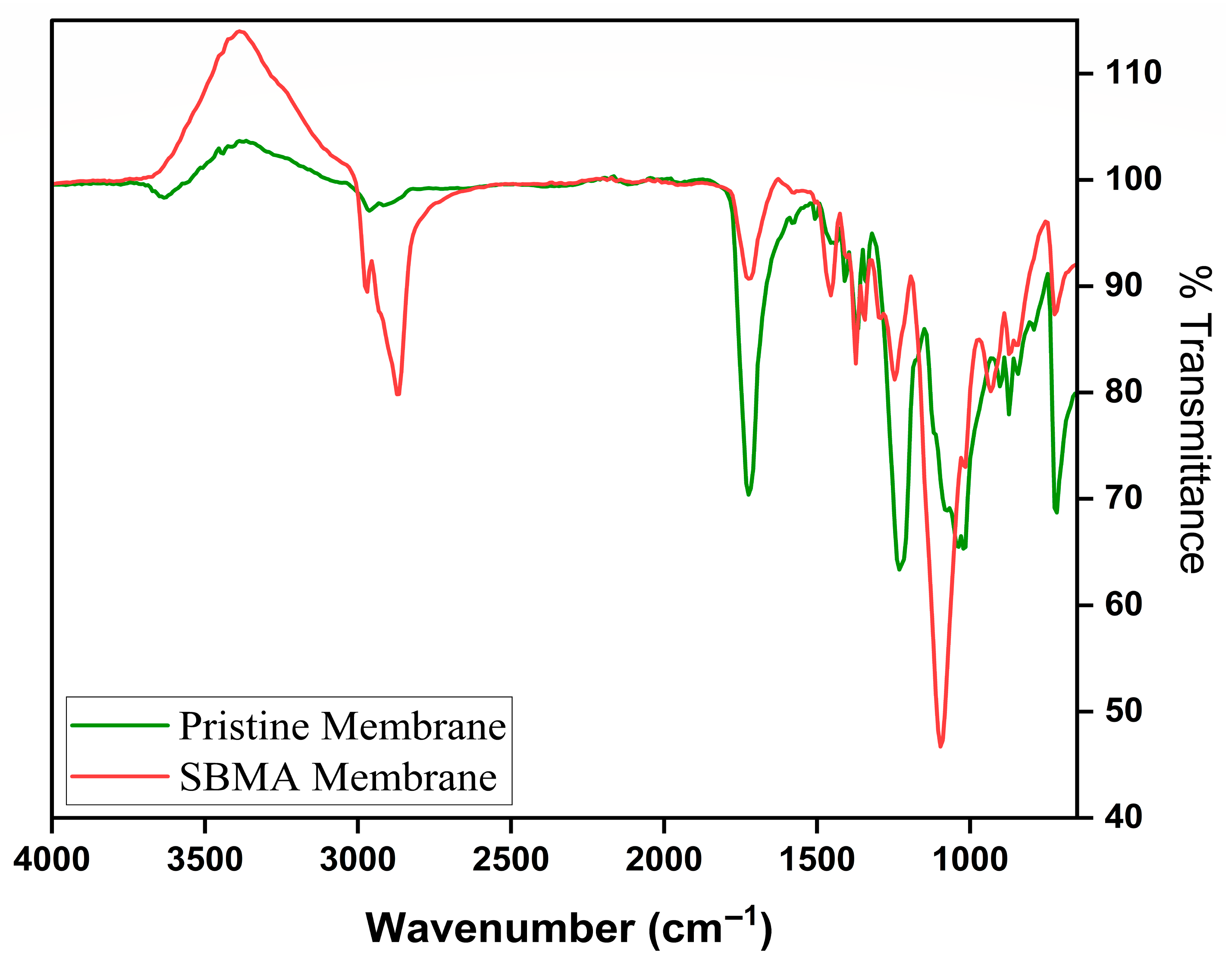
3.2. Characterization of Pristine and SBMA Modified Membrane
3.3. Performance of Magnetic Particle as Draw Solute in Forward Osmosis
3.4. Performance of Regenerated Magnetic Nanoparticle as Draw Solute
3.5. Total Dissolved Solids
3.6. Mechanism of Magnetic Nanoparticle as a Draw Solute
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiménez, S.; Micó, M.M.; Arnaldos, M.; Medina, F.; Contreras, S. State of the art of produced water treatment. Chemosphere 2018, 192, 186–208. [Google Scholar] [CrossRef]
- Amakiri, K.T.; Canon, A.R.; Molinari, M.; Angelis-Dimakis, A. Review of oilfield produced water treatment technologies. Chemosphere 2022, 298, 134064. [Google Scholar] [CrossRef] [PubMed]
- Olajire, A.A. Recent advances on the treatment technology of oil and gas produced water for sustainable energy industry-mechanistic aspects and process chemistry perspectives. Chem. Eng. J. Adv. 2020, 4, 100049. [Google Scholar] [CrossRef]
- Soni, S.; Jha, A.B.; Dubey, R.S.; Sharma, P. Nanowonders in agriculture: Unveiling the potential of nanoparticles to boost crop resilience to salinity stress. Sci. Total Environ. 2024, 925, 171433. [Google Scholar] [CrossRef] [PubMed]
- Green, A.; DeSutter, T.M.; Meehan, M.A.; Daigh, A.L.M.; O’Brien, P.L. Produced water’s impact on soil properties: Remediation challenges and opportunities. Agrosystems Geosci. Environ. 2020, 3, e20042. [Google Scholar] [CrossRef]
- Tornero, V.; Hanke, G. Chemical contaminants entering the marine environment from sea-based sources: A review with a focus on European seas. Mar. Pollut. Bull. 2016, 112, 17–38. [Google Scholar] [CrossRef]
- Honda, M.; Suzuki, N. Toxicities of Polycyclic Aromatic Hydrocarbons for Aquatic Animals. Int. J. Environ. Res. Public Health 2020, 17, 1363. [Google Scholar] [CrossRef]
- Akinsanya, B.; Ayanda, I.O.; Onwuka, B.; Saliu, J.K. Bioaccumulation of BTEX and PAHs in Heterotis niloticus (Actinopterygii) from the Epe Lagoon, Lagos, Nigeria. Heliyon 2020, 6, e03272. [Google Scholar] [CrossRef]
- Eldos, H.I.; Zouari, N.; Saeed, S.; Al-Ghouti, M.A. Recent advances in the treatment of PAHs in the environment: Application of nanomaterial-based technologies. Arab. J. Chem. 2022, 15, 103918. [Google Scholar] [CrossRef]
- Mangotra, A.; Singh, S.K. Volatile organic compounds: A threat to the environment and health hazards to living organisms—A review. J. Biotechnol. 2024, 382, 51–69. [Google Scholar] [CrossRef]
- Antia, M.; Ezejiofor, A.N.; Obasi, C.N.; Orisakwe, O.E. Environmental and public health effects of spent drilling fluid: An updated systematic review. J. Hazard. Mater. Adv. 2022, 7, 100120. [Google Scholar] [CrossRef]
- Madduri, S.B.; Kommalapati, R.R. Harnessing Novel Reduced Graphene Oxide-Based Aerogel for Efficient Organic Contaminant and Heavy Metal Removal in Aqueous Environments. Nanomaterials 2024, 14, 1708. [Google Scholar] [CrossRef]
- Madduri, S.; Elsayed, I.; Hassan, E.B. Novel oxone treated hydrochar for the removal of Pb(II) and methylene blue (MB) dye from aqueous solutions. Chemosphere 2020, 260, 127683. [Google Scholar] [CrossRef] [PubMed]
- Tong, T.; Elimelech, M. The Global Rise of Zero Liquid Discharge for Wastewater Management: Drivers, Technologies, and Future Directions. Environ. Sci. Technol. 2016, 50, 6846–6855. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, Y.; Lee, W. Zero-liquid discharge (ZLD) technology for resource recovery from wastewater: A review. Sci. Total Environ. 2019, 681, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Delanka-Pedige, H.M.K.; Zhang, Y.; Young, R.B.; Wang, H.; Hu, L.; Danforth, C.; Xu, P. Safe reuse of treated produced water outside oil and gas fields? A review of current practices, challenges, opportunities, and a risk-based pathway for produced water treatment and fit-for-purpose reuse. Curr. Opin. Chem. Eng. 2023, 42, 100973. [Google Scholar] [CrossRef]
- Hammond, G.P.; O’Grady, Á. Indicative energy technology assessment of UK shale gas extraction. Appl. Energy 2017, 185, 1907–1918. [Google Scholar] [CrossRef]
- Johnston, J.E.; Werder, E.; Sebastian, D. Wastewater Disposal Wells, Fracking, and Environmental Injustice in Southern Texas. Am. J. Public Health 2016, 106, 550–556. [Google Scholar] [CrossRef]
- Panagopoulos, A.; Michailidis, P. Membrane Technologies for Sustainable Wastewater Treatment: Advances, Challenges, and Applications in Zero Liquid Discharge (ZLD) and Minimal Liquid Discharge (MLD) Systems. Membranes 2025, 15, 64. [Google Scholar] [CrossRef]
- Hu, Y.; Cai, X.; Xue, Y.; Du, R.; Ji, J.; Chen, R.; Sano, D.; Li, Y.-Y. Recent developments of anaerobic membrane bioreactors for municipal wastewater treatment and bioenergy recovery: Focusing on novel configurations and energy balance analysis. J. Clean. Prod. 2022, 356, 131856. [Google Scholar] [CrossRef]
- Mohammadifakhr, M.; de Grooth, J.; Roesink, H.D.W.; Kemperman, A.J.B. Forward Osmosis: A Critical Review. Processes 2020, 8, 404. [Google Scholar] [CrossRef]
- Chung, T.-S.; Luo, L.; Wan, C.F.; Cui, Y.; Amy, G. What is next for forward osmosis (FO) and pressure retarded osmosis (PRO). Sep. Purif. Technol. 2015, 156, 856–860. [Google Scholar] [CrossRef]
- Alsvik, I.L.; Hägg, M.-B. Pressure Retarded Osmosis and Forward Osmosis Membranes: Materials and Methods. Polymers 2013, 5, 303–327. [Google Scholar] [CrossRef]
- Abounahia, N.; Ibrar, I.; Kazwini, T.; Altaee, A.; Samal, A.K.; Zaidi, S.J.; Hawari, A.H. Desalination by the forward osmosis: Advancement and challenges. Sci. Total Environ. 2023, 886, 163901. [Google Scholar] [CrossRef] [PubMed]
- Feria-Díaz, J.J.; Correa-Mahecha, F.; López-Méndez, M.C.; Rodríguez-Miranda, J.P.; Barrera-Rojas, J. Recent Desalination Technologies by Hybridization and Integration with Reverse Osmosis: A Review. Water 2021, 13, 1369. [Google Scholar] [CrossRef]
- Sun, M.; Wang, X.; Winter, L.R.; Zhao, Y.; Ma, W.; Hedtke, T.; Kim, J.-H.; Elimelech, M. Electrified Membranes for Water Treatment Applications. ACS EST Eng. 2021, 1, 725–752. [Google Scholar] [CrossRef]
- Hafiz, M.; Hassanein, A.; Talhami, M.; Al-Ejji, M.; Hassan, M.K.; Hawari, A.H. Magnetic nanoparticles draw solution for forward osmosis: Current status and future challenges in wastewater treatment. J. Environ. Chem. Eng. 2022, 10, 108955. [Google Scholar] [CrossRef]
- Hafiz, M.; Talhami, M.; Ba-Abbad, M.M.; Hawari, A.H. Optimization of Magnetic Nanoparticles Draw Solution for High Water Flux in Forward Osmosis. Water 2021, 13, 3653. [Google Scholar] [CrossRef]
- Yavuz, C.T.; Mayo, J.T.; Yu, W.W.; Prakash, A.; Falkner, J.C.; Yean, S.; Cong, L.; Shipley, H.J.; Kan, A.; Tomson, M.; et al. Low-Field Magnetic Separation of Monodisperse Fe3O4 Nanocrystals. Science 2006, 314, 964–967. [Google Scholar] [CrossRef]
- Medved, I.; Černý, R. Osmosis in porous media: A review of recent studies. Microporous Mesoporous Mater. 2013, 170, 299–317. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Shaterabadi, Z.; Nabiyouni, G.; Soleymani, M. Correlation between effects of the particle size and magnetic field strength on the magnetic hyperthermia efficiency of dextran-coated magnetite nanoparticles. Mater. Sci. Eng. C 2020, 117, 111274. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Dong, Y.; Yu, C.; Liu, Y.; Teng, X. A novel Nafion-g-PSBMA membrane prepared by grafting zwitterionic SBMA onto Nafion via SI-ATRP for vanadium redox flow battery application. J. Membr. Sci. 2018, 554, 324–330. [Google Scholar] [CrossRef]
- Hartanto, Y.; Corvilain, M.; Mariën, H.; Janssen, J.; Vankelecom, I.F.J. Interfacial polymerization of thin-film composite forward osmosis membranes using ionic liquids as organic reagent phase. J. Membr. Sci. 2020, 601, 117869. [Google Scholar] [CrossRef]
- Kim, I.; Kang, S.M. Formation of Amphiphilic Zwitterionic Thin Poly(SBMA-co-TFEMA) Brushes on Solid Surfaces for Marine Antifouling Applications. Langmuir 2024, 40, 3213–3221. [Google Scholar] [CrossRef]
- Farahbakhsh, J.; Golgoli, M.; Khiadani, M.; Najafi, M.; Suwaileh, W.; Razmjou, A.; Zargar, M. Recent advances in surface tailoring of thin film forward osmosis membranes: A review. Chemosphere 2024, 346, 140493. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, R.; Liu, K.; Zhang, Y.; Shi, X.; Sand, W.; Hou, B. Application of nanomaterials in antifouling: A review. Nano Mater. Sci. 2024, 6, 672–700. [Google Scholar] [CrossRef]
- Dey, P.; Izake, E.L. Magnetic nanoparticles boosting the osmotic efficiency of a polymeric FO draw agent: Effect of polymer conformation. Desalination 2015, 373, 79–85. [Google Scholar] [CrossRef]
- Gong, Y.; Fan, M.; Gao, F.; Hong, J.; Liu, S.; Luo, S.; Yu, J.; Huang, J. Preparation and characterization of amino-functionalized magnetic nanogels via photopolymerization for MRI applications. Colloids Surf. B Biointerfaces 2009, 71, 243–247. [Google Scholar] [CrossRef]
- Deng, S.; Bai, R.; Chen, J.P.; Jiang, Z.; Yu, G.; Zhou, F.; Chen, Z. Produced water from polymer flooding process in crude oil extraction: Characterization and treatment by a novel crossflow oil–water separator. Sep. Purif. Technol. 2002, 29, 207–216. [Google Scholar] [CrossRef]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic Iron Oxide Nanoparticles: Synthesis, Stabilization, Vectorization, Physicochemical Characterizations, and Biological Applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Khan, R.; Daverey, A. Synthesis and characterization of magnetic nanoparticles, and their applications in wastewater treatment: A review. Environ. Technol. Innov. 2021, 24, 101924. [Google Scholar] [CrossRef]
- Xu, P.; Zeng, G.M.; Huang, D.L.; Feng, C.L.; Hu, S.; Zhao, M.H.; Lai, C.; Wei, Z.; Huang, C.; Xie, G.X.; et al. Use of iron oxide nanomaterials in wastewater treatment: A review. Sci. Total Environ. 2012, 424, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; He, Q.; Jiang, C. Magnetic Iron Oxide Nanoparticles: Synthesis and Surface Functionalization Strategies. Nanoscale Res. Lett. 2008, 3, 397. [Google Scholar] [CrossRef]
- Laurent, S.; Dutz, S.; Häfeli, U.O.; Mahmoudi, M. Magnetic fluid hyperthermia: Focus on superparamagnetic iron oxide nanoparticles. Adv. Colloid Interface Sci. 2011, 166, 8–23. [Google Scholar] [CrossRef]
- Lu, A.-H.; Salabas, E.L.; Schüth, F. Magnetic Nanoparticles: Synthesis, Protection, Functionalization, and Application. Angew. Chem. Int. Ed. 2007, 46, 1222–1244. [Google Scholar] [CrossRef]
- Reddy, D.H.K.; Yun, Y.-S. Spinel ferrite magnetic adsorbents: Alternative future materials for water purification? Coord. Chem. Rev. 2016, 315, 90–111. [Google Scholar] [CrossRef]
- Chen, S.; Li, L.; Zhao, C.; Zheng, J. Surface hydration: Principles and applications toward low-fouling/nonfouling biomaterials. Polymer 2010, 51, 5283–5293. [Google Scholar] [CrossRef]
- Han, L.; Tan, Y.Z.; Xu, C.; Xiao, T.; Trinh, T.A.; Chew, J.W. Zwitterionic grafting of sulfobetaine methacrylate (SBMA) on hydrophobic PVDF membranes for enhanced anti-fouling and anti-wetting in the membrane distillation of oil emulsions. J. Membr. Sci. 2019, 588, 117196. [Google Scholar] [CrossRef]

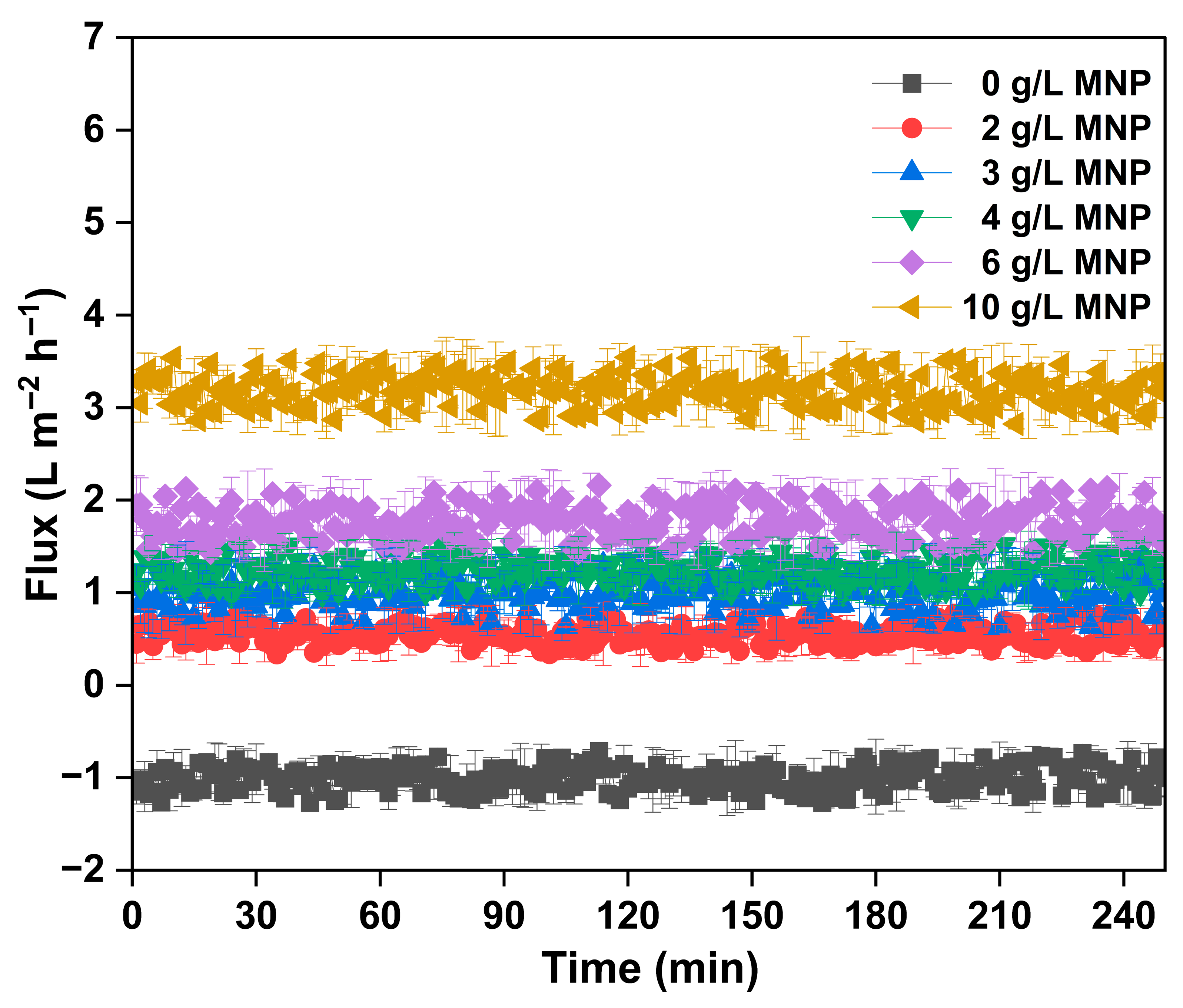
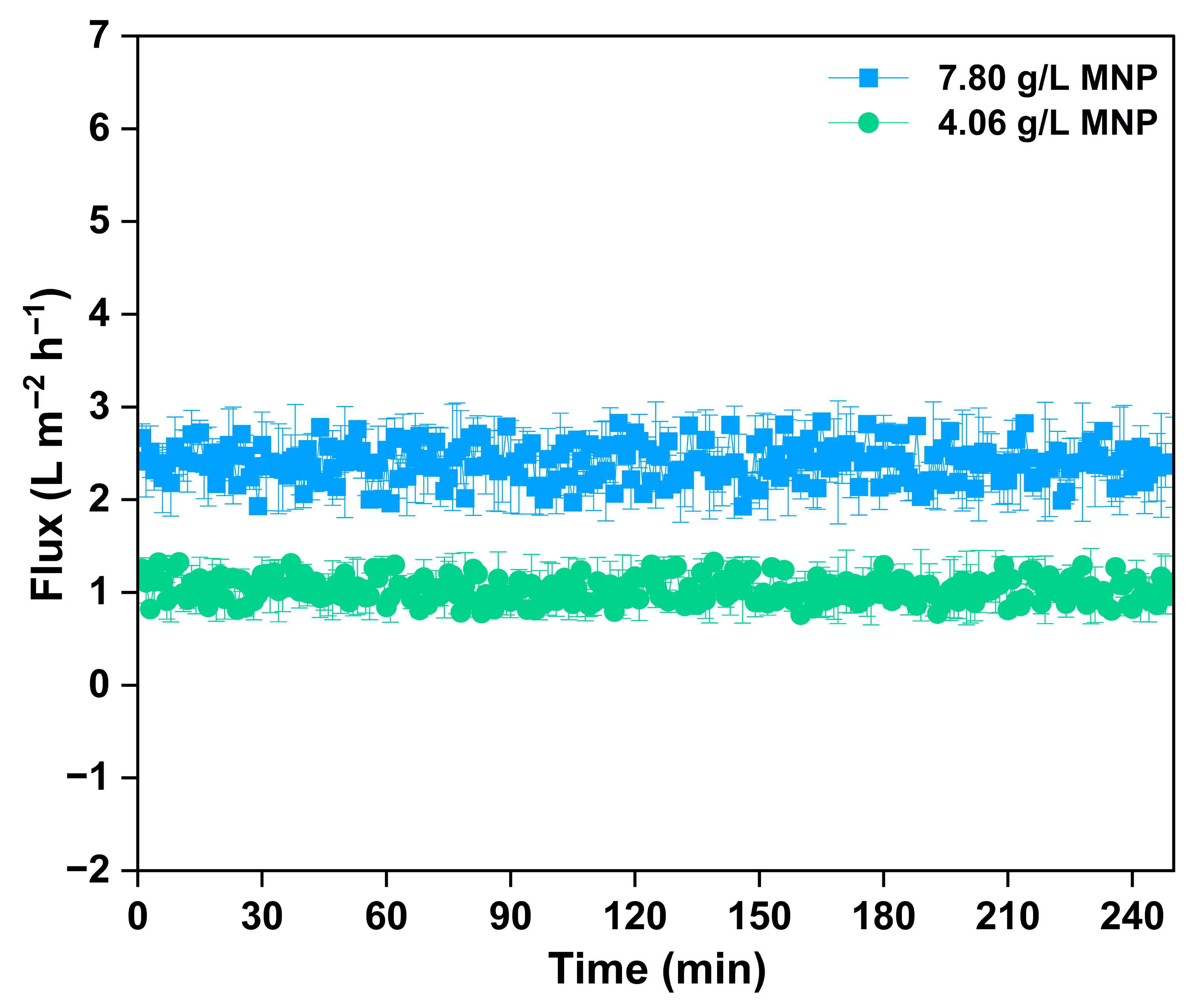

| MNP Dosage (g/L) | TDS (mg/L) |
|---|---|
| 0 | 2.5 ± 0.3 |
| 2 | 48.0 ± 2.4 |
| 3 | 103.9 ± 5.7 |
| 4 | 151.6 ± 6.5 |
| 6 | 191.8 ± 4.3 |
| 10 | 227.1 ± 9.1 |
| MNP Dosage (g/L) | TDS (mg/L) |
|---|---|
| 4.06 | 144.8 ± 7.2 |
| 7.8 | 206.4 ± 5.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madduri, S.B.; Kommalapati, R.R. Functionalized Magnetic Nanoparticles as Recyclable Draw Solutes for Forward Osmosis: A Sustainable Approach to Produced Water Reclamation. Separations 2025, 12, 199. https://doi.org/10.3390/separations12080199
Madduri SB, Kommalapati RR. Functionalized Magnetic Nanoparticles as Recyclable Draw Solutes for Forward Osmosis: A Sustainable Approach to Produced Water Reclamation. Separations. 2025; 12(8):199. https://doi.org/10.3390/separations12080199
Chicago/Turabian StyleMadduri, Sunith B., and Raghava R. Kommalapati. 2025. "Functionalized Magnetic Nanoparticles as Recyclable Draw Solutes for Forward Osmosis: A Sustainable Approach to Produced Water Reclamation" Separations 12, no. 8: 199. https://doi.org/10.3390/separations12080199
APA StyleMadduri, S. B., & Kommalapati, R. R. (2025). Functionalized Magnetic Nanoparticles as Recyclable Draw Solutes for Forward Osmosis: A Sustainable Approach to Produced Water Reclamation. Separations, 12(8), 199. https://doi.org/10.3390/separations12080199







