Carbon Dioxide Absorption by Polyethylene Glycol Dimethyl Ether Modified by 2-methylimidazole
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
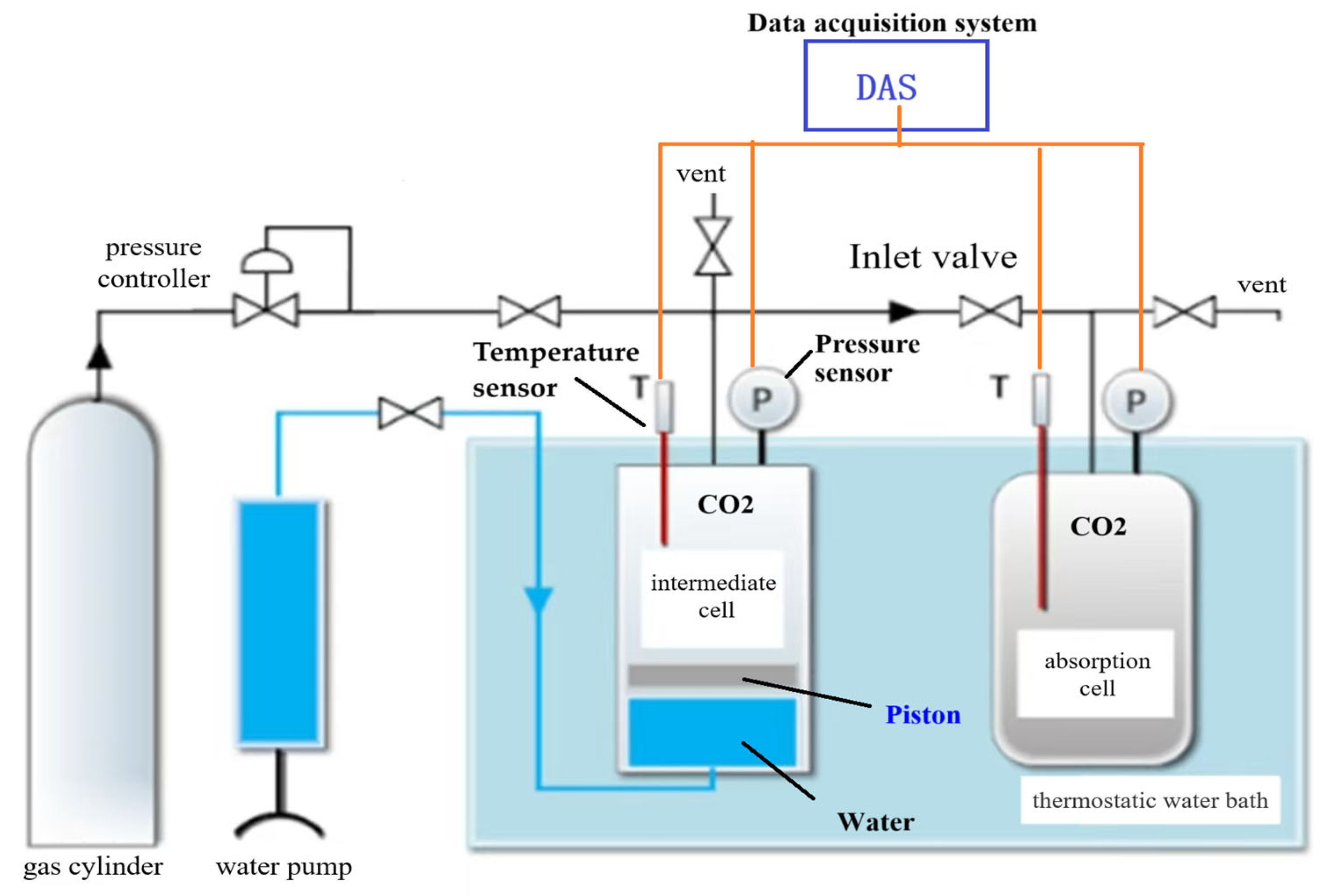
2.2. Experimental Apparatus and Procedure
2.3. Experimental Data Processing
3. Experimental Results and Discussion
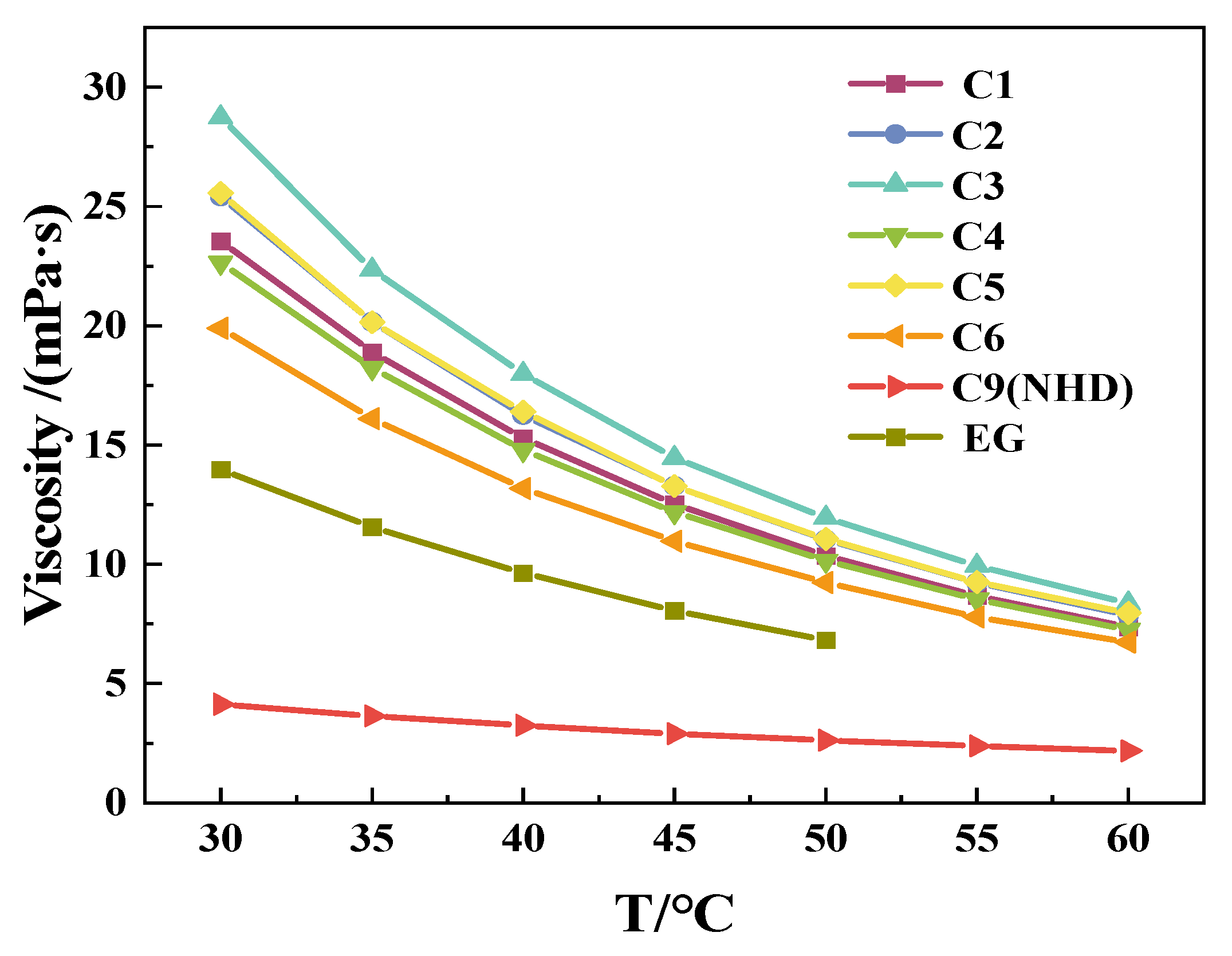
3.1. Viscosity of the Mixed Solution
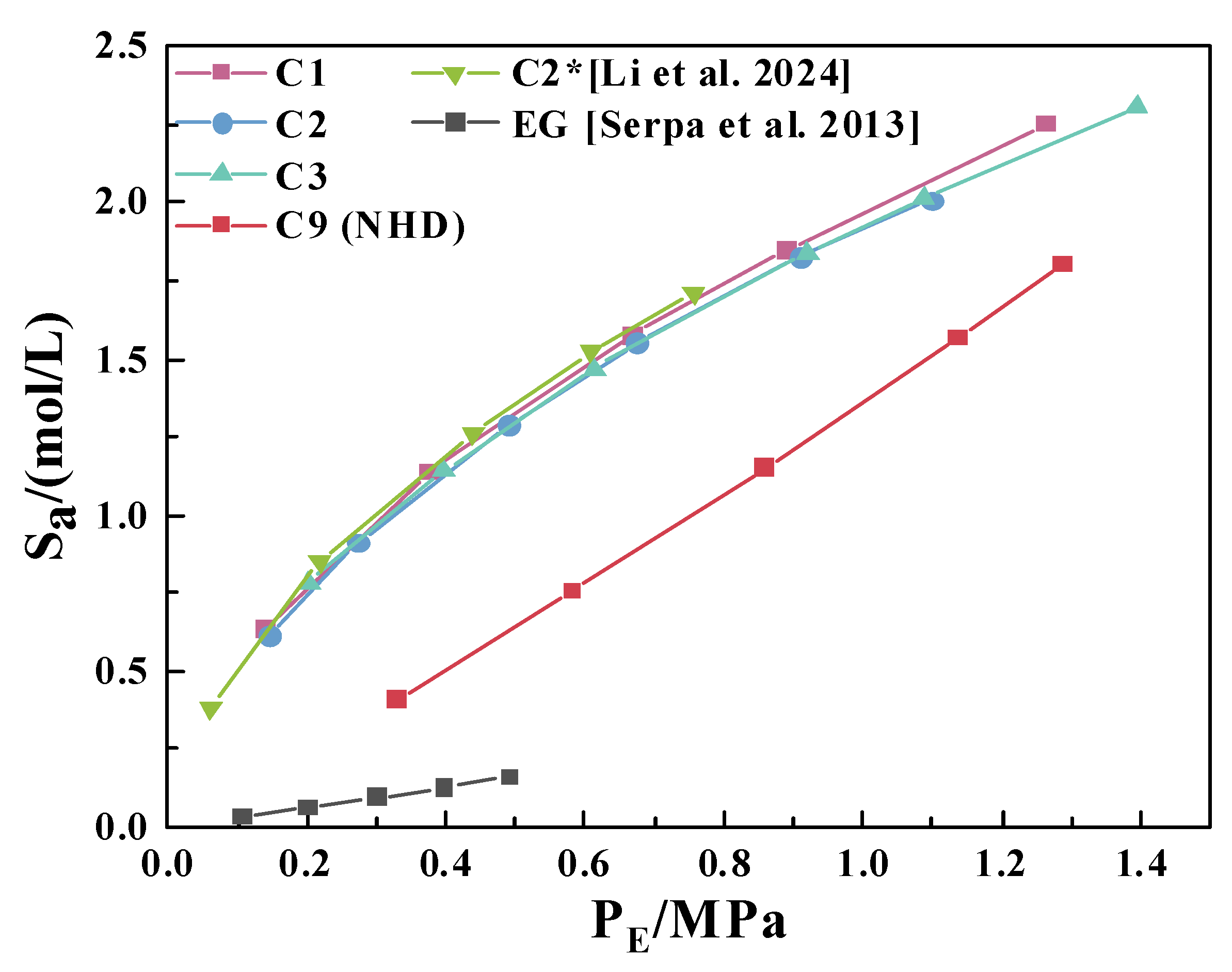
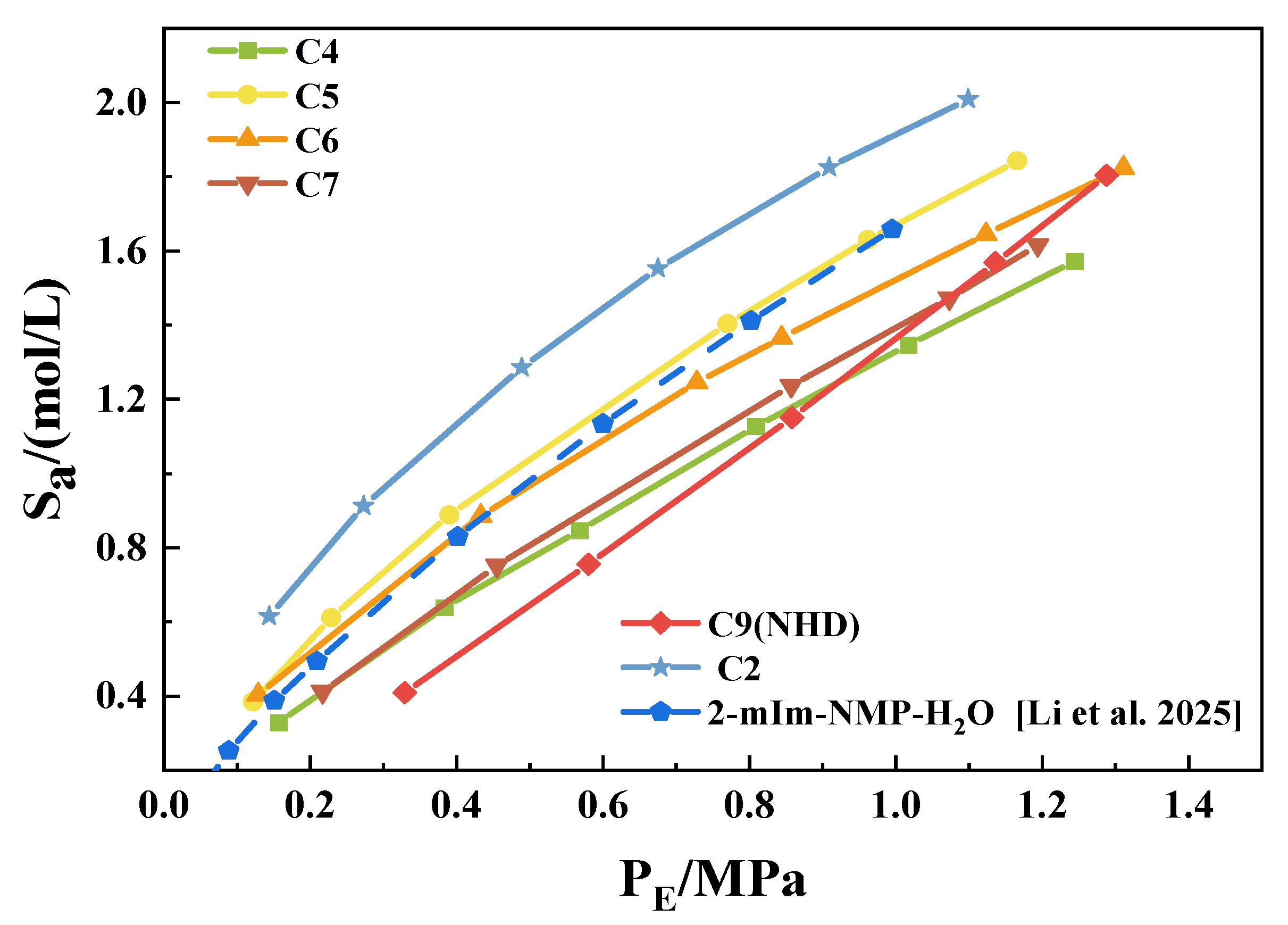
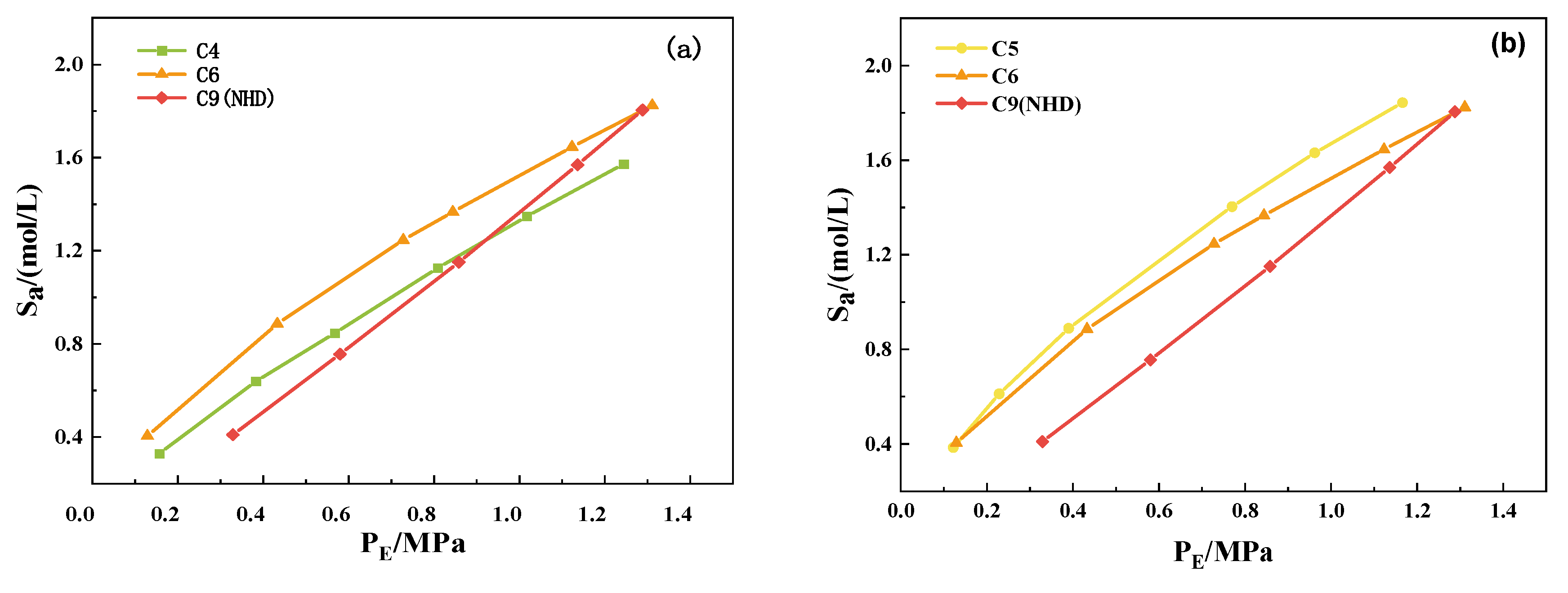
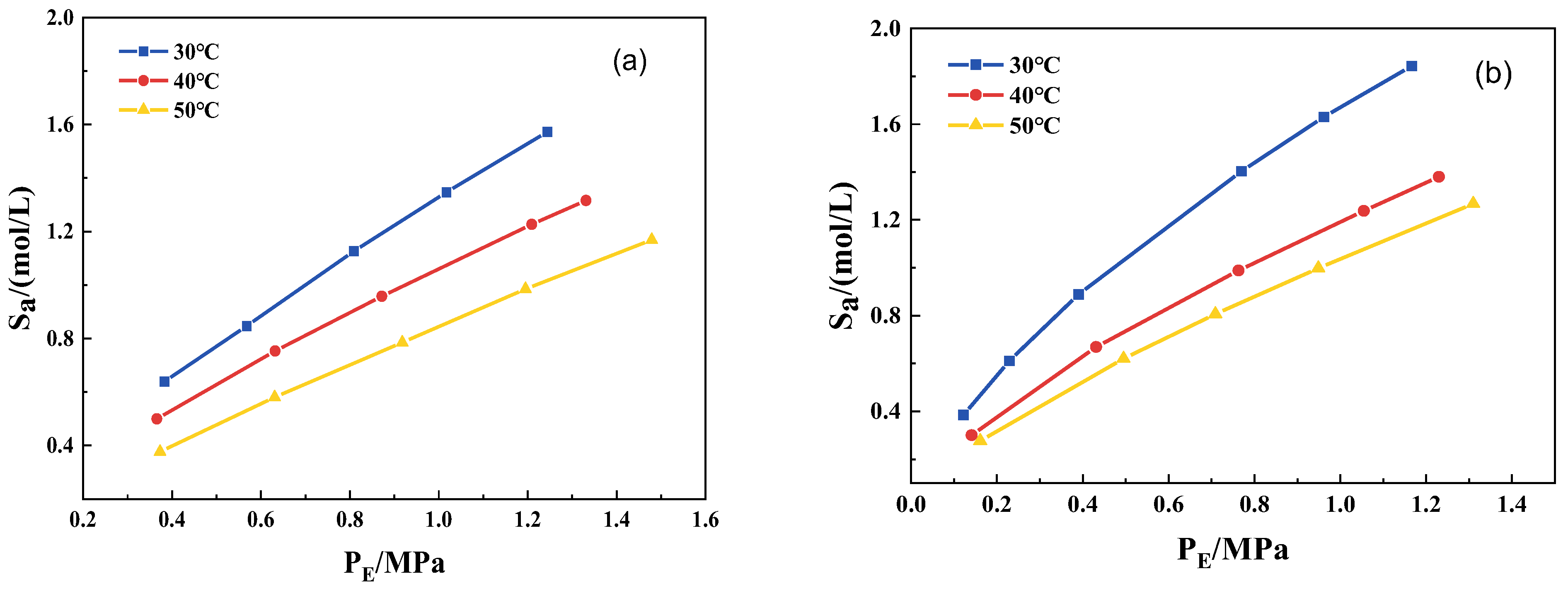
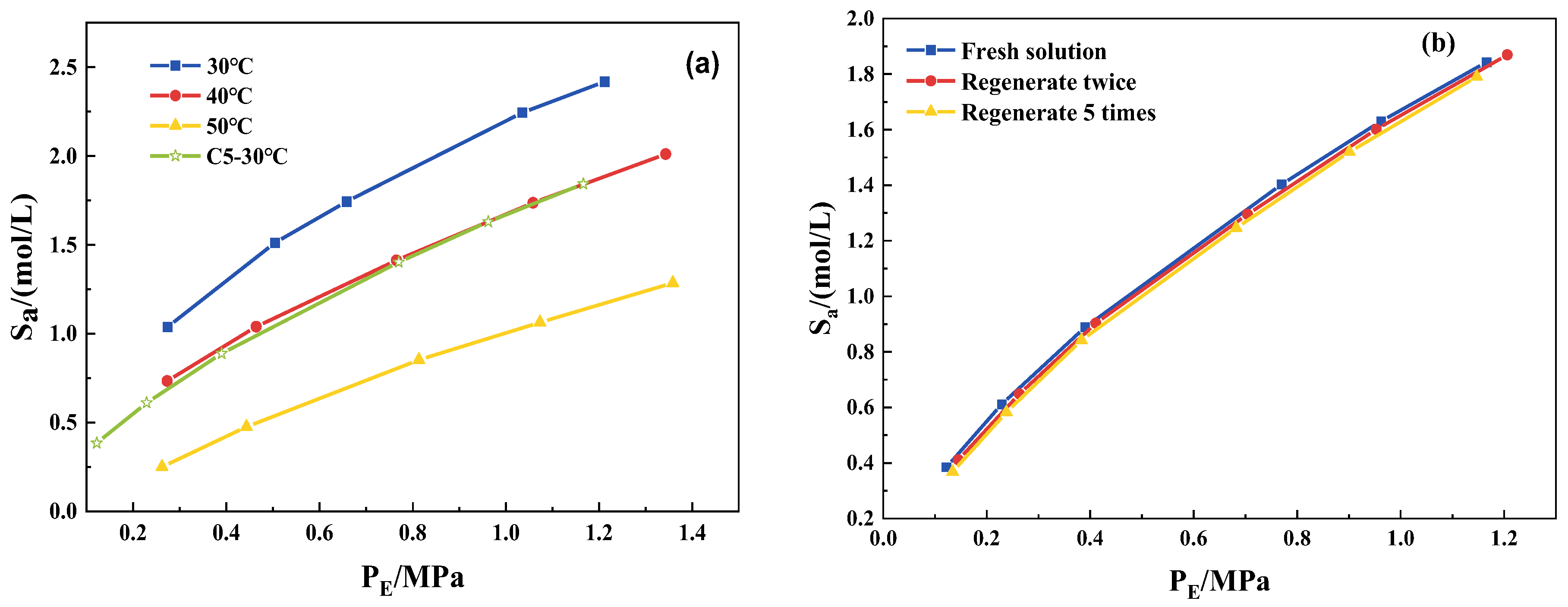
3.2. CO2 Solubility in 2-mIm–EG Solution
3.3. CO2 Absorption Properties of NHD-2-mIm–EG Mixed Solution
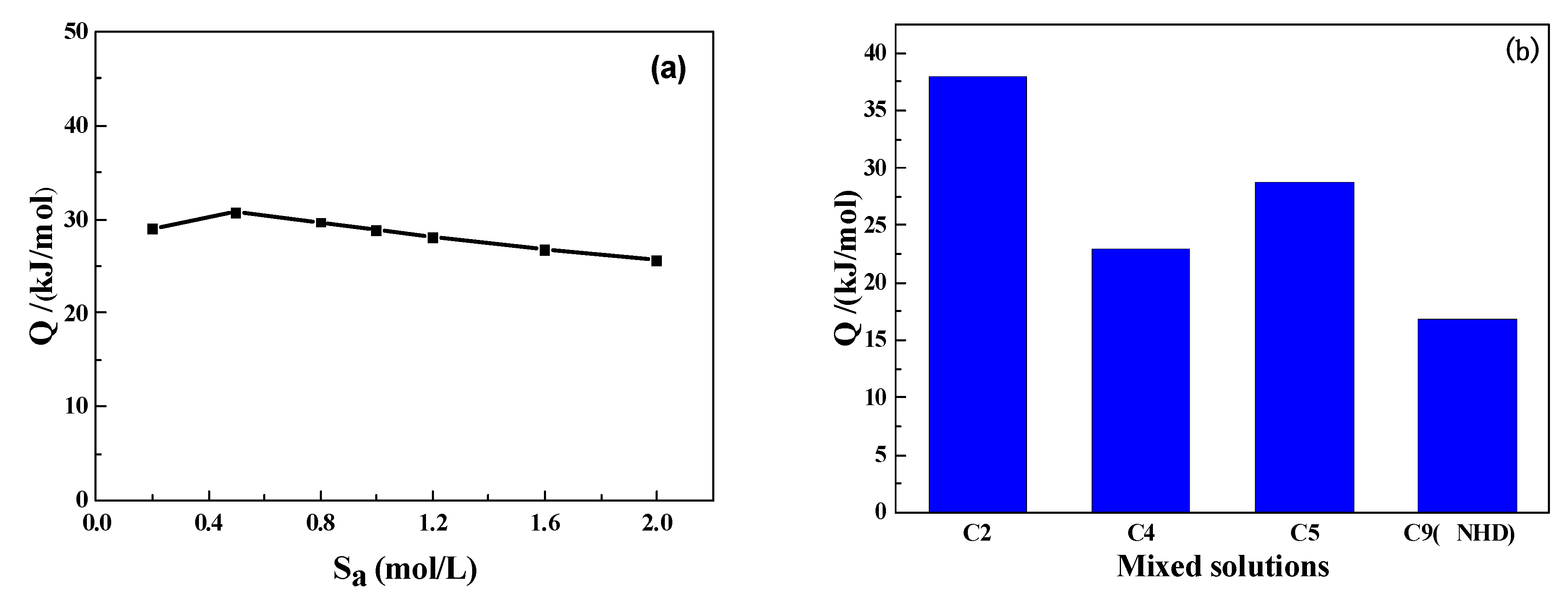
3.4. CO2 Isothermal Absorption Curves and Desorption Heat Consumption of Mixed Solutions
3.5. Mixed Solution Selectivity
3.6. Water Resistance and the Recyclability of a Mixed Solution
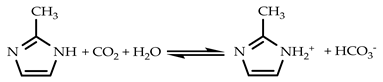
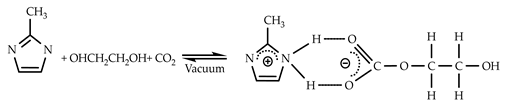
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Luo, Y.M.; Lu, S.J.; Liu, L.; Kang, G.J.; Yang, F.; Zhu, W.J.; Ma, Y.H.; Huang, X.Z.; Chen, Z.; Li, J.H. Mechanistic study and performance enhancement of CO2 absorption using DEHA as a viscosity modifier in biphasic solvent systems. Carbon Capture Sci. Technol. 2025, 15, 100392. [Google Scholar] [CrossRef]
- Zou, J.; Wu, Z.; Wand, J. Energy conservation technology based on magnetic levitation heat pump in oilfields. Xinjiang Oil Gas 2023, 19, 88–94. [Google Scholar]
- Cui, Y.K.; Guo, D.F.; Chen, X.Y.; Zhou, Y.L.; Wang, Z.H.; Wang, S.J. The impact of directly introducing aromatic nitrogen heterocycles on the performance of CO2 capture by diethylenetriamine/diethylene glycol dimethyl ether biphasic solvents: Experimental and theoretical analysis. Chem. Eng. J. 2025, 515, 163625. [Google Scholar] [CrossRef]
- Ghasem, N. CFD simulation of CO2 absorption by water-based TiO2 nanoparticles in a high pressure stirred vessel. Sci. Rep. 2021, 11, 1984. [Google Scholar] [CrossRef]
- Yang, T.M.; Yang, F.; Rezey, R.; Gou, G.L.; Li, X.H.; Hou, J.W. Research Progress on Capture of CO2 by Alcohol Amine Absorption Method. Xinjiang Oil Gas 2024, 20, 52–60. [Google Scholar]
- Xiong, X.Q.; Liao, T.; Xing, X.K.; Zhang, Z.; Dong, Z.; Bi, Y. Study Progress on Characteristics and Separation of Produced Fluid of CO2 Flooding. Xinjiang Oil Gas 2022, 18, 33–39. [Google Scholar]
- Zhu, J.; Yan, J. Problems related to CO2 recovery in hydrogen production unit and their solutions. Xinjiang Oil Gas 2022, 18, 98–102. [Google Scholar]
- Revelli, A.L.; Mutelet, F.; Jaubert, J.N. High carbon dioxide solubilities in imidazolium-based ionic liquids and in poly (ethylene glycol) dimethyl ether. J. Phys. Chem. B. 2010, 114, 12908–12913. [Google Scholar] [CrossRef]
- Rayer, A.V.; Henni, A.; Tontiwachwuthikul, P. High pressure physical solubility of carbon dioxide (CO2) in mixed polyethylene glycol dimethyl ethers. Can. J. Chem. Eng. 2012, 90, 576–583. [Google Scholar] [CrossRef]
- Eyitayo, S.I.; Okere, C.J.; Hussain, A.; Gamadi, T.; Watson, M.C. Synergistic sustainability: Future potential of integrating produced water and CO2 for enhanced carbon capture, utilization, and storage (CCUS). J. Environ. Manag. 2024, 351, 119713. [Google Scholar] [CrossRef]
- Vega, F.; Baenae-Moreno, F.M.; Fernandez, L.M.G.; Portillo, E.; Navarrete, B.; Zhang, Z. Current status of CO2 chemical absorption research applied to CCS: Towards full deployment at industrial scale. Appl. Energy 2020, 260, 114313. [Google Scholar] [CrossRef]
- Xing, H.; Yu, F.; Li, X.; Bao, Y.; Ye, W.; Li, C.; Zheng, S.; Huang, M. Application of ionic liquids in CO2 capture and conversion: A review. Sep. Purif. Technol. 2025, 360, 130981. [Google Scholar] [CrossRef]
- Rahim, A.H.; Yunus, N.M.; Bustam, M.A. Ionic liquids hybridization for carbon dioxide capture: A review. Molecules 2023, 28, 7091. [Google Scholar] [CrossRef] [PubMed]
- Moioli, T.; Pellegrini, L.A. Fixed and Capture Level Reduction operating modes for carbon dioxide removal in a Natural Gas Combined Cycle power plant. J. Clean. Prod. 2020, 254, 120016. [Google Scholar] [CrossRef]
- Yu, Y.S.; Zhang, T.T.; Wu, X.M.; Mu, D.L.; Zhang, Z.X.; Wang, G.G. Exploiting an Alternative CO2 Absorption Process by Efficient Solvent Mixture. Ind. Eng. Chem. Res. 2015, 54, 6165–6174. [Google Scholar] [CrossRef]
- Gao, H.; Xu, B.; Liu, H.; Liang, Z. Effect of amine activators on aqueous N, N-diethylethanolamine solution for post-combustion CO2 capture. Energy Fuels 2016, 30, 7481–7488. [Google Scholar] [CrossRef]
- Meng, F.; Meng, Y.; Ju, T.; Han, S.; Lin, L.; Jiang, J. Research progress of aqueous amine solution for CO2 capture: A review, Renew. Sust. Energ. Rev. 2022, 168, 112902. [Google Scholar] [CrossRef]
- Rao, A.B.; Rubin, E.S. A Technical, Economic, and Environmental Assessment of Amine-based CO2 Capture Technology for Power Plant Greenhouse Gas Control. Environ. Sci. Technol. 2002, 36, 4467–4475. [Google Scholar] [CrossRef]
- Yang, M.K.; Han, Y.; Zou, E.B.; Chen, W.; Peng, X.W.; Dong, B.C.; Sun, C.Y.; Liu, B.; Chen, G.J. Separation of IGCC syngas by using ZIF-8/dimethylacetamide slurry with high CO2 sorption capacity and sorption speed but low sorption heat. Energy 2020, 201, 117605. [Google Scholar] [CrossRef]
- Banerjee, R.; Phan, A.; Wang, B.; Knobler, C.; Furukawa, H.; O’Keeffe, M.; Yaghi, O.M. High-Throughput Synthesis of Zeolitic Imidazolate Frameworks and Application to CO2 Capture. Science 2008, 319, 939–943. [Google Scholar] [CrossRef]
- Liu, H.; Liu, B.; Lin, L.C.; Chen, G.; Wu, Y.; Wang, J.; Gao, X.; Lv, Y.; Pan, Y.; Zhang, X.; et al. A hybrid absorption -adsorption method to efficiently capture carbon. Nat. Commun. 2014, 5, 5147. [Google Scholar] [CrossRef]
- Evjen, S.; Fiksdahl, A.; Knuutila, H.K. High-capacity amine-imidazole solvent blends for CO2 capture. Ind. Eng. Chem. Res. 2019, 58, 10533–10539. [Google Scholar] [CrossRef]
- Shannon, M.S.; Bara, J.E. Properties of Alkylimidazoles as Solvents for CO2 Capture and Comparisons to Imidazolium-Based Ionic Liquids. Ind. Eng. Chem. Res. 2011, 50, 8665–8677. [Google Scholar] [CrossRef]
- Evjen, S.; Wanderley, R.; Fiksdahl, A.; Knuutila, H.K. Viscosity, density, and volatility of binary mixtures of imidazole, 2-methylimidazole, 2,4,5-trimethylimidazole, and 1,2,4,5-tetramethylimidazole with water. J. Chem. Eng. Data. 2019, 64, 507–516. [Google Scholar] [CrossRef]
- Li, K.; Tang, H.; Li, S.; Huang, Z.; Liu, B.; Deng, C.; Sun, C.; Chen, G. Highly efficient CO2 capture using 2-methylimidazole aqueous solution on laboratory and pilot-scale. Chin. J. Chem. Eng. 2024, 67, 148–156. [Google Scholar] [CrossRef]
- Liu, H.; Guo, P.; Chen, G.J. Investigation of CO2 capture efficiency and mechanism in 2-methylimidazole-glycol solution. Sep. Purif. Technol. 2017, 189, 66–73. [Google Scholar] [CrossRef]
- Chen, W.; Chen, M.; Yang, M.; Zou, E.; Li, H.; Jia, C.; Sun, C.; Ma, Q.; Chen, G.; Qin, H. A new approach to the upgrading of the traditional propylene carbonate washing process with significantly higher CO2 absorption capacity and selectivity. Appl. Energy 2019, 240, 265–275. [Google Scholar] [CrossRef]
- Li, J.; Kan, J.; Cao, Y.; Ding, B.; Bi, Z.; Li, N.; Huang, X.; Li, Z.; Chen, G.J. Enhancing CO2 capture performance of N-methylpyrrolidone with 2-methylimidazole aqueous solution. J. Environ. Chem. Eng. 2025, 13, 116847. [Google Scholar] [CrossRef]
- Starling, K.E.; Han, M.S. Thermo data refined for LPG--14. Mixtures. Hydrocarb. Process. 1972, 5, 51. [Google Scholar]
- Pan, Y.; Li, H.; Zhang, X.X.; Tong, X.S.; Jia, C.Z.; Liu, B.; Sun, C.Y.; Yang, L.Y.; Chen, G.J. Large-scale synthesis of ZIF-67 and highly efficient carbon capture using a ZIF-67/glycol-2-methylimidazole slurry. Chem. Eng. Sci. 2015, 137, 504–514. [Google Scholar] [CrossRef]
- Serpa, F.S.; Vidal, R.S.; Amaral Filho, J.H.B.; Nascimento, J.F.; Ciambelli, J.R.P.; Figueiredo, C.M.S.; Salazar-Banda, G.R.; Santos, A.F.; Fortuny, M.; Franceschi, E.; et al. Solubility of Carbon Dioxide in Ethane-1,2-diol-Water Mixtures. J. Chem. Eng. Data 2013, 58, 3464–3469. [Google Scholar] [CrossRef]
- Gupta, M.; Silva, E.F.; Hartono, A.; Svendsen, H.F. Theoretical study of differential enthalpy of absorption of CO2 with MEA and MDEA as a function of temperature. J. Phys. Chem. B 2013, 32, 9457–9468. [Google Scholar] [CrossRef]
- Vinjarapu, S.H.B.; Regueira, T.; Neerup, R.; Solms, N.; Fosbøl, P.L. Heat of absorption of CO2 in 30wt% MEA with monoethyleneglycol and urea as vapour reduction additives. Energy 2024, 293, 130609. [Google Scholar] [CrossRef]

| Chemicals | Material Purity | Manufacturer |
|---|---|---|
| 2-methylimidazole | 98% | Shanghai Aladdin Biochemical Technology Co., Ltd., Shanghai, China |
| Ethylene glycol | Analytical Reagent, AR | Shanghai Aladdin Biochemical Technology Co., Ltd., Shanghai, China |
| Polyethylene glycol dimethyl ether | Average Mn~250 | Shanghai Aladdin Biochemical Technology Co., Ltd., Shanghai, China |
| Carbon dioxide | >99.99% | Zhongke Gas Co., Ltd., Karamay, China |
| Nitrogen | >99.99% | Zhongke Gas Co., Ltd., Karamay, China |
| Absorbents | Compositional Ratios (Mass Fraction) | |||
|---|---|---|---|---|
| NHD | 2-mIm | EG | H2O | |
| C1 | 0 | 0.33 | 0.67 | 0 |
| C2 | 0 | 0.4 | 0.6 | 0 |
| C3 | 0 | 0.43 | 0.57 | 0 |
| C4 | 0.17 | 0.33 | 0.5 | 0 |
| C5 | 0.2 | 0.4 | 0.4 | 0 |
| C6 | 0.34 | 0.33 | 0.33 | 0 |
| C7 | 0.4 | 0.3 | 0.3 | 0 |
| C8 | 0.18 | 0.36 | 0.36 | 0.1 |
| C9 | 1 | 0 | 0 | 0 |
| PE (MPa) | SNHD (mol/L) | SC5 (mol/L) | Sr = (SC5 − SNHD)/SNHD × 100% |
|---|---|---|---|
| 0.2 | 0.209 | 0.546 | 161.2% |
| 0.5 | 0.646 | 1.044 | 61.61% |
| 1 | 1.373 | 1.678 | 22.22% |
| PE (MPa) | SN2 (mol/L) | SCO2 (mol/L) | K = SCO2/SN2 |
|---|---|---|---|
| 0.294 | 0.0107 | 0.712 | 66.54 |
| 0.471 | 0.0191 | 1.00 | 52.36 |
| 0.707 | 0.0298 | 1.34 | 44.97 |
| 1.087 | 0.0485 | 1.76 | 36.29 |
| 1.228 | 0.0571 | 1.89 | 33.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Wang, Z.; Yu, H.; Ding, B.; Fei, K.; Ma, X.; Xu, B.; Zhang, Y.; Fu, X.; Ding, B.; et al. Carbon Dioxide Absorption by Polyethylene Glycol Dimethyl Ether Modified by 2-methylimidazole. Separations 2025, 12, 198. https://doi.org/10.3390/separations12080198
Wu Y, Wang Z, Yu H, Ding B, Fei K, Ma X, Xu B, Zhang Y, Fu X, Ding B, et al. Carbon Dioxide Absorption by Polyethylene Glycol Dimethyl Ether Modified by 2-methylimidazole. Separations. 2025; 12(8):198. https://doi.org/10.3390/separations12080198
Chicago/Turabian StyleWu, Yan, Zicheng Wang, Hui Yu, Bin Ding, Ke Fei, Xueli Ma, Baoshen Xu, Yonghu Zhang, Xiaoning Fu, Bowen Ding, and et al. 2025. "Carbon Dioxide Absorption by Polyethylene Glycol Dimethyl Ether Modified by 2-methylimidazole" Separations 12, no. 8: 198. https://doi.org/10.3390/separations12080198
APA StyleWu, Y., Wang, Z., Yu, H., Ding, B., Fei, K., Ma, X., Xu, B., Zhang, Y., Fu, X., Ding, B., & Li, N. (2025). Carbon Dioxide Absorption by Polyethylene Glycol Dimethyl Ether Modified by 2-methylimidazole. Separations, 12(8), 198. https://doi.org/10.3390/separations12080198