Abstract
This study provides a thorough examination of the utilization of hydrophobic deep eutectic solvents (HDESs) for the extraction of 1,2-dichloroethane (1,2-DCA) from effluent, with an emphasis on a sustainable and environmentally friendly approach. The extraction efficacy of six HDES systems was initially evaluated, and the combinations of thymol/camphor (Thy/Cam) and menthol/thymol (Men/Thy) exhibited superior performance. Subsequently, these two HDESs were chosen for a comprehensive parametric analysis. The impact of contact time demonstrated that extraction equilibrium was reached at 15 min for both systems, thereby achieving a balance between high efficiency and time efficiency. Next, the impact of the HDES-to-water mass ratio was investigated. A 1:1 ratio was determined to be the most effective, as it minimized solvent consumption and provided high efficiency. An additional examination of the molar ratios of the HDES components revealed that the 1:1 ratio exhibited the most effective extraction performance. This was due to the fact that imbalances in the solvent mixture resulted in diminished efficiency as a result of disrupted molecular interactions. The extraction efficiency was significantly influenced by the initial concentration of 1,2-DCA, with higher concentrations resulting in superior results as a result of the increased mass transfer driving forces. In general, the Men/Thy and Thy/Cam systems have shown noteworthy stability and efficiency under different conditions, which makes them highly suitable for large-scale applications.
1. Introduction
The rapid growth of the world’s population and rapid industrial revolution requires high water consumption, which has led to an increase in the amount of wastewater to be treated [1]. A major problem is the direct discharge of industrial wastewater into water bodies, which can cause serious problems to flora and fauna [2]. One of the major water pollutants is chlorinated aliphatic hydrocarbons (CAHs), including perchloroethylene (PCE), trichloroethylene (TCE), 1,1,1-trichloroethane (TCA), and 1,2-dichloroethane (1,2-DCA). Owing to their high density and poor water solubility, CAHs are persistent and potentially hazardous pollutants that form dense nonaqueous liquids in water [3]. Among CAHs, 1,2-DCA has become an attractive topic for researchers because it has not been extensively studied like other CAHs and because it is persistent, toxic, and potentially carcinogenic owing to its high demand for the synthesis of vinyl chloride monomers [3,4]. Hydrophobic deep eutectic solvents (HDESs) have recently attracted interest as environmentally friendly substitutes for the extraction of nonpolar contaminants from water. Terpene-based HDESs, derived from natural chemicals such as thymol and menthol, have favorable properties such as high hydrophobicity, low toxicity, and robust extraction efficacy.
The common use of 1,2-DCA is the production of vinyl chloride in chlorinated hydrocarbon reactions. The latter is the most important precursor in the production of polyvinyl chloride (PVC). Wastewater from PVC production is characterized by several pollutants, including 1,2-DCA [5]. The World Health Organization has set a permissible limit for 1,2-DCA in drinking water at 0.03 mg/L. The U.S. Environmental Protection Agency has also set a maximum level for 1,2-DCA in drinking water of 0.05 mg/L [6]. However, lower levels of 1,2-DCA in water are recommended.
1,2-DCA has attracted much attention in recent years because it is considered a hazardous substance for both the environment and human health. The properties of 1,2-DCA, such as high solubility, difficult adsorption, and stability, make it very difficult to deal with contaminated soil and groundwater [7]. The symmetrical structure with a single C-C bond, as well as the high energy for dissociation of the C-Cl bond and the high activation energy required for dechlorination, indicate that strong dissociation energies are required for the degradation of 1,2-DCA [3,7]. Therefore, it is important to find an effective and inexpensive method to remove 1,2-DCA from aquifers.
Researchers have attempted several methods to reduce or remove 1,2-DCA from wastewater. Lee et al. [8] investigated the adsorption of 1,2-DCA from water using activated carbon produced from beer lees. This study demonstrates the effectiveness of activated carbon in the removal of 1,2-DCA and highlights the potential of this low-cost adsorbent for water remediation. However, the strong attraction of water to 1,2-DCA interrupts adsorption. In addition, these physical treatments only transfer contaminants from the water to another phase and must be disposed of or further treated [9]. Another method is zero-valent iron or nano iron (nZVI), which has been used by many researchers to degrade 1,2-DCA. According to Baric et al. [10], this method is effective in treating chlorinated ethenes and ethanes (for example, PCE, TCE, and other chlorinated compounds) by degrading these pollutants into non-toxic compounds. In contrast, the degradation of 1,2-DCA has been shown to be resistant to reduction by nZVI. However, nZVI can be coupled with other compounds, such as dithionite, and it was found that coupled nZVI-dithionite was able to degrade >90% of 1,2-DCA over the course of a year. However, this method requires a long time to achieve a highly effective degradation [10,11]. Eydivand and Nikazar [12] used an advanced oxidation method to remove 1,2-DCA from simulated wastewater. They investigated different oxidation methods, including ultraviolet (UV) irradiation, H2O2/UV, and photocatalytic methods (using TiO2 and ZnO as photocatalysts), to degrade 1,2-DCA in a simulated synthetic solution to optimize the method. The removal of 1,2-DCA reached 90% using the TiO2/UV method. Jeong et al. [3] utilized Fenton oxidation (a powerful advanced oxidation process that generates hydroxyl radicals) to degrade 1,2-DCA. They investigated several reagents (dithionite, hydrosulfide, sulfite, persulfate (PS), sulfate radicals, and hydroxyl radicals), of which the hydroxyl radicals generated by the Fenton reaction were the most suitable oxidizing agents and degraded 92% of 1,2-DCA. Although this method showed good results in the removal of 1,2 DCA, it has some disadvantages, such as the toxicity of hydrogen peroxide and hydroxyl radicals, the need for acidic pH, the production of iron sludge, and the need for a high chemical input [13,14]. Liu et al. [15] investigated the degradation of 1,2-DCA with advanced reduction processes (ARPs). In their study, 1,2-DCA was successfully degraded with various ARPs by combining UV irradiation with different reagents (dithionite, sulfite, sulfide, and iron). More than 90% of the initial 1,2-DCA was removed within 20 min under alkaline conditions (pH 8.2, 9.0, and 11.0), while it took 130 min to achieve the same removal at pH 7.0. However, despite these positive results, there are no reports on the large-scale use of this technology [16]. Membrane filtration is a method used to remove contaminants from water. Yazdanbakhsh et al. [5] attained a very high removal of 1,2-DCA (96.7) from water using a nanofiltration membrane cell followed by microfiltration.
Biodegradation techniques have also been used to remove 1,2-DCA. According to some studies, 1,2-DCA can act as both an electron donor and an acceptor during aerobic and anaerobic reductive dechlorination, respectively [7]. Marines et al. [17] investigated the feasibility of chlorinated solvent removal by biostimulated and bioaugmented biological processes in permeable reactive barriers at a laboratory scale under anaerobic and aerobic conditions. They obtained 100% removal of 1,2-DCA during the anaerobic process, whereas the aerobic process showed a slightly lower 1,2-DCA removal efficiency of 98% after 75 days of testing. Despite the high biodegradation efficiency and effective dechlorination of 1,2-DCA to non-toxic ethylene [18], numerous variables, such as temperature, pH, and nutritional status, affect the biodegradation process. These variables are difficult to manage in real-world situations and change throughout the season. In addition, the biodegradation process is time-consuming, and the resulting byproducts are not always desired [7]. Table 1 shows the comparison of various methods used for the removal of CAHs. The table signifies the strength of various approaches based on key performance indicators such as distribution coefficients (D), selectivity (S), absorption rate (AR), and absorption capacity (AC).

Table 1.
Comparison of various methods used for the removal of CAHs.
Liquid–liquid extraction (LLE) was utilized effectively in this study to remove 1,2-DCA from polluted water. This technique is user-friendly, can handle thermally unstable and high-boiling substances with ease, and is known for its high efficiency and selectivity. Additionally, it is economically dependable and can be seamlessly scaled up. Therefore, this method is widely used at the laboratory scale and in industrial processes [29,30,31]. LLE involves the extraction of a solute or an analyte from a nonpolar solvent and an aqueous polar solution. It utilizes the principle of differential solubility and partition equilibrium to extract the desired material from two different immiscible solvents [32].
Various solvents, such as hydrocarbons, esters, alcohols, halogens, and ketones, can be used in LLE applications. Although they have good properties (readily available, high solvent power), they have some disadvantages, such as high volatility, high cost, and toxicity [31]. However, there are environmentally friendly alternatives, such as ionic liquids (ILs) and deep eutectic solvents (DESs) [33]. Both have interesting properties, such as a low melting point, low vapor pressure, non-flammability, and biodegradability [34]. In addition, DESs have advantages over ILs because they are cheaper, can usually be synthesized easily, are less toxic, and are often biodegradable, resulting in DESs receiving more attention from researchers [35]. In 2015, a new subclass of DESs with hydrophobic properties was discovered as an effective method for extracting nonpolar organic and inorganic molecules from aqueous solutions [36]. The first HDES was introduced by van Osch et al. [37] and consists of various quaternary ammonium salts with decanoic acid. HDESs are formed by mixing two or more (usually hydrophobic) components, resulting in a eutectic composite with a melting point lower than that of one of its components.
HDESs represent a novel class of green solvents specifically designed for the efficient extraction of non-polar pollutants from aqueous environments. Their low water solubility, environmental compatibility, and ease of synthesis make them attractive alternatives to conventional solvents and ionic liquids. In this class, terpene-based HDESs, which consist of natural compounds such as thymol, menthol, and camphor, have particularly promising properties due to their strong hydrophobic interactions, low viscosity, and biodegradability. These properties improve mass transfer during extraction and minimize the environmental and safety concerns associated with conventional solvents. In this study, both terpenes and other hydrophobic HDESs were evaluated for their efficacy in the removal of 1,2-DCA, a persistent and toxic pollutant.
The aim of this study is to develop an efficient method for the removal of 1,2-DCA from aqueous media using HDESs. First, six HDESs were investigated for the extraction of 1,2-DEA. All the HDESs were prepared at a molar ratio of 1:1. After the initial screening, the best HDES with the highest extraction efficiency was selected for further investigation. This study aimed to optimize the use of HDESs by investigating the effects of various parameters, including the contact time, molar ratio of the HDES, mass ratio of HDES to aqueous solution, and initial concentration of 1,2-DCA. A thorough investigation of these parameters will improve our understanding of the extraction process and ensure its effectiveness and sustainability. This approach aims to provide an environmentally friendly and effective solution for the removal of 1,2-DCA from contaminated water.
2. Materials and Methods
Table 2 shows the list of chemicals used in this experiment, along with their molecular weights, CAS No., sources, and mass fractions. The chemicals were used as received without further purification. Naphthalene was used as an internal standard material.

Table 2.
Name, CAS No., supplier, and purity of the chemicals.
2.1. Preparation of HDESs
The HDESs were prepared by mixing hydrogen bond donor (HBD) and hydrogen bond acceptor (HBA). Coumarin and camphor can act as HBAs, while menthol, thymol, decanoic acid, and hydrocinnamic acid can act as both HBAs and HBDs. The HDESs were prepared by mixing and stirring the components at a constant speed of 350 rpm and a temperature of 80 °C under magnetic stirring until a homogeneous liquid was formed [38,39]. All HDESs were prepared in a 1:1 molar ratio. Optimized molecular structure of the individual components of the HDESs are shown in Figure 1.
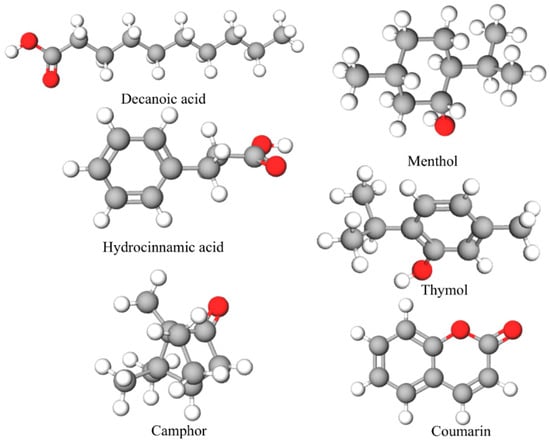
Figure 1.
Optimized molecular structure of the individual components of the HDESs.
2.2. Experimental Procedure
A liquid–liquid extraction process was implemented in this investigation to effectively eliminate 1,2-DCA from water through the utilization of HDESs. A schematic of the experimental procedure is shown in Figure 2. Initially, a stock solution of 2.5 g of 1,2-DCA per 100 mL of deionized water was prepared for the screening of six distinct HDESs. This solution was treated with a Millipore Q-water system. The Eppendorf Thermomixer was utilized to mix the feed solution with the HDES at a 1:1 mass ratio during the extraction procedure. The selected mass ratio provides an optimal balance between solvent consumption and extraction efficiency. Excessive use of HDESs may slightly improve extraction but increases costs and environmental impact. Conversely, too little solvent limits mass transfer and phase separation. Previous studies have shown that the 1:1 ratio is effective for HDES–water systems due to favorable dispersion and reduced emulsion formation [33,34]. The mixer was operated at 2000 rpm and 25 °C for 30 min. A speed of 2000 rpm was chosen to ensure sufficient shear and mixing intensity to overcome interfacial resistance and allow rapid mass transfer, while avoiding the formation of emulsions that could hinder separation [33]. Extraction was performed at 25 °C to match standard environmental conditions and maintain consistency with previous HDES-based extraction studies. This temperature reduces volatility-related losses of 1,2-DCA and maintains the structural integrity of the terpene-based solvents. Following the mixing process, the samples were centrifuged at 5000 rpm for 10 min to facilitate phase separation, which resulted in the formation of two distinct layers. This speed was sufficient to overcome the differences in density and viscosity between the HDES and water and to ensure complete stratification into two separate layers.
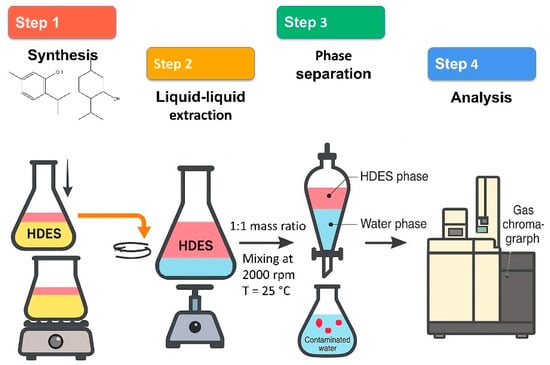
Figure 2.
Schematic representation of the experimental procedure.
The HDES-phase samples were diluted with toluene, while the water-phase samples were diluted with toluene and ethanol. Samples from both the HDES and the water phases were collected. Following this, the samples were subjected to gas chromatography (GC) analysis, with naphthalene serving as an internal standard due to its thermal stability within the 300–400 °C range [40]. The results were validated by measuring average uncertainties, and each measurement was conducted in triplicate to assure accuracy.
The extraction efficiency (E %) of 1,2-DCA was evaluated using Equation (1):
where is the initial 1,2-DCA concentration in the raffinate phase and is the final 1,2-DCA concentration in the raffinate phase.
GC was used to quantitatively analyze the presence of 1,2-DCA in both the HDES and the water phases. The study was performed with a Shimadzu GC-2010 Pro instrument, which was outfitted with a flame ionization detector and an HP-5 column under the operating conditions listed in Table 3. The HP-5 column is composed of 5% diphenyl and 95% dimethylpolysiloxane. It has a length of 30 m, an inner diameter of 0.32 mm, and a film thickness of 0.25 µm. Helium was used as the carrier gas in split mode.

Table 3.
Gas chromatography operating conditions.
In order to avoid contamination and maintain the accuracy of the data, the GC liner was thoroughly cleansed following each analysis, specifically to eliminate any remaining HDES components. Naphthalene was chosen as the internal standard because of its high temperature stability and minimal interaction with 1,2-DCA, which makes it well-suited for precise calibration and quantification. Reliable measurements were aided by the use of naphthalene, which was free from interference in the target analyte matrix. The calibration curves were generated by comparing the response of naphthalene to 1,2-DCA. These curves showed a high level of linearity, with correlation coefficients of more than 0.99. This guaranteed accurate measurement of 1,2-DCA concentrations in the samples. Figure 3 displays the GC calibration curve for 1,2-DCA and naphthalene. Karl Fischer titration (Aquamax Karl-Fischer, GR Scientific Ltd., Halle, Germany) was used to quantify the water content of pure HDES and after extraction. Briefly, around 100 mg of each HDES was weighted and injected into the Karl-Fisher titrator cell. The titration was repeated three times, and an average value was calculated.
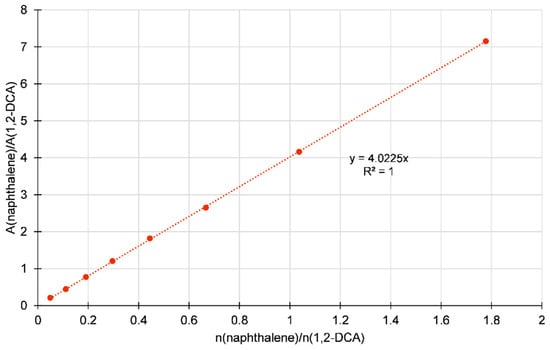
Figure 3.
GC calibration curve of 1,2-DCA and naphthalene.
2.3. Selection of HDESs
The selection of HDESs for this study was conducted in a systematic manner to guarantee their efficacy in extracting 1,2-DCA from effluent. These particular HDESs were selected due to their known hydrophobicity and their capacity to generate stable eutectic mixtures at room temperature, a critical factor in the efficient separation of phases. Terpenes, including thymol and menthol, were chosen for their well-documented ability to produce stable HDESs. This property is further enhanced when combined with carboxylic acids, such as decanoic acid [41,42]. The selection process was influenced by the previous literature, which emphasized the advantages of utilizing natural, biodegradable components that are consistent with the principles of green chemistry. The hydrophobic nature of terpene-based components, such as thymol and menthol, facilitates the efficient separation of phases from the aqueous solution, while the reduced viscosity they provide enhances mass transfer during the extraction process [43]. Table 4 displays the list of HDESs that were evaluated in this study.

Table 4.
List of prepared HDESs and their water content.
3. Results and Discussion
3.1. Cross-Contamination of HDESs with Water
When evaluating the suitability of HDESs for wastewater treatment applications, it is crucial to consider the potential for water contamination. The study focused on examining the interaction between HDESs and water during the LLE process. The HDESs were combined with water in a 50% v/v ratio and stirred for 2 h to ensure complete interaction between the two phases. Afterwards, there was a period of settling to naturally separate the phases. After completing this procedure, the HDES-phase samples were carefully examined to assess the level of water contamination. Figure 4 illustrates that the majority of HDESs exhibited minimal water absorption, suggesting excellent hydrophobicity and stability throughout the extraction process. Thy/Dec exhibited a significantly greater water absorption level in comparison to the other samples, with a water content of around 3.6%. It appears that while this HDES may have impressive extraction capabilities, its higher water solubility could potentially affect its separation efficiency and long-term stability in industrial applications. The other HDESs displayed water content below 3%, confirming their classification as neutral solvents with low water solubility.
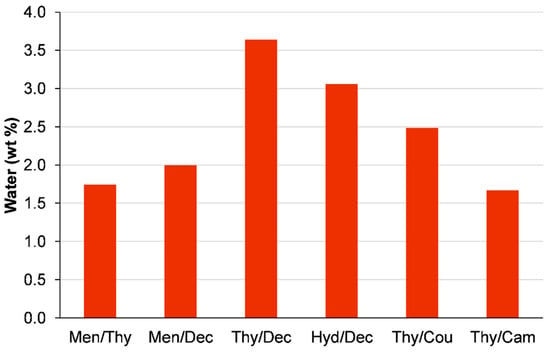
Figure 4.
Water solubility of the prepared HDESs. HDES-to-water volume ratio = 1:1; stirring at 350 rpm for 2 h at 25 °C.
This research aligns with previous studies on terpene-based HDESs, which typically show minimal interaction with water [41,44,45,46]. It is evident from the results that most of the tested HDESs exhibited a remarkable ability to retain their hydrophobic properties, even when exposed to water for extended periods of time. This feature is especially beneficial for processes that involve frequent reuse of solvents. It helps to minimize the loss of solvents and ensures consistent extraction efficiency over time.
3.2. Screening of HDESs
During the initial screening phase, we evaluated six HDESs systems to determine their effectiveness in extracting 1,2-DCA from wastewater. The combinations that were tested include Men/Thy, Men/Dec, Thy/Dec, Hyd/Dec, Thy/Cou, and Thy/Cam. The extraction efficiencies showed variation, with Men/Thy and Thy/Cam demonstrating the highest performance, achieving efficiencies of 86.42% and 87.32%, respectively (Figure 5). These results highlight the superior extraction capabilities of these two HDES systems, making them prime candidates for further study.
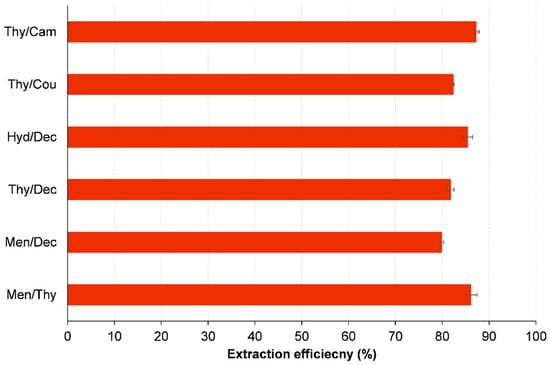
Figure 5.
Extraction efficiency (%) of 1,2-DCA by various HDESs. Initial concentration of 1,2-DCA is 5000 ppm; HDES-to-water mass ratio = 1:1; vortex mixing, 2000 rpm for 30 min at 298.15 K; centrifugation at 5000 rpm for 10 min.
The improved performance of Men/Thy and Thy/Cam can be ascribed to the distinctive molecular interactions present in these solvent pairs. The potential synergistic effect of combining menthol and thymol is believed to be due to the unique properties of each compound. Menthol, known for its flexibility, and thymol, with its aromatic structure, are believed to generate an optimal microenvironment that enhances the extraction of 1,2-DCA. The Thy/Cam system leverages the aromatic interactions of thymol and the strong hydrophobic character of camphor to enhance its ability to solubilize and extract the target contaminant. Based on the encouraging preliminary findings, the Men/Thy and Thy/Cam configurations were chosen for a more comprehensive parametric analysis. This analysis aims to enhance their performance by optimizing various parameters across different conditions.
3.3. Eeffect of Contact Time
To enhance the efficiency of 1,2-DCA extraction, the influence of contact time was assessed using the top two HDESs from the screening phase, namely Thy/Men and Thy/Cam. For each system, extraction efficiency was determined across a range of contact times, spanning from 5 to 60 min. The results for the Thy/Men system demonstrated a steady increase in efficiency from 85.51% at 5 min to a peak of 86.64% at 45 min. However, any further improvement beyond 15 min was limited, with efficiency stabilizing around 86%, suggesting that the extraction process achieved equilibrium relatively quickly, as can be seen in Figure 6.
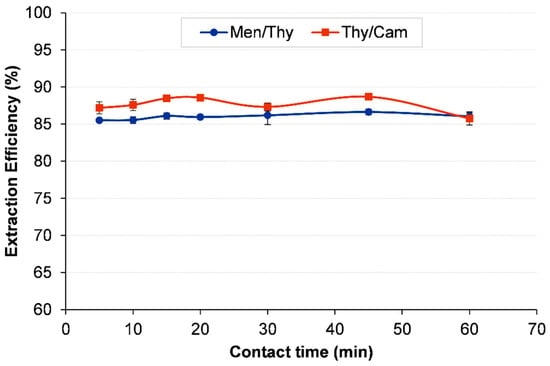
Figure 6.
Effect of contact time on the extraction of 1,2-DCA using Men/Thy and Thy/Cam HDESs. Initial concentration of 1,2-DCA is 5000 ppm; HDES-to-water mass ratio = 1:1; vortex mixing, 2000 rpm for 30 min at 298.15 K; centrifugation at 5000 rpm for 10 min.
In contrast, the Thy/Cam system exhibited a more noticeable rise in extraction efficiency, reaching a maximum of 88.69% at 45 min before slightly decreasing to 85.77% at 60 min. The highest efficiency (88.57%) was attained at just 15 min, after which only slight gains were observed with extended contact times. Considering that both systems showed minimal improvement beyond 15 min while maintaining high extraction efficiency, this contact time was deemed optimal for further studies. Choosing 15 min balances the extraction performance with time efficiency, making the process effective and practical for real-world applications.
3.4. Eeffect of HDES-to-Water Mass Ratio
The ratio of HDES to water is essential in determining the efficiency of 1,2-dichloroethane extraction. In this study, we examined various mass ratios for the HDES systems Thy/Men and Thy/Cam in order to determine the most effective ratio for achieving maximum extraction. The results are depicted in Figure 7. For the Thy/Men system, the 1:1 mass ratio resulted in an efficiency of 86.42%, which showed a slight improvement when the HDES concentration was increased to a 2:1 and 3:1 ratio, reaching up to 87.26%. Nevertheless, the efficiency experienced a significant drop when the HDES concentration was reduced to ratios of 1:2, 1:3, and 1:4. This suggests that a decrease in HDES content has a detrimental impact on the solvent’s ability to extract 1,2-dichloroethane. Although there was only a slight improvement at higher ratios, the 1:1 ratio was selected as the optimal choice due to its high efficiency and minimal solvent usage.
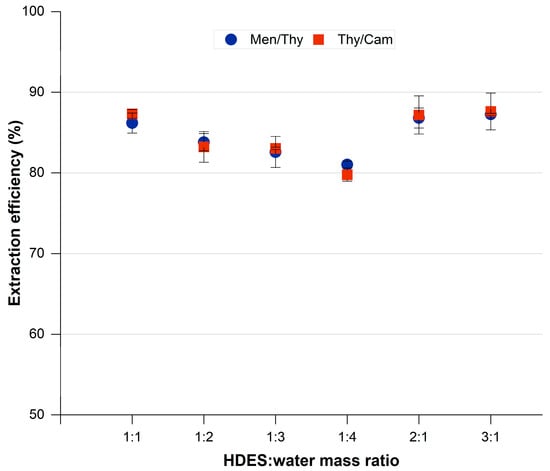
Figure 7.
Effect of water-to-HDES mass ratio on the extraction of 1,2-DCA. Initial concentration of 1,2-DCA is 5000 ppm; vortex mixing, 2000 rpm for 15 min at 298.15 K; centrifugation at 5000 rpm for 10 min.
For the Thy/Cam system, it is worth noting that the 1:1 ratio demonstrated an efficiency of 87.32%. By increasing the HDES concentration to ratios of 2:1 and 3:1, there were only slight improvements observed, with the highest efficiencies reaching 87.61%. On the other hand, reducing the HDES content resulted in a significant decrease in extraction performance. The efficiency dropped to as low as 79.78% when using the 1:4 ratio. After careful evaluation of performance and solvent economy, the optimal condition for this system was determined to be a 1:1 ratio. This ratio ensures a high extraction efficiency while minimizing the excessive use of HDESs, making it a practical choice for further parametric studies and real-world applications.
3.5. Eeffect of HDES Molar Ratio
The extraction effectiveness of hydrophobic deep eutectic solvents (HDESs) is usually influenced by the molar ratio of their components, since it affects the interactions between the solvent components and the contaminant being targeted. The Thy/Men system had the maximum extraction efficiency of 86.42% while using a 1:1 molar ratio. The appropriate chemical interactions between menthol and thymol in this balanced ratio enabled the solvent combination to effectively remove 1,2-dichloroethane. The extraction efficiency decreased when the ratio was altered to have an excess of either menthol (1:2 and 1:3) or thymol (2:1 and 3:1), as reported in Figure 8. The decrease in solubility is probably caused by an uneven distribution of the solvent molecules, where an excess of one component hinders the formation of optimal hydrogen bonding and van der Waals interactions necessary for efficient dissolution of the contaminant.
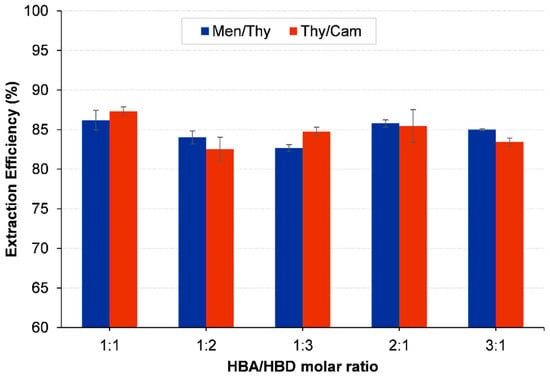
Figure 8.
Effect of HDES molar ratio on the extraction of 1,2-DCA. Initial concentration of 1,2-DCA is 5000 ppm; HDES-to-water mass ratio = 1:1; vortex mixing, 2000 rpm for 15 min at 298.15 K; centrifugation at 5000 rpm for 10 min.
In the Thy/Cam system, similar behavior was observed, with the highest efficiency attained at a 1:1 molar ratio, resulting in an extraction efficiency of 87.32%. The efficiency decreased significantly when the ratio was adjusted to favor either thymol or camphor. For example, when the molar ratio was 1:2 (with a preference for camphor), the efficiency decreased to 82.37%, and at a ratio of 3:1 (with a preference for thymol), it decreased to 83.43%. The decrease in efficiency resulting from modified ratios can be ascribed to the disturbance of the ideal solvent environment. An excessive quantity of either component might result in a dilution effect or an imbalance in the eutectic mixture, which diminishes the HDES’s capacity to generate stable microenvironments for extracting the contaminant. The findings highlight the need to maintain a balanced 1:1 molar ratio in order to preserve the structural integrity and extraction capabilities of the HDESs.
3.6. Effect of Initial Concentration of 1,2-DCA
The efficiency of the HDES extraction process for 1,2-dichloroethane (1,2-DCA) is strongly influenced by the initial concentration of the contaminant in the aqueous phase. In the Thy/Men system, the extraction efficiency increased as the 1,2-DCA concentration rose from 2000 ppm to 7000 ppm. At lower concentrations, the efficiency was only 50.13%, but it improved significantly at higher concentrations, reaching 86.47% at 5000 ppm and 91.82% at 7000 ppm (see Figure 9). This suggests that the HDES becomes more effective at extracting the contaminant as its concentration increases, likely due to the increased driving force for mass transfer.
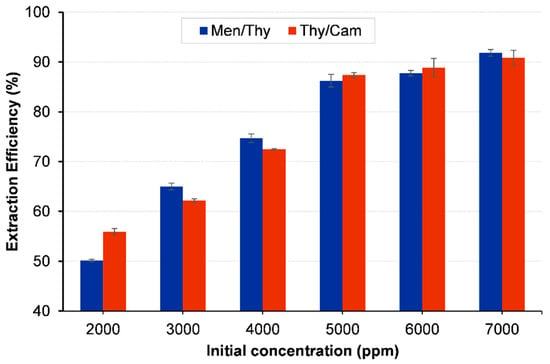
Figure 9.
Effect of initial concentration of 1,2-DCA on the extraction efficiency. Vortex mixing, 2000 rpm for 15 min at 298.15 K; centrifugation at 5000 rpm for 10 min.
A similar trend was observed in the Thy/Cam system. Efficiency improved from 55.92% at 2000 ppm to 90.86% at 7000 ppm. At concentrations below 4000 ppm, the extraction performance was moderate, but it increased sharply once the concentration reached 5000 ppm and above, where efficiencies exceeded 87%. This indicates that the HDES systems can effectively capture more 1,2-DCA when it is present in greater quantities. This behavior is likely due to the enhanced partitioning of 1,2-DCA into the HDES phase as its concentration rises, leading to more efficient extraction. These findings suggest that higher initial concentrations of 1,2-DCA favor the extraction process, making these HDESs particularly suitable for applications involving high contaminant loads.
3.7. Extraction Mechanism
COSMO-RS (Conductor-like Screening Model for Real Solvents) is a quantum chemistry-based model used to predict the thermodynamic properties of liquids, especially liquid–liquid equilibria. It integrates quantum chemical calculations with statistical thermodynamics and provides insights into molecular interactions and solubility behavior without the need for experimental data [47]. In the context of COSMO-RS, two important results are the sigma profile and the sigma potential.
The sigma profile represents the distribution of surface screening charge densities (σ) across a molecule. Peaks in this profile indicate the presence of electron-rich (negative σ) or electron-poor (positive σ) regions and thus reflect hydrogen bond acceptor (HBA) or donor (HBD) sites. On the other hand, the sigma potential curve provides information about the chemical potential of a molecule in an environment with different surface polarity and thus characterizes the affinity of the molecule for different types of interactions, such as polar or non-polar [48]. Both profiles can be divided into three regions: positive polarity (σ < −0.0084 eÅ2), non-polar (−0.0084 eÅ2 ≤ σ ≤ 0.0084 eÅ2), and negative polarity (σ > 0.0084 eÅ2). The boundaries of the individual regions are highlighted by the dashed lines. The interactions between the molecules of a system are taken into account by comparing the peak area between the curves at negative and positive σ values.
In this study, the COSMO-RS model was used to investigate the extraction mechanism of 1,2-dichloroethane (1,2-DCA) from aqueous media using two hydrophobic deep eutectic solvents (HDESs): thymol/camphor (Thy/Cam) and menthol/thymol (Men/Thy). Figure 10 shows the sigma profiles of 1,2-DCA, water, and the two DESs, while Figure 11 shows their respective sigma potentials.
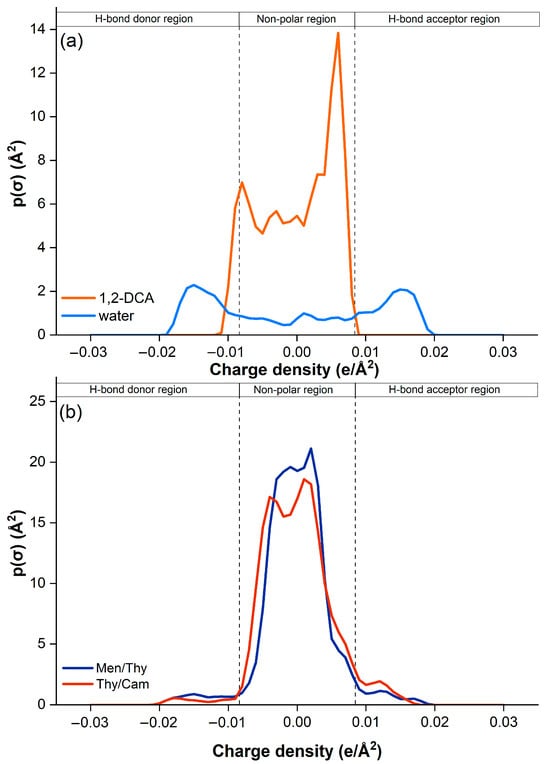
Figure 10.
COSMO-RS-predicted sigma profiles of (a) 1,2-DCA and water, and (b) DESs.
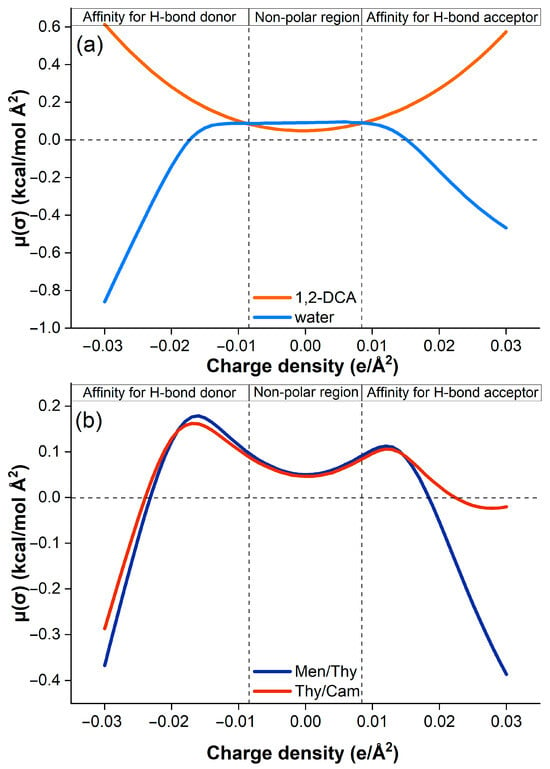
Figure 11.
COSMO-RS-predicted sigma potentials of (a) 1,2-DCA and water, and (b) DESs.
Figure 10a first shows that 1,2-DCA has a relatively narrow sigma distribution dominated by non-polar and weakly polar regions, with a negligible capacity for hydrogen bonding. In contrast, water shows a very sharp and narrow peak in the negative sigma region, indicating its strong hydrogen bond donor (HBD) character. On the other hand, Figure 10b shows that both Thy/Cam and Men/Thy exhibit a broader sigma distribution extending into both the HBA and the HBD regions. This reflects their amphiphilic nature, possessing both hydrophilic (polar) and lipophilic (non-polar) properties, as well as their ability to participate in a range of non-covalent interactions, which is critical for efficient extraction of weakly polar compounds such as 1,2-DCA [49]. Indeed, Figure 11a confirms this characteristic where the sigma potential of 1,2-DCA is rather flat and close to zero, indicating a weak affinity for polar environments and weak hydrogen bonding. Water, on the other hand, has a steep sigma potential, which favors strongly polar environments and underlines its high HBD character.
Figure 11b also illustrates the interaction potential of the two HDESs. Both Thy/Cam and Men/Thy show more favorable sigma potentials over a wider range of σ values, especially in the non-polar and weakly polar regions. This confirms their compatibility with 1,2-DCA, such that strong van der Waals and dispersion interactions dominate the extraction process.
Furthermore, the good extraction performance of the terpene-based DESs obtained in this work can be explained by the molecular structures of the DES components. In fact, thymol contains an aromatic ring and a hydroxyl group, which enable both hydrophobic interactions and hydrogen bonding. Camphor, a bicyclic ketone, contributes with its strong hydrophobicity to enhance the ability of Thy/Cam DES to interact with and solubilize 1,2-DCA. Similarly, menthol, which is present in the Men/Thy DES, introduces a flexible, aliphatic hydroxyl structure that interacts with thymol to create a less viscous and more dynamic microenvironment.
Compared to other HDESs, the broad sigma profiles and moderate sigma potentials of Men/Thy and Thy/Cam enable more effective uptake of 1,2-DCA molecules, which is mainly driven by hydrophobic and dispersion forces. Their ability to solubilize 1,2-DCA while excluding water (due to minimal hydrophilic domains) leads to superior extraction efficiency, which is confirmed by the previously reported experimental results.
In summary, the qualitative results of COSMO-RS confirm the experimental performance of HDESs. The unique molecular properties and interaction profiles of Men/Thy and Thy/Cam make them highly effective for the extraction of weakly polar pollutants such as 1,2-DCA from aqueous environments, which is consistent with the environmentally friendly and sustainability goals of this study.
4. Conclusions
This study systematically evaluated the performance of solvents known as HDESs in extracting 1,2-DCA from wastewater. After screening six HDES systems, menthol/thymol (Men/Thy) and thymol/camphor (Thy/Cam) were found to be the most effective, leading to further investigation of their performance under different operating conditions. A detailed study of the parameters revealed that both systems achieved optimal extraction efficiency within 15 min, indicating rapid equilibrium and suitability for time-sensitive processes. The study also found that a 1:1 mass ratio of HDES to water was the most efficient, allowing for high extraction performance while minimizing solvent use, which is crucial for sustainable operation in practical applications.
Further analysis of the HDES molar ratios revealed that a 1:1 ratio was optimal for both Men/Thy and Thy/Cam systems. Deviations from this balance resulted in decreased efficiency due to disruptions in the solvent’s molecular interactions, which are critical for the selective capture of 1,2-DCA. The study also found that higher initial concentrations of 1,2-DCA in the wastewater led to increased extraction efficiencies. The study suggests that as the contaminant concentration increases, the driving force for mass transfer strengthens, leading to improved partitioning into the HDES phase. These findings demonstrate that both Men/Thy and Thy/Cam HDES systems exhibit robust performance across a range of conditions, making them viable options for large-scale industrial applications in wastewater treatment. Unlike traditional solvents, these HDESs combine high efficiency with environmental sustainability, offering a greener alternative for the chemical industry. It is worth noting that although the regeneration of HDESs is not addressed in this work, it can be easily performed by distillation due to the large difference between the boiling points of 1,2-dichloroethane (83.5 °C) and the individual HDES components (menthol: 212 °C, thymol: 232 °C, and camphor: 209 °C). The rotary evaporator can be used on a laboratory scale.
In conclusion, although this study makes a significant contribution to environmentally friendly solvent-based contaminant extraction, the transition from laboratory scale to industrial application still requires more comprehensive studies, especially in terms of HDES recovery, toxicity, and techno-economic feasibility. Future work should focus on reconciling the development of HDESs with industrial process integration, circular economy principles, and regulatory requirements to ensure both performance and sustainability.
Author Contributions
Conceptualization, I.W. and M.K.H.-K.; methodology, I.W., A.O., and L.E.B.; software, I.W. and M.K.H.-K.; validation, I.W., E.A., and M.K.H.-K.; formal analysis, I.W. and L.E.B.; investigation, M.K.H.-K., L.E.B., and I.W.; resources, E.A. and M.K.H.-K.; data curation, I.W., E.A., and M.K.H.-K.; writing—I.W. and M.K.H.-K.; writing—review and editing, I.W. and M.K.H.-K.; supervision, M.K.H.-K.; project administration, E.A., S.M., and M.K.H.-K.; funding acquisition, E.A., S.M., and M.K.H.-K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Plan for Science, Technology and Innovation (NPSTI, KACST) of Saudi Arabia, which supported this work through project number 14-ENV-1934-02.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Larsen, T.A.; Hoffmann, S.; Lüthi, C.; Truffer, B.; Maurer, M. Emerging solutions to the water challenges of an urbanizing world. Science 2016, 352, 928–933. [Google Scholar] [CrossRef]
- Al-Baldawi, I.A. Removal of 1, 2-Dichloroethane from real industrial wastewater using a sub-surface batch system with Typha angustifolia L. Ecotoxicol. Environ. Saf. 2018, 147, 260–265. [Google Scholar] [CrossRef]
- Jeong, W.-G.; Kim, J.-G.; Baek, K. Removal of 1, 2-dichloroethane in groundwater using Fenton oxidation. J. Hazard. Mater. 2022, 428, 128253. [Google Scholar] [CrossRef]
- Wu, S.; Wang, P.; Fang, N.; Wang, Y.; Ding, S.; Chu, Y. Insight into catalytic performance and reaction mechanism for 1,2-dichloroethane elimination over MnCoOx-supported catalyst. Fuel 2024, 360, 130551. [Google Scholar] [CrossRef]
- Yazdanbakhsh, A.R.; Jaafarzadeh, N.; Hematian, S.; Sheikhmohammadi, A. Removal of 1,2-dichloroethane from industrial wastewater with membrane filtration. J. Health Field 2014, 2, 8–15. [Google Scholar]
- Motomizu, S.; Sakai, T. On-line sample pretreatment: Extraction and preconcentration. In Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2008; Volume 54, pp. 159–201. [Google Scholar]
- Zhou, Z.; Huang, J.; Danish, M.; Zeng, G.; Yang, R.; Gu, X.; Ali, M.; Lyu, S. Insights into enhanced removal of 1, 2-dichloroethane by amorphous boron-enhanced Fenton system: Performances and mechanisms. J. Hazard. Mater. 2021, 420, 126589. [Google Scholar] [CrossRef]
- Lee, H.-H.; Hirano, Y.; Murayama, N.; Matsumoto, S.; Shibata, J. Adsorption properties of activated carbon prepared from waste beer lees by KOH activation and CO2 activation. Resour. Process. 2007, 54, 19–24. [Google Scholar] [CrossRef]
- Reyes, J.; Arriola, E.; Hernández, J.A.; Fuentes, G.A. Liquid-phase chloroform hydrodechlorination catalyzed by Pd/TiO2–Na. Appl. Catal. A Gen. 2015, 497, 211–215. [Google Scholar]
- Baric, M.; Majone, M.; Beccari, M.; Papini, M.P. Coupling of polyhydroxybutyrate (PHB) and zero valent iron (ZVI) for enhanced treatment of chlorinated ethanes in permeable reactive barriers (PRBs). Chem. Eng. J. 2012, 195, 22–30. [Google Scholar] [CrossRef]
- Nunez Garcia, A.; Boparai, H.K.; O’Carroll, D.M. Enhanced dechlorination of 1,2-dichloroethane by coupled nano iron-dithionite treatment. Environ. Sci. Technol. 2016, 50, 5243–5251. [Google Scholar] [CrossRef]
- Eydivand, S.; Nikazar, M. Degradation of 1,2-dichloroethane in simulated wastewater solution: A comprehensive study by photocatalysis using TiO2 and ZnO nanoparticles. Chem. Eng. Commun. 2015, 202, 102–111. [Google Scholar] [CrossRef]
- Büyüksönmez, F.h.; Hess, T.F.; Crawford, R.L.; Watts, R.J. Toxic effects of modified Fenton reactions on Xanthobacter flavus FB71. Appl. Environ. Microbiol. 1998, 64, 3759–3764. [Google Scholar] [CrossRef]
- Bello, M.M.; Raman, A.A.A.; Asghar, A. A review on approaches for addressing the limitations of Fenton oxidation for recalcitrant wastewater treatment. Process Saf. Environ. Prot. 2019, 126, 119–140. [Google Scholar] [CrossRef]
- Liu, X.; Vellanki, B.P.; Batchelor, B.; Abdel-Wahab, A. Degradation of 1, 2-dichloroethane with advanced reduction processes (ARPs): Effects of process variables and mechanisms. Chem. Eng. J. 2014, 237, 300–307. [Google Scholar] [CrossRef]
- Capodaglio, A.G. Critical perspective on advanced treatment processes for water and wastewater: AOPs, ARPs, and AORPs. Appl. Sci. 2020, 10, 4549. [Google Scholar] [CrossRef]
- De Marines, F.; Cruciata, I.; Di Bella, G.; Di Trapani, D.; Giustra, M.G.; Calabrisotto, L.S.; Lucchina, P.G.; Quatrini, P.; Viviani, G. Degradation of 1,2-dichloroethane in real polluted groundwater by using enriched bacterial consortia in aerobic and anaerobic laboratory-scale conditions. Int. Biodeterior. Biodegrad. 2023, 183, 105644. [Google Scholar] [CrossRef]
- Wu, Z.; Yu, X.; Liu, G.; Li, W.; Lu, L.; Li, P.; Xu, X.; Jiang, J.; Wang, B.; Qiao, W. Sustained detoxification of 1, 2-dichloroethane to ethylene by a symbiotic consortium containing Dehalococcoides species. Environ. Pollut. 2023, 325, 121443. [Google Scholar] [CrossRef]
- Draga, M.; Ciucanu, I.; Agotici, V.; Fernandez, A.; Barna, R. Quantitative evaluation of perchlorethylene in groundwater before and after its oxidation by helical solid sorbent extraction and gas chromatography. Chemosphere 2009, 75, 1210–1214. [Google Scholar] [CrossRef]
- Dolatabadi, M.; Ghorbanian, A.; Ahmadzadeh, S. Degradation of high-concentration of perchloroethylene from aqueous solution using electro-fenton process. J. Environ. Health Sustain. Dev. 2022, 7, 1676–1683. [Google Scholar] [CrossRef]
- Liu, X.; Wu, M.; Zhao, J. Removal of trichloroethylene from water by bimetallic Ni/Fe nanoparticles. Water 2022, 14, 1616. [Google Scholar] [CrossRef]
- Dominguez, C.M.; Rodriguez, V.; Montero, E.; Romero, A.; Santos, A. Methanol-enhanced degradation of carbon tetrachloride by alkaline activation of persulfate: Kinetic model. Sci. Total Environ. 2019, 666, 631–640. [Google Scholar] [CrossRef]
- Li, B.; Li, L.; Lin, K.; Zhang, W.; Lu, S.; Luo, Q. Removal of 1, 1, 1-trichloroethane from aqueous solution by a sono-activated persulfate process. Ultrason. Sonochem. 2013, 20, 855–863. [Google Scholar] [CrossRef]
- Almomani, F.; Rene, E.R.; Veiga, M.C.; Bhosale, R.R.; Kennes, C. Treatment of waste gas contaminated with dichloromethane using photocatalytic oxidation, biodegradation and their combinations. J. Hazard. Mater. 2021, 405, 123735. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, Y.; Cao, Z.; Li, Y.; Bai, Y.; Zhang, X.; Li, T.; Ren, B. Absorption of ethylene dichloride with imidazolium-based ionic liquids. J. Mol. Liq. 2023, 376, 121449. [Google Scholar] [CrossRef]
- Gui, C.; Li, G.; Lei, Z.; Wei, Z.; Dong, Y. Experiment and molecular mechanism of two chlorinated volatile organic compounds in ionic liquids. Ind. Eng. Chem. Res. 2023, 62, 1160–1171. [Google Scholar] [CrossRef]
- Song, M.; Gui, C.; Lei, Z. Experimental and Simulation Studies on the Capture of Chlorinated Volatile Organic Compounds by Ionic Liquids. Ind. Eng. Chem. Res. 2023, 62, 10184–10194. [Google Scholar] [CrossRef]
- Yang, C.; Yu, X.; Wang, L.; Shi, M.; He, G.; Li, Q. Experimental measurement and thermodynamic modelling of liquid-liquid equilibria for the separation of 1,2-dichloroethane from cyclohexane using various extractants. J. Mol. Liq. 2018, 252, 263–270. [Google Scholar] [CrossRef]
- Rawa-Adkonis, M.; Wolska, L.; Przyjazny, A.; Namieśnik, J. Sources of errors associated with the determination of PAH and PCB analytes in water samples. Anal. Lett. 2006, 39, 2317–2331. [Google Scholar] [CrossRef]
- Tshepelevitsh, S.; Hernits, K.; Jenčo, J.; Hawkins, J.M.; Muteki, K.; Solich, P.; Leito, I. Systematic optimization of liquid–liquid extraction for isolation of unidentified components. ACS Omega 2017, 2, 7772–7776. [Google Scholar] [CrossRef]
- Almohasin, J.A.; Balag, J.; Miral, V.G.; Moreno, R.V.; Tongco, L.J.; Lopez, E.C.R. Green Solvents for Liquid–Liquid Extraction: Recent Advances and Future Trends. Eng. Proc. 2023, 56, 174. [Google Scholar]
- Rudrapal, M.; Kothawade, A.P.; Ezzat, S.M.; Egbuna, C. Bioanalysis: Methods, techniques, and applications. In Analytical Techniques in Biosciences; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–24. [Google Scholar]
- Wazeer, I.; Mulyono, S.; Halilu, A.; Hizaddin, H.F.; Hashim, M.A.; Hadj-Kali, M.K. Comparative analysis of lead and cadmium extraction capacities of hydrophobic deep eutectic solvents. J. Ind. Eng. Chem. 2025, 143, 303–318. [Google Scholar] [CrossRef]
- Wazeer, I.; Hizaddin, H.F.; Wen, N.X.; El Blidi, L.; Hashim, M.A.; Hadj-Kali, M.K. Extraction of phenol as pollutant from aqueous effluents using hydrophobic deep eutectic solvents. Water 2023, 15, 4289. [Google Scholar] [CrossRef]
- Cichowska-Kopczyńska, I.; Nowosielski, B.; Warmińska, D. Deep eutectic solvents: Properties and applications in CO2 separation. Molecules 2023, 28, 5293. [Google Scholar] [CrossRef]
- Lee, J.; Jung, D.; Park, K. Hydrophobic deep eutectic solvents for the extraction of organic and inorganic analytes from aqueous environments. TrAC Trends Anal. Chem. 2019, 118, 853–868. [Google Scholar] [CrossRef]
- Van Osch, D.J.; Zubeir, L.F.; Van Den Bruinhorst, A.; Rocha, M.A.; Kroon, M.C. Hydrophobic deep eutectic solvents as water-immiscible extractants. Green Chem. 2015, 17, 4518–4521. [Google Scholar] [CrossRef]
- Xing, J.; Liu, X.; Dai, Y.; Zhang, Y.; Su, Z.; Chen, Z.; Gao, J.; Wang, Y.; Cui, P. Phase behavior and extraction mechanism of ethanol in alcohol ester mixture separated by deep eutectic solvents. J. Mol. Liq. 2022, 368, 120694. [Google Scholar] [CrossRef]
- Zafarani-Moattar, M.T.; Shekaari, H.; Dizaj, A.S. Investigation of solute-solvent interactions in binary and quaternary solutions containing lithium perchlorate, propylene carbonate, and the deep eutectic solvent (choline chloride/ethylene glycol) at T=(288.15 to 318.15) K. J. Mol. Liq. 2020, 319, 114090. [Google Scholar] [CrossRef]
- Adeyemi, A.; Bergman, J.; Branalt, J.; Sävmarker, J.; Larhed, M. Continuous flow synthesis under high-temperature/high-pressure conditions using a resistively heated flow reactor. Org. Process Res. Dev. 2017, 21, 947–955. [Google Scholar] [CrossRef]
- Wazeer, I.; Hizaddin, H.F.; Mulyono, S.; Hashim, M.A.; Hadj-Kali, M.K. Removal of cresol contaminants from aqueous media using hydrophobic deep eutectic solvents. J. Mol. Liq. 2024, 407, 125082. [Google Scholar] [CrossRef]
- Vlachoudi, D.; Chatzimitakos, T.; Athanasiadis, V.; Bozinou, E.; Lalas, S.I. Enhanced extraction of carotenoids from tomato industry waste using menthol/fatty acid deep eutectic solvent. Waste 2023, 1, 977–992. [Google Scholar] [CrossRef]
- Fan, C.; Sebbah, T.; Liu, Y.; Cao, X. Terpenoid-capric acid based natural deep eutectic solvent: Insight into the nature of low viscosity. Clean. Eng. Technol. 2021, 3, 100116. [Google Scholar] [CrossRef]
- Ribeiro, B.D.; Florindo, C.; Iff, L.C.; Coelho, M.A.; Marrucho, I.M. Menthol-based eutectic mixtures: Hydrophobic low viscosity solvents. ACS Sustain. Chem. Eng. 2015, 3, 2469–2477. [Google Scholar] [CrossRef]
- Martins, M.A.; Crespo, E.A.; Pontes, P.V.; Silva, L.P.; Bülow, M.; Maximo, G.J.; Batista, E.A.; Held, C.; Pinho, S.P.; Coutinho, J.A. Tunable hydrophobic eutectic solvents based on terpenes and monocarboxylic acids. ACS Sustain. Chem. Eng. 2018, 6, 8836–8846. [Google Scholar] [CrossRef]
- Almustafa, G.; Sulaiman, R.; Kumar, M.; Adeyemi, I.; Arafat, H.A.; AlNashef, I. Boron extraction from aqueous medium using novel hydrophobic deep eutectic solvents. Chem. Eng. J. 2020, 395, 125173. [Google Scholar] [CrossRef]
- Klamt, A. Conductor-like screening model for real solvents: A new approach to the quantitative calculation of solvation phenomena. J. Phys. Chem. 1995, 99, 2224–2235. [Google Scholar] [CrossRef]
- Klamt, A.; Eckert, F. COSMO-RS: A novel and efficient method for the a priori prediction of thermophysical data of liquids. Fluid Phase Equilib. 2000, 172, 43–72. [Google Scholar] [CrossRef]
- Stefanovic, R.; Ludwig, M.; Webber, G.B.; Atkin, R.; Page, A.J. Nanostructure, hydrogen bonding and rheology in choline chloride deep eutectic solvents as a function of the hydrogen bond donor. Phys. Chem. Chem. Phys. 2017, 19, 3297–3306. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).