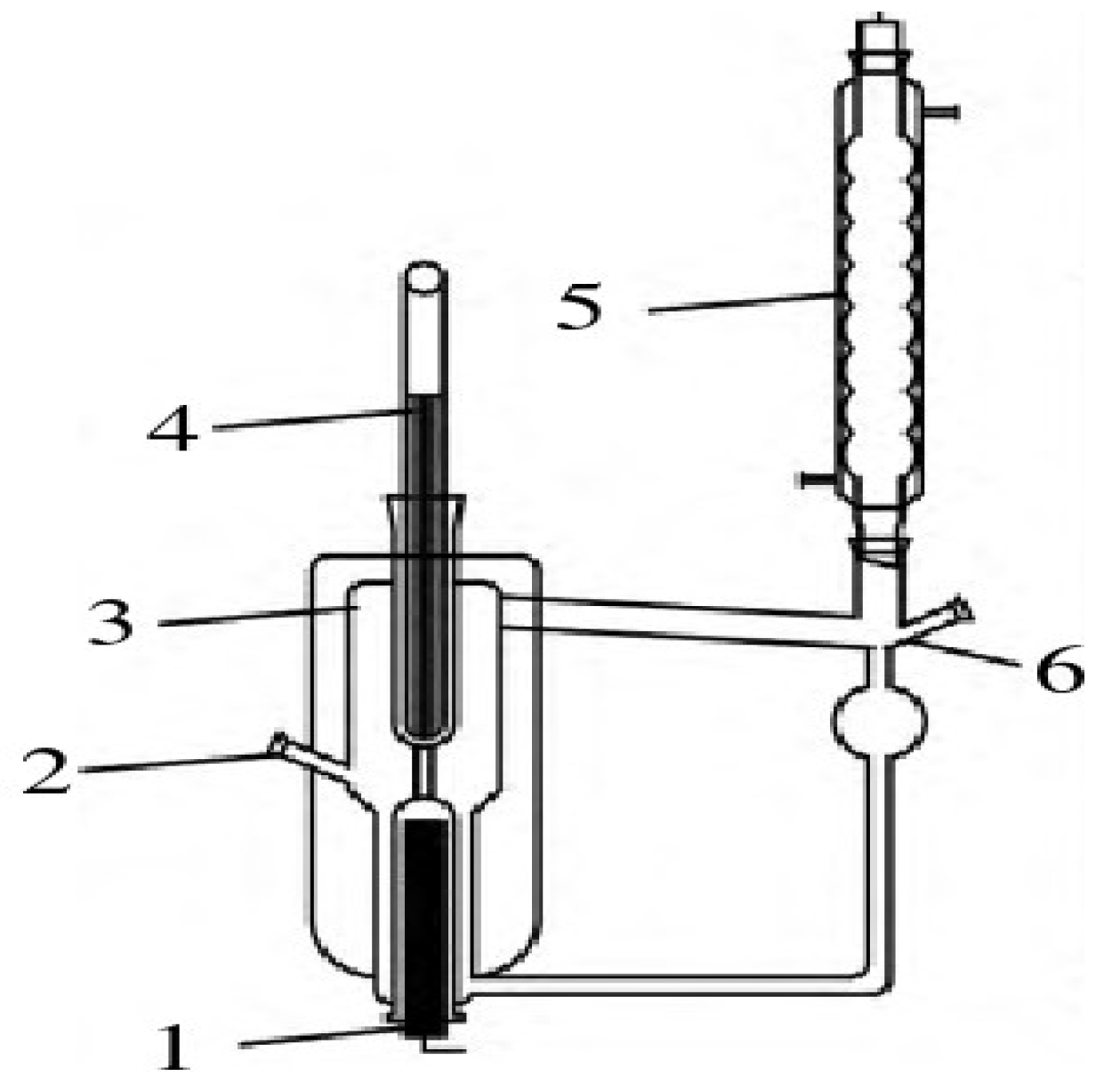
1. Introduction
EM, also known as methyl cellosolve, is an important chemical raw material [
1], Due to the presence of both hydrophobic and hydrophilic functional groups in this compound, it is widely utilized in diverse industrial and commercial applications, including household products, paints, inks [
2], coatings, cleaning solutions and biochemical applications [
1,
3]. Since the H2O/EM system forms a completely miscible binary azeotrope, specialized distillation methods must be employed for separation, including extractive distillation [
4,
5], azeotropic distillation [
6,
7], and salting-out distillation [
8]. In this study, 1-pentanol, 1-hexanol, and 1-heptanol were selected as azeotropic agents to separate the H
2O/EM azeotropic mixture. The advantages of azeotropic distillation in this separation process lie in its ability to break azeotropic limitations, technological maturity, and high purity of the final product. Compared to alternative technologies such as extraction and membrane separation, azeotropic distillation demonstrates strong competitiveness due to its reliability and high product purity.
Currently, there are no experimental reports on VLE data for entrainer screening in the H
2O/EM azeotropic system. To separate the azeotropic mixture using azeotropic distillation and selected entrainers, binary isobaric VLE data for the systems are required (H
2O/EM, EM/1-pentanol, EM/1-hexanol, EM/1-heptanol, H
2O/1-pentanol, H
2O/1-hexanol and H
2O/1-heptanol). Numerous studies have reported binary VLE data for the H
2O/EM azeotropic system. Bejarano [
9] studied the system under varying pressures (74.5 kPa, 101.3 kPa, and 134.0 kPa). Ochi K [
10] reported data for the H
2O/1-hexanol system under the 101.3 kPa, Krishnaiah reported the vapor liquid equilibrium data for EM/1-pentanol at 40 kPa and 95 kPa [
11], and in NIST, there are binary VLE data for H
2O/1-pentanol at 101.3 kPa. Notably, the NIST database currently lacks data for the following binary systems at 101.3 kPa (EM/1-pentanol, EM/1-hexanol, EM/1-heptanol, and H
2O/1-heptanol).
In this work, binary VLE data were measured at 101.3 kPa for the following systems (H
2O/EM, EM/1-pentanol, EM/1-hexanol, EM/1-heptanol, H
2O/1-pentanol, H
2O/1-hexanol and H
2O/1-heptanol). The measured binary VLE data were validated for thermodynamic consistency using the Herington area test [
12]. The experimental data were correlated with the Non-Random Two-Liquid (NRTL) [
13], Universal Quasi-Chemical (UNIQUAC) [
14], and Wilson activity coefficient models [
15,
16]. The azeotropic distillation process was simulated using Aspen Plus to evaluate the separation performance and determine the optimal operating parameters.
3. Experimental Results and Discussion
3.1. VLE Data of Binary Systems
Under low-pressure or medium-pressure conditions, the expressions for VLE [
27,
28] are as follows:
Among them,
xi and
yi represent the mole fractions of component
i in the liquid and vapor phases, respectively,
P denotes the system pressure, and
Pis is the saturation vapor pressure of pure component
i, which can be calculated using the Extended Antoine Equation [
29] from Aspen V12 Plus. Compared to the standard Antoine equation [
30], the additional parameters introduced in the Extended Antoine Equation enhance its flexibility and allow it to describe the entire vapor pressure curve.
Table 3 lists the Antoine parameters for
C1 to
C9. Extended Antoine Equation Calculation:
Among them,
T (K) represents the system temperature, and
P (kPa) denotes the system pressure. Based on the calculations using the above formulas and parameters,
Table 4,
Table 5,
Table 6,
Table 7,
Table 8,
Table 9 and
Table 10 present the experimentally obtained binary VLE data and activity coefficients (
γi). As observed in
Table 8,
Table 9 and
Table 10, activity coefficients (
γ) exhibit abnormally high values in some cases. This can be attributed to the significant difference between the strong hydrogen bonding among H
2O molecules and the pronounced hydrophobicity of the long alkyl chain of alcohol molecules. This leads directly to highly non-ideal behavior in the liquid phase and a strong tendency for liquid-phase splitting (partial miscibility). In dilute aqueous solutions, alcohol molecules exhibit extremely high fugacity, indicating a substantial tendency to “escape” from the liquid phase, which is reflected as activity coefficients γ >> 1.
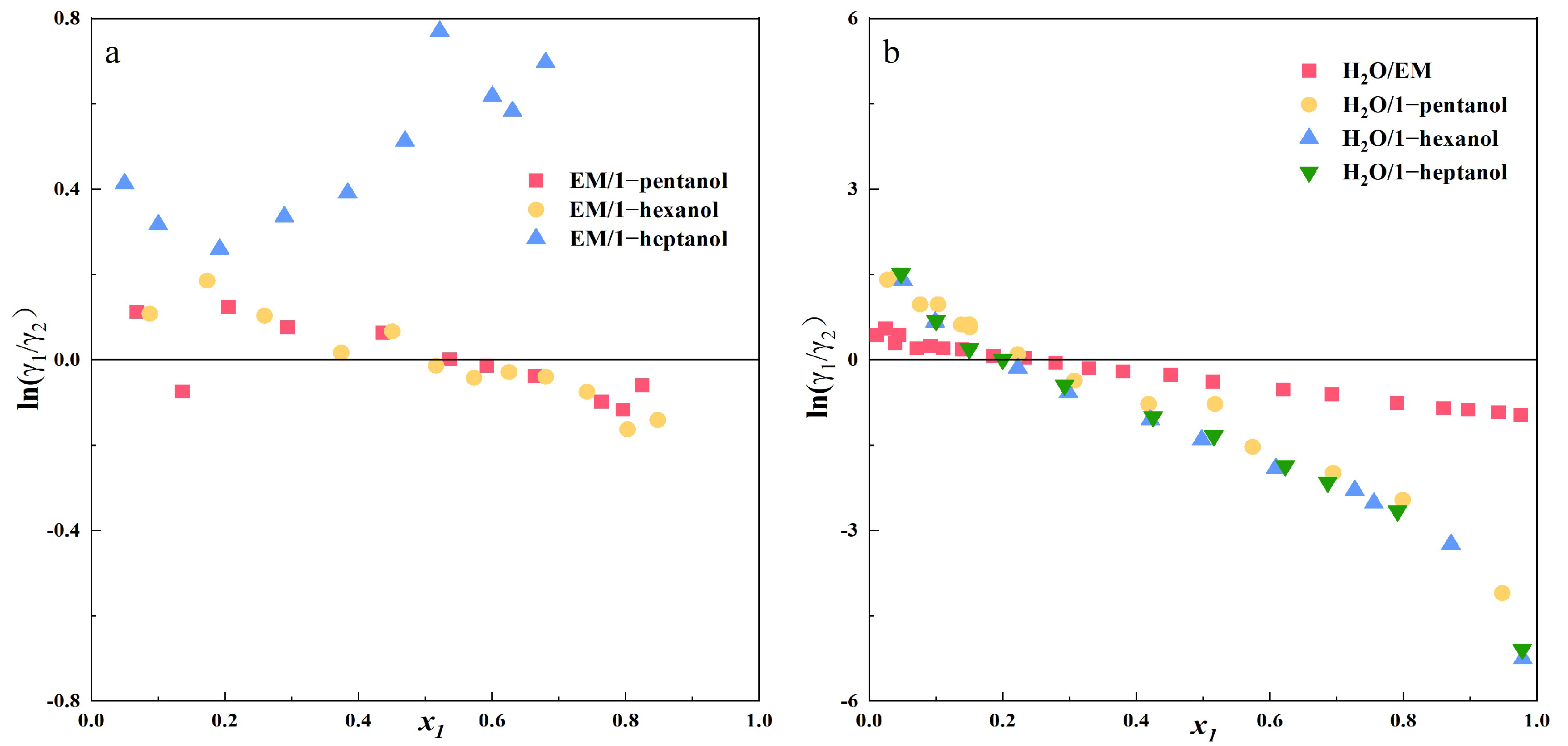
3.2. Thermodynamic Consistency Test for the Experimental Data (Herington Integral Method)
To ensure the accuracy and reliability of the measured VLE data, thermodynamic consistency tests must be performed on the experimental data. Common methods include the integral test, differential test, and slope method. To assess adherence to the Gibbs-Duhem equation, the thermodynamic consistency of the experimental phase equilibrium data was rigorously evaluated via Herington area test [
31]. The Herington method is a semi-empirical approach that involves plotting
x1 against ln(
γ1/
γ2) (as shown in
Figure 3) and defining and examining the relationship between
D and
J. The specific steps are as follows:
Among them, Tmax (K) corresponds to the maximum temperature of the system. Tmin (K) corresponds to the minimum temperature of the system.
The calculation results are presented in
Table 11,
D −
J represents the criterion for evaluating thermodynamic consistency using the Herington integral test method. If |
D −
J| < 10, the data exhibits reasonable reliability. As shown in
Table 11, the measured VLE data for the binary system demonstrate reliable consistency.
3.3. Correlation of Binary VLE Data
To validate experimental data, the VLE data obtained from experiments were correlated using the NRTL, UNIQUAC, and Wilson activity coefficient models, which appear in Equations (7)–(9). The calculated correlated binary interaction parameters are listed in
Table 12, and a comparison between experimental and predicted data is shown in
Figure 4. To quantify model prediction fidelity, root mean square deviations (RMSD) of vapor-phase mole fractions and equilibrium temperatures were computed via Equations (10) and (11), serving as key metrics for thermodynamic model validity.
NRTL:
where
.
UNIQUAC:
where
.
Wilson:
where
.
The experimental results indicate that the investigated H2O–long-chain alcohol system exhibits highly non-ideal behavior. During the correlation process, although the NRTL model demonstrated the best correlation accuracy within the experimental data range, the optimized binary interaction parameters exhibited abnormally large absolute values. This clearly reveals the inherent mathematical limitations of local composition models in describing such extremely non-ideal systems characterized by strong asymmetry and a tendency for liquid-phase splitting. These abnormal parameters likely result from over-parameterization, where the model forces a perfect fit to a limited dataset, leading to physically meaningless values. Consequently, the obtained model parameters should be used strictly for interpolation within the experimental data range. Extrapolation for predictions is strongly discouraged, as it would yield unreliable results.
As indicated by the binary correlated interaction parameters and root mean square deviations (RMSD) listed in the table, all three activity coefficient models can satisfactorily correlate the H2O/EM system. The figure demonstrates that each model is capable of describing this thermodynamic system with reasonable accuracy. Moreover, 1-pentanol, 1-hexanol, and 1-heptanol are all able to break the azeotropic system of H2O/EM. Data indicate that the mass fractions of H2O in their azeotropes with water are approximately 56%, 68%, and 83%, respectively.
From the experimental data, the azeotropic temperature of H
2O and EM is 99.9 °C, and the boiling point of the new azeotrope formed by the azeotrope and H
2O should be lower than that of the original azeotrope, while the temperature difference between the new azeotrope formed by 1-hexanol and 1-heptanol and H
2O is too small compared with the original azeotropic temperature, which will cause separation difficulties. Secondly, the larger the proportion of H
2O in the azeotropic composition, the less azeotropic dose required to remove the H
2O from the system, that is, the less separation load, which is more conducive to reducing the energy consumption of the subsequent distillation column. It can be seen from
Table 13 that the azeotropic composition of the three azeotropic agents accounts for a large proportion of H
2O. In summary, 1-pentanol is the best choice among the three azeotropes.
3.4. Molecular Interaction Simulation
Molecular interaction energy simulations depicted in
Figure 5 enabled the calculation of Δ
E via Equation (2), yielding values of −6.348 kJ/mol for H
2O/EM, 3.058 kJ/mol for EM/1-pentanol, 3.115 kJ/mol for EM/1-hexanol, 3.162 kJ/mol for EM/1-heptanol, −4.691 kJ/mol for H
2O/1-pentanol, −4.701 kJ/mol for H
2O/1-hexanol, and −4.694 kJ/mol for H
2O/1-heptanol. Systems exhibiting negative deviations (H
2O/EM, H
2O/1-pentanol, H
2O/1-hexanol, and H
2O-1-heptanol; all Δ
E < 0) corresponded to minimum H
2O activity coefficients of 0.9962, 0.6867, 0.7580. Conversely, systems showing positive deviations (EM/1-pentanol, EM/1-hexanol, and EM/1-heptanol; all Δ
E > 0) displayed maximum EM activity coefficients of 1.1857, 1.2116. Critically, these molecular simulation outcomes demonstrate quantitative consistency with experimental VLE data across all investigated systems.
5. Conclusions
VLE data for seven binary systems were measured at atmospheric pressure using a modified Othmer still, filling a significant data gap. All experimental data passed the thermodynamic consistency test via Herington’s area method. Using the Aspen Plus process simulation software, the parameters for the NRTL, UNIQUAC, and Wilson activity coefficient models were regressed, yielding parameter sets applicable at atmospheric pressure. The results indicate that all three activity coefficient models can satisfactorily correlate the H2O/EM system. Molecular interaction energy simulations agreed with the experimental VLE data, confirming the reliability of the data provided in this work. The analysis shows that 1-pentanol is the optimal choice among the three evaluated entrainers.
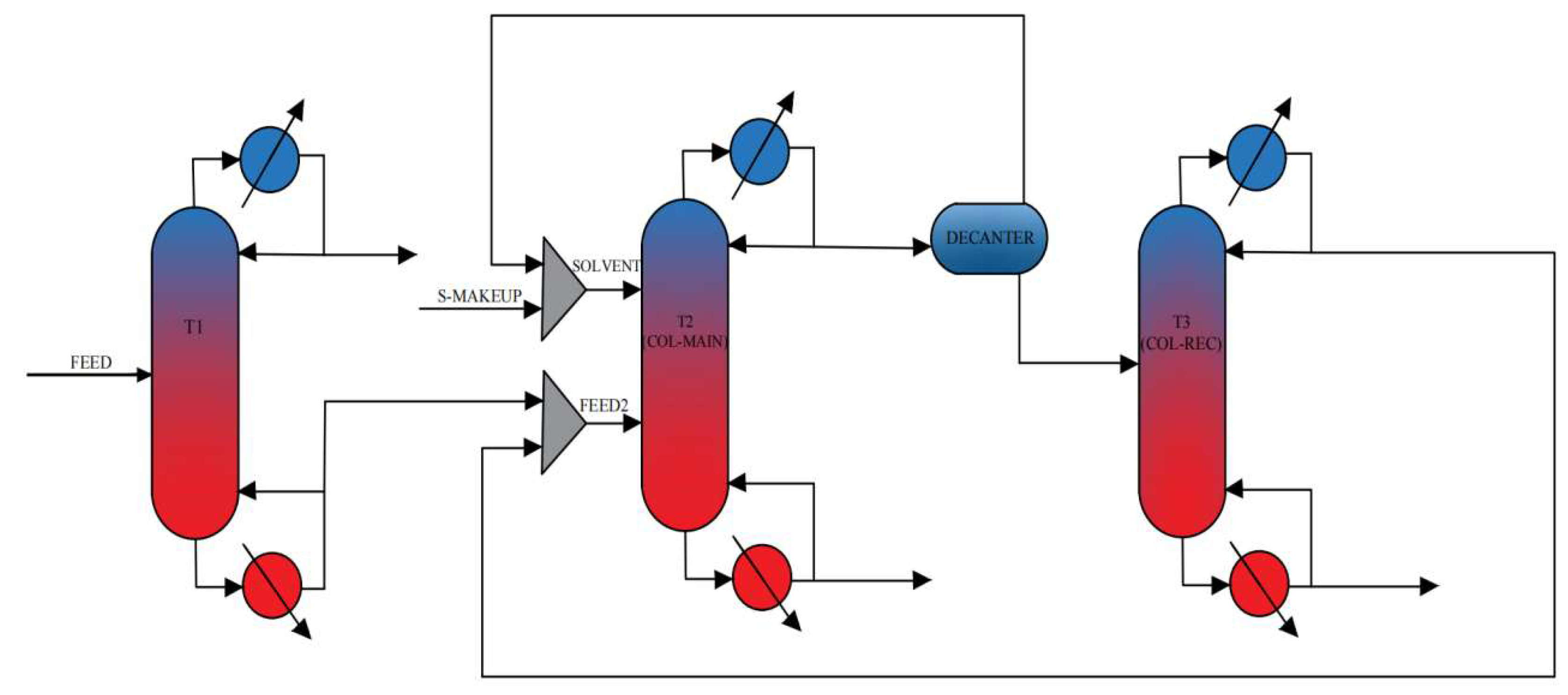
Subsequently, the azeotropic distillation process was simulated using Aspen Plus to evaluate the separation performance and identify the optimal operating parameters. This simulation provided the foundation for conceptual process design and preliminary thermodynamic assessment. Upon finalizing the conceptual design, addressing operational challenges such as emulsion formation and entrainer loss will become critical obstacles to overcome during subsequent detailed engineering design and pilot-scale scaling. These issues represent key focus areas for future work.