Pilot-Scale Phycocyanin Extraction by the Green Two-Step Ultrasound-Based UltraBlu Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Organism and Culture Conditions
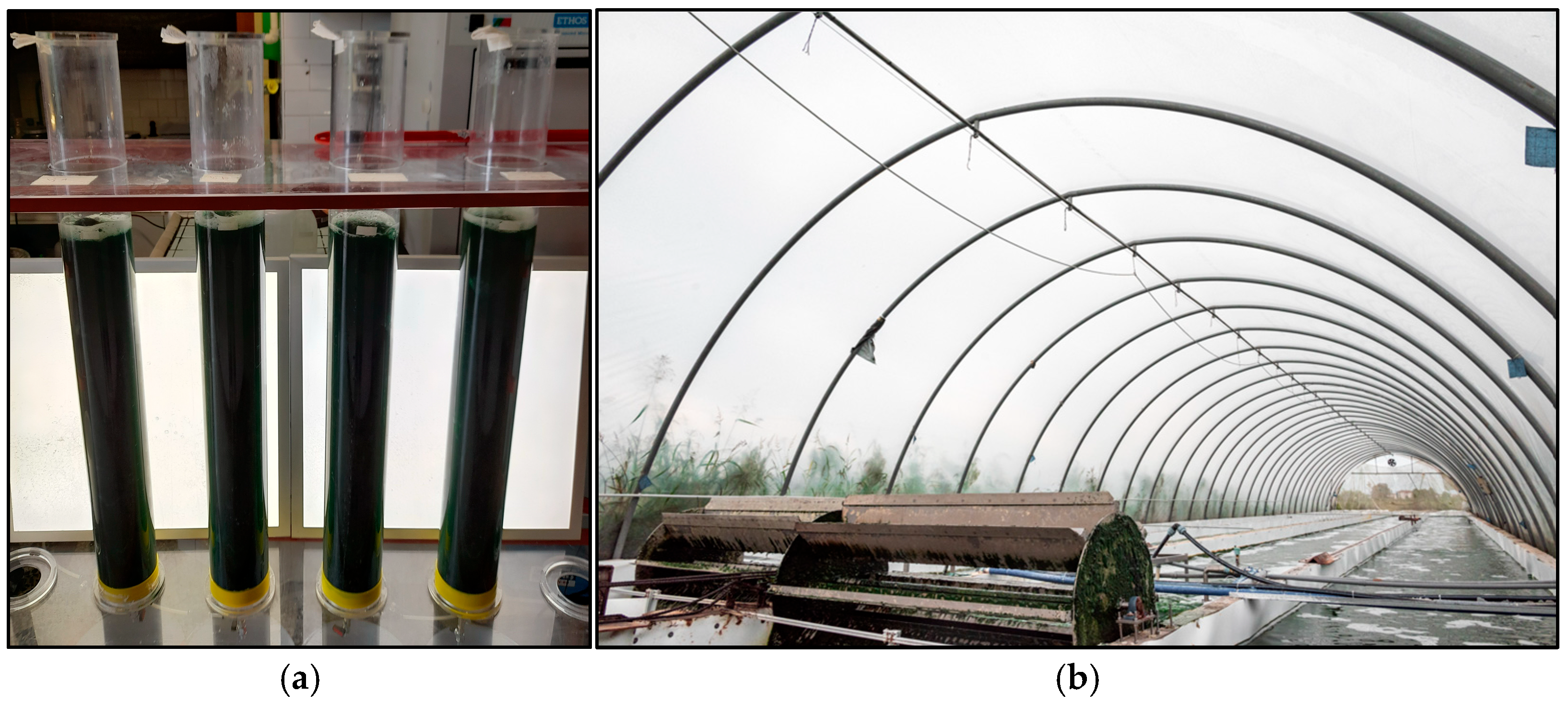
2.1.1. Laboratory Scale
2.1.2. Pilot Scale
2.2. Phycocyanin Extraction Procedures
2.2.1. One-Step Conventional Ultrasound-Assisted Extraction Procedures for PC Control-Extraction and Optimization of Large-Scale Cell Lysis
Control Extraction Procedure
Optimization of Cell Lysis on Lab-Scale Processing 3–4 L of Biomass Suspension
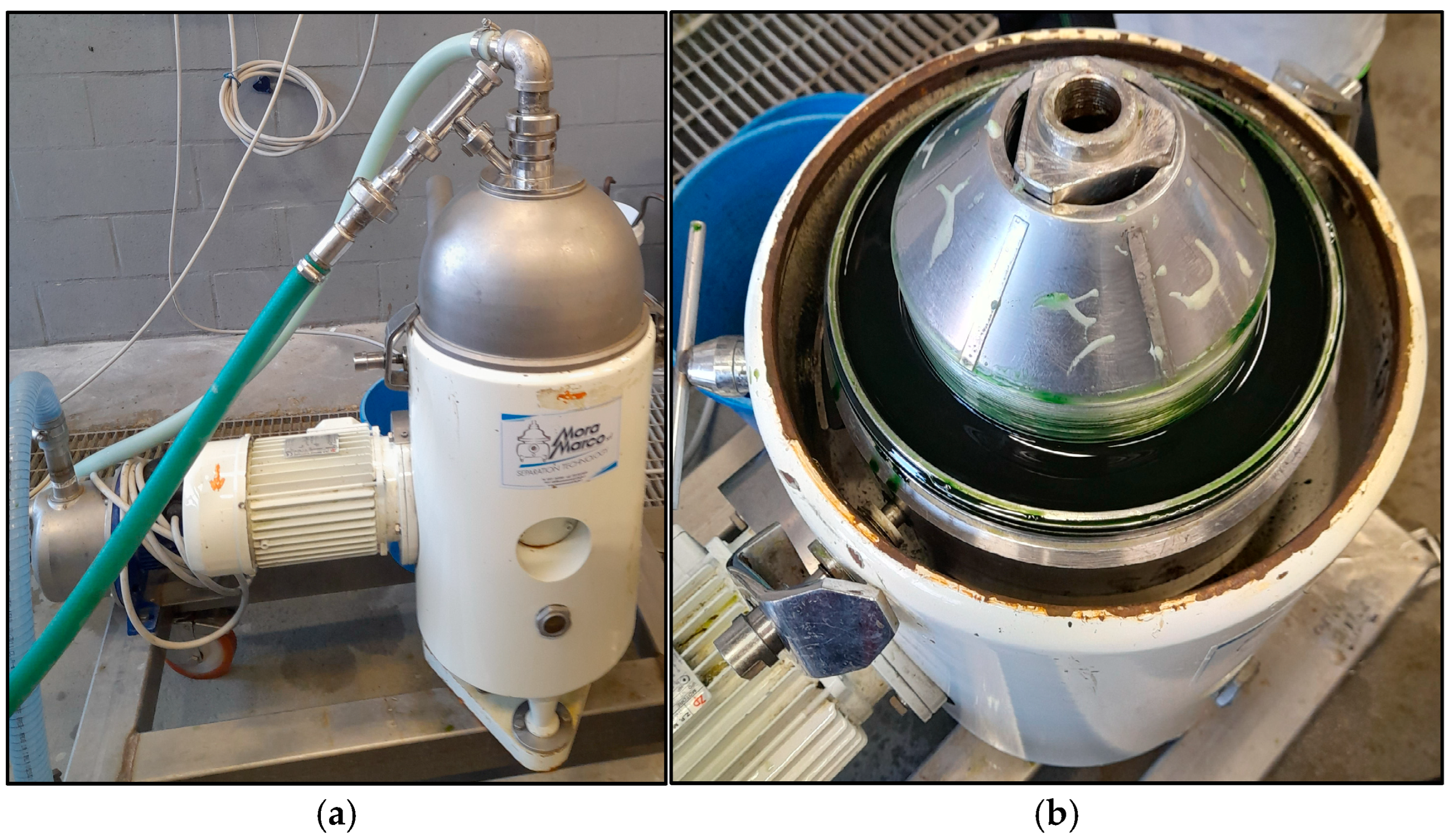
Optimization of Cell Lysis on Pilot-Scale
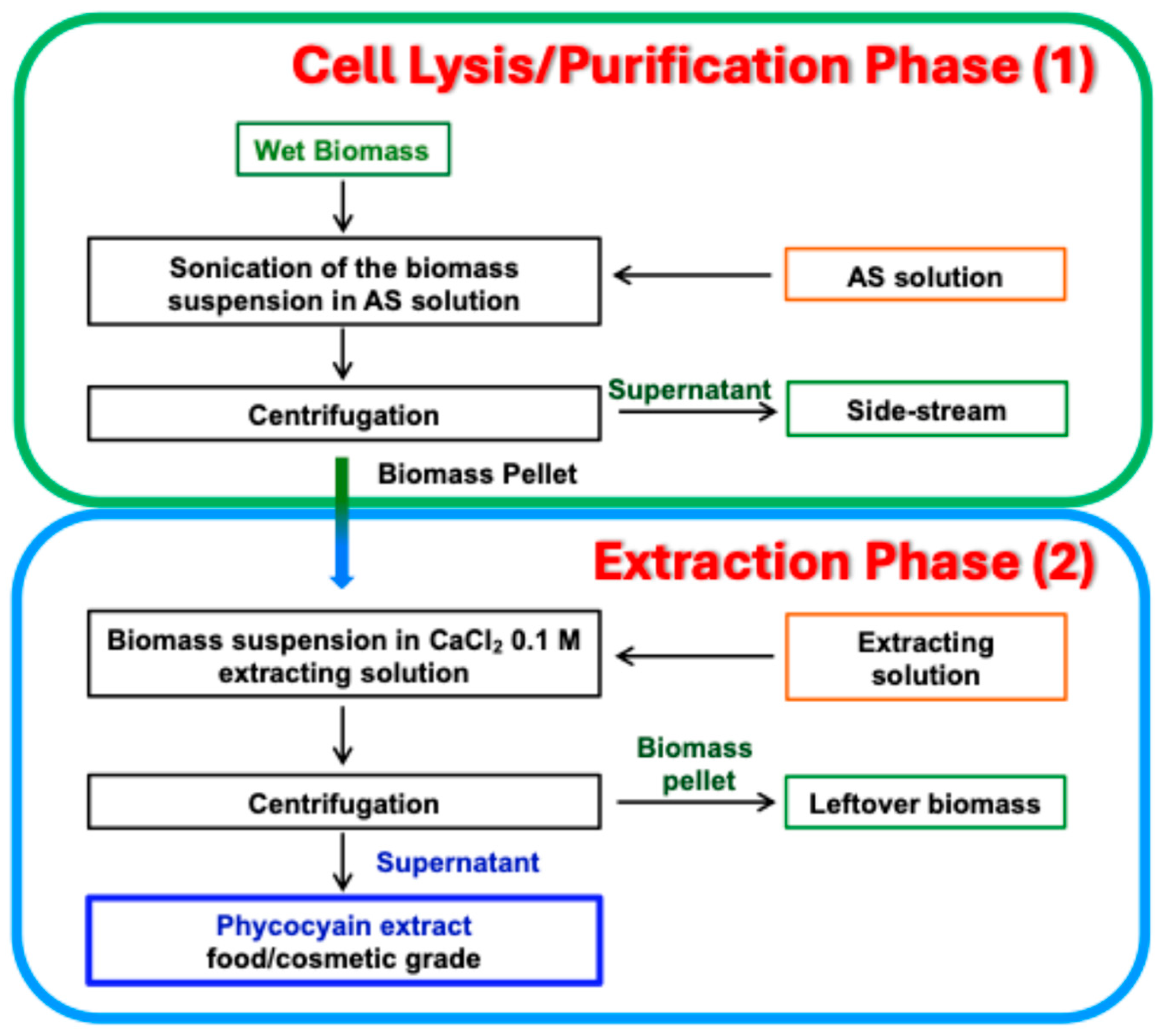
2.2.2. UltraBlu Extraction Process
Laboratory-Scale UltraBlu Extraction Process
- Step 1—Cell lysis/purification phase. Biomass was suspended in AS 1.2 M (final volume 3 L) and ultrasonicated at A 100% under stirring for 2 h (ultrasonication time), while being kept in an ice-water bath (temperature continually checked by a temperature probe). The lysed biomass was separated from the AS solution by centrifuging.
- Step 2—Extraction phase. The lysed biomass was resuspended in CaCl2 0.1 M extracting solution (final volume 1.7 L) and stirred for 2 h (extraction time). Finally, the supernatant (crude extract) was recovered by centrifuging and PBP content and PC purity determined by spectrophotometric analysis.
Pilot-Scale UltraBlu Extraction Process
- Step 1—Cell lysis/purification phase. Biomass was suspended in AS 1.2 M (final volume 50 L) and ultrasonicated at A 100% under stirring for 1:30 h (ultrasonication time), while being kept in an ice-water bath (temperature continually checked by a temperature probe). The lysed biomass was separated from the AS solution by centrifuging.
- Step 2—Extraction phase. The lysed biomass was resuspended in CaCl2 0.1 M extracting solution (final volume 50 L) and stirred for 2 h (extraction time). Finally, the supernatant (crude extracts) was recovered by centrifuging and PBP content and PC purity determined by spectrophotometric analysis after having filtered the solution with a 0.45 μm or 0.22 μm pore-size membrane to eliminate chlorophyll contamination.
2.3. Spectrophotometric Analyses
3. Results
3.1. Laboratory-Scale Optimization of PC Extraction by UltraBlu Process
3.2. Pilot-Scale PC Extraction by UltraBlu Process
4. Discussion
PC Extraction by UltraBlu Process
5. Conclusions
6. Patents
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bürck, M.; Fratelli, C.; Campos Assumpção de Amarante, M.; Braga, A.R.C. Unveiling the potential of Spirulina biomass—A Glimpse into the future circular economy using green and blue ingredients. Biomass 2024, 4, 704–719. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, Y.; Zhang, R.; Cai, T.; Cai, Y. Medical application of Spirulina platensis derived C-phycocyanin. Evid.-Based Compl. Alt. 2016, 2016, 7803846. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Wu, Y.; Wang, G.; Jia, T.; Zhang, Y. Purification and bioactivities of phycocyanin. Crit. Rev. Food Sci. 2017, 57, 3840–3849. [Google Scholar] [CrossRef]
- Ashaolu, T.J. The powerful phycobiliproteins-phycocyanin and phycoerythrin: Pleiotropic applications and biofunctional uses. Algal Res. 2024, 82, 103636. [Google Scholar] [CrossRef]
- Mao, M.; Han, G.; Zhao, Y.; Xu, X.; Zhao, Y. A review of phycocyanin: Production, extraction, stability and food applications. Int. J. Biol. Macromol. 2024, 280, 135860. [Google Scholar] [CrossRef]
- Minić, S.; Gligorijević, N.; Veličković, L.; Nikolić, M. Narrative review of the current and future perspectives of Phycobiliproteins’ applications in the Food Industry: From natural colors to alternative proteins. Int. J. Mol. Sci. 2024, 25, 7187. [Google Scholar] [CrossRef] [PubMed]
- Aoki, J.; Koshikawa, R.; Asayama, M. Recent progress in the cyanobacterial products and applications of phycocyanins. World J. Microb. Biot. 2025, 41, 84. [Google Scholar] [CrossRef]
- Nowicka-Krawczyk, P.; Mühlsteinová, R.; Hauer, T. Detailed characterization of the Arthrospira type species separating commercially grown taxa into the new genus Limnospira (Cyanobacteria). Sci. Rep. 2019, 9, 694. [Google Scholar] [CrossRef] [PubMed]
- Vieira, V.V.; Benemann, J.; Vonshak, A.; Belay, A.; Ras, M.; Unamunzaga, C.; Cadoret, J.P.; Rizzo, A. Spirulina in the 21st century: Five reasons for success in Europe. J. Appl. Phycol. 2025, 37. [Google Scholar] [CrossRef]
- Chini Zittelli, G.; Lauceri, R.; Faraloni, C.; Silva Benavides, A.M.; Torzillo, G. Valuable pigments from microalgae: Phycobiliproteins, primary carotenoids, and fucoxanthin. Photochem. Photobiol. Sci. 2023, 22, 1733–1789. [Google Scholar] [CrossRef]
- Lehto, S.; Buchweitz, M.; Klimm, A.; Straßburger, R.; Bechtold, C.; Ulberth, F. Comparison of food colour regulations in the EU and the US: A review of current provisions. Food Addit. Contam. Part A 2017, 34, 335–355. [Google Scholar] [CrossRef]
- Lauceri, R.; Cavone, C.; Chini Zittelli, G.; Kamburska, L.; Musazzi, S.; Torzillo, G. High purity grade phycocyanin recovery by decupling cell lysis from the pigment extraction: An innovative approach. Food Bioprocess Technol. 2023, 16, 111–121. [Google Scholar] [CrossRef]
- TechSci Reasearch. Phycocyanin Market—Global Industry Size, Share, Trends, Opportunity, and Forecast, Segmented by Nature (Organic, Conventional), by Form (Powder, Liquid), by Application (Food & Beverages, Nutraceuticals, Animal Feed, Cosmetics & Personal Care, Others), by Region and Competition, 2020–2030. Available online: https://www.techsciresearch.com/report/phycocyanin-market/19026.html (accessed on 6 June 2025).
- Athiyappan, K.D.; Routray, W.; Paramasivan, B. Phycocyanin from Spirulina: A comprehensive review on cultivation, extraction, purification, and its application in food and allied industries. Food Humanit. 2024, 2, 100235. [Google Scholar] [CrossRef]
- Tan, H.T.; Yusof, F.M.; Khaw, Y.S.; Ahmad, S.A.; Shaharuddin, N.A. Uncovering research trends of phycobiliproteins using bibliometric approach. Plants 2021, 10, 2358. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Coutinho, J.A.; Weingarten, M. Sustainable recovery of microbial-derived natural pigments using deep eutectic solvents: Advances, potential, and challenges. Sep. Purif. Technol. 2025, 361, 131413. [Google Scholar] [CrossRef]
- Masoumi, S.; Zokaei, M.; Ahmadvand, A.; Ghalamkarpour, N.; Asadimanesh, N.; Azarimatin, A.; Fattah, K.; Payandeh, Z.; Rostami, M.; Farjami, A.; et al. Microalgae: A Treasure Trove of Anticancer Nutraceuticals and Promising Therapeutic Mechanisms. Adv. Biomed. Res. 2025, 14, 5. [Google Scholar] [CrossRef] [PubMed]
- Usai, L.; Torre, S.; Aktay, N.; Dunford, N.T.; Citi, V.; Flori, L.; Nieri, P.; Lutzu, G.A. Recent advancements in production and extraction methods of phycobiliprotein c-phycocyanin by arthrospira (spirulina) platensis: A mini review. Curr. Microbiol. 2024, 81, 428. [Google Scholar] [CrossRef]
- Jaeschke, D.P.; Teixeira, I.R.; Marczak, L.D.F.; Mercali, G.D. Phycocyanin from Spirulina: A review of extraction methods and stability. Food Res. Int. 2021, 143, 110314. [Google Scholar] [CrossRef]
- Gomes-Diaz, J.S.; Teixeira, J.A.; Rocha, C.M. Recent advances in the valorization of algae polysaccharides for food and nutraceutical applications: A review on the role of green processing technologies. Food Bioprocess Technol. 2022, 15, 1948–1976. [Google Scholar] [CrossRef]
- Yu, Z.; Zhao, W.; Sun, H.; Mou, H.; Liu, J.; Yu, H.; Dai, L.; Kong, Q.S. Phycocyanin from microalgae: A comprehensive review covering microalgal culture, phycocyanin sources and stability. Food Res. Int. 2024, 186, 114362. [Google Scholar] [CrossRef]
- Deniz, I.; Ozen, M.O.; Yesil-Celiktas, O. Supercritical fluid extraction of phycocyanin and investigation of cytotoxicity on human lung cancer cells. J. Supercrit. Fluids 2016, 108, 13–18. [Google Scholar] [CrossRef]
- Teixeira, I.R.; Marczak, L.D.F.; Mercali, G.D.; Jaeschke, D.P. Saline extraction assisted by ultrasound: A method to obtain purified phycocyanin. J. Biotechnol. 2024, 384, 38–44. [Google Scholar] [CrossRef]
- Vernes, L.; Abert-Vian, M.; El Maâtaoui, M.; Tao, Y.; Bornard, I.; Chemat, F. Application of ultrasound for green extraction of proteins from spirulina. Mechanism, optimization, modeling, and industrial prospects. Ultrason. Sonochem. 2019, 54, 48–60. [Google Scholar] [CrossRef]
- Kannaujiya, V.K.; Sundaram, S.; Sinha, R.P. Phycobiliproteins: Recent Developments and Future Applications; Springer Nature: Singapore, 2017; 147p. [Google Scholar] [CrossRef]
- İlter, I.; Akyıl, S.; Demirel, Z.; Koç, M.; Conk-Dalay, M.; Kaymak-Ertekin, F. Optimization of phycocyanin extraction from Spirulina platensis using different techniques. J. Food Compos. Anal. 2018, 70, 78–88. [Google Scholar] [CrossRef]
- Bachchhav, M.B.; Kulkarni, M.V.; Ingale, A.G. Process-intensified extraction of phycocyanin followed by β-carotene from Spirulina platensis using ultrasound-assisted extraction. Sep. Sci. Technol. 2020, 55, 932–944. [Google Scholar] [CrossRef]
- Sommer, M.C.; Balazinski, M.; Rataj, R.; Wenske, S.; Kolb, J.F.; Zocher, K. Assessment of phycocyanin extraction from Cyanidium caldarium by spark discharges, compared to freeze-thaw cycles, sonication, and pulsed electric felds. Microorganisms 2021, 9, 1452. [Google Scholar] [CrossRef]
- Zarrouk, C. Contribution a l’etude d’une Cyanophycee. Influence de Divers Facteurs Physiques et Chimiques sur la croissance et la photosynthese de Spirulina maxima. Ph. D. Thesis, University of Paris, Paris, France, 1966. [Google Scholar]
- Bennett, A.; Bogorad, L. Complementary chromatic adaptation in a filamentous blue-green alga. J. Cell Biol. 1973, 58, 419–435. [Google Scholar] [CrossRef] [PubMed]
- Boussiba, S.; Richmond, A.E. Isolation and characterization of phycocyanins from the blue-green alga Spirulina platensis. Arch. Microbiol. 1979, 120, 155–159. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.; Yu, W.; Wang, G.; Cifuentes, A.; Ibañez, E.; Lu, W. Microalgal proteins: Extraction, interfacial properties, bioactivities, and future perspectives—A review. Food Chem. 2025, 486, 144680. [Google Scholar] [CrossRef]
- Obeid, S.; Rida, H.; Peydecastaing, J.; Takache, H.; Ismail, A.; Pontalier, P.Y. Coupling ultrasound and membrane filtration for the fractionation of Spirulina platensis sp. and the recovery of phycocyanin and pigment-free proteins. Biotechnol. Lett. 2025, 47, 8. [Google Scholar] [CrossRef]
- Khalid, S.; Chaudhary, K.; Aziz, H.; Amin, S.; Sipra, H.M.; Ansar, S.; Rasheed, H.; Naeem, M.; Onyeaka, H. Trends in extracting protein from microalgae Spirulina platensis, using innovative extraction techniques: Mechanisms, potentials, and limitations. Crit. Rev. Food Sci. 2024, 65, 4293–4309. [Google Scholar] [CrossRef] [PubMed]



| Extraction Tests of PBPs: TEstr 1 = 2 h | Control: Lab-Scale One-Step Direct Ultrasound-Assisted Extraction V = 5–6 mL; TUS 2 = 8 min; TEstr = 2 h | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A. platensis Strain | Process | Scale | Volume Processed | R 3 | TUS (h:mm) | PBPs 4 Yield % (d.w.) ± SD (no. = 2) | P 5 ± SD (no. = 2) | R | PBPs Yield % (d.w.) ± SD (no. = 2) | P ± SD (no. = 2) |
| M2M | One-step 6 | Lab-scale | 700 mL | 461 | 0:12 | Yrel 7 = 98.7 ± 0.7 | 1.82 ± 0.00 | 711 | Y 8 = 23.9 ± 1.3 | 1.77 ± 0.01 |
| M2M | One-step | Lab-scale | 700 mL | 223 | 0:15 | Yrel = 89.7± 01 | 1.67 ± 0.01 | 401 | Y = 23.5 ± 0.1 | 1.72 ± 0.02 |
| M2M | One-step | Lab-scale | 3 L | 313 | 1:45 | Yrel = 90.7± 0.7 | 1.54 ± 0.01 | 1560 | Y = 22.1 ± 0.3 | 1.63 ± 0.00 |
| M2M | One-step | Lab-scale | 4 L | 258.4 | 2:30 | Yrel = 82.5 ± 2.4 | 1.73 ± 0.05 | 1292 | Y = 30.1 ± 1.0 | 1.75 ± 0,01 |
| M2M | One-step | Lab-scale | 4 L | 145.6 | 2:30 | Yrel = 82.4 ± 3.3 | 1.80 ± 0.01 | 728 | Y = 24.3 ± 1.0 | 1.82 ± 0.01 |
| Algaria-001 | UltraBlu 9 | Lab-scale | 3 L (Step 1) 1.7 L (Step 2) | 96.2 (Step 1) 54.5 (Step 2) | 2:00 | Yrel = 86.1 (no. = 1) | 2.58 (no. = 1) | 280 | Y = 23.9 ± 0.5 | 0.84 ± 0.01 |
| Algaria-001 | One-step | Pilot-scale | 50 L | 575 | 1:30 | Yrel = 95.8 ± 3.3 | 0.79 ± 0.00 | 575 | Y = 10.6 ± 0.5 | 0.81 ± 0.01 |
| Algaria-001 | One-step | Pilot-scale | 50 L | 61.3 | 1:30 | Yrel = 92.4 ± 1.1 | 0.67 ± 0.00 | 307 | Y = 10.6 ± 0.1 | 0.66 ± 0.01 |
| Algaria-001 | UltraBlu | Pilot-scale | 50 L | 266 | 1:30 | Yrel = 59.2 (no. = 1) | 1.72 (no. = 1) | 194 | Y = 8.7 ± 0.2 | 0.61 ± 0.00 |
| Algaria-001 | UltraBlu | Pilot-scale | 50 L | 70.5 | 1:30 | Yrel = 76.1 (no. = 1) | 1.41 (no. = 1) | 572 | Y = 8.1 ± 1.4 | 0.65 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lauceri, R.; Pignataro, M.; Giorgi, A.; Idà, A.; Kamburska, L. Pilot-Scale Phycocyanin Extraction by the Green Two-Step Ultrasound-Based UltraBlu Process. Separations 2025, 12, 194. https://doi.org/10.3390/separations12080194
Lauceri R, Pignataro M, Giorgi A, Idà A, Kamburska L. Pilot-Scale Phycocyanin Extraction by the Green Two-Step Ultrasound-Based UltraBlu Process. Separations. 2025; 12(8):194. https://doi.org/10.3390/separations12080194
Chicago/Turabian StyleLauceri, Rosaria, Melissa Pignataro, Antonio Giorgi, Antonio Idà, and Lyudmila Kamburska. 2025. "Pilot-Scale Phycocyanin Extraction by the Green Two-Step Ultrasound-Based UltraBlu Process" Separations 12, no. 8: 194. https://doi.org/10.3390/separations12080194
APA StyleLauceri, R., Pignataro, M., Giorgi, A., Idà, A., & Kamburska, L. (2025). Pilot-Scale Phycocyanin Extraction by the Green Two-Step Ultrasound-Based UltraBlu Process. Separations, 12(8), 194. https://doi.org/10.3390/separations12080194








