The Role of Freezing Temperature in Modulating Chitosan Gel Structure and Evaporation Performance for Seawater Desalination
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Crosslinking Solution
2.3. Preparation of CPG Gel Evaporators
2.4. Preparation of CPG Gels and PPy Particles
2.5. Characterization of Gel Evaporators
2.6. Evaporation Test
2.7. Swelling Test
2.8. Light Absorption Test
2.9. Long-Term Evaporation Test
3. Results and Discussion
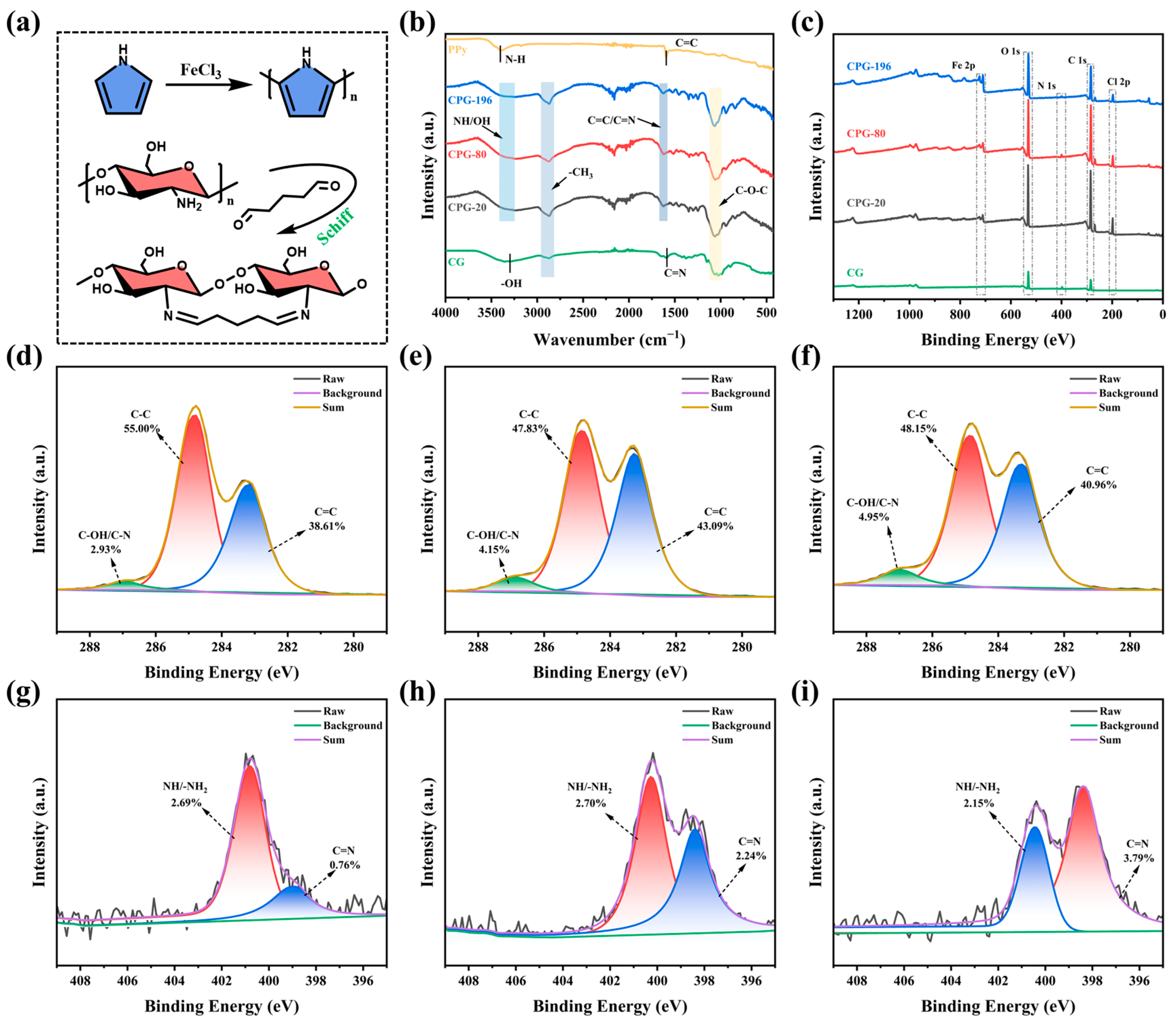
3.1. Characterization of CPG-X Gel Evaporators
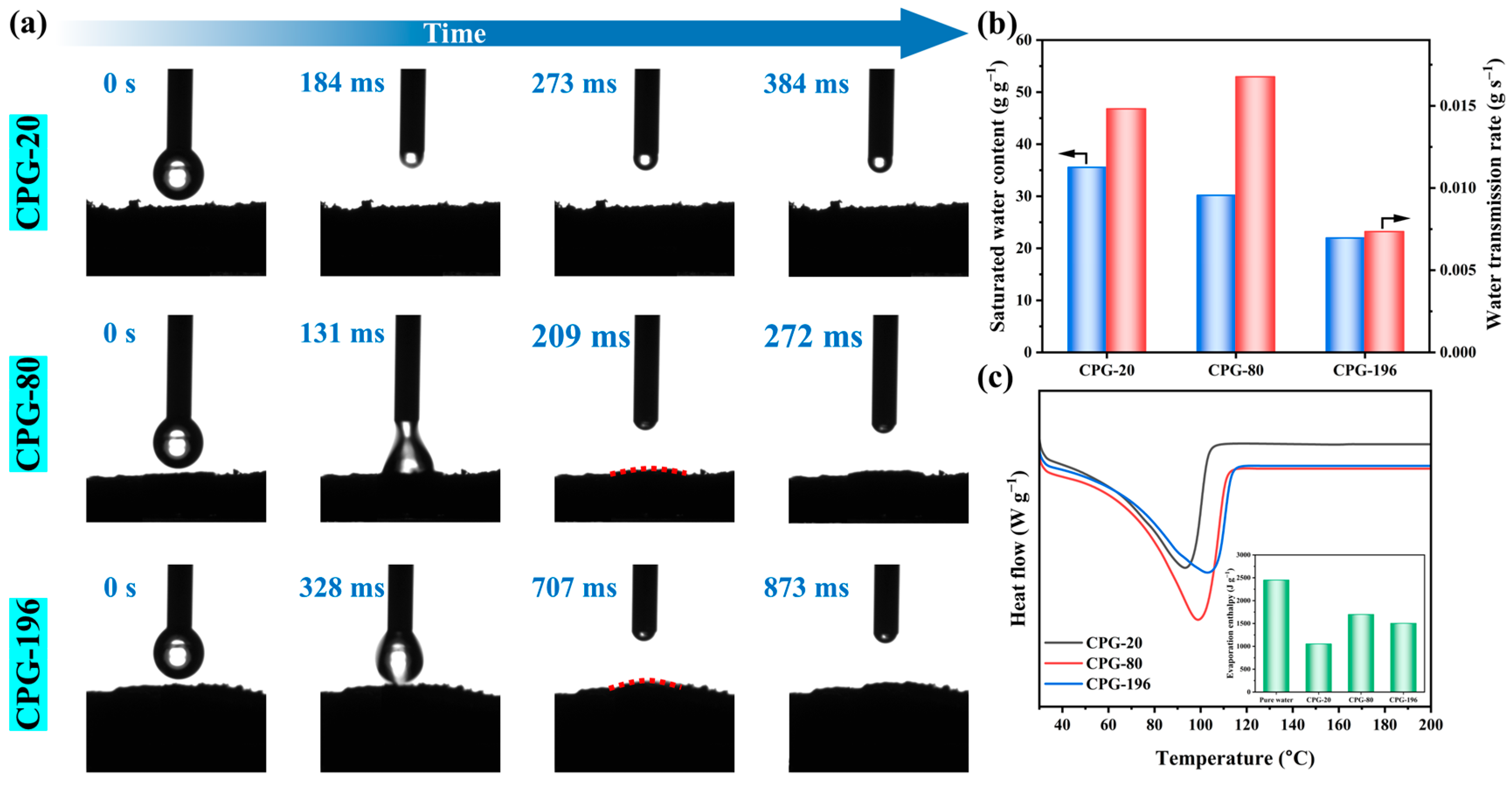
3.2. Water Transport and Evaporation Enthalpy
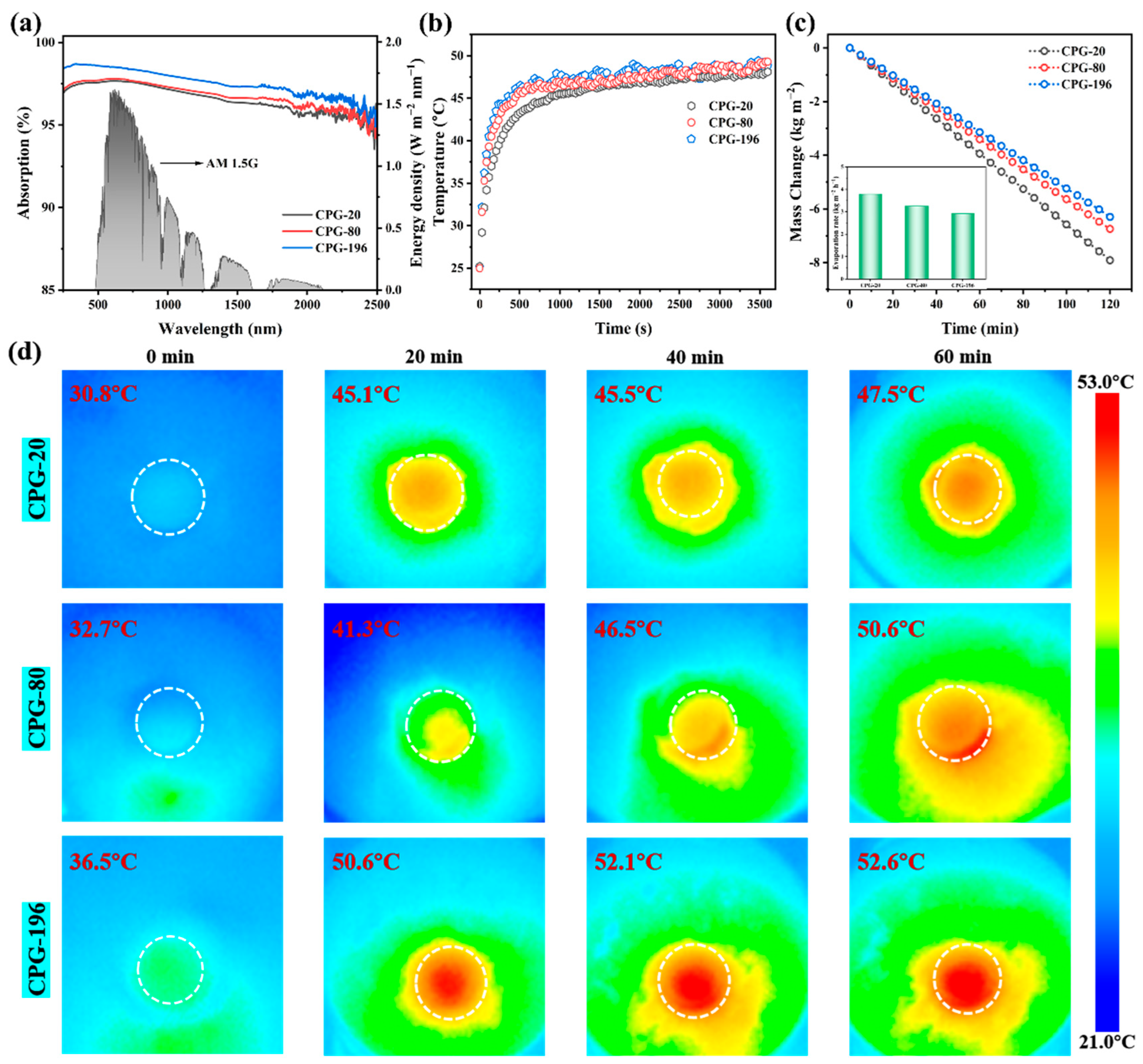
3.3. Photothermal Conversion Performance and Evaporation Performance
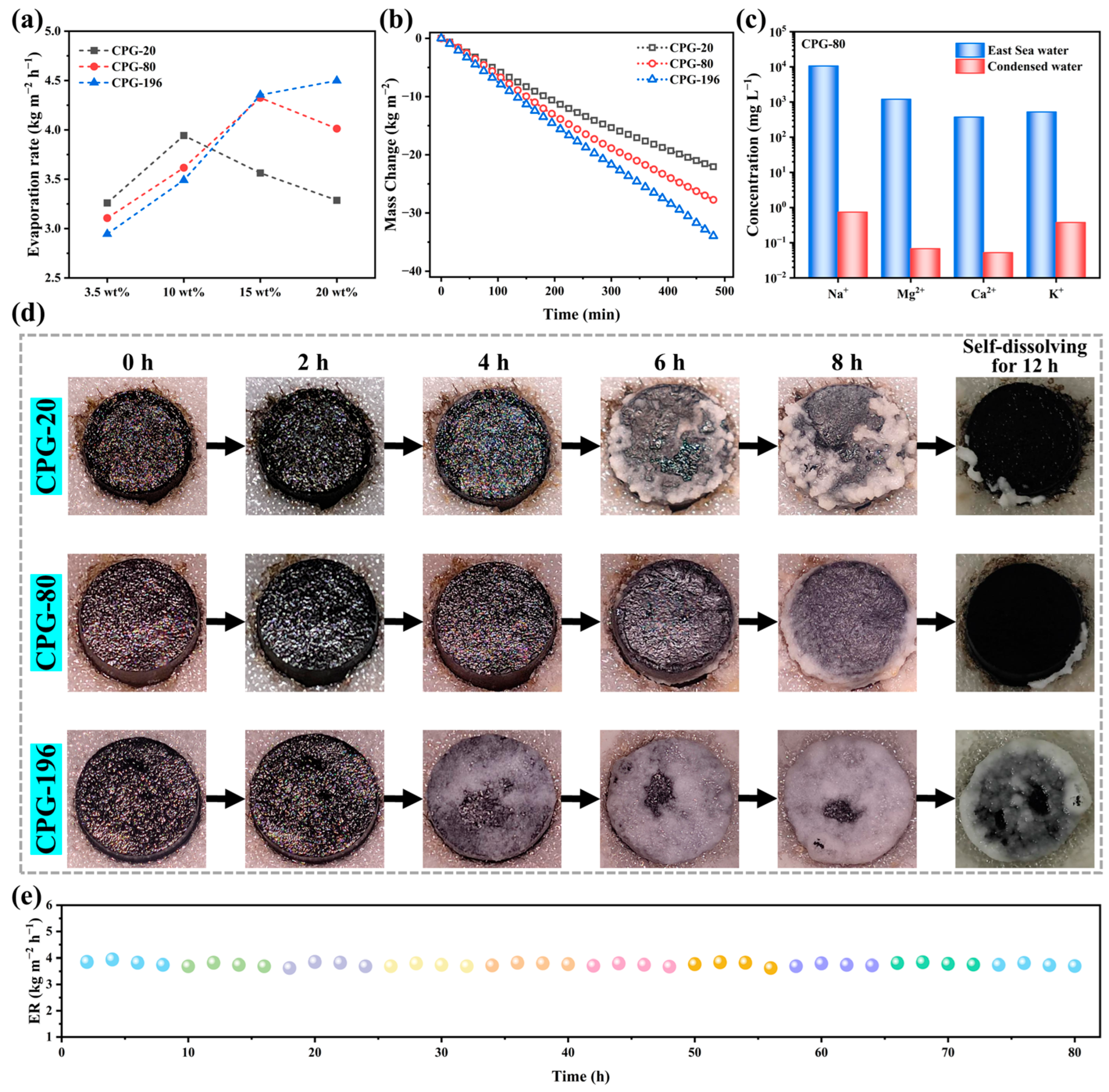
3.4. Seawater Evaporation Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, L.; Tan, Y.; Ji, D.; Zhu, B.; Zhang, P.; Xu, J.; Gan, Q.; Yu, Z.; Zhu, J. Self-assembly of highly efficient, broadband plasmonic absorbers for solar steam generation. Sci. Adv. 2019, 2, e1501227. [Google Scholar] [CrossRef]
- Ghasemi, H.; Ni, G.; Marconnet, A.M.; Loomis, J.; Yerci, S.; Miljkovic, N.; Chen, G. Solar steam generation by heat localization. Nat. Commun. 2014, 5, 4449. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Wurtsbaugh, W.A.; Miller, C.; Null, S.E.; DeRose, R.J.; Wilcock, P.; Hahnenberger, M.; Howe, F.; Moore, J. Decline of the world’s saline lakes. Nat. Geosci. 2017, 10, 816–821. [Google Scholar] [CrossRef]
- Gao, M.; Zhu, L.; Peh, C.K.; Ho, G.W. Solar absorber material and system designs for photothermal water vaporization towards clean water and energy production. Energy Environ. Sci. 2019, 12, 841–864. [Google Scholar] [CrossRef]
- Zhao, F.; Guo, Y.; Zhou, X.; Shi, W.; Yu, G. Materials for solar-powered water evaporation. Nat. Rev. Mater. 2020, 5, 388–401. [Google Scholar] [CrossRef]
- Ahmed, F.E.; Hashaikeh, R.; Hilal, N. Hybrid technologies: The future of energy efficient desalination—A review. Desalination 2020, 495, 114659. [Google Scholar] [CrossRef]
- Zhang, D.; Min, C.; Yu, H.; Wang, J.; Xue, P.; Yu, D.; Chen, L.; Rong, Z.; Zhang, Q.; Wan, R. Development tendency analysis and early warning of resource and environmental carrying capacity based on system dynamics model in Qaidam salt Lake, China. Ecol. Indic. 2025, 175, 113516. [Google Scholar] [CrossRef]
- Wang, X.; Bu, X.; Wang, J.; Du, L.; Hong, Z.; Shi, G.; Baqiatullah. Study on the coordination and factors affecting the coupling of resource and environmental carrying capacity and regional economy in ecologically fragile areas. Ecol. Indic. 2024, 167, 112656. [Google Scholar] [CrossRef]
- Liu, S.; Li, S.; Lin, M. Understanding Interfacial Properties for Enhanced Solar Evaporation Devices: From Geometrical to Physical Interfaces. ACS Energy Lett. 2023, 8, 1680–1687. [Google Scholar] [CrossRef]
- Tao, P.; Ni, G.; Song, C.; Shang, W.; Wu, J.; Zhu, J.; Chen, G.; Deng, T. Solar-driven interfacial evaporation. Nat. Energy 2018, 3, 1031–1041. [Google Scholar] [CrossRef]
- Liu, H.; Chen, L.; Dong, C.; Zhang, L. Solar-driven hydrogel-based interfacial evaporators: From principles to material manipulations. Appl. Therm. Eng. 2025, 258, 124639. [Google Scholar] [CrossRef]
- Ren, Y.; Zhou, R.; Dong, T.G.; Lu, Q. Wood-inspired polypyrrole/cellulose aerogels with vertically aligned channels prepared by facile freeze-casting for efficient interfacial solar evaporation. J. Mater. Chem. A 2023, 11, 17748. [Google Scholar] [CrossRef]
- Li, N.; Shao, K.; He, J.; Wang, S.; Li, S.; Wu, X.; Li, J.; Guo, C.; Yu, L.; Murto, P.; et al. Solar-Powered Interfacial Evaporation and Deicing Based on a 3D-Printed Multiscale Hierarchical Design. Small 2023, 19, 2301474. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Yin, L.; Gong, K.; Wu, Q. 3D Vascular-structured Flame-retardant Cellulose-based photothermal aerogel for Solar-driven interfacial evaporation and wastewater purification. Chem. Eng. J. 2023, 464, 142616. [Google Scholar] [CrossRef]
- Yang, Z.-Y.; Liu, L.-Q.; Liu, S.-Q.; Wang, G.; Zeng, Z.-X.; Zhu, L.-J. Thermal and electrical conductive 2D CuO evaporator for all-day steam generation. Chem. Eng. J. 2023, 475, 145919. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, J.; Liu, J.; Guo, S.; Liu, J.; Lu, S.; Song, B. Pyramid-shaped solar evaporator with high-efficient interfacial evaporation and salt harvesting capability. J. Clean. Prod. 2024, 434, 139956. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, D.; Yu, H.; Wu, X.; Lu, Y.; Yang, X.; Owens, G.; Xu, H. Recent innovations in 3D solar evaporators and their functionalities. Sci. Bull. 2024, 69, 3590–3617. [Google Scholar] [CrossRef]
- Xu, P.; Gao, J.; Xia, M.; He, Q.; Cao, Y.; Ding, Y.; Chen, Y. Same volume, larger output: 3D trapezoidal multi-interface solar evaporator for concentrated seawater desalination and wastewater treatment. Desalination 2024, 581, 117581. [Google Scholar] [CrossRef]
- Zhang, J.H.; Mittapally, R.; Oluwade, A.; Chen, G. Mechanisms and scale-up potential of 3D solar interfacial-evaporators. Energy Environ. Sci. 2025, 18, 5524–5538. [Google Scholar] [CrossRef]
- Zhang, X.; Deng, K.; Zhang, C.; Shang, B.; Liu, X. A mild, versatile and time-saving interfacial gelation blackening strategy for fabricating high-quality 3D porous solar steam evaporators. J. Mater. Chem. A 2022, 10, 9482–9487. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Wang, X.; Xu, Y.; Li, Y. Structural and functional tailoring of interfacial solar evaporators using 3D printing. Mater. Today 2025, 83, 484–512. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Q.; Zhang, H.; Zuo, X.; Yang, Q.; Tang, H.; Jin, S.; Li, G. A 3D aerogel evaporator for efficient solar interfacial evaporation: Breaking through the upper limit of steam production rate. Chem. Eng. J. 2024, 499, 156520. [Google Scholar] [CrossRef]
- Faivre, J.; Sudre, G.; Montembault, A.; Benayoun, S.; Banquy, X.; Delair, T.; David, L. Bioinspired microstructures of chitosan hydrogel provide enhanced wear protection. Soft Matter 2018, 14, 2068–2076. [Google Scholar] [CrossRef]
- Friedman, M.; Juneja, V.K. Review of Antimicrobial and Antioxidative Activities of Chitosans in Food. J. Food Prot. 2010, 73, 1737–1761. [Google Scholar] [CrossRef]
- Poon, L.; Wilson, L.D.; Headley, J.V. Chitosan-glutaraldehyde copolymers and their sorption properties. Carbohydr. Polym. 2014, 109, 92–101. [Google Scholar] [CrossRef]
- Sharma, M.; Sharma, A.K.; Shukla, S.K. Potentiometric sensing of ibuprofen over ferric oxide doped chitosan grafted polypyrrole-based electrode. Int. J. Biol. Macromol. 2024, 268, 131598. [Google Scholar] [CrossRef]
- Thambiliyagodage, C.; Jayanetti, M.; Mendis, A.; Ekanayake, G.; Liyanaarachchi, H.; Vigneswaran, S. Recent Advances in Chitosan-Based Applications—A Review. Materials 2023, 16, 2073. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jiang, M.; Zhang, Y.; Cao, Q.; Wang, X.; Han, Y.; Sun, G.; Li, Y.; Zhou, J. Novel lignin–chitosan–PVA composite hydrogel for wound dressing. Mater. Sci. Eng. C 2019, 104, 110002. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, X.; Nilghaz, A.; Wu, Z.; Wang, T.; Zhu, B.; Tang, G.; Su, B.; Tian, J. Large-scale production of spent coffee ground-based photothermal materials for high-efficiency solar-driven interfacial evaporation. Chem. Eng. J. 2023, 455, 140361. [Google Scholar] [CrossRef]
- Tessema, A.A.; Wu, C.-M.; Motora, K.G.; Lee, W.-H.; Peng, Y.-T. A review on state of art of photothermal nanomaterials for interfacial solar water evaporation and their applications. Desalination 2024, 591, 117998. [Google Scholar] [CrossRef]
- Irshad, M.S.; Arshad, N.; Asghar, M.S.; Hao, Y.; Alomar, M.; Zhang, S.; Zhang, J.; Guo, J.; Ahmed, I.; Mushtaq, N.; et al. Advances of 2D-Enabled Photothermal Materials in Hybrid Solar-Driven Interfacial Evaporation Systems toward Water-Fuel-Energy Crisis. Adv. Funct. Mater. 2023, 33, 2304936. [Google Scholar] [CrossRef]
- Li, Y.; Shi, Y.; Wang, H.; Liu, T.; Zheng, X.; Gao, S.; Lu, J. Recent advances in carbon-based materials for solar-driven interfacial photothermal conversion water evaporation: Assemblies, structures, applications, and prospective. Carbon Energy 2023, 5, e331. [Google Scholar] [CrossRef]
- Song, R.; Zhang, N.; Wang, P.; Ding, H.; Wang, J.; Li, S. A self-floating Janus PPy@Ni sponge salt-resisting solar evaporator for efficient interfacial evaporation. Appl. Surf. Sci. 2023, 616, 156448. [Google Scholar] [CrossRef]
- Yan, L.; Ma, Y.; Cao, X.; Jing, Y.; Su, M.; Li, J.; Zhu, Z.; Liang, W.; Sun, H.; Li, A. PC@PPy porous membrane prepared by breath figure method with superior mechanical property for efficient solar interfacial evaporation. Chem. Eng. J. 2023, 469, 144059. [Google Scholar] [CrossRef]
- Yin, M.; Xiao, C.; Jin, Y.; He, Y.; Zhang, Y.; Chen, L. Bionic solar-Driven interfacial evaporator for synergistic photothermal-Photocatalytic activities and salt collection during desalination. Chem. Eng. J. 2024, 499, 156282. [Google Scholar] [CrossRef]
- Yang, R.; Li, H.; Huang, M.; Yang, H.; Li, A. A review on chitosan-based flocculants and their applications in water treatment. Water Res. 2016, 95, 59–89. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Han, X.; Xu, D.; Zhang, Z.; Zhu, L.; Wang, S. All-in-one chitosan-based aerogel with a semi-clad structure for solar-driven interfacial evaporation. Desalination 2024, 575, 117333. [Google Scholar] [CrossRef]
- Cao, C.; Li, Y. Highly stretchable calcium ion/polyacrylic acid hydrogel prepared by freezing–thawing. J. Mater. Sci. 2020, 55, 5340–5348. [Google Scholar] [CrossRef]
- Li, B.; Lin, M.; Cheng, C.; Li, X. Three-dimensional freeze-casting solar evaporator for highly efficient water evaporation and high salinity desalination. Desalination 2024, 580, 117542. [Google Scholar] [CrossRef]
- Hu, Y.; Luo, Q.; Du, X.; Li, J.; Hao, X. Film electrode by incorporating polypyrrole/carbon black into cross-linked binders of chitosan/cationic polyacrylamide for selective chloride extraction in wastewater. Sep. Purif. Technol. 2024, 330, 125434. [Google Scholar] [CrossRef]
- Upadhyay, J.; Kumar, A.; Gupta, K.; Mandal, M. Investigation of physical and biological properties of polypyrrole nanotubes–chitosan nanocomposites. Carbohydr. Polym. 2015, 132, 481–489. [Google Scholar] [CrossRef]
- Garnica-Palafox, I.M.; Sánchez-Arévalo, F.M. Influence of natural and synthetic crosslinking reagents on the structural and mechanical properties of chitosan-based hybrid hydrogels. Carbohydr. Polym. 2016, 151, 1073–1081. [Google Scholar] [CrossRef]
- Beppu, M.M.; Vieira, R.S.; Aimoli, C.G.; Santana, C.C. Crosslinking of chitosan membranes using glutaraldehyde: Effect on ion permeability and water absorption. J. Membr. Sci. 2007, 301, 126–130. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, F.; Li, C.; Gilbert, R.G. Molecular structural differences between maize leaf and endosperm starches. Carbohydr. Polym. 2017, 161, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Y.; Huang, Q.; Chen, Z.; Wang, W.; Li, W. Tailorable Lignocellulose-Based Aerogel to Achieve the Balance between Evaporation Enthalpy and Water Transport Rate for Efficient Solar Evaporation. ACS Appl. Mater. Interfaces 2023, 15, 11827–11836. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, J.; Ding, L.; Zhu, X.; Sun, R.; Chang, K. Interfacial Assembled Hydrogel Evaporator for Highly Efficient Thermal Management and Photothermal Coupled Water Splitting Reaction. Adv. Funct. Mater. 2024, 34, 2411387. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, J.; Bai, Y.; Li, F. The Role of Freezing Temperature in Modulating Chitosan Gel Structure and Evaporation Performance for Seawater Desalination. Separations 2025, 12, 193. https://doi.org/10.3390/separations12080193
Cai J, Bai Y, Li F. The Role of Freezing Temperature in Modulating Chitosan Gel Structure and Evaporation Performance for Seawater Desalination. Separations. 2025; 12(8):193. https://doi.org/10.3390/separations12080193
Chicago/Turabian StyleCai, Jiaonan, Yong Bai, and Fang Li. 2025. "The Role of Freezing Temperature in Modulating Chitosan Gel Structure and Evaporation Performance for Seawater Desalination" Separations 12, no. 8: 193. https://doi.org/10.3390/separations12080193
APA StyleCai, J., Bai, Y., & Li, F. (2025). The Role of Freezing Temperature in Modulating Chitosan Gel Structure and Evaporation Performance for Seawater Desalination. Separations, 12(8), 193. https://doi.org/10.3390/separations12080193




