Recycling and Separation of Valuable Metals from Spent Cathode Sheets by Single-Step Electrochemical Strategy
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Reagents
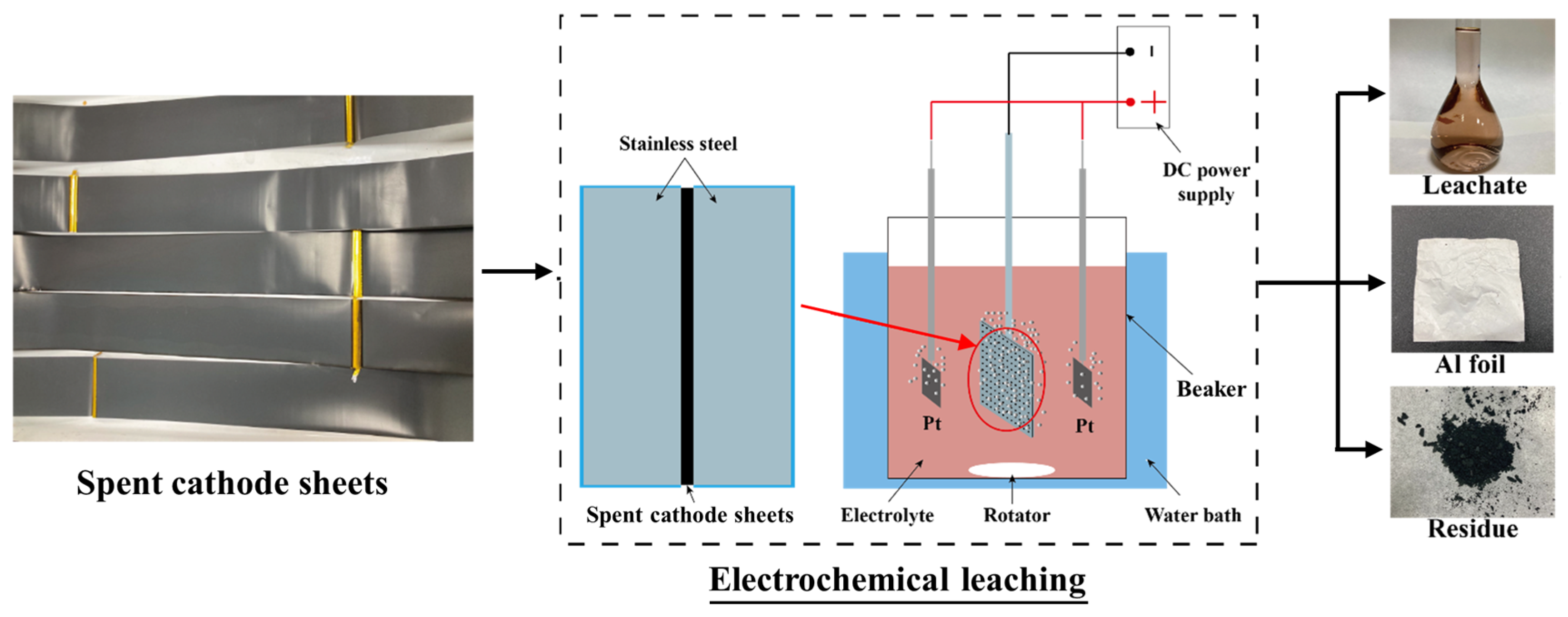
2.2. Electrochemical Leaching Process
2.3. Measurement and Characterization
3. Results and Discussion
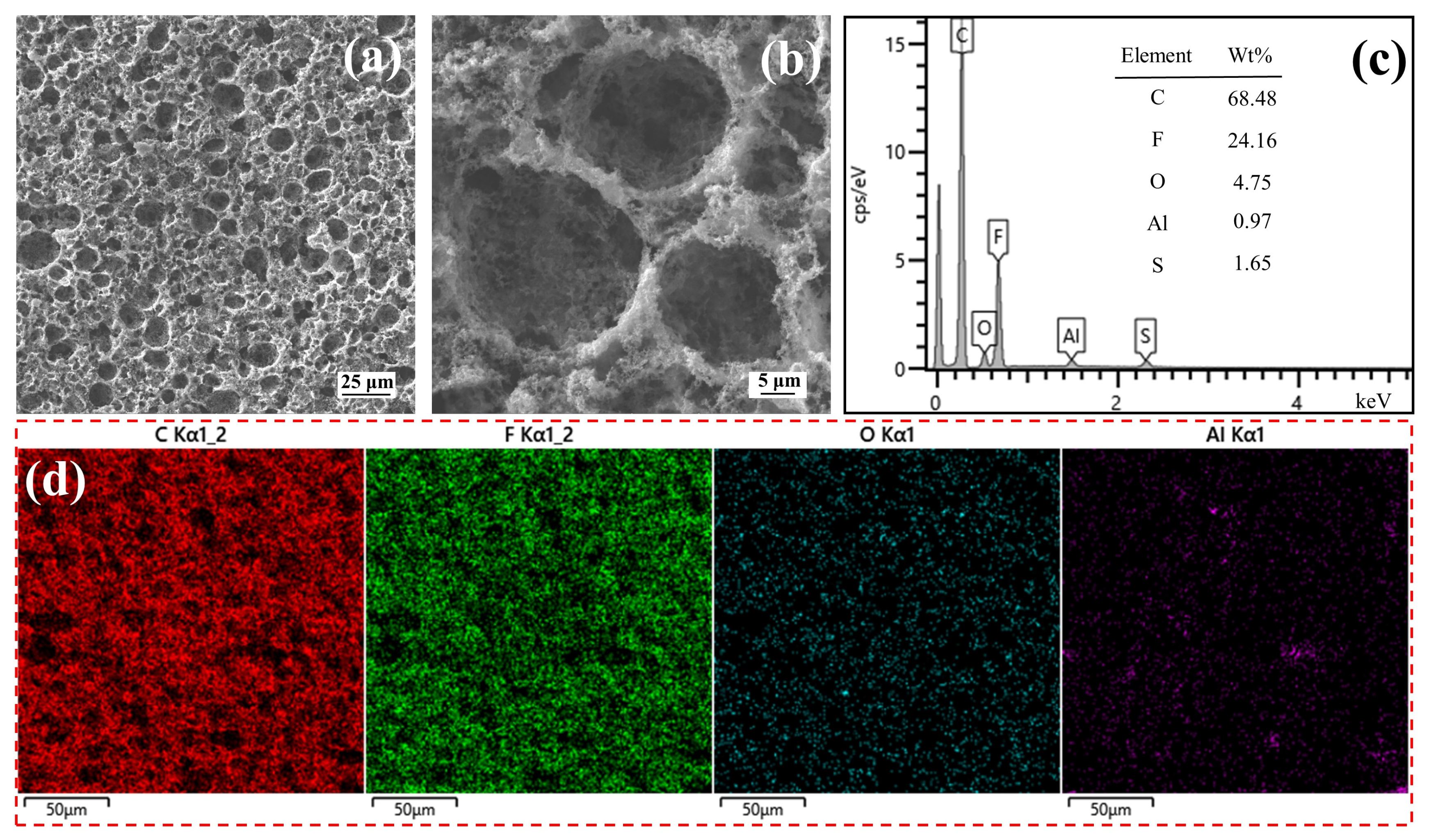
3.1. Characterization of the Spent CSs
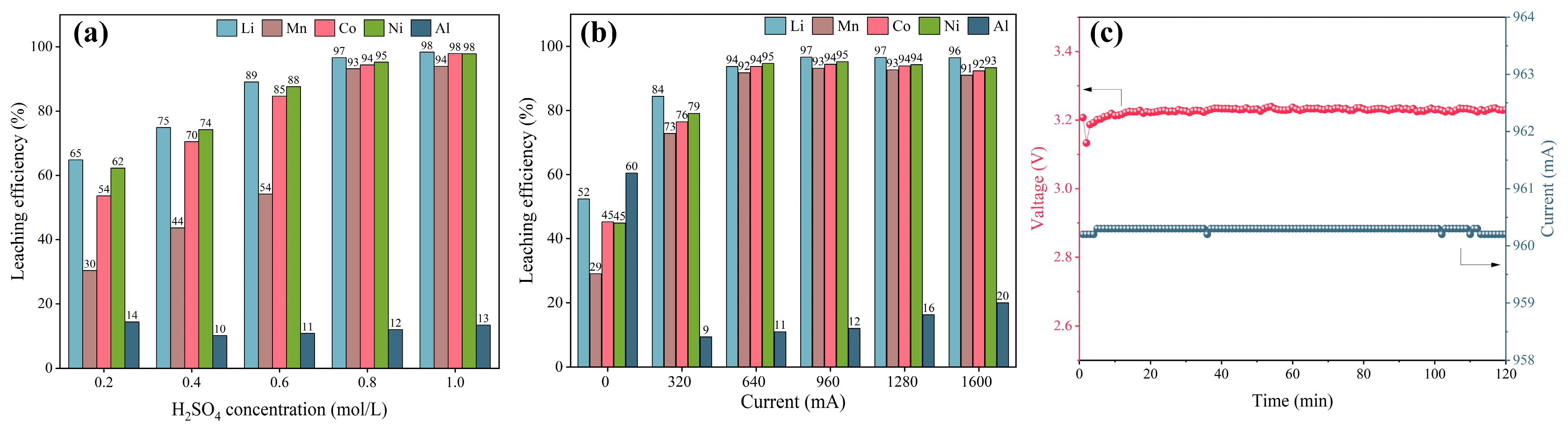
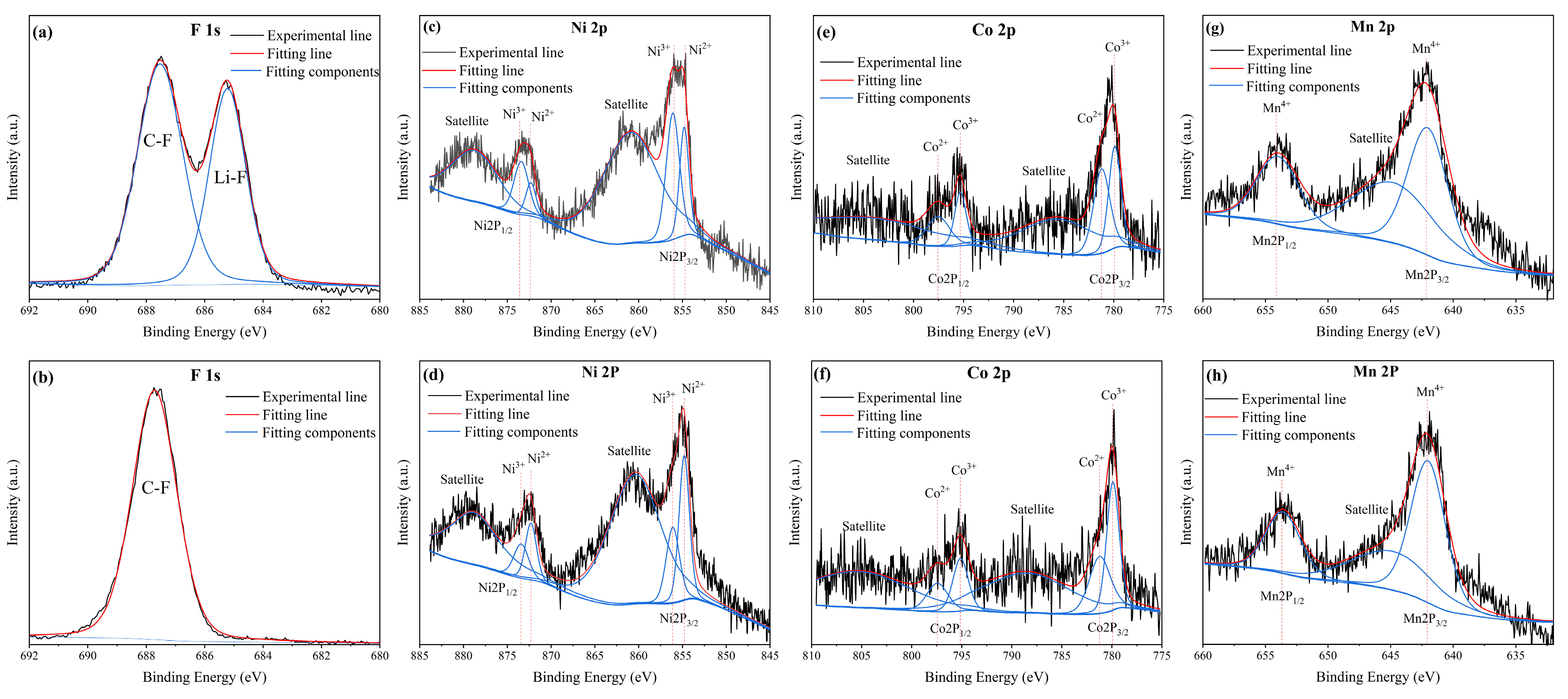
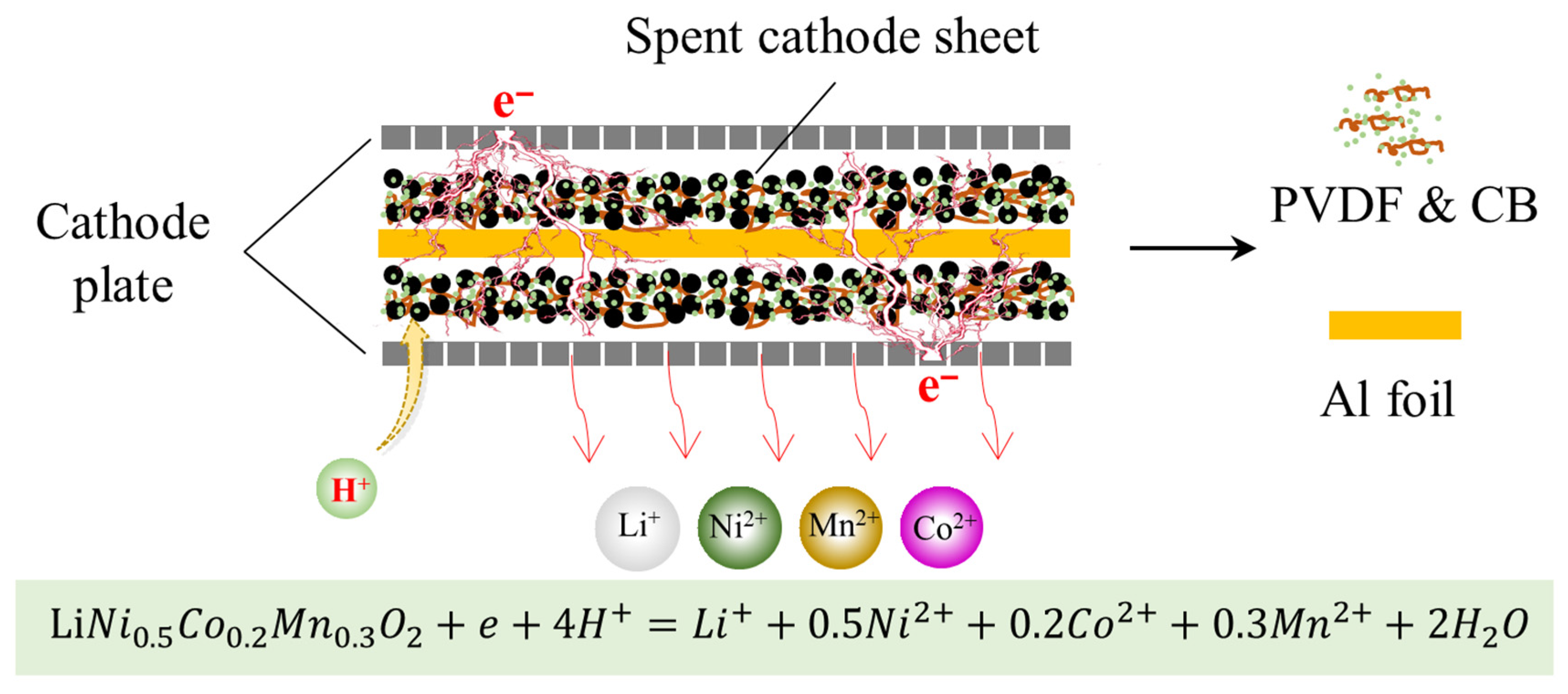
3.2. Electrochemical Leaching and Separation Valuable Metals from Spent CSs
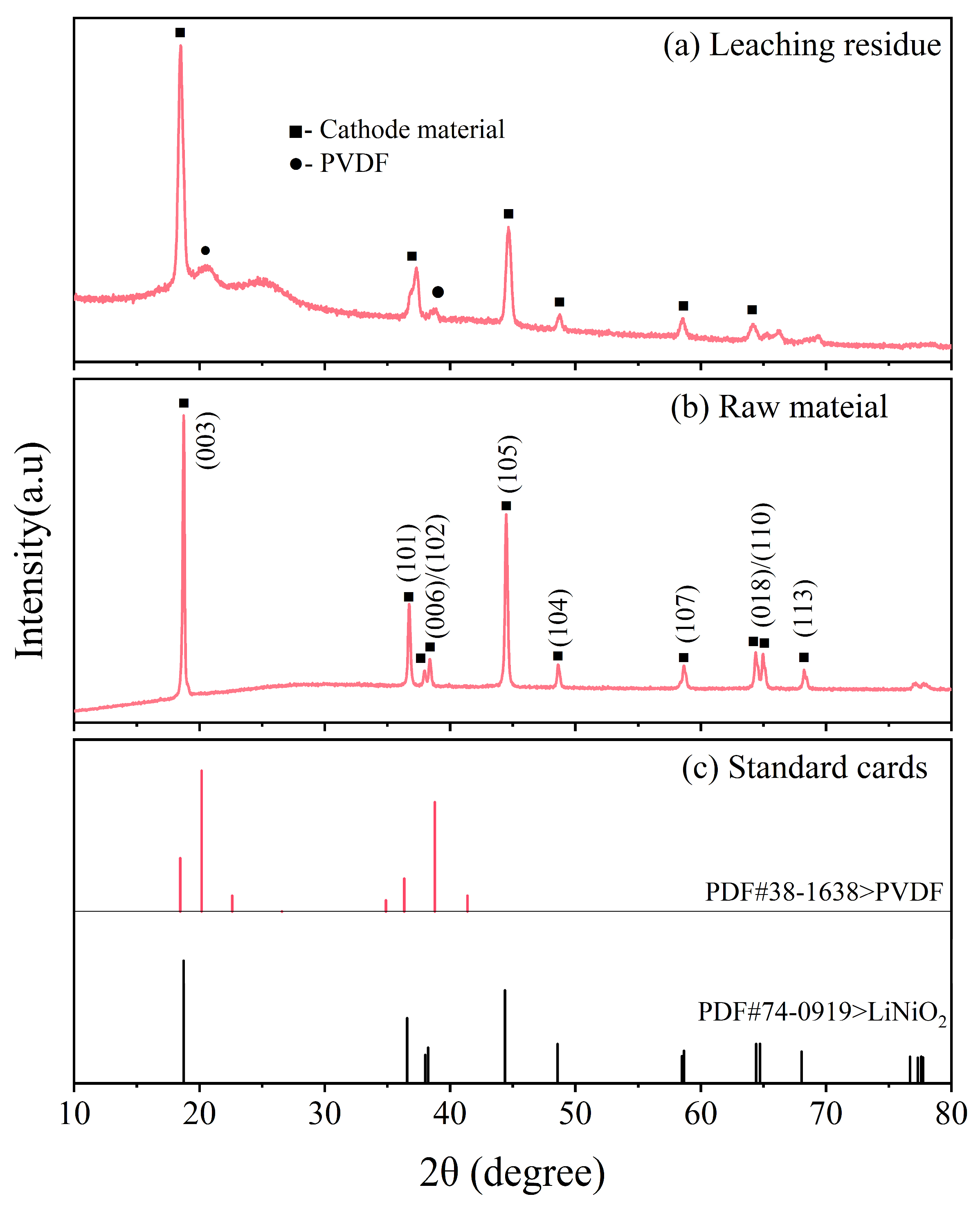
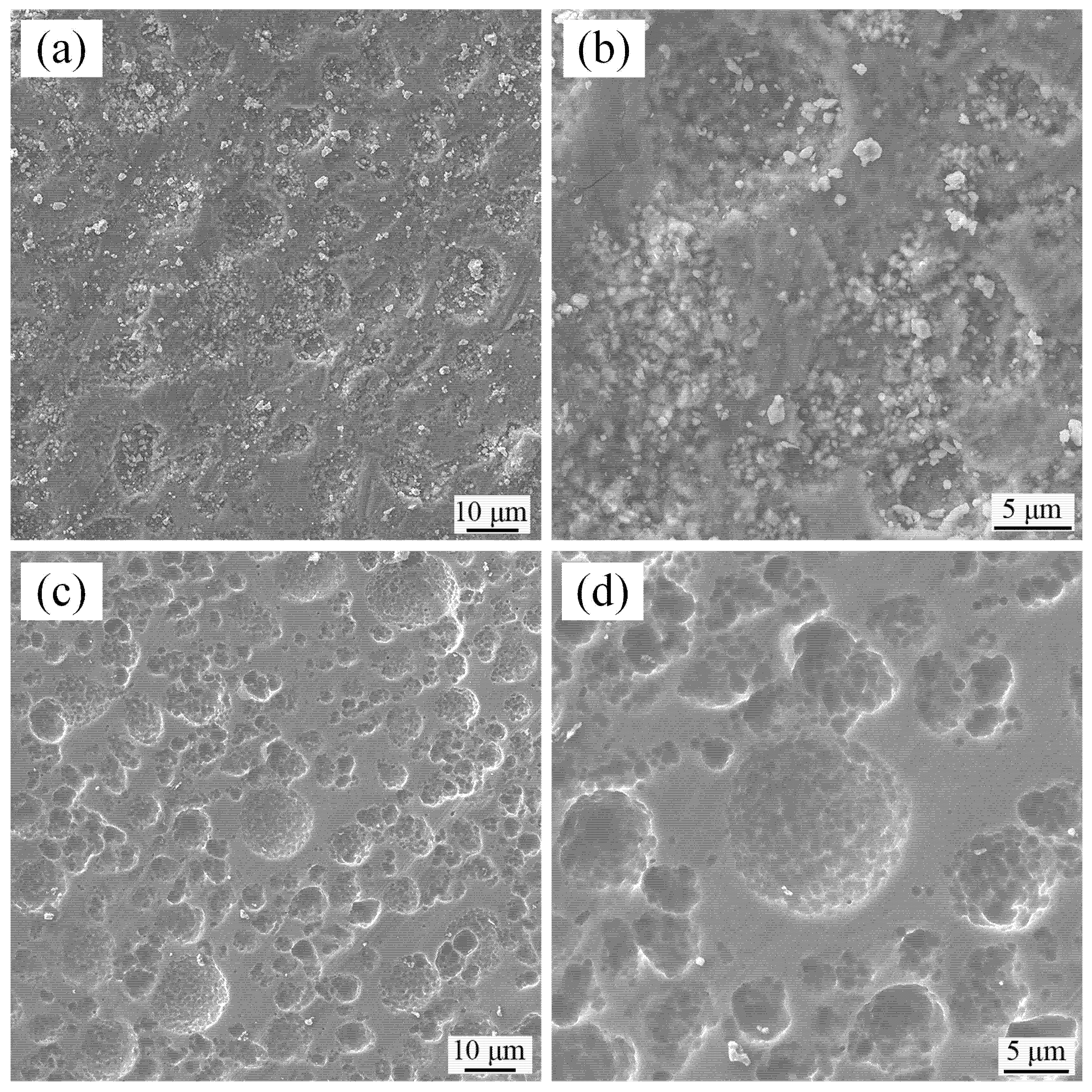
3.3. Properties of the Leaching Residue
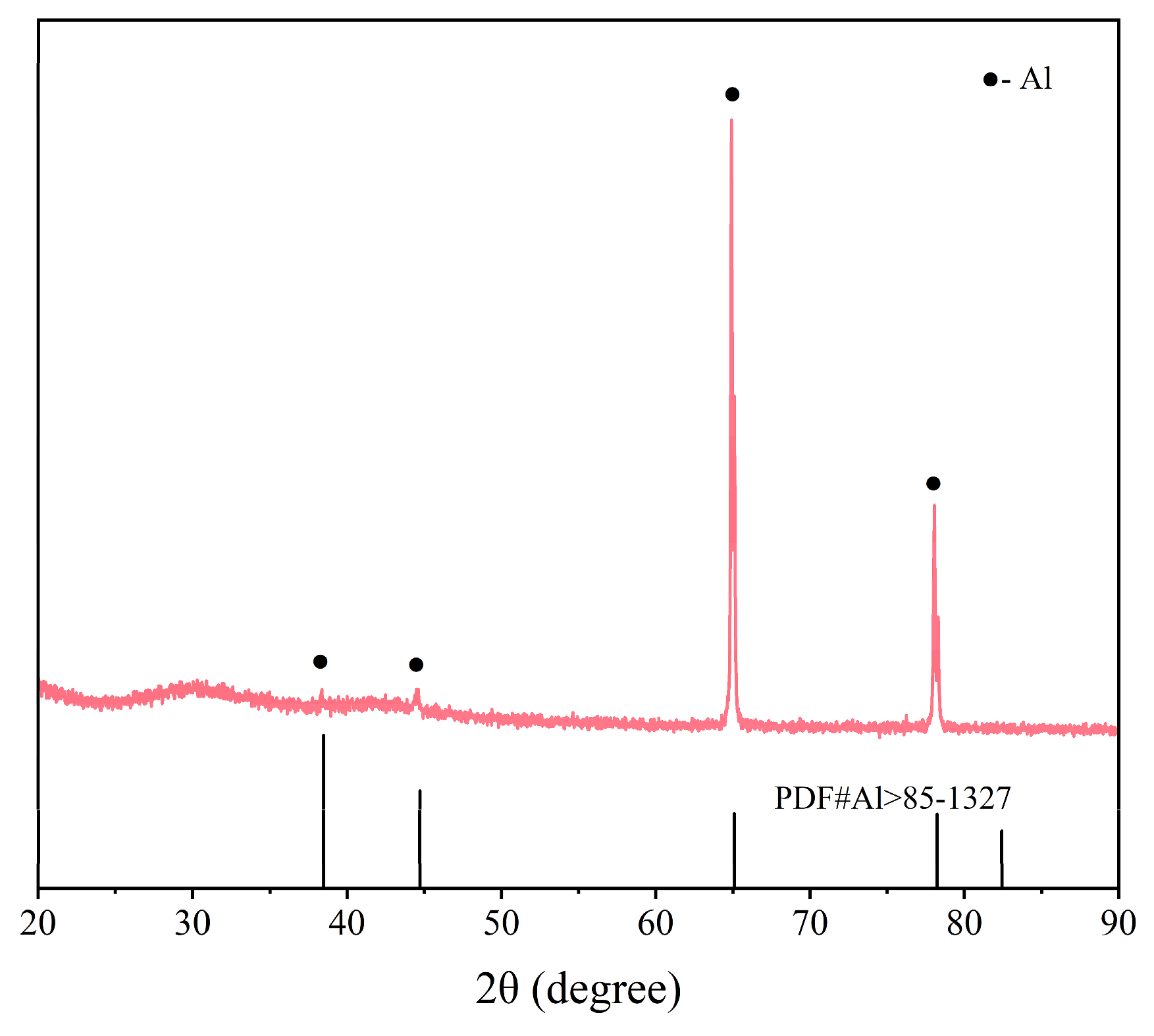
3.4. Properties of the Recovered Al Foils
3.5. Electrochemical Leaching Mechanisms and Valuable Metals Recovery Process
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Malik, M.; Chan, K.H.; Azimi, G. Review on the synthesis of LiNixMnyCo1-x-yO2 (NMC) cathodes for lithium-ion batteries. Mater. Today Energy 2022, 28, 101066. [Google Scholar] [CrossRef]
- He, Y.; Yuan, X.; Zhang, G.; Wang, H.; Zhang, T.; Xie, W.; Li, L. A critical review of current technologies for the liberation of electrode materials from foils in the recycling process of spent lithium-ion batteries. Sci. Total Environ. 2021, 766, 142382. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.; Wang, Z.; Cao, H.; Sun, Y.; Zhang, Y.; Sun, Z. A critical review and analysis on the recycling of spent lithium-ion batteries. ACS Sustain. Chem. Eng. 2018, 6, 1504–1521. [Google Scholar] [CrossRef]
- Natarajan, S.; Aravindan, V. Burgeoning Prospects of spent lithium-ion batteries in multifarious applications. Adv. Energy Mater. 2018, 8, 1802303. [Google Scholar] [CrossRef]
- Fan, M.; Chang, X.; Guo, Y.J.; Chen, W.P.; Yin, Y.X.; Yang, X.; Meng, Q.; Wan, L.J.; Guo, Y.G. Increased residual lithium compounds guided design for green recycling of spent lithium-ion cathodes. Energy Environ. Sci. 2021, 14, 1461–1468. [Google Scholar] [CrossRef]
- Fu, Y.; Schuster, J.; Petranikova, M.; Ebin, B. Innovative recycling of organic binders from electric vehicle lithium-ion batteries by supercritical carbon dioxide extraction. Resour. Conserv. Recycl. 2021, 172, 105666. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, J.; Chen, Y.; Wang, C. High value-added regeneration of anode materials from retired lithium-ion batteries: Structural design and synthesis process. Fuel Process. Technol. 2023, 250, 107909. [Google Scholar] [CrossRef]
- Golmohammadzadeh, R.; Faraji, F.; Rashchi, F. Recovery of lithium and cobalt from spent lithium ion batteries (LIBs) using organic acids as leaching reagents: A review. Resour. Conserv. Recycl. 2020, 136, 418–435. [Google Scholar] [CrossRef]
- Tao, R.; Xing, P.; Li, H.; Sun, Z.; Wu, Y. Recovery of spent LiCoO2 lithium-ion battery via environmentally friendly pyrolysis and hydrometallurgical leaching. Resour. Conserv. Recycl. 2022, 176, 105921. [Google Scholar] [CrossRef]
- Zhu, L.; Chen, M. Development of a two-stage pyrolysis process for the end-of-life nickel cobalt manganese lithium battery recycling from electric vehicles. Sustainability 2020, 12, 9164. [Google Scholar] [CrossRef]
- Shoaei, A.; Firoozi, S. Recovery of lithium, cobalt and nickel from the spent NMC li-ion battery by reduction roasting, selective leaching and precipitation. Waste Manag. 2025, 200, 114749. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Wei, S.; Yongjie, B.; Chenyang, Z.; Shaole, S.; Yuehua, H. Recovering valuable metals from spent lithium ion battery via a combination of reduction thermal treatment and facile acid leaching. ACS Sustain. Chem. Eng. 2018, 6, 10445–10453. [Google Scholar] [CrossRef]
- Yu, M.; Zhang, Z.; Xue, F.; Yang, B.; Guo, G.; Qiu, J. A more simple and efficient process for recovery of cobalt and lithium from spent lithium-ion batteries with citric acid. Sep. Purif. Technol. 2019, 215, 398–402. [Google Scholar] [CrossRef]
- Chen, X.; Luo, C.; Zhang, J.; Kong, J.; Zhou, T. Sustainable recovery of metals from spent lithium-ion batteries: A green process. ACS Sustain. Chem. Eng. 2015, 3, 3104–3113. [Google Scholar] [CrossRef]
- Liu, H.; Liu, G.; Feng, Y.; Gu, J.; Wang, S.; Tang, Y.; Yuan, H.; Chen, Y. Reaction behaviors and mechanisms of in-situ carbothermal reduction in spent lithium batteries. Sep. Purif. Technol. 2025, 362, 131708. [Google Scholar] [CrossRef]
- Li, J.; Wang, G.; Xu, Z. Environmentally-friendly oxygen-free roasting/wet magnetic separation technology for in situ recycling cobalt, lithium carbonate and graphite from spent LiCoO2/graphite lithium batteries. J. Hazard. Mater. 2016, 302, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Xiao, L.; Tang, Y.; Chen, Y.; Ye, L.; Zhu, Y. Study on the reduction roasting of spent LiNixCoyMnzO2 lithium-ion battery cathode materials. J. Therm. Anal. Calorim. 2019, 136, 1323–1332. [Google Scholar] [CrossRef]
- Liu, X.; Ma, Y.; Zhou, X.; Yang, J.; Kang, H.; He, Y.; Tang, J.; Su, F.; Yang, W.; Zhang, Y. Reduction mechanism of bamboo powder pyrolysis in selective lithium extraction from spent lithium-ion batteries. J. Environ. Chem. Eng. 2023, 11, 110172. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, Y.; Huang, W.; Omran, M.; Zhang, F.; Gao, L.; Chen, G. Reductive roasting of cathode powder of spent ternary lithium-ion battery by pyrolysis of invasive plant crofton weed. Renew. Energy 2023, 206, 86–96. [Google Scholar] [CrossRef]
- Xue, Y.; Wang, Y. Green electrochemical redox mediation for valuable metal extraction and recycling from industrial waste. Green Chem. 2020, 22, 6288–6309. [Google Scholar] [CrossRef]
- Wei, N.; He, Y.; Zhang, G.; Feng, Y.; Li, J.; Liu, Z.; Ding, L.; Xie, W. Green recycling of valuable metals from spent cathode materials by water electrolysis. J. Environ. Chem. Eng. 2023, 11, 111150. [Google Scholar] [CrossRef]
- Wei, N.; He, Y.; Zhang, G.; Li, J.; Su, P. A Sustainable strategy for recovering spent cathode materials based on in-situ thermal reduction and electrically driven leaching. Sep. Purif. Technol. 2025, 354, 129543. [Google Scholar] [CrossRef]
- Chu, W.; Zhang, Y.; Chen, L.; Wu, K.; Huang, Y.; Jia, Y. Comprehensive recycling of Al foil and active materials from the spent lithium-ion battery. Sep. Purif. Technol. 2021, 269, 118704. [Google Scholar] [CrossRef]
- Meng, Q.; Zhang, Y.; Dong, P. Use of electrochemical cathode-reduction method for leaching of cobalt from spent lithium-ion batteries. J. Clean. Prod. 2018, 180, 64–70. [Google Scholar] [CrossRef]
- Zhao, J.; Qu, J.; Qu, X.; Gao, S.; Wang, D.; Yin, H. Cathode electrolysis for the comprehensive recycling of spent lithium-ion batteries. Green Chem. 2022, 24, 6179–6188. [Google Scholar] [CrossRef]
- Yu, J.; Tan, Q.; Li, J. Exploring a green route for recycling spent lithium-ion batteries: Revealing and solving deep screening problem. J. Clean. Prod. 2020, 255, 120269. [Google Scholar] [CrossRef]
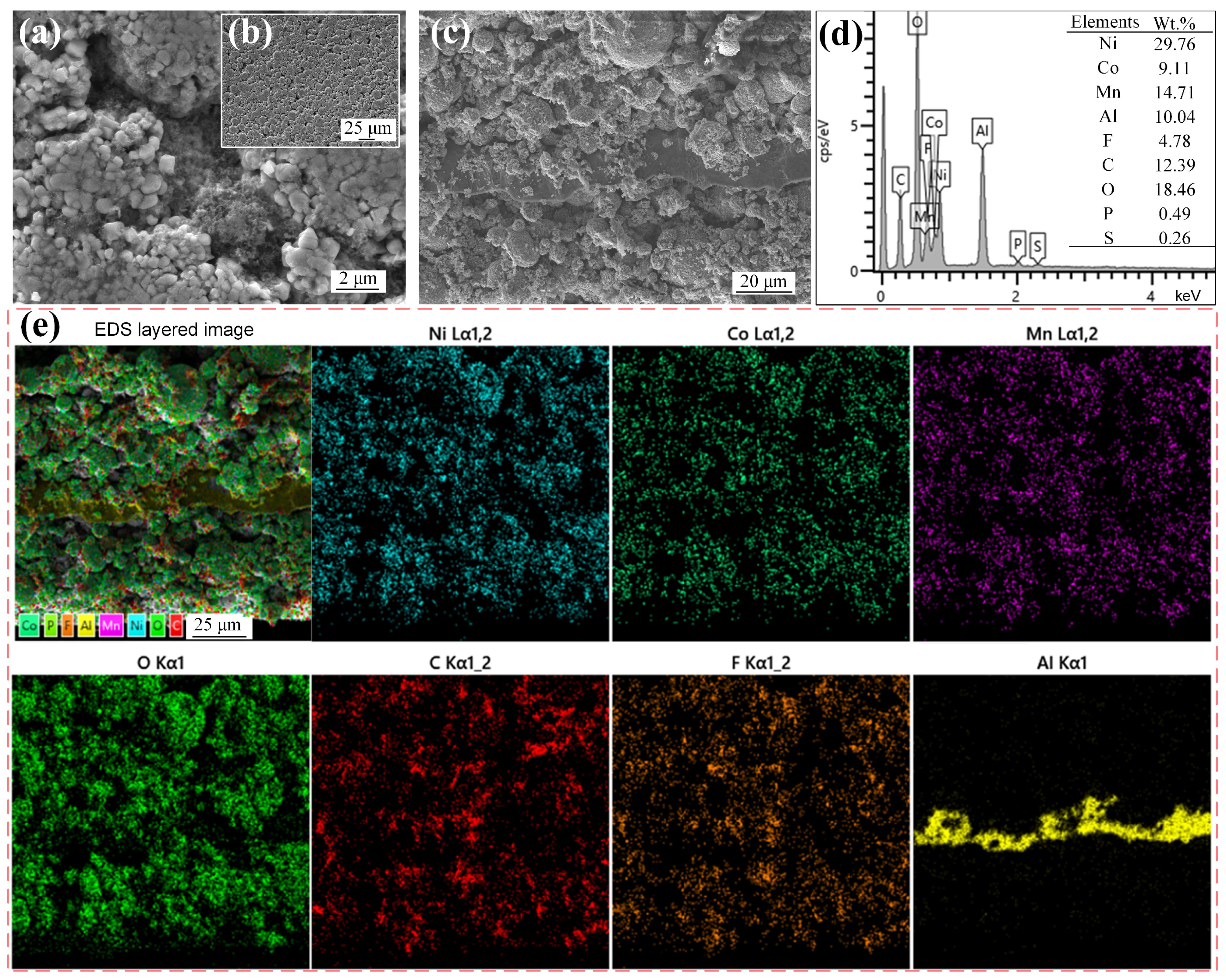
| Element | Li | Ni | Co | Mn | Al |
|---|---|---|---|---|---|
| Content wt.% | 6.69 | 28.26 | 11.10 | 15.62 | 7.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, N.; He, Y.; Zhang, G.; Li, J.; Zhang, F. Recycling and Separation of Valuable Metals from Spent Cathode Sheets by Single-Step Electrochemical Strategy. Separations 2025, 12, 178. https://doi.org/10.3390/separations12070178
Wei N, He Y, Zhang G, Li J, Zhang F. Recycling and Separation of Valuable Metals from Spent Cathode Sheets by Single-Step Electrochemical Strategy. Separations. 2025; 12(7):178. https://doi.org/10.3390/separations12070178
Chicago/Turabian StyleWei, Neng, Yaqun He, Guangwen Zhang, Jiahao Li, and Fengbin Zhang. 2025. "Recycling and Separation of Valuable Metals from Spent Cathode Sheets by Single-Step Electrochemical Strategy" Separations 12, no. 7: 178. https://doi.org/10.3390/separations12070178
APA StyleWei, N., He, Y., Zhang, G., Li, J., & Zhang, F. (2025). Recycling and Separation of Valuable Metals from Spent Cathode Sheets by Single-Step Electrochemical Strategy. Separations, 12(7), 178. https://doi.org/10.3390/separations12070178







