1. Introduction
Rare earth metals (REMs) are critical for high-tech industries, including defense, laser technology, electronics, medicine, and steel alloying [
1].
Primary REM sources are minerals such as loparite, monazite, and bastnasite. However, increasing attention is being given to the possibility of involving production wastes in processing, corresponding to the principles of green chemistry, aimed at minimizing the environmental impact of the chemical industry and preventing the formation of waste [
2,
3]. The use of secondary sources of REMs, such as red mud [
4], phosphogypsum [
5], nickel-metal hydride and lithium-ion batteries [
6,
7], will not only reduce the load on the environment, but also provide the output of byproducts, contributing to the sustainable use of resources and rational utilization of industrial waste [
8,
9].
Methods for extracting metals, including REMs, from process solutions include precipitation [
10,
11], solvent extraction [
12], flotation-extraction [
13] and sorption using various sorbents [
14,
15]. Solvent extraction and solid-phase extraction [
16] are highly selective but primarily recover heavy REMs, leaving light REMs in solution. Moreover, solvent extraction uses toxic, volatile organic solvents, posing fire and explosion risks. Sorption processes, such as ion-exchange methods, offer an alternative approach for REM extraction.
Adsorption processes are widely used for metal ion recovery from process solutions, due to their high sorption capacity, extraction efficiency and selectivity toward target components [
17]. This approach eliminates the necessity of using toxic solvents, overcoming a key limitation of solvent extraction and enabling sustainable REM recovery.
1.1. Sorption on Cation-Exchange Resins
The extraction of REMs by means of cation-exchange resins from acidic solutions of various compositions has been adequately addressed in the extant literature, due to their versality and effectiveness. Cation-exchange resins demonstrate significant sorption capacities and high REM extraction efficiencies.
Araucz et al. [
18] investigated the potential application of Purolite S957 and Diphonix Resin
® cation-exchange resins for extracting rare earth metals from aqueous solutions obtained during Ni-MH battery reprocessing in the presence of citric acid. The effects of metal and citric acid concentrations, solution pH, solid-to-liquid ratio, contact time, and temperature were investigated. Langmuir and Freundlich isotherm models were used to describe the sorption process. The maximum adsorption capacity obtained from the Langmuir isotherm was 46.63 mg/g for Ni (II) and 60.75 mg/g for La (III) on Purolite S957, and 46.55 mg/g for Ni (II) and 60.12. mg/g for La (III) on Diphonix Resin
®. The adsorption character was defined as spontaneous and endothermic.
A series of experiments on La (III) sorption on macroporous resins containing iminodiacetic acid (Lewatit TP 207) and aminomethylphosphonic acid (Lewatit TP 260) groups were carried out in [
19]. The parameters investigated included initial La (III) concentration, pH, temperature, and contact time. In an experiment with 0.1 g of resin and 5 mL of a solution with a concentration of 0.5 × 10
−3 mol/L La (III), equilibrium was reached in 30 min. The optimum pH was 1.5–4.6 for Lewatit 207 and approximately 5.2 for Lewatit TP 260. The sorption capacities of Lewatit TP 207 and Lewatit TP 260 resins were 114.7 and 106.7 mg/g, respectively. The linear forms of adsorption isotherms were plotted using Langmuir and Freundlich models. It was found that the sorption process of La (III) on Lewatit TP 207 resin agrees with the Langmuir adsorption model, while the Freundlich model reliably describes the sorption on Lewatit TP 260. Thermodynamic analysis indicated an endothermic and spontaneous process, with decreasing Gibbs free energy at higher temperatures, suggesting improved sorption efficiency.
The adsorption and desorption process of Er(III) ions on D113-III resin was investigated in [
20]. The resin exhibited a maximum sorption capacity of 250 mg/g at T = 298 K and pH = 6.04. Negative Gibbs free energy values, calculated using the Langmuir model, indicated a spontaneous and endothermic sorption process. It was found that Er(III) ions can be extracted from the solid phase using a 4.0 mol/L HCl solution.
Botelho Junior et al. [
21] investigated the kinetics and thermodynamics of lanthanum and cerium sorption using chelating resins. The study was carried out for three chelating resins Dowex M1495, Lewatit TP 207 and Dowex XUS43605 with different functional groups. The effect of solution pH and solid-to-liquid ratio was evaluated. Thermodynamic analysis indicated a spontaneous and endothermic sorption process for lanthanum and cerium.
The regeneration of ion-exchange resins can be accomplished with both conventional acid solutions and environmentally friendly complexing agents. For instance, Kurkinen et al. [
22] developed a “resin-in-leach” (RIL) method to extract REMs from phosphogypsum using Purolite S940 resin in dilute H
2SO
4 solutions. The effectiveness of alkaline solutions of the chelating agents, including N,N-dicarboxymethylglutamic acid (GLDA), methylglycindiacetic acid (MGDA), oxalic acid, citric acid and iminodiacetate, was evaluated. The chelating agent MGDA provided the production of REM compounds with high purity (up to 99.01%).
Despite the extensive research conducted on the extraction of REMs by cation-exchange resins, the existing literature lacks sufficient data on the thermodynamic parameters (equilibrium constants, Gibbs energy change, enthalpy, and entropy) of sorption. A notable oversight is the inadequate consideration of the potential separation of neighboring rare earth metals of the light group from concentrated phosphoric acid solutions, a subject of both scientific interest and practical significance.
1.2. Sorption on Anion-Exchange Resins
Anion-exchange resins have not been extensively utilized in REM extraction processes; however, their efficacy has been demonstrated in the separation of thorium, uranium, chromium, and certain other metals.
Uranium adsorption on the strongly basic anion-exchange resin Purolite A400 was studied by the authors of [
23]. The effects of initial uranium concentration, pH, phase contact time and temperature were evaluated. Purolite A400 exhibited a sorption capacity of 117.6 mg/g under optimal conditions. Thermodynamic data best fit the Langmuir isotherm model.
Stroganova et al. [
24] analyzed the sorption of Ge(IV) and Cu(II) ions on weakly basic anion-exchange resin AN-31 from chloride solutions. The dependence of equilibrium parameters of sorption of Ge(IV) and Cu(II) ions on the concentration of chloride ions was shown. Chloride concentration affected sorption capacity and extracted the complex structure, as determined by diffuse reflectance spectroscopy, sorption isotherms, and isomolar ion distribution diagrams. The synergistic effect of sorption of Cu(II) ions in the presence of Ge(IV) ions was established. Selective sorption of Cu(II) towards Ge(IV) ions was achieved in chloride solutions of 1 mol/L concentration at molar ratios of Ge(IV):Cu(II) from 1:3 to 1:1.
The study on the extraction of heavy metal ions—copper (II), nickel (II), cobalt (II) and zinc (II)—from model sulfate solutions by a new anion exchanger based on epoxidized vinyl ester of monoethanolamine, allylglycidyl ether and polyethyleneamine was carried out in [
25]. The influence of metal salt concentration, solution pH, and contact time on the resin’s sorption capacity was evaluated. Optimal pH values for maximum sorption were 4.1 for Cu(II), 6.1 for Ni(II), 0.8 for Zn(II), and 5.4 for Co(II). It was found that the sorption capacity under optimal conditions reaches the following values: 705.2 mg/g for Cu(II), 598.8 mg/g for Ni(II), 536.4 mg/g for Zn(II), 436.0 for Co(II). Metal recovery rates of 75.6–80.7% confirm the possibility of using the resin for group sorption of heavy metal ions in hydrometallurgical processes.
Bekchanov at al. developed an anion-exchange resin from plasticized polyvinyl chloride and polyethylene polyamine to remove Cr(VI) from aqueous solutions [
26]. X-ray diffraction, FT-IR spectroscopy, and scanning electron microscopy revealed the resin’s weakly basic amino groups and macroporous structure. Thermodynamic studies showed that the sorption process fit the Freundlich isotherm model. The resin’s maximum sorption capacity for Cr(VI) was 218.4 mg/g. Thermodynamic calculations indicated a spontaneous and endothermic sorption process.
The study [
27] examined the sorption of perrhenate ions (ReO
4−) on an anion-exchange resin synthesized from epichlorohydrin oligomer and 4-vinylpyridine. The effects of rhenium concentration (0.102–1.024 g/L), solution pH, and contact time on the sorption characteristics of the synthesized material were studied. At optimal pH (5.1), the resin achieved a sorption capacity of 371.6 mg/g and 99% perrhenate sorption efficiency.
However, in conjunction with the more thoroughly studied positively charged complexes, REMs have the capacity to form negatively charged complexes with both organic and inorganic ligands. For instance, Hubicka et al. investigated the use of strongly basic gel and macroporous polystyrene anion-exchange resins for the sorption and separation of REM complexes with iminodiacetic acid [
28]. The complexes were identified as Ln(IMDA)
−, and the resins were converted to acetate form for optimal separation efficiency. Due to the different affinity of lanthanum and neodymium complexes with imidoacetic acid to different types of anion exchangers, the separation of these lanthanides on gel and macroporous anion-exchange resin is possible. Separation efficiency followed the order: strongly basic gel anion-exchange resins > strongly basic macroporous resins > weakly basic macroporous resins.
1.3. Problem Statement
The separation of REMs is a challenging scientific task due to their similar chemical properties [
29]. Their similar chemical behavior complicates lanthanide separation from multicomponent mineral and industrial sources [
30,
31].
To address this challenge, the subtle structural differences between lanthanides, especially in physical properties such as ionic radii, must be exploited.
These differences are manifested in ionic radii and electronic structure, primarily 4f-orbitals [
32]. Chemical bonding in REM complexes is predominantly ionic due to large lanthanide ion sizes, fully occupied 5s
25p
6 shells resembling noble gas configurations, and shielded 4f-orbitals. However, available 4f-orbitals enable covalent bonding with donor ligands, resulting in different coordination numbers in REM compounds.
REM compounds with inorganic ligands are typically unstable in aqueous solutions due to high instability constants. However, in acidic leaching solutions from mineral and industrial sources, lanthanides are capable of forming relatively stable anionic complexes. Thus, in sulfuric acid solutions from phosphogypsum leaching, lanthanides form sulfate complexes, whose stage of coordination is directly determined by the concentration of sulfuric acid.
At an excess of sulfate ions, stable anionic sulfate complexes of the second coordination step are formed: [Ln(SO4)2]−. In low-pH phosphoric acid solutions, formed during apatite processing for fertilizer production, REMs form stable hydrogen phosphate complexes, leading to their high solubility (~0.1 wt %).
The determination of the composition of complex compounds and their stability in aqueous solutions was given special attention at the initial stages of the development of REM coordination chemistry, which is confirmed by a large number of works devoted to this subject. However, the necessity to create effective methods of REM separation has led to the development of thermochemical studies of lanthanide complex compounds, in order to clarify the comprehensive set of thermodynamic characteristics.
Thermodynamic differences in ion-exchange adsorption provide a foundation for the effective REM separation. Considering the characteristics of sulfuric and phosphoric acid leaching solutions of apatite raw materials, the thermodynamic properties of the ion-exchange process of light-group REM extraction using cation- and anion-exchange resins were examined.
2. Materials and Methods
In the present work, the sorption of sulfate and phosphate complexes of Pr(III), Nd(III) and Sm(III) from sulfuric and phosphoric acid solutions was studied using ion-exchange resins with characteristics listed in
Table 1. Resin selection was based on prior data demonstrating high REM sorption capacities by ion-exchange resin matrices.
To study the sorption of REM disulfate and hydrogen phosphate complexes, anion-exchange resins were converted to the SO42− form and cation-exchange resins to the H+ form. For this purpose, the anion-exchange resin was treated in 2 mol/L sodium sulfate solution for 24 h, followed by treatment with 2 mol/L sulfuric acid for 2 h after phase separation. The resin converted to the exchangeable form SO42− was washed with distilled water until neutral medium was reached. Preliminary preparation of cation-exchange resins was carried out in a similar way, using sulfuric acid with a concentration of 2 mol/L as the eluent solution.
Experimental Study of the Sorption Process Under Static Conditions
The selection of acidic solutions containing sulfate and phosphate ions is explained by the composition of phosphoric acid and sulfuric acid leaching solutions both directly from apatite concentrate and products of its processing.
The composition of the phosphoric acid solutions used simulates the basic composition of the technological phosphoric acid solution containing H
3PO
4 4.5 mol/L, H
2SO
4 0.19 mol/L and REM from 3.8 to 200.7 mmol/L, which are in the form of cationic complexes of the composition [Ln(H
2PO
4)]
2+ [
33].
Sulfate solutions with pH = 2 contained H
2SO
4, 1 mol/L MgSO
4 and REM from 10.4 to 321.1 mmol/L in the form of anionic complexes of the composition: [Ln(SO
4)
2]
− [
34].
The sorption process of Pr, Nd and Sm was studied by the method of variable concentrations under static conditions at a constant temperature of 298 K and a stirring speed of 120 rpm. Constant experimental conditions were ensured using a thermostated GFL Shaking Incubator 3032 mixing cabinet.
The ion-exchange resins were in contact with the solutions for a minimum of six hours, which ensured that equilibrium was reached in the heterogeneous system.
The extraction of REMs from sulfate solutions was performed using anion-exchange resins Rosek-21 and PurometTM MTA6002PF, from phosphoric acid media using cation-exchange resins PurometTM MTS9500, PurometTM MTS9570, SupergelTM SGC650, PurometTM MTS9560 and PurometTM MTS9580.
The solid-to-liquid phase ratios were determined to be 1:1 for sulfate solutions and 1:5 for phosphoric acid solutions.
Resin sorption capacity (q, mol/kg) and sorption efficiency (α, %) were calculated using Formula (1) and presented in
Table 2.
Results showed that anion-exchange resin capacity was significantly lower than cation-exchange resin capacity. However, anion-exchange resins remained suitable for REM sorption from sulfuric acid leaching solutions. Among the cation-exchange resins, MTS9500, MTS9570 and SGC650 exhibited the highest recovery rates. Consequently, subsequent experimental investigations were conducted using anion-exchange resins Rosek-21 and PurometTM MTA6002PF (MTA6002PF) and cation-exchange resins PurometTM MTS9500 (MTS9500), PurometTM MTS9570 (MTS9570) and SupergelTM (SGC650).
3. Results and Discussion
3.1. Sorption of REMs on Anion-Exchange Resins
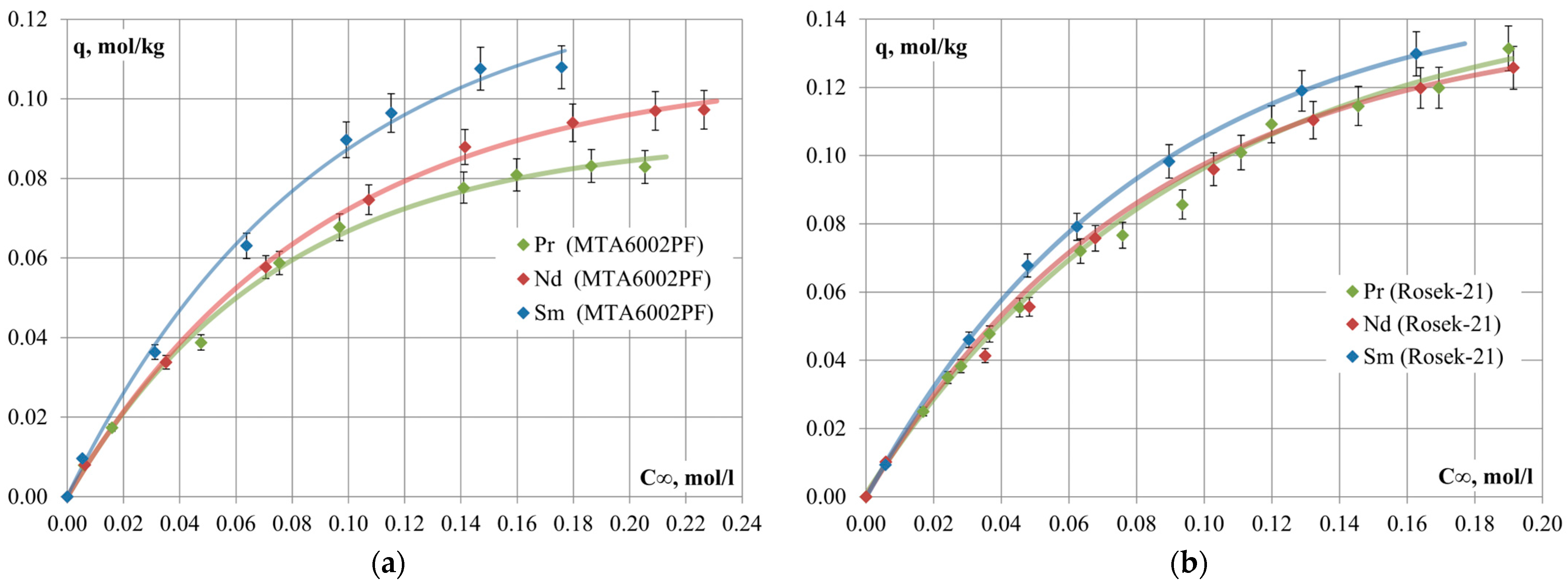
The sorption isotherms of Pr, Nd and Sm on Rosek-21 and PurometTM MTA6002PF anion-exchange resins are presented in
Figure 1.
The equivalent exchange of SO
42− functional groups of the polymer matrix for REM complex ions from sulfate solutions was expressed by the following equation [
34]:
where
and
] determine the concentrations of
and
in the solid matrix of polymer resin.
The experimental ion-exchange isotherms were described through the application of the fundamental law of thermodynamics, specifically the mass action equation [
35], under the assumption that the solid phase is ideal and the values of activity coefficients in it are equal to one:
Linearization of the mass action equation and application of mathematical transformations allowed for obtaining a modified ion-exchange equation similar to the Langmuir equation, which describes a specific exchange process involving complex REM ions:
where the value of the total sorbent capacity (q
∞) is expressed through the equilibrium ion concentrations in the solid phase of the resin:
Considering the weak dependence of activity coefficients on the nature of ions and the strong dependence on charge, the average ionic activity coefficient was calculated using reference values [
36].
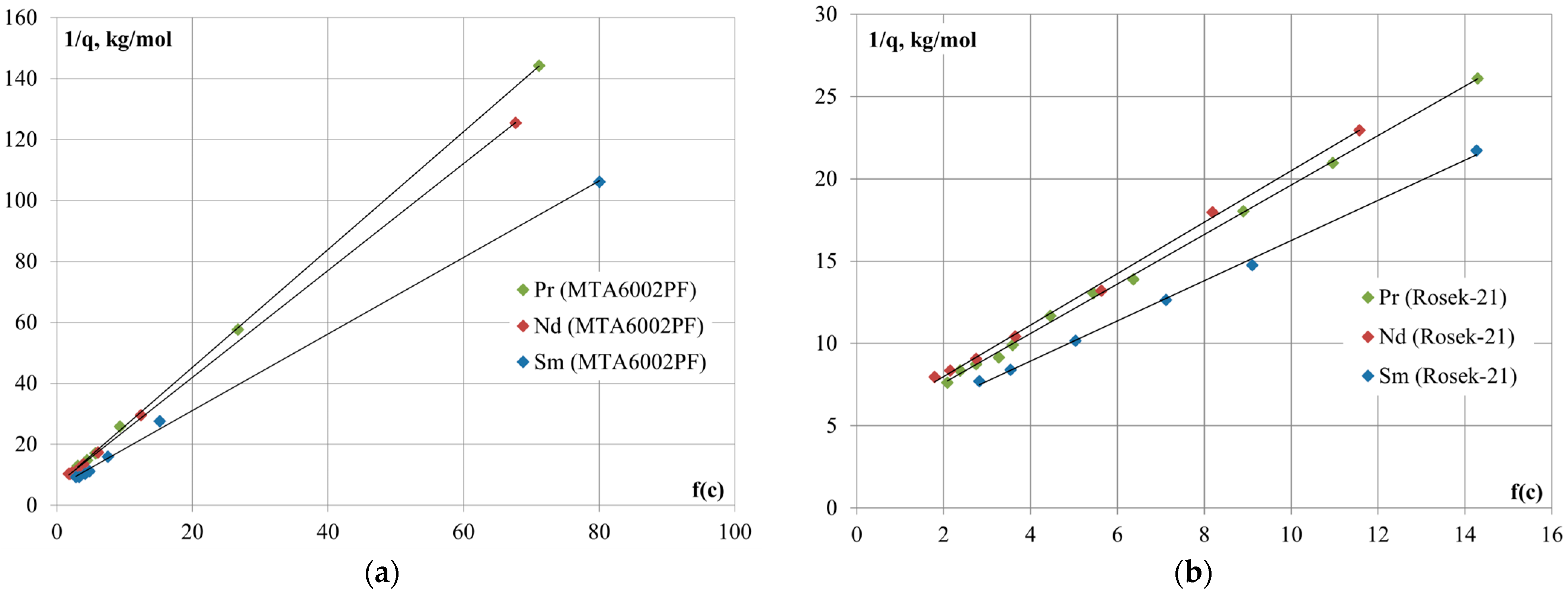
In order to determine the ion-exchange equilibrium constant, a graphical dependence of the reverse sorption value or equilibrium concentration of REM ions in the solid phase
on the concentration argument
was plotted (
Figure 2).
The constants and Gibbs energies (∆G
298) of the ion-exchange equilibrium were calculated from the tangent of the slope angle of the linear forms of the sorption isotherms (
Table 3).
According to the calculated values of the effective equilibrium constants of the sorption process, the selectivity series of Rosek-21 and MTA6002PF anion-exchange resins follows the order: [Sm(SO4)2]− > [Nd(SO4)2]− > [Pr(SO4)2]−. The values of effective equilibrium constants, which are almost equal to one for the anion-exchange resin Rosek-21, are consistent with the low values of REM sorption values.
3.2. Sorption of REMs on Cation-Exchange Resins
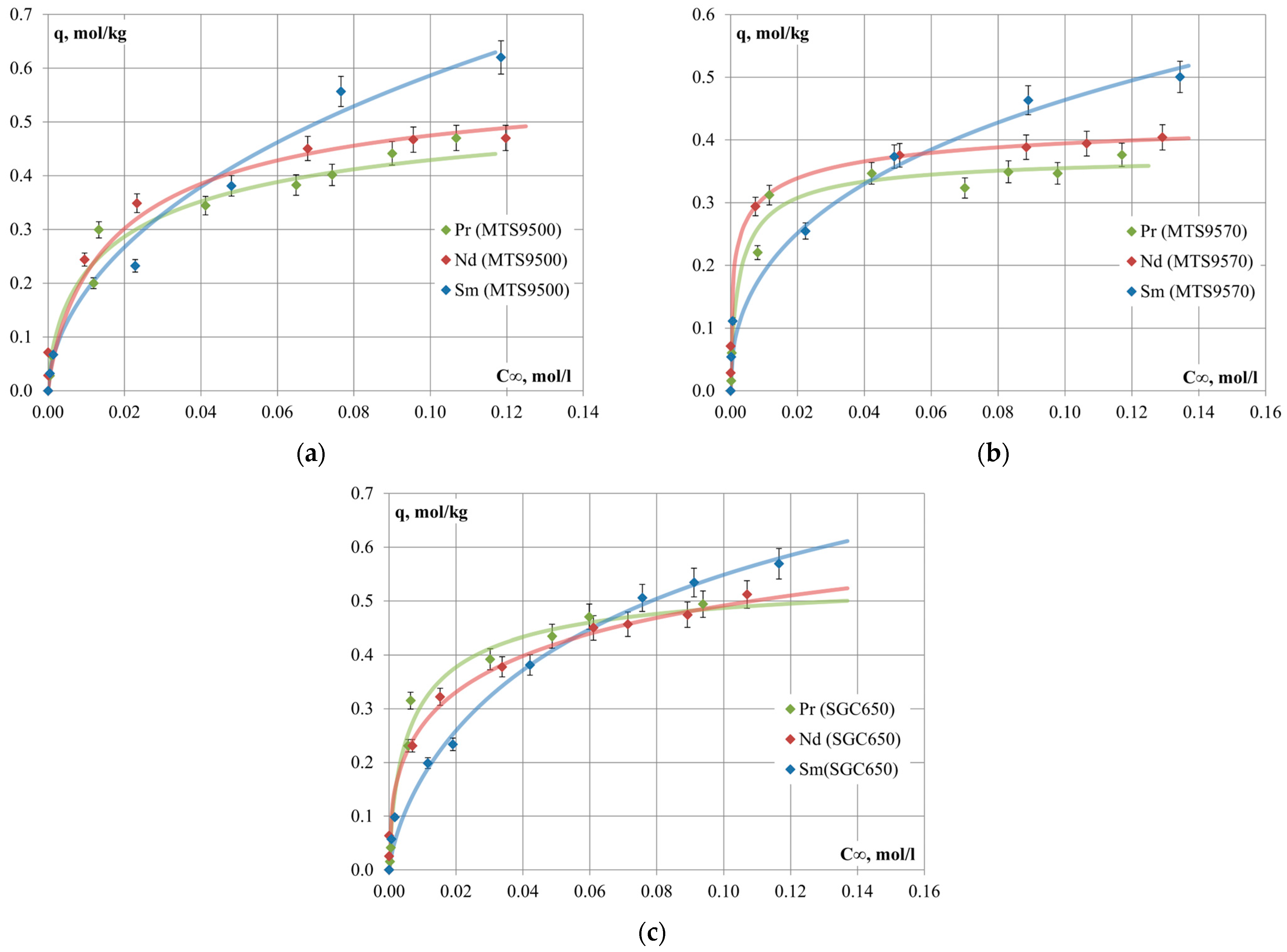
The sorption isotherms of Pr, Nd and Sm on Puromet
TM MTS9500, Puromet
TM MTS9570 and Supergel
TM SGC650 cation-exchange resins are presented in
Figure 3.
For the process of ion exchange from phosphoric acid solutions involving cation-exchange functional H
+ groups of the resin, expressed by Equation (7),
ion-exchange equilibrium constant takes the following form:
Linearizing this equation taking into account the ideality of the solid phase and transforming the total sorption capacity through the value of the sum of the equivalent concentrations of hydrogen ions and dihydrophosphate ions of REMs in the solid phase of the ion-exchange resin,
the following expression was obtained:
The values of activity coefficients were calculated by the Lebedev–Kulyak equation for the solution ionic strength range of 0–2.5 mol/kg.
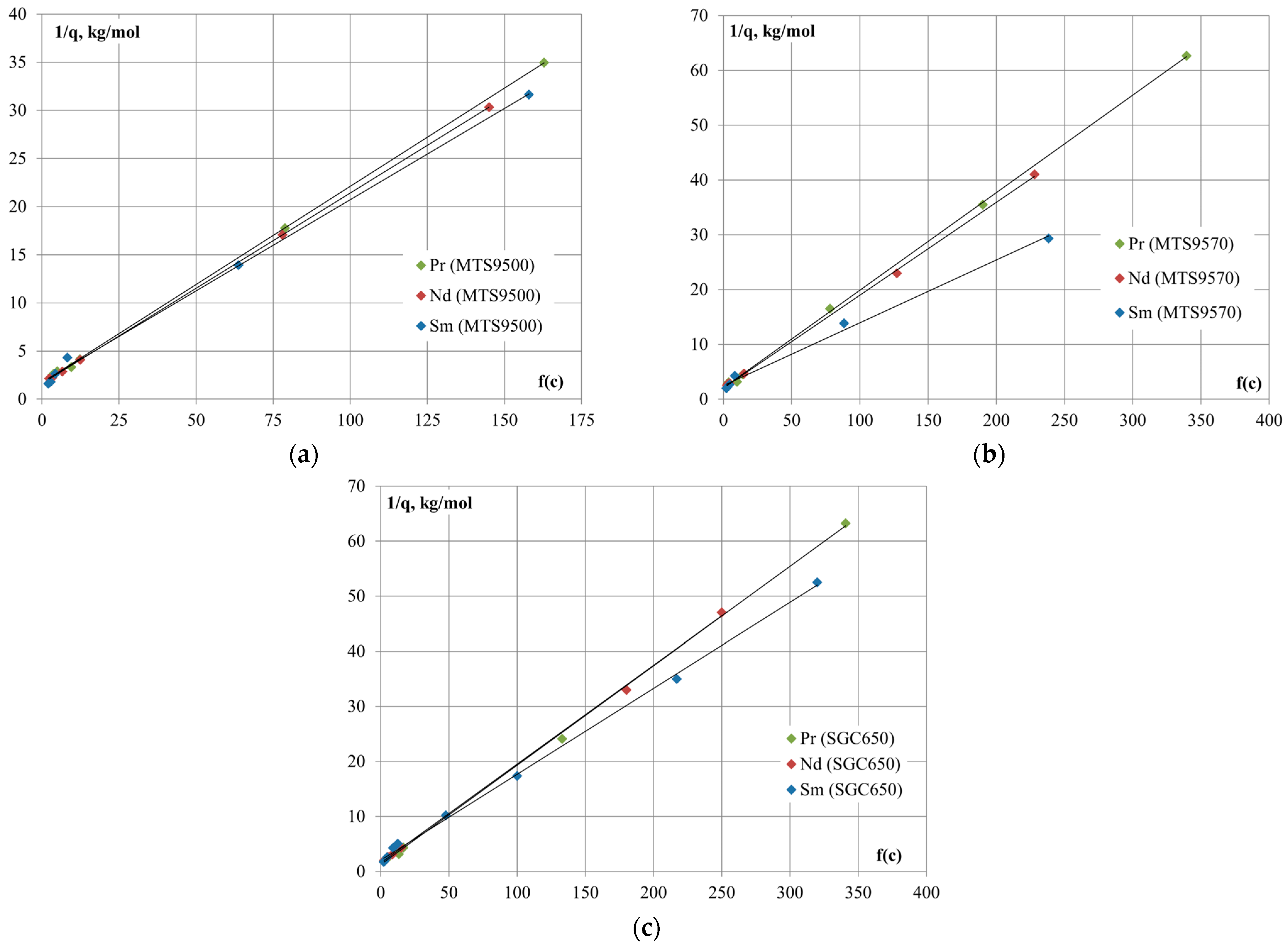
Graphical forms of linear sorption isotherms are presented in
Figure 4, using which the values of ion-exchange equilibrium constants were calculated from the dependence of the value of reverse sorption
on the concentration argument
.
The values of effective equilibrium constant and Gibbs energies of ion exchange on cation-exchange resins are presented in
Table 4.
The process of ion exchange with the participation of dihydrophosphate complexes of REM is characterized by negative values of Gibbs energy, which is consistent with the shift of equilibrium in the direction of displacement of hydrogen ions from the solid matrix of the resin into the aqueous solution of phosphoric acid and high values of REM sorption degree. According to the calculated values of apparent equilibrium constants, the selectivity series of cation-exchange resins follows this order: [Sm(H2PO4)]2+ > [Nd(H2PO4)]2+ > [Pr(H2PO4)]2+. The process of REM extraction on cation-exchange resin MTS9570 is characterized by the highest differences in the values of thermodynamic parameters, which indicates the possibility of REM separation within the group.
3.3. Spectral Characteristics of Ion-Exchange Resins
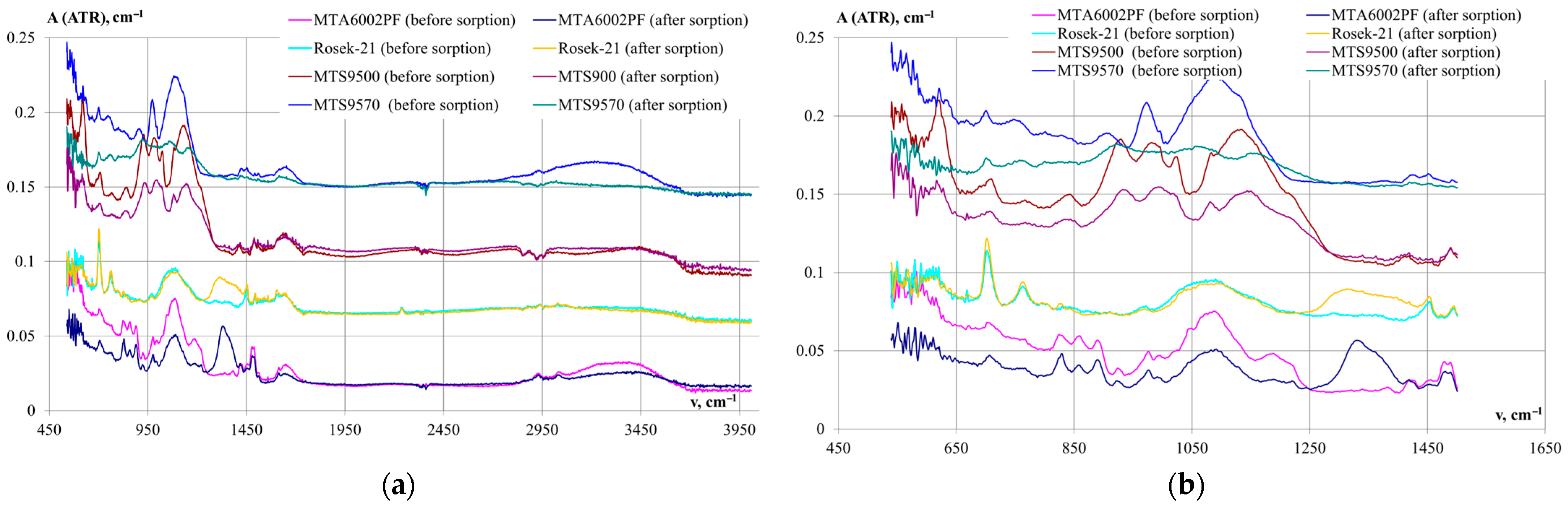
The IR spectra of ion-exchange resins before and after REM extraction from sulfate and phosphoric acid solutions were studied in this work.
In the lumen geometry, the samples are opaque, even with press thinning. The transmittance region starts at 3600 cm
−1, so the detection of changes by this technique is uninformative. The ATR technique shows differences between the original and modified samples.
Figure 5 show the IR spectra of anion- and cation-exchange resin samples before and after praseodymium sorption.
The spectra of anion-exchange resins before and after praseodymium sorption were identical up to the characteristic frequencies of 1200 and above 1400 cm−1, indicating an unchanged styrene-divinylbenzene polymer matrix.
In the range of 1318–1350 cm−1, in the IR spectrum of the samples after sorption, a broad characteristic absorption band of medium intensity is observed, which is characteristic of the SO42− ion with Td-symmetry (tetrahedral). The preservation of Td-symmetry in the sulfate ion, which is a ligand in the composition of the REM complex ion, characterizes the chemical bonding in the complex, very close to the ionic type.
Thus, the results of spectral analysis confirm the presence of the sulfate ion and, consequently, the REM complex ion, in the ion-exchange resin phase.
The IR spectra of the cation-exchange resins before praseodymium sorption show several characteristic frequencies inherent to the phosphonic group R2(HO)P = O (where R is an aromatic radical): 1138, 1086 and 925, 980 cm−1 in the spectrum of the MTS9500 cation-exchange resin and 1146, 1082 and 925, 980 cm−1 on the spectrum of the MTS9570 cation-exchange resin. The absorbance in the region of 1021 cm−1 of cation-exchange resin MTS9500 indicates the presence of R(HO)2P = O groups in the sample.
It is shown that after sorption in the spectrum of the MTS9500 sample, the values of characteristic frequencies are sustained, accompanied by a slight decrease in intensity and an increase in band width. Conversely, in the spectrum of the MTS9570 cation-exchange resin, the characteristic frequencies are no longer discernible, indicating the formation of a stronger bond between the ion-exchange resin matrix and the lanthanide ion.
3.4. Thermal Effect and Entropy of Ion-Exchange Process
The thermal effect of the sorption process was studied on complex neodymium ions in sulfate and phosphoric acid solutions. The obtained values of enthalpy, Gibbs energy of ion exchange, and the calculated entropy values are presented in
Table 5.
The REM adsorption process is spontaneous, exothermic, and accompanied by a decrease in entropy for anion-exchange resins.
The positive values of entropy and enthalpy in the REM sorption process of cationic resins can be explained by the loss of the hydrate shell of complex ions and by differences in the structure and functional groups of the resins.
4. Conclusions
The utilization of secondary sources and waste materials, such as extraction phosphoric acid and phosphogypsum, which are byproducts of apatite concentrate processing, offers a promising approach to the recovery of rare earth metals (REMs).
The process of ion exchange of the light group of REMs (Nd, Pr, Sm) from sulfate and phosphoric acid solutions, modeling the process of sulfuric acid leaching solutions from apatite concentrate and phosphogypsum, was studied. In concentrated phosphoric acid solutions, REMs are present in the form of cationic complexes [Ln(H2PO4)]2+, for the extraction of which cation-exchange resins (PurometTM MTS9500, PurometTM MTS9570, SupergelTM SGC650) are used. In sulfate solutions, REMs can form anionic complexes [Ln(SO4)2]−, which requires the use of anion-exchange resins (Rosek-21, PurometTM MTA6002PF).
The experimental sorption isotherms were described by a thermodynamic model based on linearization of the mass action law equation obtained for the corresponding ion-exchange equations and mathematically transformed into a linear form.
Low values of the equilibrium constants (in the range of 1.06 to 3.92), and consequently of the Gibbs energy changes (in the range of −0.14 to −3.38 kJ/mol), indicate a low selectivity of anion-exchange resins with respect to individual REMs, but do not exclude the use of anion-exchange resins for the extraction of the sum of the light group of REMs.
The process of light-group REM extraction on cation-exchange resins from solutions modeling the composition of extraction phosphoric acid is characterized by significant differences in the values of equilibrium constants (in the range of 12.77 to 115.29) and Gibbs energy changes (in the range of −6.31 to −11.76 kJ/mol). The highest values of equilibrium constants were obtained for the cation-exchange resin MTS9570 (34.39 for praseodymium, 39.19 for neodymium and 115.29 for samarium), which allows a more complete extraction of REMs. However, the most significant discrepancies in the values of ion-exchange equilibrium constants were observed for SGC650 resin, which suggests that this resin is particularly effective for the separation of REMs into individual lanthanides.
The low values of equilibrium constants and Gibbs energies also indicate the possibility of regeneration of ion-exchange resins by sulfuric acid solution due to the shift in ion-exchange reaction equilibrium.
Spectral analysis of ion-exchange resins before and after sorption of REMs confirmed the presence of sulfate and phosphate complexes in the resin matrix and clarified the mechanism of ion exchange.
Based on the values of the Gibbs energy values and the experimentally determined enthalpy of the ion-exchange process, entropy values were calculated. The process of REM extraction on anion-exchange resins is characterized by negative enthalpy and entropy values. REM sorption on cation-exchange resins MTS9570 and SGC650 is endothermic with positive entropy values, which may be due to differences in the structure of the ion-exchange resins and the destruction of hydrate shells of REM complex ions.
The obtained results emphasize the potential of ion-exchange resins for REM extraction from sulfate and phosphoric acid media, offering an environmentally friendly alternative to liquid extraction. This approach enables the elimination of organic solvents, which are frequently utilized in substantial quantities during liquid extraction processes. The utilization of these solvents can result in environmental contamination and the formation of potentially explosive mixtures. Therefore, the method of ion-exchange extraction of REMs facilitates the efficient extraction of target components from process solutions while concomitantly contributing to sustainable development and the reduction in the environmental impact of the chemical industry.
Author Contributions
Conceptualization, O.V.C.; methodology, O.V.C. and M.A.P.; validation, M.A.P. and Y.A.M.; formal analysis, M.A.P. and Y.A.M.; investigation, Y.A.M., N.A.N. and M.D.B.; writing—original draft preparation, Y.A.M. and M.A.P.; writing—review and editing, O.V.C.; visualization, Y.A.M.; supervision, O.V.C.; project administration, M.A.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the current article.
Acknowledgments
The research was carried out with the involvement of the laboratory facilities of the Empress Catherine II Saint Petersburg Mining University Scientific Center “Issues of Processing Mineral and Technogenic Resources” and the Collective Use Center. Samples of ion-exchange resins were provided by the Representative Office of Purolite Ltd. in the CIS, Moscow, Russia.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Diao, H.; Yang, H.; Tan, T.; Ren, G.; You, M.; Wu, L.; Yang, M.; Bai, Y.; Xia, S.; Song, S.; et al. Navigating the Rare Earth Elements Landscape: Challenges, Innovations, and Sustainability. Miner. Eng. 2024, 216, 108889. [Google Scholar] [CrossRef]
- Petrov, D.S.; Danilov, A.S. Hydrochemistry and Ecology of Aquatic Ecosystems in Influence Zones of Mineral Fertilizers Production. Gorn. Zh. 2023, 2023, 83–88. [Google Scholar] [CrossRef]
- Matveeva, V.A.; Smirnov, Y.D.; Suchkov, D.V. Industrial Processing of Phosphogypsum into Organomineral Fertilizer. Environ. Geochem. Health 2022, 44, 1605–1618. [Google Scholar] [CrossRef] [PubMed]
- Kozyrev, B.A.; Sizyakov, V.M.; Arsentyev, V.A. Principles of Rational Processing of Red Mud with the Use of Carboxylic Acids. Non-Ferr. Met. 2022, 53, 30–34. [Google Scholar] [CrossRef]
- Litvinova, T.; Gerasev, S.; Sergeev, V.; Lidanovskiy, E. Rare Earth Metal Ion-Associates in Ln3+—CO32−—H2O System. Metals 2025, 15, 239. [Google Scholar] [CrossRef]
- Salehi, H.; Maroufi, S.; Mofarah, S.S.; Nekouei, R.K.; Sahajwalla, V. Recovery of Rare Earth Metals from Ni-MH Batteries: A Comprehensive Review. Renew. Sustain. Energy Rev. 2023, 178, 113248. [Google Scholar] [CrossRef]
- Zhao, Y.; Pohl, O.; Bhatt, A.I.; Collis, G.E.; Mahon, P.J.; Rüther, T.; Hollenkamp, A.F. A Review on Battery Market Trends, Second-Life Reuse, and Recycling. Sustain. Chem. 2021, 2, 167–205. [Google Scholar] [CrossRef]
- Kutepova, N.A.; Kutepov, Y.I.; Kudashov, E.S.; Daniliev, S.M. Strength of Phosphogypsum Mixed with Nepheline Slime in Construction of Embankments of Gypsum Ponds. Min. Inform. Anal. Bull. 2020, 2020, 67–78. [Google Scholar] [CrossRef]
- Lebedev, A.B.; Utkov, V.A. Chemical Interactions of Red Mud during the Cleaning of an Industrial Gases Ejected to the Atmosphere from Harmful Impurities. Russ. Metall. 2020, 2020, 1653–1657. [Google Scholar] [CrossRef]
- Litvinova, T.E.; Gerasev, S.A. Behaviour of Cerium (III) Phosphate in a Carbonate-Alkaline Medium. J. Min. Inst. 2024, 271, 181–188. [Google Scholar]
- Takano, M.; Asano, S.; Goto, M. Recovery of Nickel, Cobalt and Rare-Earth Elements from Spent Nickel–Metal-Hydride Battery: Laboratory Tests and Pilot Trials. Hydrometallurgy 2022, 209, 105826. [Google Scholar] [CrossRef]
- Lutskiy, D.S.; Ignatovich, A.S. Study on Hydrometallurgical Recovery of Copper and Rhenium in Processing of Substandard Copper Concentrates. J. Min. Inst. 2021, 251, 723–729. [Google Scholar] [CrossRef]
- Dzhevaga, N.; Lobacheva, O.; Smirnova, O.; Yakovlev, G. Reduction in Technogenic Burden on the Environment by Flotation Recovery of Rare Earth Elements from Diluted Industrial Solutions. Appl. Sci. 2021, 11, 7452. [Google Scholar] [CrossRef]
- Tumialán, P.E.E.; Martinez, N.T.; Hinostroza, C.B. Acid Mine Water Treatment Using Neutralizer with Adsorbent Material. J. Min. Inst. 2024, 267, 381–387. [Google Scholar]
- Liu, K.; Liu, Y.; Wu, Y.; Liu, J.; Shuai, Q.; Huang, L.; Hu, Z.; Yamauchi, Y. Advances in reticular materials for sustainable rare earth element recovery. Coord. Chem. Rev. 2025, 522, 216199. [Google Scholar] [CrossRef]
- Lutskiy, D.S.; Lukyantseva, E.S.; Mikheeva, V.Y.; Grigorieva, L.V. Investigation of the Extraction of Samarium and Gadolinium from Leaching Solutions of Phosphorus-Containing Raw Materials Using Solid Extractants. Arab. J. Basic Appl. Sci. 2023, 30, 68–73. [Google Scholar] [CrossRef]
- Toli, A.; Mikeli, E.; Marinos, D.; Balomenos, E.; Panias, D. Assessing the Efficiency of Ion Exchange Resins for the Recovery of Scandium from Sulfuric Acid Leaching Solutions. Separations 2023, 10, 366. [Google Scholar] [CrossRef]
- Araucz, K.; Aurich, A.; Kołodyńska, D. Novel Multifunctional Ion Exchangers for Metal Ions Removal in the Presence of Citric Acid. Chemosphere 2020, 251, 126331. [Google Scholar] [CrossRef]
- Esma, B.; Omar, A.; Amine, D.M. Comparative Study on Lanthanum(III) Sorption onto Lewatit TP 207 and Lewatit TP 260. J. Radioanal. Nucl. Chem. 2014, 299, 439–446. [Google Scholar] [CrossRef]
- Xiong, C.; Meng, Y.; Yao, C.; Shen, C. Adsorption of Erbium(III) on D113-III Resin from Aqueous Solutions: Batch and Column Studies. J. Rare Earths 2009, 27, 923–931. [Google Scholar] [CrossRef]
- Botelho Junior, A.B.; Pinheiro, É.F.; Espinosa, D.C.R.; Tenório, J.A.S.; Baltazar, M.d.P.G. Adsorption of Lanthanum and Cerium on Chelating Ion Exchange Resins: Kinetic and Thermodynamic Studies. Sep. Sci. Technol. 2022, 57, 60–69. [Google Scholar] [CrossRef]
- Kurkinen, S.; Virolainen, S.; Sainio, T. Recovery of Rare Earth Elements from Phosphogypsum Waste in Resin-in-Leach Process by Eluting with Biodegradable Complexing Agents. Hydrometallurgy 2021, 201, 105569. [Google Scholar] [CrossRef]
- Masoud, A.M. Sorption Behavior of Uranium from Sulfate Media Using Purolite A400 as a Strong Base Anion Exchange Resin. Int. J. Environ. Anal. Chem. 2022, 102, 3124–3146. [Google Scholar] [CrossRef]
- Stroganova, E.A.; Bezryadin, S.G.; Larina, T.V. The Equilibrium of Germanium(IV) and Copper(II) Ions Sorption from Chloride Solutions on the Anion-Exchange Resin AN-31. Adsorption 2019, 26, 349–359. [Google Scholar] [CrossRef]
- Serikbaeva, K.T.; Kovrigina, T.V.; Ergozhin, E.E.; Chalov, T.K.; Nikitina, A.I. Sorption of Heavy Metal Ions by New Complexing Anion Exchanger on the Basis of Epoxy Derivative of Vinyl Ether of Monoethanolamine, Allyl Glycidyl Ether and Polyethylene Imine. Life Sci. J. 2014, 11, 120–124. [Google Scholar]
- Bekchanov, D.; Mukhamediev, M.; Lieberzeit, P.; Babojonova, G.; Botirov, S. Polyvinylchloride-Based Anion Exchanger for Efficient Removal of Chromium (VI) from Aqueous Solutions. Polym. Adv. Technol. 2021, 32, 3995–4004. [Google Scholar] [CrossRef]
- Ergozhin, E.E.; Chalov, T.K.; Nikitina, A.I.; Pidakhmet, A. Anionite, Based on Epichlorohydrin Oligomer and 4-Vinylpyridine for Extraction of Rhenium (VII) Ions. Tsvetn. Met. 2014, 8, 321–325. [Google Scholar]
- Hubicka, H.; Kolodyńska, D. Investigation into the Use of Macroporous Anion Exchangers for the Sorption and Separation of Iminodiacetate Complexes of Lanthanum(III) and Neodymium(III). Adsorpt. Sci. Technol. 2000, 18, 719–726. [Google Scholar] [CrossRef]
- Mwewa, B.; Tadie, M.; Ndlovu, S.; Simate, G.S.; Matinde, E. Recovery of Rare Earth Elements from Acid Mine Drainage: A Review of the Extraction Methods. J. Environ. Chem. Eng. 2022, 10, 107704. [Google Scholar] [CrossRef]
- Kaczor-Kurzawa, D.; Wysocka, I.; Porowski, A.; Drzewicz, P.; Vassileva, E. The Occurrence and Distribution of Rare Earth Elements in Mineral and Thermal Waters in the Polish Lowlands. J. Geochem. Explor. 2022, 237, 106984. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, G.; Sun, M.; Liang, X.; He, H.; Zhu, J.; Takahashi, Y. Environmental Risk Assessment of the Potential “Chemical Time Bomb” of Ion-Adsorption Type Rare Earth Elements in Urban Areas. Sci. Total Environ. 2022, 822, 153305. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Q.; Mao, S.; Cheng, W. A Theoretical Study on the Electronic Structure and Floatability of Rare Earth Elements (La, Ce, Nd and Y) Bearing Fluorapatite. Minerals 2019, 9, 500. [Google Scholar] [CrossRef]
- Singh, D.K.; Anitha, M.; Yadav, K.K.; Kotekar, M.K.; Vijayalakshmi, R.; Singh, H. Simultaneous Recovery of Yttrium and Uranium Using D2EHPA–TBP and DNPPA–TOPO from Phosphoric Acid. Desalin. Water Treat. 2012, 38, 236–244. [Google Scholar] [CrossRef]
- Cheremisina, O.V.; Chirkst, D.E.; Ponomareva, M.A. Thermodynamic Study of Cerium Sorption onto Anionite from Sulfate Media. Russ. J. Phys. Chem. A 2013, 87, 288–295. [Google Scholar] [CrossRef]
- Kokotov, Y.A.; Pasechnik, V.A. Equilibria and Kinetics of Ion Exchange; Chemistry, Leningrad, 1970; Available online: https://search.rsl.ru/ru/record/01007403310?ysclid=mconlcfr1f978875393 (accessed on 2 July 2025).
- Ravdel, A.A.; Ponomareva, A.M. Brief Reference Book of Physical and Chemical Quantities; Ivan Fedorov: St. Petersburg, Russia, 2003; Available online: https://rusneb.ru/catalog/010003_000061_31d5cfaee9ae4e8376f9b5b339e5e08f/?ysclid=mconngbazq874913693 (accessed on 2 July 2025).
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).