1. Introduction
Heracleum sosnowskyi Manden. (
H. sosnowskyi), commonly known as Sosnowsky’s hogweed, is a monocarpic perennial plant in the Apiaceae family, native to the eastern and central Caucasus, Transcaucasia, and northeastern Turkey [
1,
2]. Initially introduced to Central and Eastern Europe as a promising high-yield fodder crop, it rapidly established itself as an invasive and problematic weed. It has since spread extensively, particularly across abandoned lands, field margins, and roadsides [
2,
3]. Capable of reaching heights of up to 4.5 m,
H. sosnowskyi often forms dense monodominant communities and even pure monocultural stands that displace native vegetation [
4].
H. sosnowskyi is capable of forming stands that vary widely in both size and density. These stands can range from just a few square meters to several hectares in extent. In larger infestations, plant density may range from sparse growth (1–3 adult individuals per 10 m
2) to dense stands where the species occupies almost the entire ground surface (more than 20 adult individuals per 10 m
2) [
5].
Today,
H. sosnowskyi is recognized as one of the most damaging invasive plant species. Its primary threat lies in its aggressive competitive behavior, which suppresses the growth of native flora [
6]. Additionally, the plant poses a health risk to both humans and animals due to its phototoxic properties; contact with the plant followed by sunlight exposure can cause severe skin burns [
4,
7]. Since the 1980s,
H. sosnowskyi has been officially recognized as a dangerous weed in the Baltic states [
8,
9], and in 2016, it was listed as an invasive species of concern by the European Union [
10].
Controlling the spread of
H. sosnowskyi remains a major challenge due to its adaptability, prolific reproduction, and efficient seed dispersal mechanisms [
9]. Management strategies include manual removal, burning, hot water treatments, repeated cutting, herbicide application, and methods aimed at reducing seed viability [
2,
8,
11]. However, these approaches often require substantial financial resources and may negatively impact native ecosystems.
Despite its invasive nature,
H. sosnowskyi also offers potential benefits, particularly as a source of biologically active compounds. Among biologically active compounds, furanocoumarins are the most prominent and represent the primary active substances found in the plant [
4].
Furanocoumarins are phototoxic compounds naturally produced by a limited number of plant families, primarily as a defense mechanism against herbivores and pathogens. These families include Apiaceae, Fabaceae, Moraceae, and Rutaceae, with smaller contributions from Amaranthaceae, Asteraceae, Cyperaceae, Meliaceae, Pittosporaceae, Rosaceae, and Solanaceae [
12,
13]. Among them, the Apiaceae and Rutaceae families contain the largest number of furanocoumarin-producing species and are considered the most important sources for both pharmacological and toxicological applications [
13]. Structurally, furanocoumarins are classified into two types: linear and angular. Linear furanocoumarins, such as psoralen, have the furan ring attached at the 6,7 positions of the coumarin backbone, while in angular furanocoumarins, such as angelicin, the furan ring is attached at the 7,8 positions. These compounds are responsible for causing skin irritation, blistering, and, in more severe cases, photosensitivity reactions in humans and animals following exposure to both the plant and sunlight.
However, beyond their toxic effects, furanocoumarins also exhibit a range of beneficial biological activities. They have been shown to positively influence the central nervous and circulatory systems and demonstrate antioxidant, anti-inflammatory, antibacterial, antifungal, and bone health-promoting properties [
14,
15,
16].
Thanks to their photochemical properties, furanocoumarins are widely used in PUVA (psoralen and ultraviolet A) therapy, particularly for the treatment of skin conditions such as vitiligo and psoriasis [
13]. Additionally, they are believed to possess anticancer properties, with a notable selectivity for tumor cells over normal cells, and may serve as natural alternatives to synthetic photosensitizers in cancer therapy and other medical applications involving photoactive compounds [
14,
17,
18,
19]. These effects are largely attributed to their unique chemical structure and vary from compound to compound.
Solid–liquid extraction using organic solvents remains the most widely used approach for isolating furanocoumarins from plant materials. Conventional techniques such as maceration [
19,
20], Soxhlet extraction [
21,
22], ultrasonication [
3,
22], and hydrodistillation [
23] are commonly employed for this purpose. However, more advanced methods—including accelerated solvent extraction, supercritical fluid extraction, and microwave-assisted extraction (MAE)—are gaining attention for their improved efficiency and environmental benefits [
4,
13].
Among these, MAE has emerged as a particularly promising technique, especially with the advent of specialized microwave systems that enable precise control over power, temperature, sample agitation, and pressure within sealed vessels [
24]. Unlike traditional heating, microwaves directly heat the entire sample volume, bypassing the container and enabling rapid, uniform heating of the extraction mixture. This results in significantly shorter extraction times (typically 5–30 min) and extraction yields that are comparable to, or even exceed, those achieved through Soxhlet extraction. Additionally, MAE offers the advantages of lower energy consumption, reduced operational costs, and decreased solvent usage, thereby minimizing waste generation [
25].
Despite the proven effectiveness of MAE in extracting furanocoumarins from various plant sources, its application to H. sosnowskyi has not yet been reported. Therefore, the aim of this study is to investigate and optimize the use of MAE for extracting furanocoumarins from H. sosnowskyi, while also developing an efficient method for extract purification and compound separation.
2. Materials and Methods
Methanol (≥99.9%) was obtained from Fisher Chemical (Pittsburgh, PA, USA), acetone (≥99.8%) from Honeywell, and n-hexane (≥98%) from Supelco. Coumarin (≥98%) was purchased from Thermo Scientific Chemicals (Waltham, MA, USA).
Raw plant material of H. sosnowskyi was collected in the Vilnius District in 2024. Prior to analysis, the leaves were air-dried and ground using a porcelain mortar.
A coumarin stock solution (1 mg/mL) was prepared by weighing the appropriate amount of coumarin and dissolving it in acetone. Working solutions of coumarin were prepared by diluting the stock solution with acetone to the desired concentrations.
Microwave-assisted extraction was carried out using a Monowave 450 microwave reactor (Anton Paar, Graz, Austria). Unless otherwise specified, 0.1 g of plant material and a measured volume of the selected solvent were placed in a 30 mL G30 Wide Neck glass vial sealed with a silicone septum and snap cap. The “heat as fast as possible” mode was used to rapidly reach the target temperature. Extraction was carried out for a predetermined duration, after which the vial was cooled to 50 °C. Prior to chromatographic analysis, the resulting extracts were filtered using Whatman® Grade 114 qualitative filter paper.
Gas chromatography–mass spectrometry (GC-MS) analysis was performed using a PerkinElmer Clarus 580 series gas chromatograph equipped with a programmable temperature vaporizing (PTV) injector and coupled to a PerkinElmer Clarus 560 S mass spectrometer (PerkinElmer, Springfield, IL, USA). The system was fitted with an Elite-5MS capillary column (30 m × 0.25 mm i.d., 0.25 µm film thickness). Helium served as the carrier gas at a constant flow rate of 1.9 mL/min. Injections were made in split mode (10:1).
The oven temperature was programmed to rise from 40 °C to 250 °C at a rate of 10 °C/min and then held at 250 °C for 5 min. The transfer line temperature was set to 280 °C. The mass spectrometer operated with an electron ionization (EI) source at 70 eV and a source temperature of 180 °C. Full scan mode was used for data acquisition across the m/z range of 45–500. Compound identification was performed by comparing their retention indices (RIs) and mass spectra with entries in the NIST (National Institute of Standards and Technology) library. Compounds with a match factor score greater than 800, indicating a good to excellent match [
26], were considered positively identified.
Quantification of furanocoumarins was performed using a PerkinElmer Clarus 580 gas chromatograph equipped with a flame ionization detector (FID). The detector was maintained at 250 °C, with hydrogen and air flow rates of 40 mL/min and 400 mL/min, respectively. Chromatographic separation was achieved on an Elite-200 capillary column (30 m × 0.25 mm i.d., 0.25 µm film thickness; PerkinElmer). The injector temperature was set to 250 °C. The oven temperature program was the same as for the GC-MS: from 40 °C to 250 °C at 10 °C/min, held for 5 min. Nitrogen was used as the carrier gas at a flow rate of 2 mL/min.
For GC-FID analysis, compound identification was based on the analysis of fractions collected during SPE. These fractions were first characterized by GC-MS to determine their composition. Fractions containing a single target compound were then analyzed by GC-FID to establish the corresponding retention times.
Quantitative determination of furanocoumarins was carried out using a calibration curve constructed with standard solutions of coumarin. The curve was based on five calibration points, each measured in triplicate, and demonstrated linearity over the concentration range of 10–1000 µg/mL, with a correlation coefficient of 0.9982. The linear regression equation for the calibration curve was y = 649.2x + 3617.4. The limit of detection (LOD), defined as the concentration producing a signal three times higher than the baseline noise, was 1.1 µg/mL. The limit of quantification (LOQ), defined as the concentration corresponding to a signal ten times the baseline noise, was 3.8 µg/mL.
Confidence intervals (CIs) for the furanocoumarins were calculated using the following formulas:
where
xi is an individual measurement,
is the sample mean based on
n = 3 replicate measurements,
t is the critical
t-value at the 95% confidence level, and
S is the sample standard deviation.
Solid-phase extraction (SPE) was carried out using Strata Eco-Screen cartridges (1 g/3 mL, 500 mg sodium sulfate, Phenomenex). Stepwise elution was carried out by increasing the polarity of the solvent mixture, consisting of n-hexane and acetone. The optimized procedure involved conditioning the cartridge with 5 mL of n-hexane, loading 1 mL of a 5-times preconcentrated H. sosnowskyi extract in hexane, and collecting the following fractions:
Fraction 1: 4 mL of n-hexane;
Fraction 2: 10 mL of 2% acetone in n-hexane;
Fractions 3–9: 1 mL of 5% acetone in n-hexane each;
Fractions 10–19: 1 mL of 10% acetone in n-hexane each.
3. Results and Discussion
3.1. Chemical Composition of H. sosnowskyi Extracts
Microwave-assisted extraction was chosen as a rapid and efficient method for isolating biologically active compounds from H. sosnowskyi leaves. Among the critical factors influencing MAE efficiency is the choice of solvent, which directly impacts the solubility of target compounds, mass transfer kinetics, and the solvent’s ability to penetrate the plant matrix.
To extract biologically active compounds from H. sosnowskyi, solvents of varying polarity—water, methanol, acetone, and hexane—were tested. For each extraction, 0.1 g of dried, ground H. sosnowskyi leaves was mixed with 2 mL of the chosen solvent and subjected to microwave heating at 50 °C for 10 min. The resulting extracts were filtered and analyzed using GC-MS.
For the water-based extract, an additional extraction step was performed prior to GC-MS analysis, involving shaking the sample with 2 mL of hexane for 5 min to isolate organic compounds. Methanol, acetone, and hexane extracts revealed similar major components. The compounds identified in the methanol extract with peak areas greater than 1% and a match score above 800 are listed in
Table 1. Water proved to be an ineffective solvent for this purpose, as only trace amounts of angelicin and methoxsalen were detected.
Four of the main compounds listed in
Table 1—angelicin, psoralen, methoxsalen, and bergapten—are furanocoumarins. These compounds are widely used in medicine due to their broad spectrum of pharmacological activities [
27,
28,
29,
30]. Consequently, further work focused on optimizing the separation of furanocoumarins.
3.2. Optimization of MAE Conditions for Furanocoumarins
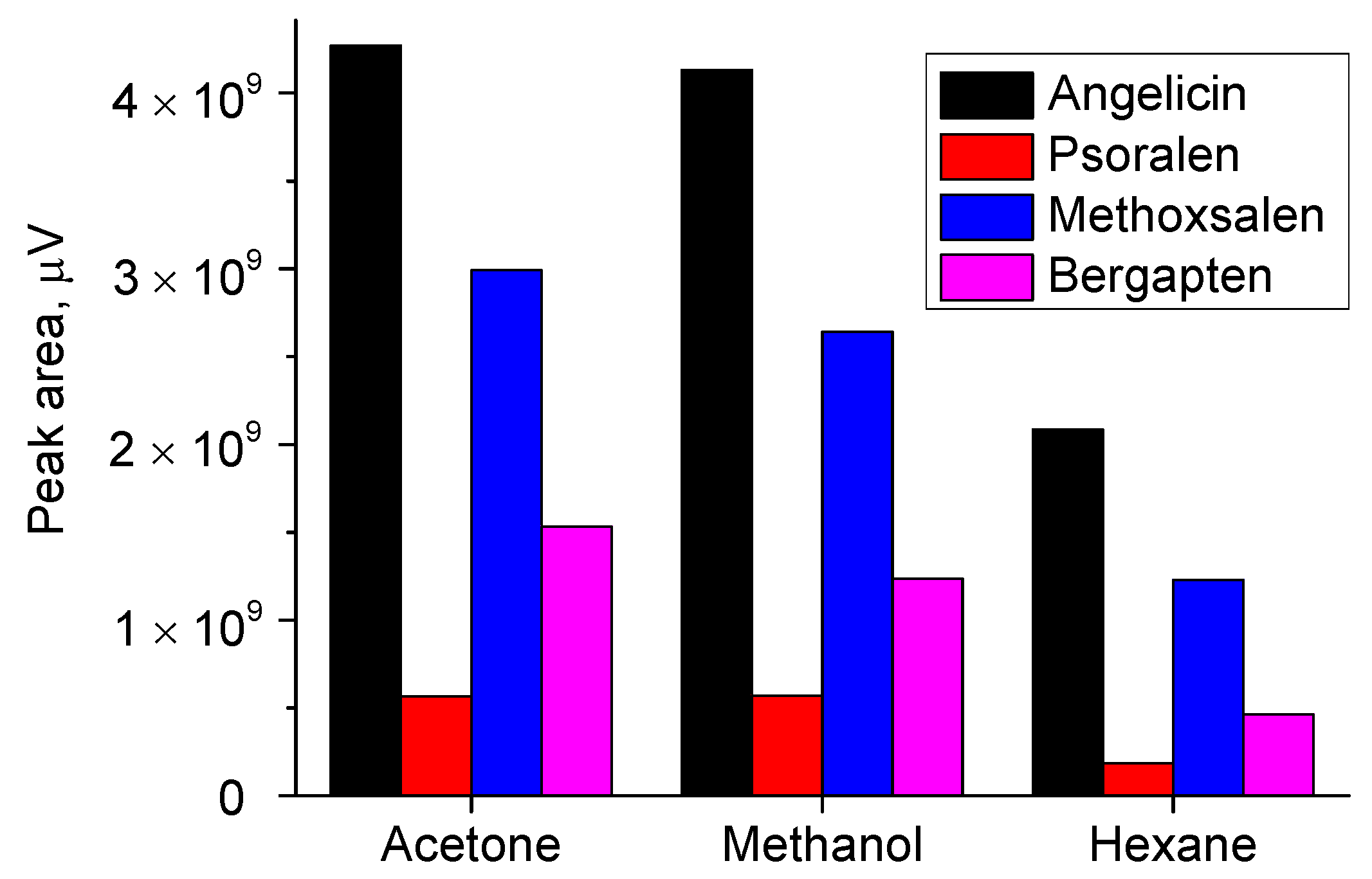
All four furanocoumarins were detected in each of the three tested organic solvent extracts. Among them, methanol and acetone extracts contained comparable and higher amounts of furanocoumarins than the hexane extract (
Figure 1).
On the other hand, the chromatogram of the hexane extract displayed fewer peaks, primarily corresponding to the target compounds. In comparison, the chromatograms of the acetone and methanol extracts were more complex (
Figure 2), as these solvents also extracted a wider range of polar organic substances. Since the ultimate goal was to isolate furanocoumarins, which is more readily achieved when fewer interfering compounds are present, hexane was selected as the preferred extraction solvent. An additional advantage of using hexane is its compatibility with the subsequent purification step by solid-phase extraction. To ensure effective retention of the target compounds on a normal-phase sorbent, the use of a nonpolar solvent like hexane is particularly beneficial.
Extraction temperature is another key factor influencing the efficiency of MAE. Raising the temperature can enhance the solvent’s extraction power by reducing its surface tension and viscosity, which in turn improves solute solubility, matrix wetting, and solvent penetration. A temperature range of 50 °C to 170 °C was tested. As shown in
Figure 3, the extraction yield increased with rising temperature up to a point, with the highest yields observed between 70 °C and 90 °C.
However, when the extraction temperature exceeded 90 °C, the peak areas of the target compounds began to decrease, likely due to their thermal degradation. This assumption is supported by a comparison of chromatograms obtained at 70 °C and 170 °C extraction temperature (
Figure 4), which reveals the appearance of new, prominent peaks at the higher temperature. These peaks—corresponding to p-cymene (5.71 min), benzeneacetaldehyde (6.01 min), (1S)-2,6,6-trimethylbicyclo[3.1.1]hept-2-ene (6.04 min), isoterpinolene (6.67 min), and β-bourbonene (10.91 min)—indicate the formation of degradation products originating from higher-molecular-weight compounds.
Therefore, an extraction temperature of 70 °C was selected for further experiments.
Hexane is a non-polar solvent with a low dielectric constant (1.89 at 20 °C) [
31], which limits its ability to absorb microwave radiation efficiently. As a result, it heats more slowly than polar solvents such as methanol or acetone, potentially contributing to its lower extraction efficiency for the target compounds.
However, it is well established that the presence of moisture can enhance the recovery of compounds from plant matrices [
32]. Water within plant cells absorbs microwave energy, leading to internal superheating and cell disruption. This process facilitates the release of compounds from the matrix, thereby improving extraction efficiency [
33].
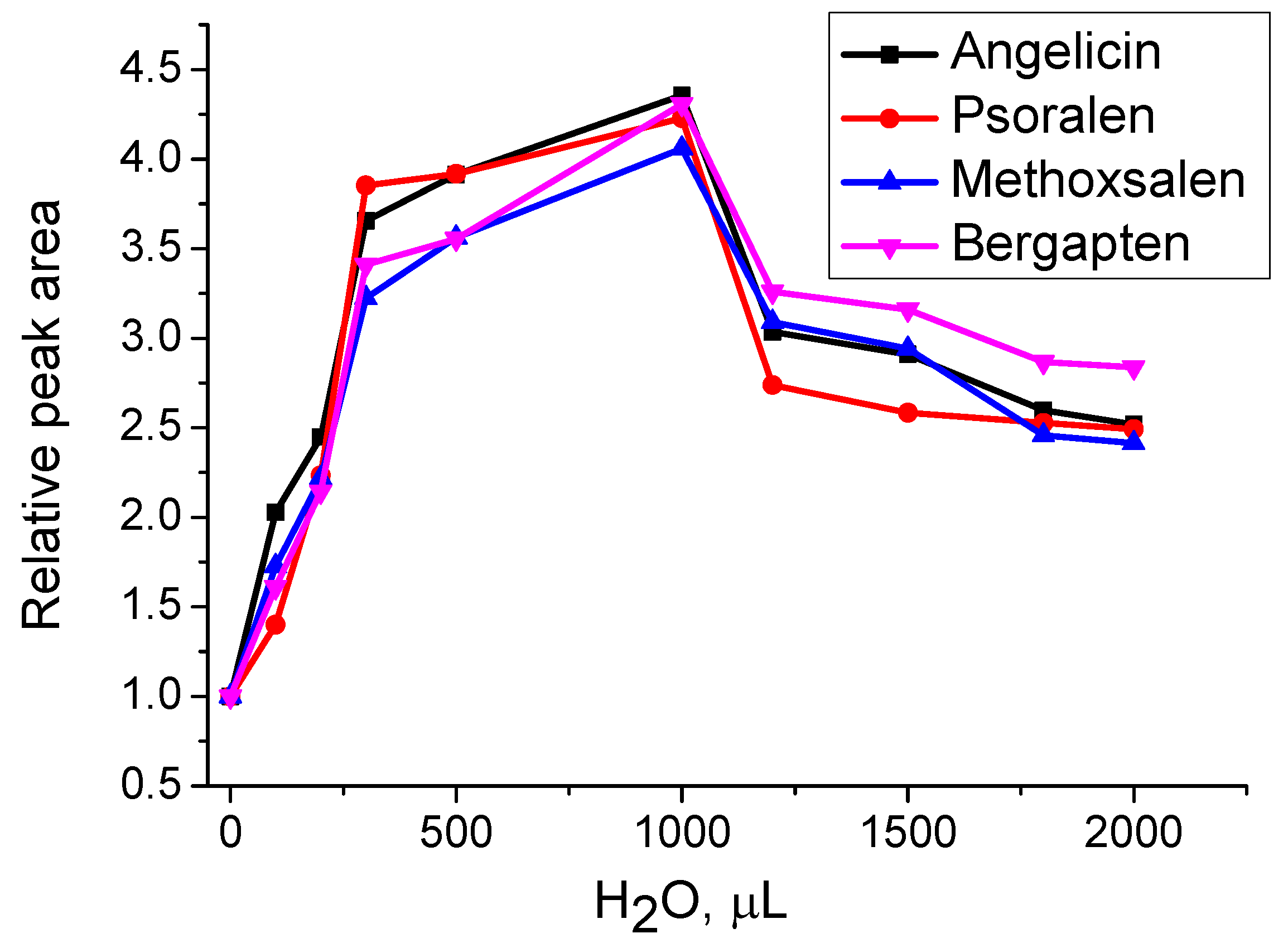
To investigate the effect of water on the extraction of furanocoumarins using hexane, varying amounts of water (up to 2 mL) were added to 0.1 g of
H. sosnowskyi leaf material along with 2 mL of hexane. MAE was performed at 70 °C for 10 min. As shown in
Figure 5, the highest extraction yield was achieved with the addition of 1 mL of water. Beyond this point, increasing the water content led to a decline in extraction efficiency. This is likely because small amounts of water are absorbed by the plant material, improving hexane’s contact with the matrix. In contrast, excess water may form a separate phase, reducing the direct interaction between hexane and the plant tissue.
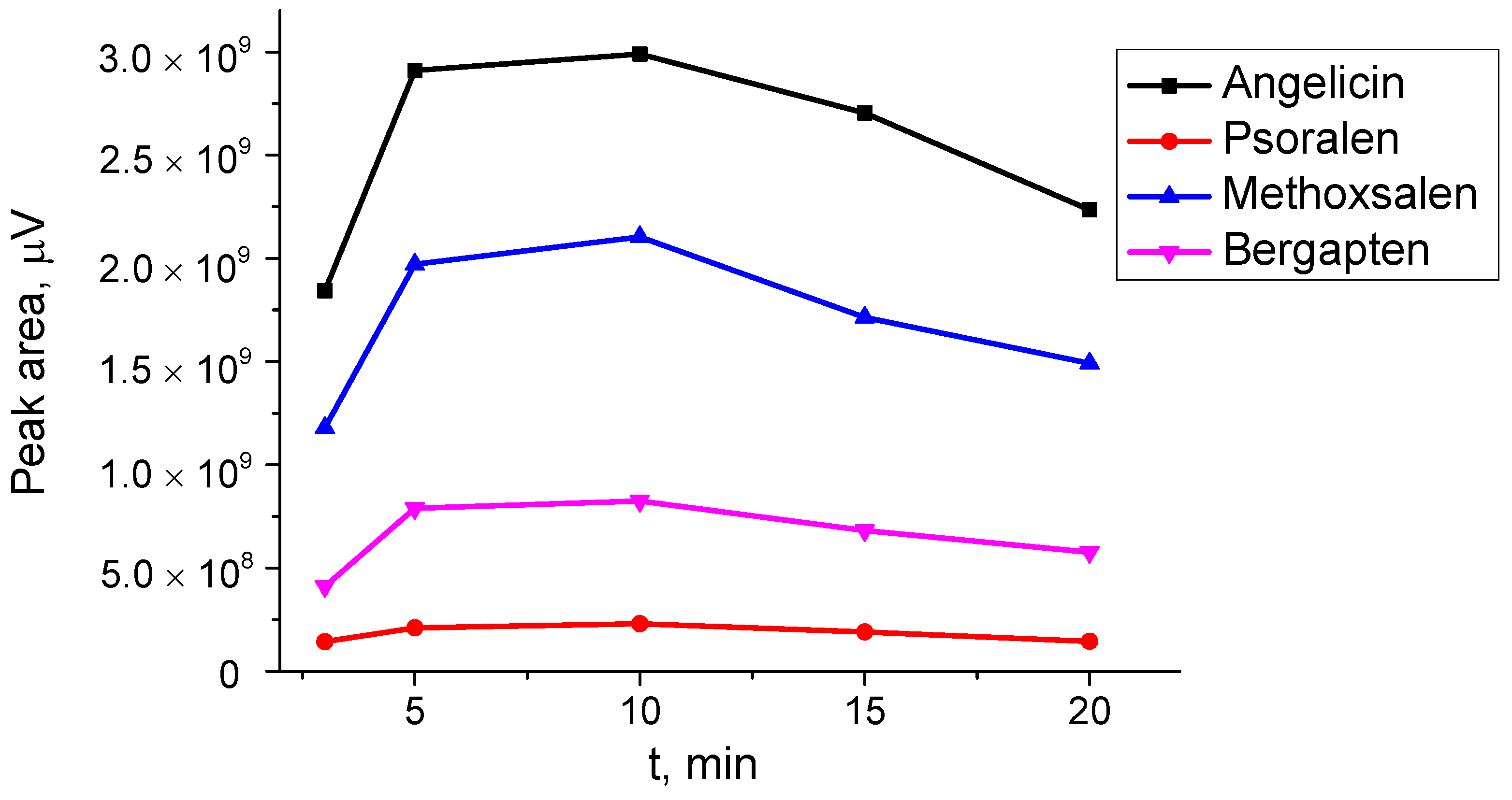
One of the advantages of MAE over conventional extraction methods is its short extraction time, typically ranging from a few to several minutes. An investigation of extraction times from 3 to 20 min (
Figure 6) showed that the highest extraction yield was achieved after 10 min. However, as extraction time continued to increase, the peak areas of the target compounds began to decrease, likely due to thermal degradation.
The hexane-to-solid ratio was further optimized. For this, 1 mL of water and 2 mL of hexane were added to 0.025–0.3 g of plant material, and MAE was performed under the previously optimized conditions. The extraction yield of furanocoumarins increased with up to 0.1 g of plant material. Between 0.1 and 0.2 g, the peak areas of the target compounds remained similar, but the volume of the filtered solvent gradually decreased. With 0.3 g of H. sosnowskyi, all the solvent was absorbed by the plant material. Therefore, the optimal hexane-to-solid ratio was determined to be 20:1 (2 mL of hexane to 0.1 g of H. sosnowskyi).
3.3. Approximate Quantification of Furanocoumarins in the Extract
For precise quantification of furanocoumarins in the extract by GC-MS, it is necessary to build calibration curves or determine response factors relative to a specific internal standard. This requires standards of the compounds of interest, which are costly. However, in our case, precise quantification was not critical, as the furanocoumarin content in H. sosnowskyi varies with the plant’s developmental stage, soil, and environmental conditions.
Thus, we opted for approximate quantification. In scientific literature, raw peak area percentages are often used for quantification, assuming that all response factors are equal to unity [
34]. Therefore, we decided to estimate the quantity of furanocoumarins by calibrating with coumarin, assuming that the response factors for the furanocoumarins of interest were similar to that of coumarin. Additionally, instead of GC-MS, we employed GC with a flame ionization detector (FID), which provides a universal response, especially for compounds with similar structures.
The results of furanocoumarin quantification are presented in
Table 2.
The total furanocoumarin content was determined to be 3.5 mg/g. As shown by the results presented,
H. sosnowskyi contains an exceptionally high amount of angelicin. This compound is particularly noteworthy because, as an angular furanocoumarin, angelicin is far less common than its linear counterparts. Angular furanocoumarins have been reported only in a limited number of species within the Fabaceae, Moraceae, and Apiaceae families. In contrast, species from the Rutaceae family, which includes citrus fruits, are known to produce only linear furanocoumarins [
35].
The prominence of angelicin in
H. sosnowskyi becomes even more evident when compared to its occurrence in other plants. According to Ref. [
36], which analyzed furanocoumarin content in various fruits and vegetables from both the Apiaceae and Rutaceae families, angelicin was detected only in
Pastinaca sativa, and at much lower levels—up to 27.8 µg/g.
Quantitative data on furanocoumarins in
H. sosnowskyi are limited. To our knowledge, only two studies have reported specific concentrations. In Ref. [
17], the juice of
H. sosnowskyi was found to contain 1332.7 mg/L of methoxsalen and 34.2 mg/L of bergapten. In another study [
37], several furanocoumarins were quantified in the fruit coats and seeds of the plant. Angelicin was present at notably high levels—ranging from 11.8 to 29.0 mg/g in the fruit coats and from 2.7 to 11.1 mg/g in the seeds, depending on the collection site. In comparison, bergapten was found at 5.0–7.1 mg/g in fruit coats and 0.8–2.7 mg/g in seeds, while methoxsalen ranged from 0.5 to 8.7 mg/g in fruit coats and from 0.1 to 1.5 mg/g in seeds—both significantly lower than angelicin.
However, to the best of our knowledge, no data have been published to date on the furanocoumarin content in the leaves of H. sosnowskyi.
3.4. Purification and Isolation of Furanocoumarins by Solid-Phase Extraction
Solid-phase extraction was used for the separation of furanocoumarins, employing a sorbent Strata Eco-Screen. Strata Eco-Screen, a normal-phase sorbent layered with sodium sulfate, was chosen for this purpose. According to the manufacturer, this sorbent is ideal for samples dissolved in organic mixtures with residual water, making it well suited for our needs. We anticipated that the separation would proceed via normal-phase chromatography, driven by polar interactions, while the sodium sulfate would remove any residual water.
Stepwise elution was carried out by increasing the polarity of the solvent mixture, consisting of n-hexane and acetone. The optimized procedure is presented in
Section 2.
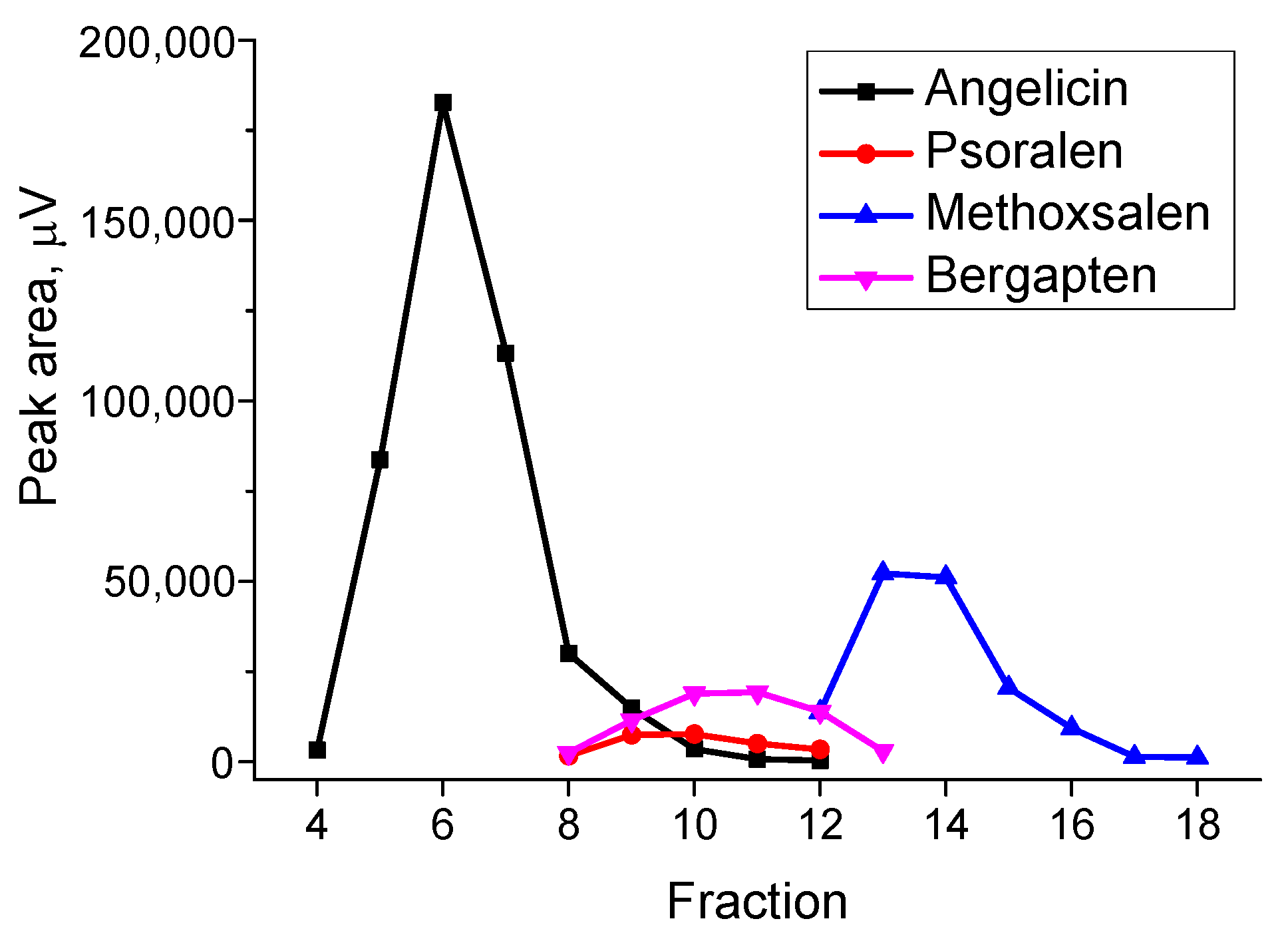
The peak areas of furanocoumarins in each fraction are shown in
Figure 7.
The recoveries of furanocoumarins were assessed by comparing the peak areas of the corresponding compounds in the chromatogram of the extract before SPE.
Angelicin (74.6%) was eluted separately from other furanocoumarins in fractions 4–7. Fraction 4 also contained 5-dodecyldihydro-2(3H)-furanone. Fractions 8–11 contained angelicin (9.5%), psoralen (57.8%), and bergapten (58.3%). Fraction 12 contained psoralen (9%), methoxsalen (7.8%), and bergapten (15.5%). Fraction 13 contained methoxsalen (29.7%) and bergapten (3.4%). In fractions 14–18, methoxsalen (46.8%) was separated.
The total recoveries of furanocoumarins in fractions 4–18 were as follows: angelicin (84.1%), psoralen (66.8%), methoxsalen (84.3%), and bergapten (77.2%).
The proposed SPE procedure effectively isolates pure angelicin (fractions 4–7) and pure methoxsalen (fractions 14–18). Of particular interest is pure angelicin, which holds significant value due to its high market price—reaching up to EUR 443 for just 10 mg [
38].
H. sosnowskyi presents a promising opportunity for beneficial use, also due to its widespread availability. It is considered that the biomass yield of
H. sosnowskyi can range from 2.3 to 4.6 kg/m
2; even at a minimal estimate of 2 kg/m
2, 20 tons of biomass per hectare can be obtained from
H. sosnowskyi [
4].
However, despite the potential economic value and ecological benefits (e.g., reduced herbicide use and invasive species management), the harvesting and processing of
H. sosnowskyi come with variable costs. These depend on factors such as population size, location, and accessibility. Mechanized harvesting can help reduce expenses compared to manual methods using tools like scythes or trimmers [
39]. Conversely, the plant’s phototoxic properties can drive up costs, as handling requires protective clothing, trained personnel, and strict adherence to safety protocols.
Additional purification steps would be required to isolate pure psoralen and bergapten. However, mixtures of furanocoumarins may also hold practical value, as all the furanocoumarins identified in
H. sosnowskyi are well known for their medical applications—particularly in combination with ultraviolet A therapy for the treatment of various skin disorders. They are also believed to have promising anticancer properties, with a notable selectivity for tumor cells over normal cells [
18]. These effects are largely attributed to their unique chemical structure and vary from compound to compound. It is therefore plausible that not only individual furanocumarines but also natural mixtures obtained via SPE may exhibit synergistic therapeutic effects.
4. Conclusions
H. sosnowskyi is considered one of the most dangerous invasive plant species. Its control is challenging and costly. However, the abundant availability of H. sosnowskyi also presents an opportunity for beneficial use.
Our study demonstrated that H. sosnowskyi leaves are a rich source of biologically active furanocoumarins, specifically angelicin, psoralen, methoxsalen, and bergapten. Among these, angelicin was found in particularly high quantities—exceeding 2 mg per gram of dried leaf material. Using solid-phase extraction, more than 70% of angelicin was successfully separated from other furanocoumarins. Methoxsalen content exceeded 0.7 mg per gram of dried leaves, and this compound was also obtained in pure form through the SPE procedure.
Furanocoumarins are considered to possess promising anticancer properties, largely attributed to their unique chemical structures. Since these properties can vary significantly among individual compounds, it is plausible that not only isolated furanocoumarins but also natural mixtures obtained through solid-phase extraction may exhibit synergistic therapeutic effects. However, this hypothesis requires further investigation to comprehensively assess both their efficacy and their safety.