Heterogeneous Interactions During Bubble–Oil Droplet Contact in Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Physical Property Measurements
2.2.1. Zeta Potential Measurements
2.2.2. Surface and Interfacial Tension Measurements
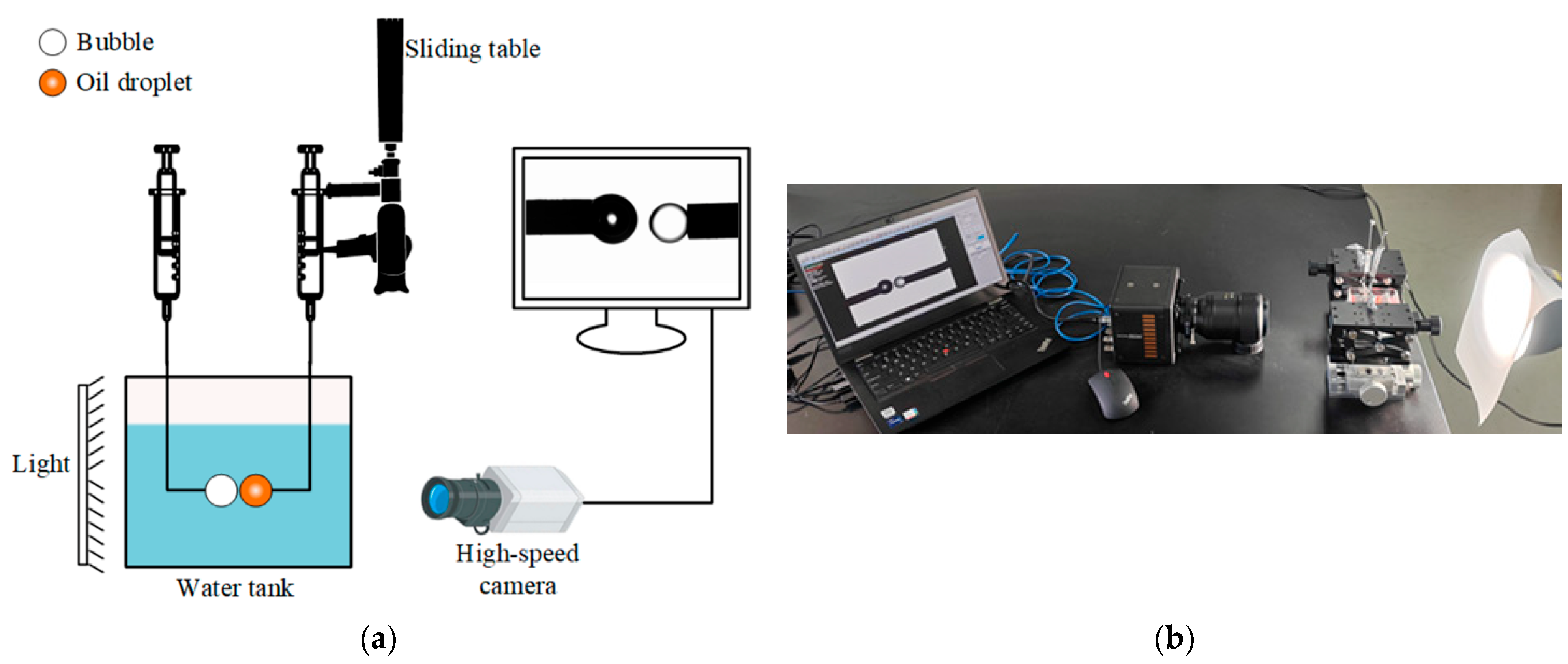
2.3. Bubble–Oil Droplet Attachment Experiments
2.4. Extended DLVO Theory
2.4.1. Van Der Waals Forces
2.4.2. Electrical Double-Layer Forces
2.4.3. Hydrophobic Forces
3. Results and Discussion
3.1. Material Property Analysis
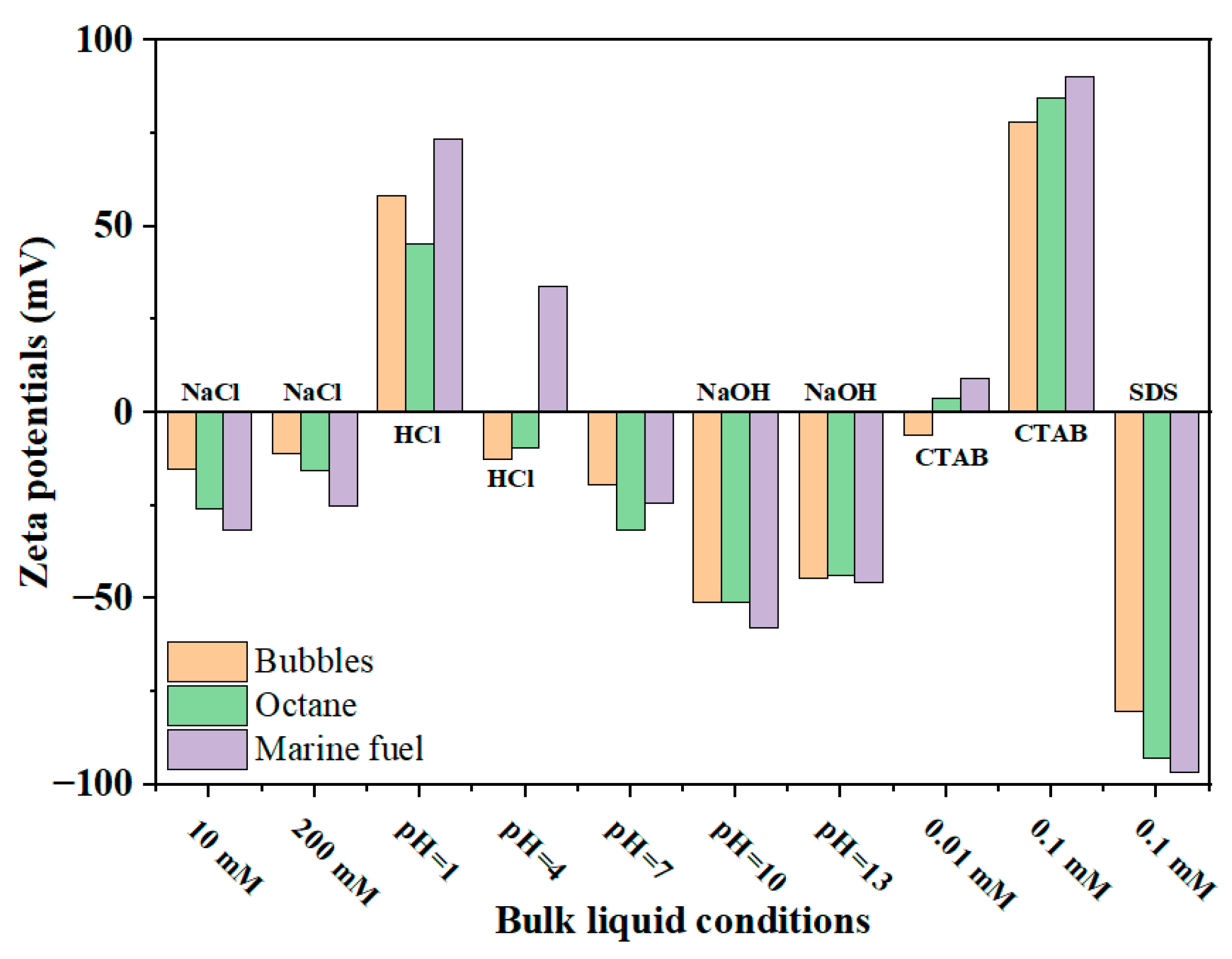
3.1.1. Zeta Potential Analysis
3.1.2. Surface and Interfacial Tension
3.2. Surface Forces in the Attachment Process of Bubbles and Oil Droplets
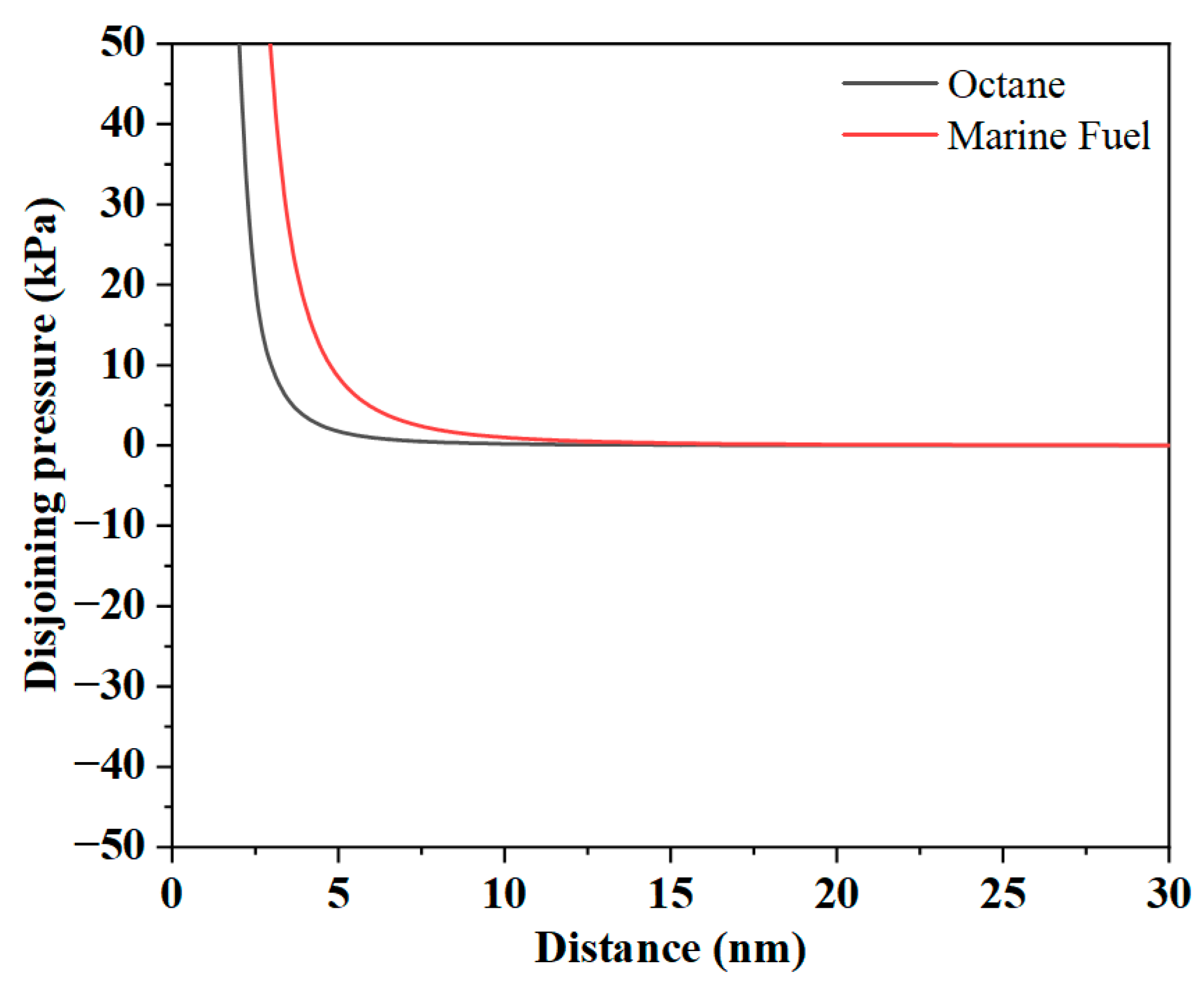
3.2.1. Van Der Waals Interaction Force Analysis
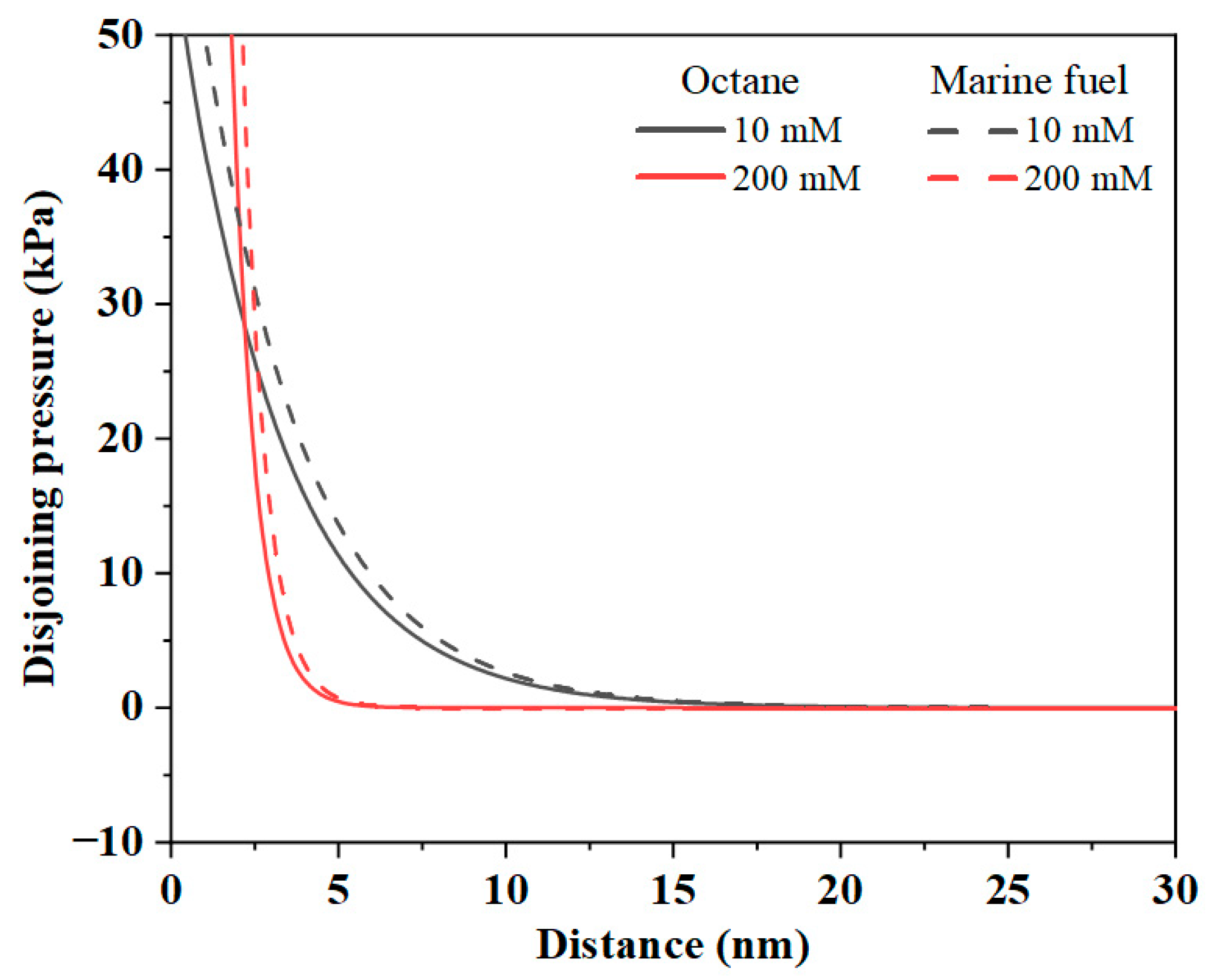
3.2.2. Electrical Double-Layer Interaction Force Analysis
3.2.3. Hydrophobic Interaction Force Analysis
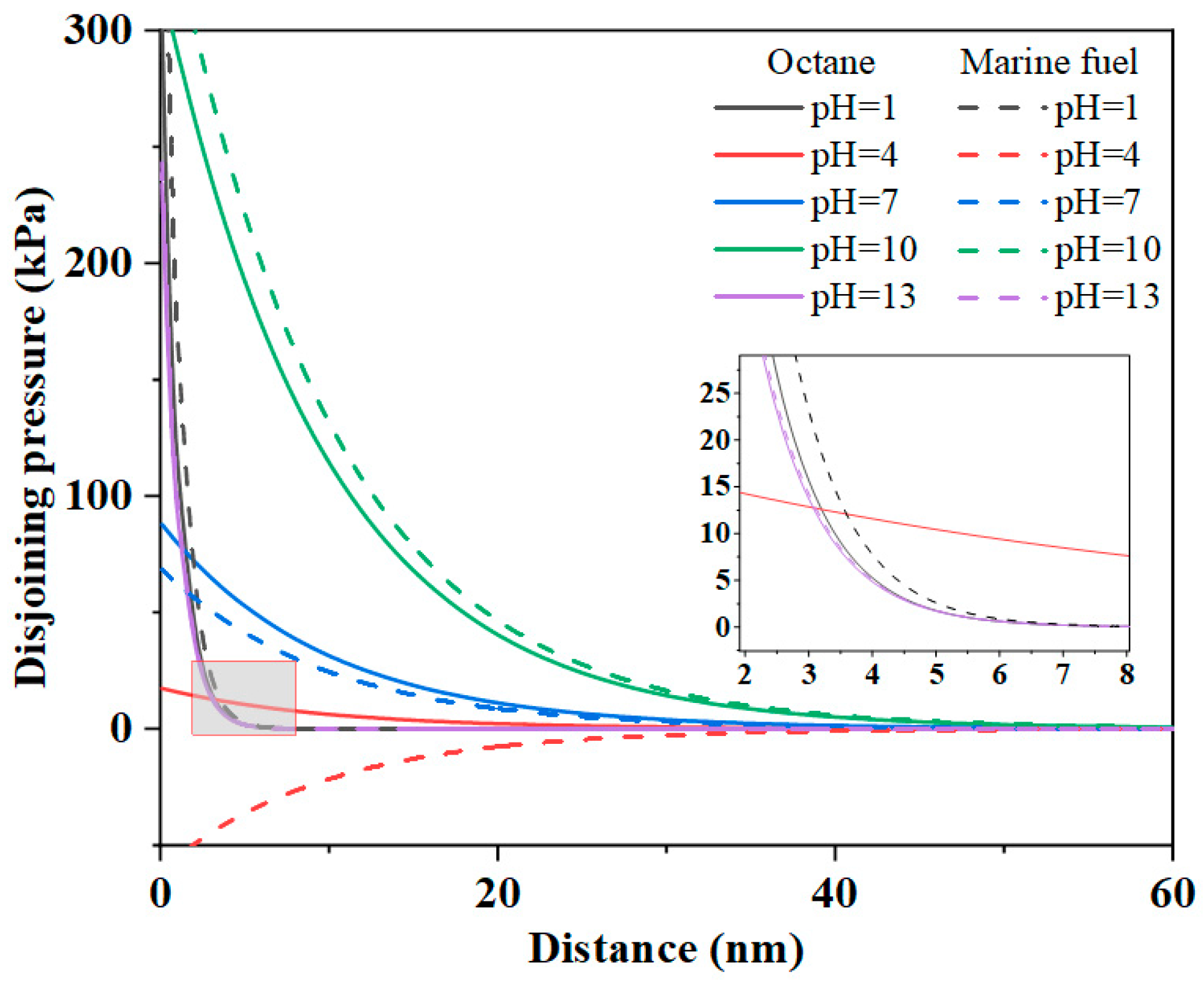
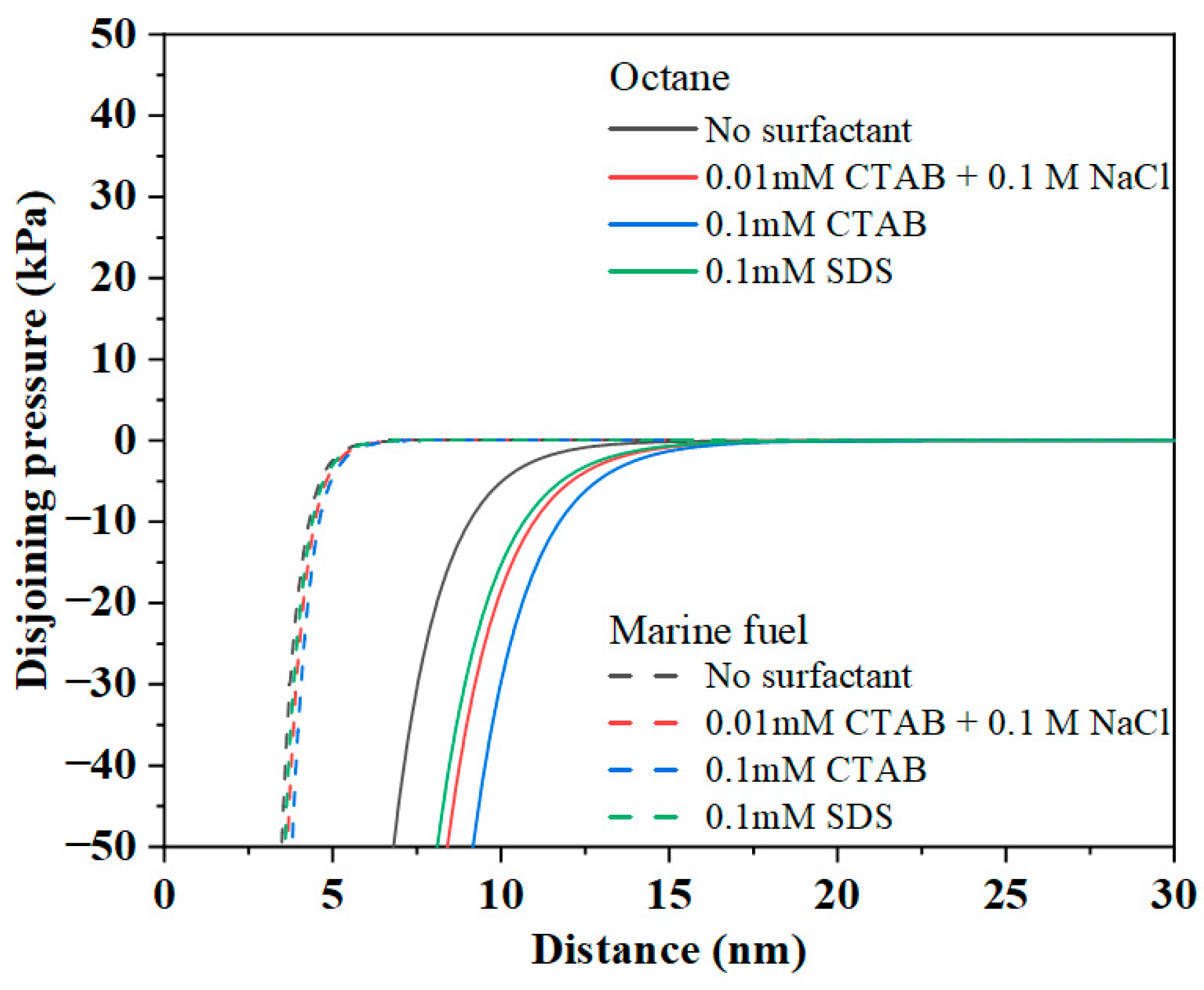
3.2.4. Total EDLVO Interaction Forces
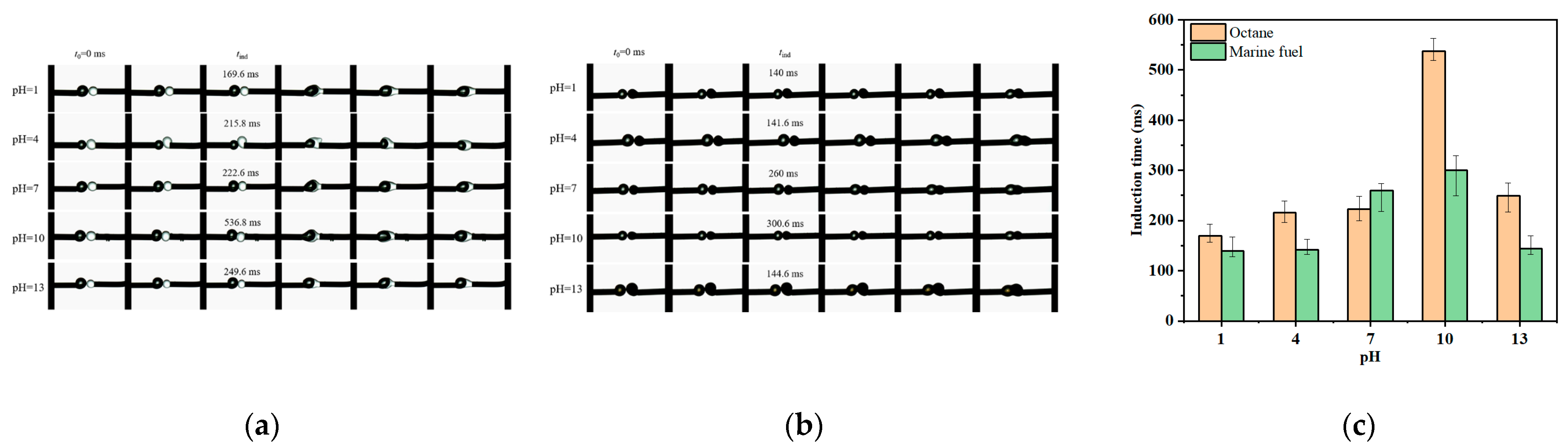
3.3. Induction Time Analysis Between Bubbles and Oil Droplets
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DLVO | Derjaguin–Landau–Verwey–Overbeek |
| EDLVO | extended DLVO |
| vdW | van der Waals |
| EDL | electric double layer |
| HB | hydrophobic interaction |
| CTAB | cetyltrimethylammonium bromide |
| SDS | sodium dodecyl sulfate |
References
- de Carvalho Neto, S.L.; Toledo Viviani, J.C.; Weschenfelder, S.E.; da Cunha, M.d.F.R.; Orlando Junior, A.E.; dos Santos Costa, B.R.; Mazur, L.P.; Marinho, B.A.; da Silva, A.; de Souza, A.A.U.; et al. Evaluation of petroleum as extractor fluid in liquid-liquid extraction to reduce the oil and grease content of oilfield produced water. Process Saf. Environ. Prot. 2022, 161, 263–272. [Google Scholar] [CrossRef]
- Ma, F.X.; Hao, B.; Xi, X.Y.; Wang, R.; Ma, P.C. Aggregation-induced demulsification technology for the separation of highly emulsified oily wastewater produced in the petrochemical industry. J. Cleaner Prod. 2022, 374, 134017. [Google Scholar] [CrossRef]
- Chakibi, H.; Hénaut, I.; Salonen, A.; Langevin, D.; Argillier, J.F. Role of bubble-drop interactions and salt addition in flotation performance. Energy Fuels 2018, 32, 4049–4056. [Google Scholar] [CrossRef]
- Lü, Y.L.; Wang, C.; Ma, Y.L.; Ye, T.X.; He, L.M. Enhancement of the attachment performance with oil droplet by coating of condensate film on the surface of air bubble. Chem. Eng. Sci. 2024, 285, 119630. [Google Scholar] [CrossRef]
- Mao, R.C.; Li, Y.D.; Liu, Y.Q.; Zhu, H.T.; Wang, N.; Yang, Q.; Lu, H. Separation characters of an axial-flow hydrocyclone with oil collecting pipe. Sep. Purif. Technol. 2023, 305, 122139. [Google Scholar] [CrossRef]
- Li, Q.; Gao, J.X.; Lu, S.B.; Zhu, H.W.; Liu, J.L.; Wang, Z.B. Numerical studies on dynamic and oil-water separation characteristics in cyclonic flotation column. Chem. Eng. Res. Des. 2023, 196, 332–341. [Google Scholar] [CrossRef]
- Arslan, M.; Usman, M.; Gamal El-Din, M. Exploring nature’s filters: Peat-mineral mix for low and high-strength oilfield produced water reclamation. Water Res. 2024, 255, 121502. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Y.Y.; Guo, Z.G.; Liu, W.M. Multifunctional polypyrrole/MXene-wrapped sponge with synergistic solar and joule-heating effect for efficient adsorption and all-weather recovery of crude oil. Chem. Eng. J. 2024, 485, 149927. [Google Scholar] [CrossRef]
- Zhang, B.B.; Li, J.; Zhang, L.H.; Wang, X.T.; Xie, S.T.; Quan, J.H. Facile fabrication of silane modified melamine sponge for highly efficient oil absorption properties. J. Water Process. Eng. 2024, 63, 105407. [Google Scholar] [CrossRef]
- Alhomadhi, E.S.; Almobaraky, M.A.; Alwosaibai, A.F. Produced oily water treatment efficiency by polyester fiber deep bed filter (phase two: Extended filter length and long duration). J. King Saud Univ. Eng. Sci. 2022, 34, 416–421. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, J.; Hou, T.; Xu, J.Y.; Wang, Y.R.; Ye, H.; Yang, B.; Li, X.L. Centrifugally spun superhydrophobic fibrous membranes with core-sheath structure assisted by hyper branched polymer and via click chemistry for high efficiency oil–water separation. Sep. Purif. Technol. 2024, 346, 127480. [Google Scholar] [CrossRef]
- Wang, X.H.; Li, X.M. Simple preparation of UV-absorbing and magnetic superhydrophobic membranes by one-step electrospinning for effective oil-water separation. Sep. Purif. Technol. 2024, 337, 126467. [Google Scholar] [CrossRef]
- Saththasivam, J.; Loganathan, K.; Sarp, S. An overview of oil-water separation using gas flotation systems. Chemosphere 2016, 144, 671–680. [Google Scholar] [CrossRef]
- Piccioli, M.; Aanesen, S.V.; Zhao, H.; Dudek, M.; Øye, G. Gas flotation of petroleum produced water: A review on status, fundamental aspects, and perspectives. Energy Fuels 2020, 34, 15579–15592. [Google Scholar] [CrossRef]
- Moosai, R.; Dawe, R.A. Gas attachment of oil droplets for gas flotation for oily wastewater cleanup. Sep. Purif. Technol. 2003, 33, 303–314. [Google Scholar] [CrossRef]
- Yan, S.L.; Yang, X.Y.; Bai, Z.S.; Xu, X.; Wang, H.L. Drop attachment behavior of oil droplet-gas bubble interactions during flotation. Chem. Eng. Sci. 2020, 223, 115740. [Google Scholar] [CrossRef]
- Verrelli, D.I.; Koh, P.T.L.; Nguyen, A.V. Particle-bubble interaction and attachment in flotation. Chem. Eng. Sci. 2011, 66, 5910–5921. [Google Scholar] [CrossRef]
- Han, Y.; Han, S.; Kim, B.; Yang, J.; Choi, J.; Kim, K.; You, K.-S.; Kim, H. Flotation separation of quartz from apatite and surface forces in bubble–particle interactions: Role of pH and cationic amine collector contents. J. Ind. Eng. Chem. 2019, 70, 107–115. [Google Scholar] [CrossRef]
- Israelachvili, J.; Pashley, R. The hydrophobic interaction is long range, decaying exponentially with distance. Nature 1982, 300, 341–342. [Google Scholar] [CrossRef]
- Wang, W.; Li, K.; Ma, M.Y.; Jin, H.; Angeli, P.; Gong, J. Review and perspectives of AFM application on the study of deformable drop/bubble interactions. Adv. Colloid Interface Sci. 2015, 225, 88–97. [Google Scholar] [CrossRef]
- Wang, C.; Lü, Y.L.; Qi, H.W.; Luo, X.M.; He, L.M. Flotation mechanism and performance of air/condensate bubbles for removing oil droplets in the presence of acetic acid. Sci. Total Environ. 2024, 927, 172311. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.N.; Pang, T.; Han, R.; Chen, S.J.; Li, Z.; Yu, Y.X.; Shi, Z.Y.; Liu, L.J.; Qu, J.Z.; Zhou, A.N. Interactions between bubble and particles of key minerals of diasporic bauxite through the extended DLVO theory. Int. J. Min. Sci. Technol. 2022, 32, 201–214. [Google Scholar] [CrossRef]
- Rajapakse, N.; Zargar, M.; Sen, T.; Khiadani, M. Effects of influent physicochemical characteristics on air dissolution, bubble size and rise velocity in dissolved air flotation: A review. Sep. Purif. Technol. 2022, 289, 120772. [Google Scholar] [CrossRef]
- Wang, C.; Lü, Y.L.; Ye, T.X.; Chen, L.; He, L.M. Investigation on the mechanism of air/condensate bubble flotation of emulsified oil droplet. Process Saf. Environ. Prot. 2023, 180, 554–565. [Google Scholar] [CrossRef]
- Yoon, R.H.; Mao, L. Application of extended DLVO theory, IV: Derivation of flotation rate equation from first principles. J. Colloid Interface Sci. 1996, 181, 613–626. [Google Scholar] [CrossRef]
- Qiu, G.; Hu, Y.; Wang, D. Particle Interactions and Fine Particle Flotation; Central South University Press: Changsha, China, 1993; pp. 31–35. [Google Scholar]
- Xie, L.; Shi, C.; Cui, X.; Huang, J.; Wang, J.Y.; Liu, Q.; Zeng, H.B. Probing the interaction mechanism between air bubbles and bitumen surfaces in aqueous media using bubble probe atomic force microscopy. Langmuir 2018, 34, 729–738. [Google Scholar] [CrossRef]
- Li, K.; Wang, W.; Xiao, F.; Ge, Y.T.; Jin, H.; Yu, Z.P.; Gong, J.; Gao, W.W.; Peng, Z.H. Atomic force microscopy study of non-DLVO interactions between drops and bubbles. Langmuir 2021, 37, 6830–6837. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.J.; Tang, L.F.; Tao, X.X.; He, H.; Yang, Z.; Chen, L. Exploration on the mechanism of oily-bubble flotation of long-flame coal. Fuel 2018, 216, 427–435. [Google Scholar] [CrossRef]
- Yang, D.L.; Zhao, Z.Q.; Gong, L.; Sun, Y.X.; Peng, X.W.; Peng, Q.Y.; Wang, T.; Liu, Q.; Zhang, H.; Zeng, H.B. Surface interaction mechanisms of air bubbles, asphaltenes and oil drops in aqueous solutions with implications for interfacial engineering processes. J. Colloid Interface Sci. 2023, 647, 264–276. [Google Scholar] [CrossRef]
- Eftekhardadkhah, M.; Øye, G. Induction and coverage times for crude oil droplets spreading on air bubbles. Environ. Sci. Technol. 2013, 47, 14154–14160. [Google Scholar] [CrossRef]
- Chen, Y.P.; Shen, C.Q.; Peterson, G.P. Hydrodynamics and morphologies of droplet coalescence. Ind. Eng. Chem. Res. 2015, 54, 9257–9262. [Google Scholar] [CrossRef]
- Yan, S.L.; Zhang, Y.; Yang, X.Y.; Huang, Y.; Bai, Z.S.; Xu, X. Interfacial behavior and internal microflow of an oil droplet during the process of the oil droplet covering a gas bubble: Without and with NaCl. Ind. Eng. Chem. Res. 2021, 60, 6006–6015. [Google Scholar] [CrossRef]


| Interfacial Tension, σw-o | No Surfactant | 0.01 M CTAB | 0.1 M CTAB | 0.1 M SDS |
|---|---|---|---|---|
| σw-octane, mN/m | 43.2 | 37.2 | 25.8 | 37.7 |
| σw-marine fuel, mN/m | 27.4 | 21.4 | 12.5 | 22.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, T.; Xiao, H.; Jiang, C.; Ma, M.; Zhang, G.; Wang, C.; Zheng, Y.; Zhao, X. Heterogeneous Interactions During Bubble–Oil Droplet Contact in Water. Separations 2025, 12, 174. https://doi.org/10.3390/separations12070174
Yang T, Xiao H, Jiang C, Ma M, Zhang G, Wang C, Zheng Y, Zhao X. Heterogeneous Interactions During Bubble–Oil Droplet Contact in Water. Separations. 2025; 12(7):174. https://doi.org/10.3390/separations12070174
Chicago/Turabian StyleYang, Tao, Hao Xiao, Chunyu Jiang, Ming Ma, Guangwen Zhang, Chun Wang, Yi Zheng, and Xiangdi Zhao. 2025. "Heterogeneous Interactions During Bubble–Oil Droplet Contact in Water" Separations 12, no. 7: 174. https://doi.org/10.3390/separations12070174
APA StyleYang, T., Xiao, H., Jiang, C., Ma, M., Zhang, G., Wang, C., Zheng, Y., & Zhao, X. (2025). Heterogeneous Interactions During Bubble–Oil Droplet Contact in Water. Separations, 12(7), 174. https://doi.org/10.3390/separations12070174






