1. Introduction
Urea holds a critically important status in both agricultural and industrial sectors. The global urea production in 2024 was approximately 239 million tons. Urea prilling, as the final stage in urea production processes, is typically accomplished through the process units. It is primarily used to convert molten urea into granular products.
Molten urea is atomized into droplets through a rotary spray nozzle or pressure nozzle at the tower top. Under gravitational force, these droplets freely descend while undergoing heat exchange with ascending cool air inside the tower, cooling to 60–80 °C within 10–15 s and solidifying into spherical particles that accumulate at the tower base [
1]. The heated air, as exhaust gas, carries fine urea dust and trace ammonia gas, which are discharged from the tower top after water-scrubbing dust removal treatment.
With the enhancement of environmental protection requirements, conventional water-scrubbing dust removal systems have become inadequate to meet ultra-low emission standards requiring dust concentrations below 10 mg/m3. Consequently, bag filters have been adopted as the terminal process for particulate control in some urea prilling towers. However, as the bag filter operates through dry filtration, it cannot effectively remove residual ammonia gas, resulting in exhaust ammonia gas concentrations of 50–100 mg/m3. Approximately 500 to 600 tons of ammonia gas are emitted annually by a urea prilling tower with an annual production capacity of 400,000 tons, leading to significant atmospheric pollution.
Adsorption, as a conventional method for gaseous pollutant removal, can be effectively applied to eliminate low-concentration ammonia gas. Activated carbon is the most commonly used material in this situation [
2]. Unfortunately, it is limited in adsorption capacity for ammonia capture. However, it can be modified by a variety of materials such as acids, metal ions, and ionic liquids to enhance the adsorption capacity. Studies have shown that the adsorption capacity of ammonia can be up to 172 mg/g [
3,
4,
5]. Moreover, activated carbon-based byproducts can also be functionalized as a soil amendment that enhances soil quality when combined with chemical fertilizers [
6,
7]. Ammonia-derived compounds including ammonium sulfate, monoammonium phosphate, and ammonium nitrate, synthesized using adsorbed ammonia gas as raw material, find extensive applications while serving as nitrogen fertilizers. Ammonia gas in urea prilling tower exhaust is adsorbed using activated carbon as an adsorbent, fixed into ammonium salts through chemical stabilization, and subsequently mixed with urea particles present in the exhaust gases. The resultant composite material containing activated carbon, ammonium salts, and urea can be repurposed as a slow-release fertilizer, achieving resource recovery and environmental synergy.
The substantial exhaust gas flow rates in urea prilling towers (exceeding 700,000 cubic meters per hour for a 400,000-ton annual capacity facility), necessitate massive adsorbent consumption, rendering this method economically unfeasible despite the end product’s commercial potential as a slow-release fertilizer due to the prohibitive costs of activated carbon [
8]. Gasified biochar, a solid byproduct derived from biomass gasification through pyrolysis under oxygen-deficient conditions, followed by incomplete combustion and gasification, primarily composed of carbon and ash constituents and currently classified as low-value biomass fly ash for disposal, presents a viable alternative. Substituting activated carbon with gasified biochar as an ammonia gas adsorbent achieves dual environmental benefits: atmospheric ammonia gas emissions are substantially reduced while enabling carbon sequestration through soil application of the biochar-based slow-release fertilizer, concurrently valorizing waste by converting both captured ammonia gas and discarded biochar into marketable slow-release fertilizer products. To date, the separation and resource utilization of pollutants from the tail gas of prilling towers have rarely been reported. In particular, the adsorption capacity of ammonia based on gasified biochar and the effectiveness of the product (slow-release fertilizer) is not yet completely known. In addition, its techno-economic performance has not been comprehensively determined and evaluated.
This work aims to systematically analyze three critical dimensions to demonstrate the technical feasibility of the proposed process: the operational status of bag filters installed atop urea prilling towers, the process flow design for biochar-based slow-release fertilizer production in 400,000-ton annual capacity prilling towers, and the effectiveness and manufacturing methodologies of biochar-based slow-release fertilizers. Consequently, a comprehensive economic analysis is conducted to evaluate process viability and finally establish the fundamental data to guide subsequent process optimization and enable large-scale engineering implementation.
2. Materials and Methods
2.1. Process Technology
The molten urea in a urea prilling tower is atomized into particles through nozzles and subsequently cooled into solid particles. During this process, urea particles are generated, with larger particles settling at the tower base as urea products, while micro-particles are discharged with the exhaust gas. Although the dust concentration is not high, the substantial total dust emissions caused by large exhaust gas volumes have attracted global attention. Prilling towers have been equipped with wet scrubbing systems at their apexes to address urea dust emissions, through which dust concentrations are reduced to 30–50 mg/m
3 while enabling urea recovery and ammonia gas emission reduction [
9]. In China, ultra-low emission policies have been imposed not only on coal-fired power plants but also on fertilizer production facilities. As the primary industrial emission sources in fertilizer plants, urea prilling towers are mandated to maintain dust concentrations ≤10 mg/m
3. Consequently, selective retrofitting has been implemented: where existing wet scrubbing systems are present, bag filters have been integrated through equipment modifications; at facilities lacking prior dust removal infrastructure, new bag filters have been installed to achieve the 10 mg/m
3 emission threshold. The 400,000-ton-annual-capacity prilling towers represent typical configurations in China, characterized by structural parameters including a 19 m main tower diameter, 14 m exhaust outlet diameter, and approximately 100 m height. These facilities operate with total flue gas volumes ranging from 700,000 to 1,000,000 cubic meters per hour, achieving urea production rates of 45–55 tons hourly.
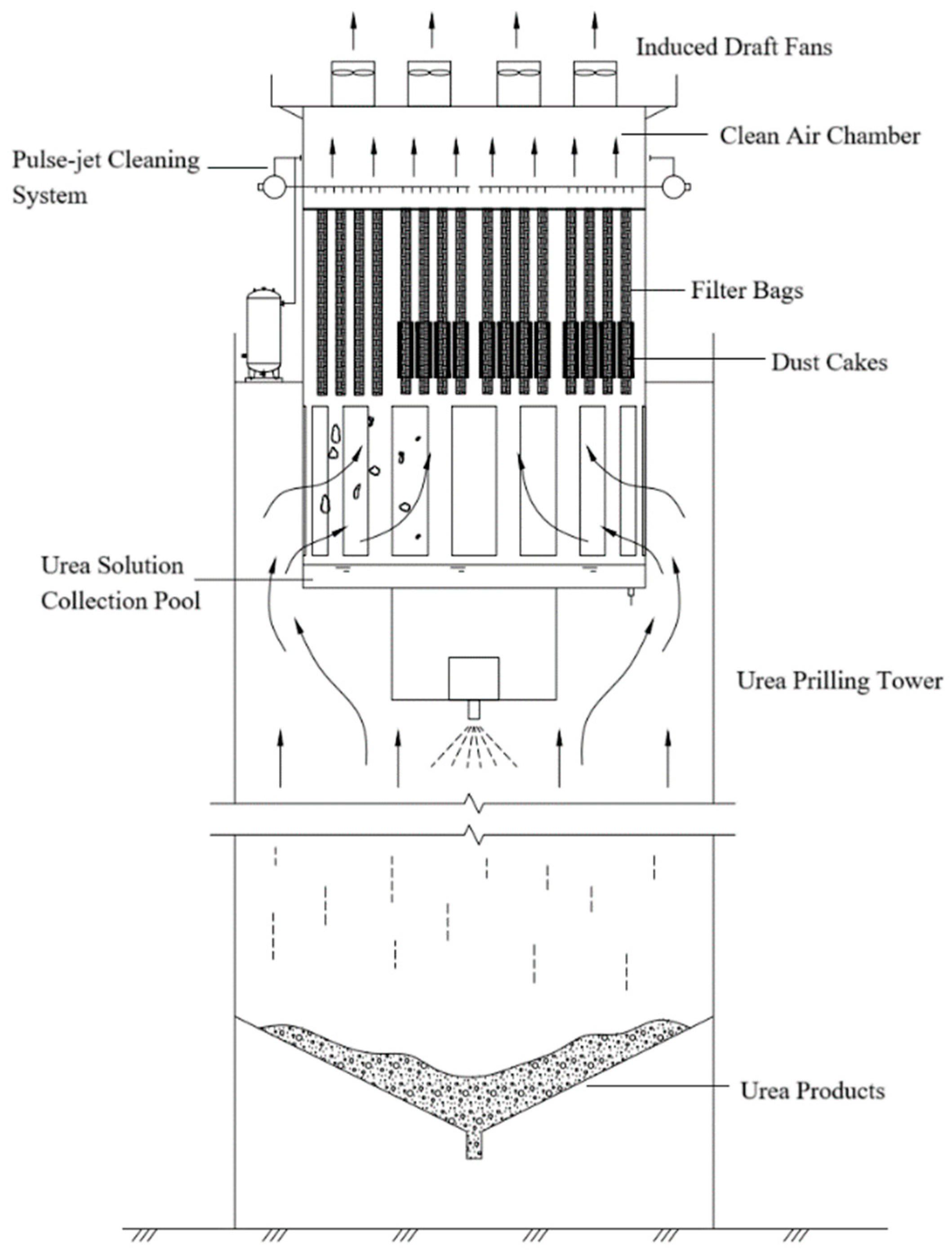
Particularly, a representative bag filter system for such towers is illustrated as follows: The bag filter is situated atop the prilling tower, with a urea solution collection pool positioned beneath it. Induced draft fans mounted at the uppermost portion convert the original natural ventilation system into forced-air operation. The clean air chamber, located below the induced draft fans, is separated from the air outlet by a tube sheet equipped with filter bags, as shown in
Figure 1.
A pulse-jet cleaning system is installed within the clean air chamber. Exhaust gases from the prilling tower are filtered through the filter bags, with cleaned gases being discharged via the induced draft fan. Particles intercepted by the filter bags accumulate externally, forming dust cakes on the bag surfaces. At predetermined intervals, these accumulated dust cakes are dislodged through reverse-pulse cleaning and subsequently deposited into the underlying solution collection pool for dissolution.
Bag filters can effectively maintain particulate emissions from urea prilling towers below 10 mg/m3. However, due to their dry filtration mechanism, ammonia gas concentrations remain unmitigated. The emissions from prilling towers are characterized by low pollutant concentrations yet substantial cumulative discharge volumes, with a single 400,000-ton-annual-capacity tower emitting over 600 tons of NH3 annually.
Such substantial ammonia gas emissions constitute a primary contributor to haze formation, as ammonia undergoes physicochemical transformations to form secondary particulates including ammonium sulfate and ammonium nitrate. These ammonia-derived compounds account for approximately 30 wt% of atmospheric PM2.5 mass concentration, peaking at 60 wt% during pollution episodes [
10]. Direct emission of ammonium not only generates secondary pollution but also results in significant resource wastage. From environmental conservation and resource sustainability perspectives, ammonia gas purification, separation, and recovery are imperative.
2.2. Gasified Biochar-Based Adsorbent for Ammonia Capture
Conventional activated carbon exhibits ammonia gas adsorption capacities ranging from 2.723 mg/g to 17.2 mg/g [
11,
12]. Gasified biochar, as a byproduct of biomass gasification with lower carbon content, exhibits poor adsorption capacity for ammonia gas. Taking a 400,000-ton-annual-capacity prilling tower as an example, with hourly gas flow rates of 700,000–1,000,000 m
3, ammonia gas concentrations of 60–100 mg/m
3, and urea dust concentrations of approximately 300 mg/m
3, the daily ammonia gas removal requirement reaches 0.91–1.51 tons, with 90% removal efficiency.
At a hypothetical adsorption capacity of 2 mg/g for gasified biochar, the total adsorbent demand would exceed 750 tons. Given the prilling tower’s vertical height surpassing 80 m, daily transportation of 750 tons of gasified biochar to the tower apex proves operationally impractical. Consequently, chemical modification of gasified biochar must be implemented to enhance its ammonia gas adsorption efficiency.
Impregnation modification is recognized as a conventional and low-cost modification method that can effectively enhance the adsorption efficiency of adsorbents. The loading of different materials has a differential effect on adsorbent performance. It has been demonstrated that the loading process leads to the generation of a large number of acidic and basic functional groups on the surface of the adsorbent, which significantly enhances its adsorption efficiency [
13]. Due to the relatively small molecular-dynamics-derived diameter of ammonia, the adsorption depends on the microporous structure of the adsorbent [
14]. Once the micropores are clogged or the pore size shrinks, the adsorption capacity of ammonia is significantly weakened [
15]. On the other hand, load-introduced organic compounds often contain polar groups that can selectively adsorb polar ammonia molecules via dipole–dipole interactions or hydrogen bonding. As a result, it prompts the adsorption mechanism on the adsorbent surface to shift from physical adsorption to chemical adsorption and enhances ammonia adsorption selectivity and stability [
16]. The adsorption capacity of ammonia gas can be significantly improved when activated carbon is modified using oxygen-rich acidic solutions [
17,
18]. Since the process aims to prepare biochar-based slow-release fertilizers, phosphoric acid groups containing PO
43− are preferentially selected for the modification of gasified biochar.
2.3. Experimental Setup and Design
The raw material biochar used was obtained from the gasification residue of biomass (mixed wood chips, provided by Shanghai Wisebond Technology Co., Ltd., Shanghai, China). The modification process was carried out using a one-step solution impregnation method. Specifically, the biochar was mixed with 30% phosphoric acid in a ratio of 1:10 wt/wt using a magnetic stirrer. The impregnation temperature was 30 °C for 20 h. Subsequently, it was filtered and dried at 120 °C for 4 h and ground to 150–200 mesh particles.
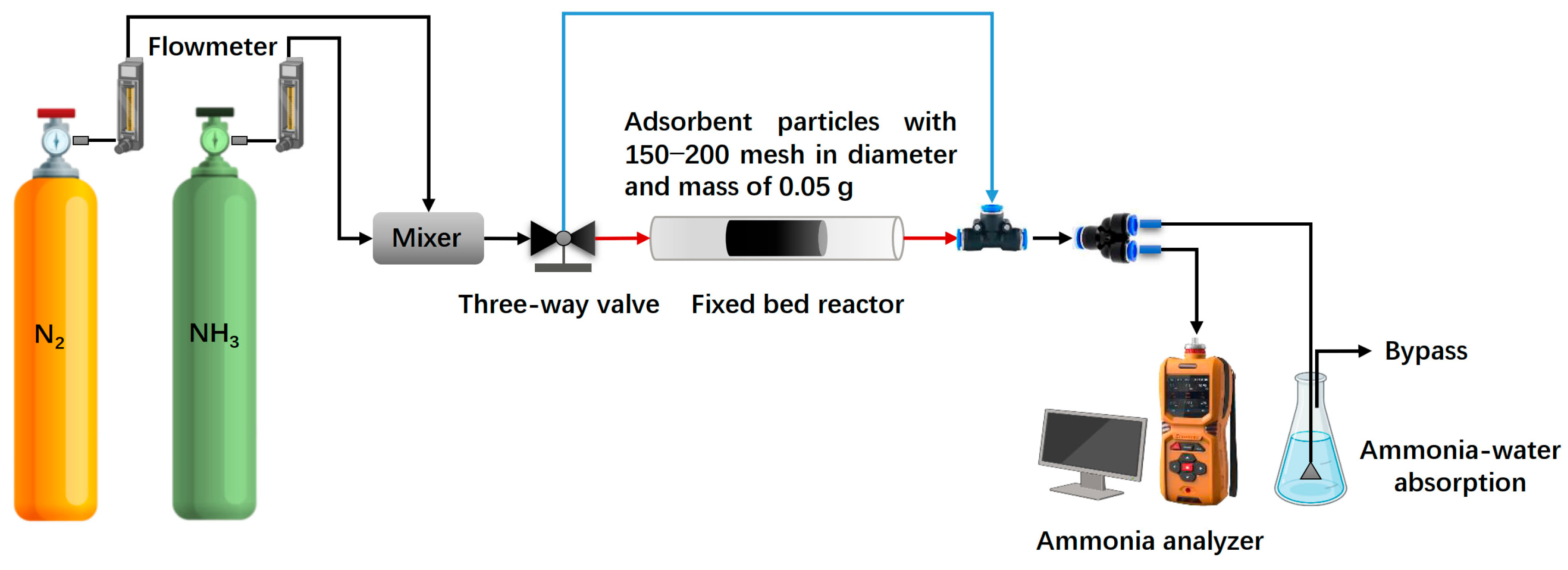
The ammonia gas adsorption performance of gasified biochar before and after phosphoric acid modification was experimentally evaluated on a constructed fixed-bed adsorption test platform. The experimental system consists of a gas mixer, a fixed-bed adsorption reactor (in diameter of 8 mm), an ammonia gas concentration analyzer, and an exhaust absorption device (
Figure 2). In this process, the experiments used a fixed-bed reactor to analogously simulate the adsorption process of an actual baghouse on a dust cake. The mass of adsorbent used was 0.05 g, the gas flow rate was 0.6 L/min, and the inlet ammonia concentration was 100 mg/m
3 to match actual operating conditions. All experiments were performed at room temperature (25 ± 2 °C) and a pressure of 101.2 ± 0.2 kPa.
The ammonia adsorption capacity was determined by the integral of the adsorption curve, which was obtained by measuring the ammonia gas concentration at the inlet and outlet of the fixed-bed reactor. Each experiment was independently repeated three times to eliminate the effect of random error.
3. Results and Discussion
3.1. Ammonia Adsorption Characteristics
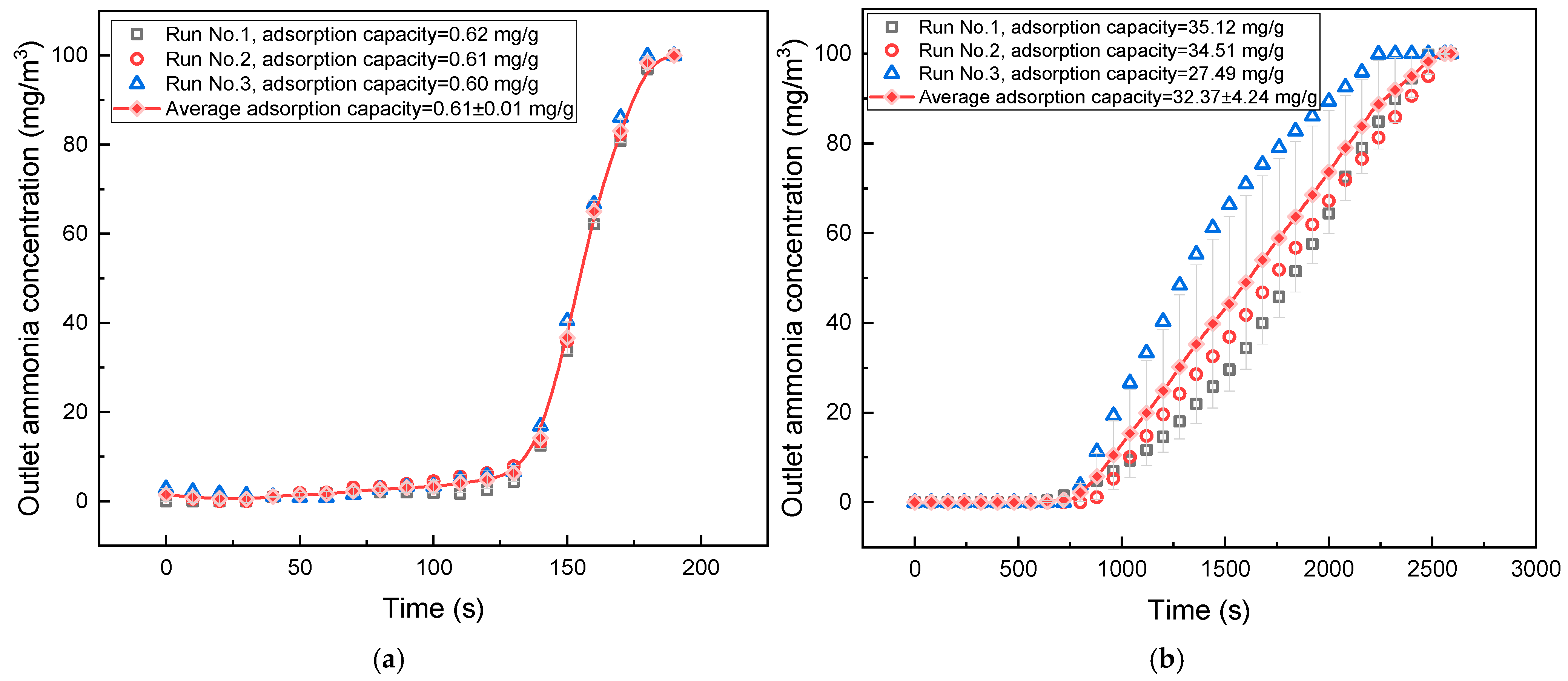
According to
Figure 3, the ammonia gas adsorption capacity of unmodified gasified biochar was measured at only 0.61 mg/g, which is significantly lower than the 2.723 mg/g adsorption capacity exhibited by conventional activated carbon. In comparison, phosphoric acid-impregnated modified gasified biochar achieved an ammonia gas adsorption capacity of 32.37 mg/g, conclusively proving the exceptional effectiveness of phosphate group functionalization in enhancing biochar’s adsorption performance.
Phosphoric acid modification introduces acidic functional groups (e.g., carboxyl, carbonyl, phenolic hydroxyl, lactone group, and carboxylic anhydride) to provide Lewis and Brønsted adsorption sites [
19,
20] and improve the adsorption selectivity of ammonia. In particular, the nitrogen atoms in ammonia have lone-pair electrons that can form hydrogen bonds with functional groups and enhance the adsorption of ammonia on the activated carbon surface [
21]. In addition, the loaded phosphate can also react with ammonia molecules to form ammonium phosphate, increasing the adsorption capacity [
22].
It should be noted that the ammonia emission standards are typically <30 mg/m3, but more stringent standards of 10 mg/m3 are also used. The effect of adsorption saturation has been considered in the experiment. In the actual baghouse, the adsorbent works through an unsteady process due to its cleaning mechanism and role. It allows fresh adsorbent to enter continuously into the urea prilling system so that ammonia adsorption can achieve undersaturation, which ultimately ensures that the ammonia emission meets technical standards. This is similar to the process for mercury removal using activated carbon in coal-fired power plants.
3.2. Process Improvement
3.2.1. Strategy and Method
Based on the experimentally demonstrated ammonia gas adsorption capacity of modified gasified biochar, the total daily biochar requirement was reduced from 750 tons to less than 50 tons (approximately 2 tons hourly), which is demonstrated to be operationally feasible for pneumatic conveying systems. Furthermore, these 50 tons of gasified biochar contain phosphoric acid required for sufficient ammonia gas reaction, indicating that the actual mass of biochar participating in the adsorption process is below 50 metric tons.
The phosphoric acid-modified gasified biochar is pneumatically conveyed to the tower apex at a rate of 50 tons/day and uniformly distributed into the urea prilling tower’s exhaust gas stream via piping networks, where it becomes deposited on the exterior surfaces of filter bags within the bag filter alongside urea dust to form composite dust cakes. During intervals between pulse-jet cleaning cycles, exhaust gas is maintained at a low flow velocity of 1 m/min through the dust cake. A fixed-bed adsorption system is formed, utilizing gasified biochar from the dust cake to adsorb and remove low-concentration ammonia gas in the exhaust gas.
Upon activation of the programmed pulse-jet cleaning system, the ammonia-adsorbed biochar/urea composite dust cakes are dislodged from the filter bag surface and deposited into the urea solution collection pool directly beneath the bag filter. Partial desorption of adsorbed ammonia gas occurs during this phase, followed by the dissolution of urea dust to form an aqueous solution. The mixed slurry formed by the combination of gasified biochar, urea, and monoammonium phosphate ultimately flows through pipelines into the dewatering system at the base of the granulation tower for dehydration.
The dehydration process separates solids from liquids in the mixed slurry, with the majority of the solution being recirculated to the urea solution collection pool at the tower apex via a pumping system, while a minor portion is combined with gasified biochar to form biochar-based slow-release fertilizer. The system maintains a single fertilizer outlet and three material inlets, comprising 48.5 tons/day of modified gasified biochar (including 8.5 tons of phosphoric acid), 1.5 tons/day of ammonia adsorbed by the biochar, and 5 tons/day of urea dust captured by the bag filter—system equilibrium is achieved when inlet and outlet mass flows balance. Under steady-state conditions, the daily fertilizer composition is determined to be 40 tons of biochar, 10 tons of monoammonium phosphate, and 5 tons of urea.
The active components (N, K
2O, and P
2O
5) in this fertilizer formulation account for only 18% by mass, failing to meet the GB/T 23348-2009 standard requiring total active content ≥ 30% [
23]. To comply with the slow-release fertilizer standard, approximately 50 tons/day of urea must be introduced into the system, which can be subsequently blended with the dehydrated fertilizer product in the granulation equipment. The complete production process flow is illustrated in
Figure 4.
3.2.2. Effectiveness Evaluation
Fertilizers play a crucial role in modern agricultural production. Traditional chemical fertilizers suffer from nutrient loss through dissolution and volatilization during direct application, necessitating the development and promotion of novel slow-release fertilizers to advance sustainable agricultural production [
24].
The encapsulation of fertilizers within carrier materials enables slow nutrient release. Biochar, characterized by its wide availability, low cost, unique structure, and chemical properties, also exhibits soil improvement and remediation capabilities, making it an excellent carrier [
25,
26,
27,
28]. Biochar-based slow-release fertilizers reduce nutrient leaching and enhance utilization efficiency. Investigations have indicated that biochar application alone increases crop yields by an average of 25.3% [
29]. Compared to standalone inorganic fertilizer use, the combined application of biochar and inorganic fertilizers further boosts yields by 10% [
30].
Current methodologies for biochar-based slow-release fertilizer production include in situ pyrolysis, coating, impregnation, granulation, co-pyrolysis, and hydrothermal carbonization [
31,
32,
33]. Due to the complexity of pyrolysis mechanisms, pretreatment processes such as pyrolysis and co-pyrolysis exhibit limited controllability, rendering post-treatment technologies—including impregnation, coating, and granulation—as the predominant approaches [
31].
Impregnation is a process where biochar is immersed in liquid fertilizer to adsorb fertilizer components by utilizing its adsorption capacity, followed by a drying treatment. Granulation is a process where one or multiple solid fertilizers are mixed with biochar and then granulated using a granulator. Both impregnation- and granulation-produced biochar-based slow-release fertilizers are recognized to exhibit enhanced fertilization efficiency [
24,
34].
The gasified biochar is initially subjected to phosphoric acid impregnation modification, followed by drying, to prepare it as an adsorbent for ammonia gas removal from prilling tower exhaust gases. Subsequently, the ammonia-adsorbed biochar is combined with urea dust collected by the bag filter and deposited into the urea solution collection pool. Monoammonium phosphate and urea readily dissolve to form an aqueous solution, while the gasified biochar undergoes secondary impregnation modification. The resultant mixture is dehydrated via a filter press to produce biochar-based slow-release fertilizer.
To achieve compliance with the total nutrient content requirement (≥30%) stipulated in GB/T 23348-2009 [
23], the nitrogen proportion could be further enhanced through post-process granulation where the dehydrated biochar-fertilizer composite is blended with solid urea prior to entering granulation equipment.
It should also be noted that according to the proposed technological process, the leaching behavior of the collected particles containing urea granules, biochar, and phosphoric acid is unavoidable, implying that the N and P of the solubilized compounds partially migrate into the water. However, they would be recovered using the recycled water as a medium. Moreover, crystallization occurs once their accumulation in the collected water reaches saturation, resulting in their reuse as a fertilizer.
3.3. Economic Assessment
The final product of the designed production process is a biochar-based slow-release fertilizer. Its economic viability is recognized as the fundamental prerequisite for industrial implementation and market penetration, beyond technical feasibility and functional efficacy. Hence, a comprehensive evaluation needs to be conducted across three critical dimensions: investment costs, operational costs, and profits.
3.3.1. Investment Costs
The investment costs are predominantly attributed to equipment expenditures. Based on existing prilling towers and bag filters, coupled with the proposed process design, the systems requiring added installation are listed in
Table 1.
A preliminary cost estimation for the entire system has been calculated at USD1.2 million. The total investment remains relatively moderate, with the dehydration system accounting for 87% of the capital expenditure, representing the most significant cost component in the process. Due to the requirement for specific moisture content in the post-dehydration material to ensure proper granulation performance, the current production process design intentionally excludes drying equipment. The necessity of integrating drying units will require subsequent determination based on comprehensive formability testing of the processed material samples.
3.3.2. Operational Costs
The operational costs of the system are primarily derived from two components: raw material costs and energy consumption costs. As previously stated, the daily fertilizer composition at the outlet comprises 40 tons of biochar, 10 tons of monoammonium phosphate, and 5 tons of urea. Since biochar is a byproduct of biomass gasification with a low market price, its selling price is set at about USD7/ton. When considering the processing cost of USD42/ton for impregnation and transportation expenses of USD21/ton, the unit price of biochar is estimated at USD70/ton. The consumption of phosphoric acid is 8.5 tons, with an estimated cost of USD1116/ton. Ammonia gas and urea are provided at no cost. Energy consumption originates primarily from the pneumatic conveying system, mixing system, dewatering system, and granulation system, with a total estimated power demand of 50 kW. Accounting for a workforce of five personnel, the operational costs are summarized in
Table 2. Note that the prices for biochar, phosphoric acid, energy, and labor are estimates based on current market prices in China. In particular, the cost of biochar as a biomass gasification residue includes the processing, logistics, and security costs.
3.3.3. Cost-Effectiveness and Cost–Benefit Analysis
The current production cost of biochar-based slow-release fertilizers with different formulations ranges from USD267–299 per ton, while the market price of commercialized biochar slow-release fertilizers is USD586–1855 per ton, significantly higher than the ~USD279 per ton price of traditional chemical fertilizers. Prohibitive pricing has led to limited applications.
This production process can produce approximately 55 tons of biochar slow-release fertilizer per day. Assuming a selling price aligned with traditional fertilizers as urea (USD279 per ton), daily revenue would reach USD15,345, with production costs of USD12,664 and a net profit of USD2681 per day. Extrapolated over 330 operating days per year, the annual net profit would be a total of 0.88 million USD.
With the existing bag filter in the prilling tower, the total estimated investment for this production process is 1.2 million USD, and the static payback period is projected to be 1.36 years.
4. Conclusions and Outlook
A novel production process for producing biochar-based slow-release fertilizers is proposed, utilizing low-concentration ammonia gas from exhaust gases and low-cost gasified biochar, based on the existing bag filter process in urea prilling towers. The feasibility of this production process is analyzed through three aspects: the operational status of the bag filter at the top of the urea prilling tower, the production process designed for producing biochar-based slow-release fertilizers in a 400,000-ton-annual-production urea prilling tower, and the effectiveness and production methods of biochar-based slow-release fertilizers. An economic analysis of the production process is also conducted. The following conclusions can be drawn:
(1) After modifying gasified biochar through phosphoric acid impregnation, its ammonia gas adsorption capacity increased from 0.61 mg/g (pre modification) to 32 mg/g. This significantly reduced the theoretical daily consumption of gasified biochar to 50 tons/day, meeting the production process design standards. The core systems of the production process include a pneumatic conveying system and a dehydration system. Based on the material balance, the daily fertilizer production comprises 40 tons of biochar, 10 tons of monoammonium phosphate, and 5 tons of urea, blended into biochar-based slow-release fertilizer.
(2) The impregnation–granulation integrated process, based on engineered design, is a widely adopted production method for biochar-based slow-release fertilizers. All products manufactured through this process demonstrate effective fertilization performance.
(3) For a single 400,000-ton/year urea prilling tower with an existing bag filter, the investment is approximately 1.2 million USD, with daily operating costs of USD 12,664 and revenue of USD 15,345. This yields a net daily profit of USD 2681, achieving a static payback period of 1.36 years and demonstrating a high return on investment.
(4) Future work is needed, including (i) detailed characterization of the adsorbent (including ultimate analysis) to assess its effectiveness and safety for use as a fertilizer; (ii) the influence of temperature as an operating parameter (usually >60 °C) to analyze the type and mechanisms of adsorption (physical or physicochemical adsorption); (iii) the validation of actual adsorption performance and effectiveness from bench-scale investigation to industrial applications (e.g., the difference between the flow distribution and the formation of preferential pathways based on densities), etc.
Author Contributions
Conceptualization, T.L., Y.M., B.C. and T.Z.; data curation, Z.Z., M.W. and F.L.; formal analysis, T.L. and X.G.; funding acquisition, Y.M. and B.C.; investigation, T.L., B.Z. and T.Z.; methodology, T.L. and B.Z.; project administration, T.L. and B.Z.; resources, Y.M., X.G. and B.C.; supervision, B.Z., B.C., Y.M. and F.L.; validation, B.Z., Z.Z., M.W. and F.L.; writing—original draft preparation, T.L. and B.Z.; writing—review and editing, T.L. and B.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was sponsored by the Henan Provincial Key Scientific and Technological Research Project (Grant No. 242102320121).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed at the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Saleh, S.N.; Barghi, S. Reduction of Fine Particle Emission from a Prilling Tower Using CFD Simulation. Chem. Eng. Res. Des. 2016, 109, 171–179. [Google Scholar] [CrossRef]
- Travlou, N.A.; Bandosz, T.J. N-Doped Polymeric Resin-Derived Porous Carbons as Efficient Ammonia Removal and Detection Media. Carbon 2017, 117, 228–239. [Google Scholar] [CrossRef]
- Wu, L.; Yang, L. A Novel Micro-Sphere Activated Carbon Synthesized from Waste Cigarette Butts for Ammonia Adsorption. Waste Manag. 2023, 168, 396–405. [Google Scholar] [CrossRef]
- Huang, C.-C.; Li, H.-S.; Chen, C.-H. Effect of Surface Acidic Oxides of Activated Carbon on Adsorption of Ammonia. J. Hazard. Mater. 2008, 159, 523–527. [Google Scholar] [CrossRef]
- Tamon, H.; Okazaki, M. Influence of Acidic Surface Oxides of Activated Carbon on Gas Adsorption Characteristics. Carbon 1996, 34, 741–746. [Google Scholar] [CrossRef]
- Wang, J.; Sun, L.; Sun, Y.; Yang, S.; Qin, Q.; Xue, Y. Long-Term Biochar-Based Fertilizer Substitution Promotes Carbon, Nitrogen, and Phosphorus Acquisition Enzymes in Dryland Soils by Affecting Soil Properties and Regulating Bacterial Community. Appl. Soil Ecol. 2025, 206, 105801. [Google Scholar] [CrossRef]
- Yan, S.; Wang, P.; Cai, X.; Wang, C.; Van Zwieten, L.; Wang, H.; Yin, Q.; Liu, G.; Ren, T. Biochar-Based Fertilizer Enhanced Tobacco Yield and Quality by Improving Soil Quality and Soil Microbial Community. Environ. Technol. Innov. 2025, 37, 103964. [Google Scholar] [CrossRef]
- Siipola, V.; Pflugmacher, S.; Romar, H.; Wendling, L.; Koukkari, P. Low-Cost Biochar Adsorbents for Water Purification Including Microplastics Removal. Appl. Sci. 2020, 10, 788. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J.; Song, C.; Wen, J. Numerical Investigation on Urea Particle Removal in a Spray Scrubber Using Particle Capture Theory. Chem. Eng. Res. Des. 2019, 145, 150–158. [Google Scholar] [CrossRef]
- Wang, J.; Zeng, S.; Chen, N.; Shang, D.; Zhang, X.; Li, J. Research progress of ammonia adsorption materials. Chin. J. Proc. Eng. 2019, 19, 14–24. [Google Scholar] [CrossRef]
- Li, C.; Zhao, S.; Li, M.; Yao, Z.; Li, Y.; Zhu, C.; Xu, S.-M.; Li, J.; Yu, J. The Effect of the Active Carbonyl Groups and Residual Acid on the Ammonia Adsorption over the Acid-Modified Activated Carbon. Front. Environ. Sci. 2023, 11, 976113. [Google Scholar] [CrossRef]
- Petit, C.; Kante, K.; Bandosz, T.J. The Role of Sulfur-Containing Groups in Ammonia Retention on Activated Carbons. Carbon 2010, 48, 654–667. [Google Scholar] [CrossRef]
- Ali, I.; Burakova, I.; Galunin, E.; Burakov, A.; Mkrtchyan, E.; Melezhik, A.; Kurnosov, D.; Tkachev, A.; Grachev, V. High-Speed and High-Capacity Removal of Methyl Orange and Malachite Green in Water Using Newly Developed Mesoporous Carbon: Kinetic and Isotherm Studies. ACS Omega 2019, 4, 19293–19306. [Google Scholar] [CrossRef] [PubMed]
- Harmanli, I.; Tarakina, N.V.; Antonietti, M.; Oschatz, M. “Giant” Nitrogen Uptake in Ionic Liquids Confined in Carbon Pores. J. Am. Chem. Soc. 2021, 143, 9377–9384. [Google Scholar] [CrossRef] [PubMed]
- Palliyarayil, A.; Prakash, P.S.; Nandakumar, S.; Kumar, N.S.; Sil, S. Palladium Nanoparticles Impregnated Activated Carbon Material for Catalytic Oxidation of Carbon Monoxide. Diam. Relat. Mater. 2020, 107, 107884. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, S.; Xu, S.; Ren, X.; Zhang, Y.; Cao, F.; Sun, Q.; Wennersten, R.; Yang, L. The Effect of Nitrogen- and Oxygen-Containing Functional Groups on C2H6/SO2/NO Adsorption: A Density Functional Theory Study. Energies 2023, 16, 7537. [Google Scholar] [CrossRef]
- Zheng, W.; Hu, J.; Rappeport, S.; Zheng, Z.; Wang, Z.; Han, Z.; Langer, J.; Economy, J. Activated Carbon Fiber Composites for Gas Phase Ammonia Adsorption. Microporous Mesoporous Mater. 2016, 234, 146–154. [Google Scholar] [CrossRef]
- Shan, X.; Zhu, S.; Zhang, W. Effect of Surface Modification of Activated Carbon on Its Adsorption Capacity for NH3. J. China Univ. Min. Technol. 2008, 18, 261–274. [Google Scholar] [CrossRef]
- Zhu, F.; Wang, Z.; Huang, J.; Hu, W.; Xie, D.; Qiao, Y. Efficient Adsorption of Ammonia on Activated Carbon from Hydrochar of Pomelo Peel at Room Temperature: Role of Chemical Components in Feedstock. J. Clean. Prod. 2023, 406, 137076. [Google Scholar] [CrossRef]
- Mochizuki, T.; Kubota, M.; Matsuda, H.; D’Elia Camacho, L.F. Adsorption Behaviors of Ammonia and Hydrogen Sulfide on Activated Carbon Prepared from Petroleum Coke by KOH Chemical Activation. Fuel Process. Technol. 2016, 144, 164–169. [Google Scholar] [CrossRef]
- Piyamawadee, C.; Sricharoenchaikul, V.; Aht-Ong, D. Bio-Composite of Nipa Palm Husk Derived Activated Carbon/Poly(Butylene Succinate): An Effective Agricultural Waste Based Adsorbent for Ammonia Removal. J. Met. Mater. Miner. 2022, 32, 27–40. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, J.; Zhang, T.C.; Ouyang, L.; Yuan, S. Synthesis of CuSiO3-Loaded P-Doped Porous Biochar Derived from Phytic Acid-Activated Lemon Peel for Enhanced Adsorption of NH3. Sep. Purif. Technol. 2022, 283, 120179. [Google Scholar] [CrossRef]
- GB/T 23348-2009; Slow Release Fertilizer. Standardization Administration of China Standards Press of China: Beijing, China, 2009.
- Chen, M.; Liu, W.; Mei, C.; Li, X.; Luo, W.; Qian, L.; Cheng, B.; Ma, H. Effects of Ca/Mg-enriched biochar fertilizer on slow-release performance and maize growth. Trans. Chin. Soc. Agric. Eng. 2023, 39, 62–70. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, R.; Cao, Y.; Wang, C. Rapid Conversion of Red Mud into Soil Matrix by Co-Hydrothermal Carbonization with Biomass Wastes. J. Environ. Chem. Eng. 2021, 9, 106039. [Google Scholar] [CrossRef]
- Zhao, X.; Qi, X.; Chen, Q.; Ao, X.; Guo, Y. Sulfur-Modified Coated Slow-Release Fertilizer Based on Castor Oil: Synthesis and a Controlled-Release Model. ACS Sustain. Chem. Eng. 2020, 8, 18044–18053. [Google Scholar] [CrossRef]
- Azeem, M.; Shaheen, S.M.; Ali, A.; Jeyasundar, P.G.S.A.; Latif, A.; Abdelrahman, H.; Li, R.; Almazroui, M.; Niazi, N.K.; Sarmah, A.K.; et al. Removal of Potentially Toxic Elements from Contaminated Soil and Water Using Bone Char Compared to Plant- and Bone-Derived Biochars: A Review. J. Hazard. Mater. 2022, 427, 128131. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Feng, Y. The Effects of Biochar Addition on Soil Physicochemical Properties: A Review. Catena 2021, 202, 105284. [Google Scholar] [CrossRef]
- Bai, S.H.; Omidvar, N.; Gallart, M.; Kämper, W.; Tahmasbian, I.; Farrar, M.B.; Singh, K.; Zhou, G.; Muqadass, B.; Xu, C.-Y.; et al. Combined Effects of Biochar and Fertilizer Applications on Yield: A Review and Meta-Analysis. Sci. Total Environ. 2022, 808, 152073. [Google Scholar] [CrossRef]
- Wang, J.; Sun, L.; Sun, Y.; Yang, S.; Qin, Q.; Xue, Y. Integrated Enzyme Activities and Untargeted Metabolome to Reveal the Mechanism That Allow Long-Term Biochar-Based Fertilizer Substitution Improves Soil Quality and Maize Yield. Environ. Res. 2025, 270, 120935. [Google Scholar] [CrossRef]
- Ndoung, O.C.N.; Figueiredo, C.C.D.; Ramos, M.L.G. A Scoping Review on Biochar-Based Fertilizers: Enrichment Techniques and Agro-Environmental Application. Heliyon 2021, 7, e08473. [Google Scholar] [CrossRef]
- Divyangkumar, N.; Panwar, N.L. Standardization, Certification, and Development of Biochar Based Fertilizer for Sustainable Agriculture: An Overview. Environ. Pollut. Manag. 2024, 1, 186–202. [Google Scholar] [CrossRef]
- Tang, X.; Li, J.H.; Yang, J.Y.; Xiang, Z.C.; He, Y.Y.; Huang, Y.Z.; Zhou, N.; Luo, W.; Zhou, Z. Agricultural Sustainability: Biochar and Bio-Based Polyurethane Coupling Coating to Prepare Novel Controlled-Release Fertilizers. Ind. Crops Prod. 2025, 223, 120296. [Google Scholar] [CrossRef]
- Niu, Z.; Liu, M.; Niu, W.; Shao, K.; Geng, J.; Tang, Z.; Huang, J.; Zhou, K. Effects of Biochar Fertilizer Ratio and Bentonite Binder on Physicochemical Properties and Slow Release Properties of Biochar Fertilizer Particles. Trans. Chin. Soc. Agric. Eng. 2020, 36, 219–227. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).