Sponge-like Modified White-Rot Fungi Adsorbent for Rapid Removal of Pb(II) and Cd(II) from Solution: Selective Performance and Mechanistic Insights
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms and Chemicals
2.2. Preparation of Modified Adsorbent
2.3. Batch Adsorption Experiments
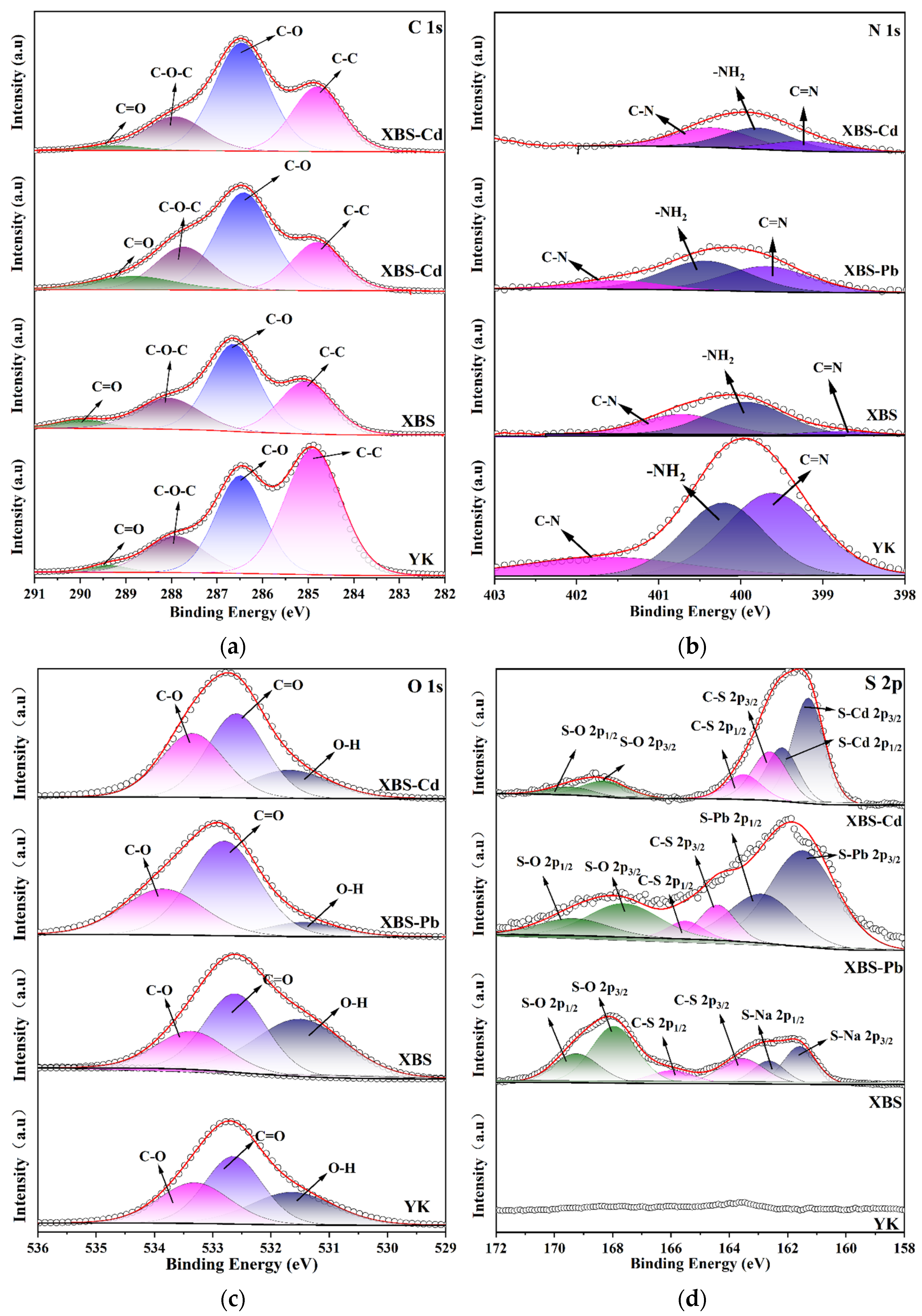
2.4. Characterization
2.5. Regeneration and Reusability Studies
3. Results and Discussion
3.1. Morphological Structural Characteristics
3.2. Pb(II) and Cd(II) Removal Performances of XBS
3.2.1. Effect of pH
3.2.2. Adsorption Kinetics
3.2.3. Adsorption Isotherm and Thermodynamics
3.2.4. Selective Adsorption Behaviors
3.3. Reproducibility and Reusability Evaluations
3.4. Material Characterization and Adsorption Mechanism
3.5. Economic Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, X.; Li, P.; Zhang, M.; Wang, J.; Cao, Y.; Zhang, T.; Zhou, G.; Li, F. Designing three-dimensional half-embedded ES-PAN/AHCNs adsorption membrane for removal of Pb(Ⅱ), Cu(Ⅱ) and Cr(Ⅲ). Colloids Surf. A Physicochem. Eng. Aspects 2021, 627, 127177. [Google Scholar] [CrossRef]
- Sun, J.; Hu, R.; Zhao, X.; Liu, T.; Bai, Z. A novel chitosan/cellulose phosphonate composite hydrogel for ultrafast and efficient removal of Pb(II) and Cu(II) from wastewater. Carbohydr. Polym. 2024, 336, 122104. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhu, Y.; Gu, H.; Lam, S.S.; Chen, X.; Sonne, C.; Peng, W. A review of phytoremediation of environmental lead (pb) contamination. Chemosphere 2024, 362, 142691. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, Y.; Zheng, H.; Zhang, B.; Zuo, Q.; Fan, K. Functionalized dual modification of covalent organic framework for efficient and rapid trace heavy metals removal from drinking water. Chemosphere 2022, 290, 133215. [Google Scholar] [CrossRef]
- Rashmi, N.; Salmataj, S.A.; Kumar, P.S.; Bhat, P. Exploring chemically and physically modified plant-based fiber biomass for biosorption in wastewater treatment: A concise review. J. Water Process Eng. 2024, 67, 106245. [Google Scholar] [CrossRef]
- Wu, J.; Cheng, X.; Li, Y.; Yang, G. Constructing biodegradable nanochitin-contained chitosan hydrogel beads for fast and efficient removal of Cu(II) from aqueous solution. Carbohydr. Polym. 2019, 211, 152–160. [Google Scholar] [CrossRef]
- Oladoye, P.O. Natural, low-cost adsorbents for toxic Pb(II) ion sequestration from (waste)water: A state-of-the-art review. Chemosphere 2022, 287, 132130. [Google Scholar] [CrossRef]
- Modwi, A.; Elamin, N.Y.; Al-Ayed, A.S.; Ismail, M.; Taha, K.K. Pb(II) ions removal via green spinel NiFe2O4 loaded on g-C3N4 nanomaterials. Nano-Struct. Nano-Objects 2023, 35, 101031. [Google Scholar] [CrossRef]
- Amarray, A.; El Ghachtouli, S.; Leroy, J.; Bonnaillie, P.; Khaless, K.; Dahbi, M.; Azzi, M. Mesoporous nanomaterials based on manganese with different interlayer alkali cations: An efficient approach for the removal of Pb(II) and Cd(II) from aqueous medium. J. Water Process Eng. 2021, 40, 101944. [Google Scholar] [CrossRef]
- Bilal, M.; Ihsanullah, I.; Younas, M.; Ul Hassan Shah, M. Recent advances in applications of low-cost adsorbents for the removal of heavy metals from water: A critical review. Sep. Purif. Technol. 2021, 278, 119510. [Google Scholar] [CrossRef]
- Yin, W.; Dai, D.; Hou, J.; Wang, S.; Wu, X.; Wang, X. Hierarchical porous biochar-based functional materials derived from biowaste for Pb(II) removal. Appl. Surf. Sci. 2019, 465, 297–302. [Google Scholar] [CrossRef]
- Shooto, N.D. Removal of toxic hexavalent chromium (Cr(VI)) and divalent lead (Pb(II)) ions from aqueous solution by modified rhizomes of Acorus calamus. Surf. Interfaces 2020, 20, 100624. [Google Scholar] [CrossRef]
- Bangaraiah, P.; Peele, K.A.; Venkateswarulu, T.C. Removal of lead from aqueous solution using chemically modified green algae as biosorbent: Optimization and kinetics study. Int. J. Environ. Sci. Technol. 2020, 18, 317–326. [Google Scholar] [CrossRef]
- Huang, F.; Gao, L.-Y.; Wu, R.-R.; Wang, H.; Xiao, R.-B. Qualitative and quantitative characterization of adsorption mechanisms for Cd2+ by silicon-rich biochar. Sci. Total Environ. 2020, 731, 139163. [Google Scholar] [CrossRef] [PubMed]
- Palabıyık, B.B.; Selcuk, H.; Oktem, Y.A. Cadmium removal using potato peels as adsorbent: Kinetic studies. Desalin. Water Treat. 2019, 172, 148–157. [Google Scholar] [CrossRef]
- Zhang, D.; Yin, C.P.; Abbas, N.; Mao, Z.C.; Zhang, Y.L. Multiple heavy metal tolerance and removal by an earthworm gut fungus Trichoderma brevicompactum QYCD-6. Sci. Rep. 2020, 10, 6940. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Wang, X.; Huang, S.; Zhang, X.; An, X.; Xu, M.; Lian, B. Characterisation of products from fungally modified wollastonite and the simulation experiment of Pb2+ fixation. Sci. Total Environ. 2021, 760, 143357. [Google Scholar] [CrossRef]
- Li, Y.; Zou, G.; Yang, S.; Wang, Z.; Chen, T.; Yu, X.; Guo, Q.; He, R.; Duan, T.; Zhu, W. Integration of bio-inspired adsorption and photodegradation for the treatment of organics-containing radioactive wastewater. Chem. Eng. J. 2019, 364, 139–145. [Google Scholar] [CrossRef]
- Enayatizamir, N.; Liu, J.; Wang, L.; Lin, X.; Fu, P. Coupling Laccase production from Trametes pubescence with heavy metal removal for Economic Waste Water Treatment. J. Water Process Eng. 2020, 37, 101357. [Google Scholar] [CrossRef]
- Sharma, K.R.; Giri, R.; Sharma, R.K. Lead, cadmium and nickel removal efficiency of white-rot fungus Phlebia brevispora. Lett. Appl. Microbiol. 2020, 71, 637–644. [Google Scholar] [CrossRef]
- Ma, X.; Li, X.; Liu, J.; Cheng, Y.; Zhai, F.; Sun, Z.; Han, L. Enhancing Salix viminalis L.-mediated phytoremediation of polycyclic aromatic hydrocarbon-contaminated soil by inoculation with Crucibulum laeve (white-rot fungus). Environ. Sci. Pollut. Res. 2020, 27, 41326–41341. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, X.; Zhang, M.; Zhu, Y.; Zhuo, R. Removal of heavy-metal pollutants by white rot fungi: Mechanisms, achievements, and perspectives. J. Clean. Prod. 2022, 354, 131681. [Google Scholar] [CrossRef]
- Priya, A.K.; Gnanasekaran, L.; Dutta, K.; Rajendran, S.; Balakrishnan, D.; Soto-Moscoso, M. Biosorption of heavy metals by microorganisms: Evaluation of different underlying mechanisms. Chemosphere 2022, 307, 135957. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Zhang, T.; Wang, F. Microbial-Based Heavy Metal Bioremediation: Toxicity and Eco-Friendly Approaches to Heavy Metal Decontamination. Appl. Sci. 2023, 13, 8439. [Google Scholar] [CrossRef]
- Fan, M.Q.; Wang, X.; Song, Q.; Zhang, L.Y.; Ren, B.; Yang, X.D. Review of biomass-based materials for uranium adsorption. J. Radioanal. Nucl. Chem. 2021, 330, 589–602. [Google Scholar] [CrossRef]
- Lu, N.; Hu, T.; Zhai, Y.; Qin, H.; Aliyeva, J.; Zhang, H. Fungal cell with artificial metal container for heavy metals biosorption: Equilibrium, kinetics study and mechanisms analysis. Environ. Res. 2020, 182, 109061. [Google Scholar] [CrossRef]
- Kordialik-Bogacka, E.; Diowksz, A. Metal uptake capacity of modified Saccharomyces pastorianus biomass from different types of solution. Environ. Sci. Pollut. Res. 2013, 21, 2223–2229. [Google Scholar] [CrossRef]
- Wang, N.N.; Xu, X.J.; Li, H.Y.; Yuan, L.Z.; Yu, H.W. Enhanced Selective Adsorption of Pb(II) from Aqueous Solutions by One-Pot Synthesis of Xanthate-Modified Chitosan Sponge: Behaviors and Mechanisms. Ind. Eng. Chem. Res. 2016, 55, 12222–12231. [Google Scholar] [CrossRef]
- Nieboer, E.; Richardson, D.H.S. The replacement of the nondescript term ‘heavy metals’ by a biologically and chemically significant classification of metal ions. Environ. Pollut. Ser. B Chem. Phys. 1980, 1, 3–26. [Google Scholar] [CrossRef]
- Lin, C.; Dong, B.; Xu, Z. Competitive adsorption of heavy metals onto xanthate-modified sludge hydrochar and its solidification as secondary minerals. Chemosphere 2024, 356, 141878. [Google Scholar] [CrossRef]
- Wang, N.; Qiu, Y.; Hu, K.; Huang, C.; Xiang, J.; Li, H.; Tang, J.; Wang, J.; Xiao, T. One-step synthesis of cake-like biosorbents from plant biomass for the effective removal and recovery heavy metals: Effect of plant species and roles of xanthation. Chemosphere 2021, 266, 129129. [Google Scholar] [CrossRef] [PubMed]
- Drumm, F.C.; Grassi, P.; Georgin, J.; Tonato, D.; Pfingsten Franco, D.S.; Chaves Neto, J.R.; Mazutti, M.A.; Jahn, S.L.; Dotto, G.L. Potentiality of the Phoma sp. inactive fungal biomass, a waste from the bioherbicide production, for the treatment of colored effluents. Chemosphere 2019, 235, 596–605. [Google Scholar] [CrossRef] [PubMed]
- Khambhaty, Y.; Mody, K.; Basha, S.; Jha, B. Kinetics, equilibrium and thermodynamic studies on biosorption of hexavalent chromium by dead fungal biomass of marine Aspergillus niger. Chem. Eng. J. 2009, 145, 489–495. [Google Scholar] [CrossRef]
- Mercier, L.; Pinnavaia, T.J. Access in mesoporous materials: Advantages of a uniform pore structure in the design of a heavy metal ion adsorbent for environmental remediation. Adv. Mater. 1997, 9, 500–503. [Google Scholar] [CrossRef]
- Benettayeb, A.; Guibal, E.; Morsli, A.; Kessas, R. Chemical modification of alginate for enhanced sorption of Cd(II), Cu(II) and Pb(II). Chem. Eng. J. 2017, 316, 704–714. [Google Scholar] [CrossRef]
- Kinraide, T.B.; Yermiyahu, U. A scale of metal ion binding strengths correlating with ionic charge, Pauling electronegativity, toxicity, and other physiological effects. J. Inorg. Biochem. 2007, 101, 1201–1213. [Google Scholar] [CrossRef]
- Sutherland, C. A method of classifying the influence of intraparticle diffusion in adsorption systems: Characteristic curves of the diffusion-chemisorption kinetic model. Environ. Toxicol. Chem. 2025, 44, 1209–1221. [Google Scholar] [CrossRef]
- Wang, A.; Zheng, Z.; Li, R.; Hu, D.; Lu, Y.; Luo, H.; Yan, K. Biomass-derived porous carbon highly efficient for removal of Pb(II) and Cd(II). Green Energy Environ. 2019, 4, 414–423. [Google Scholar] [CrossRef]
- Oliveira, M.R.F.; do Vale Abreu, K.; Romao, A.L.E.; Davi, D.M.B.; de Carvalho Magalhaes, C.E.; Carrilho, E.N.V.M.; Alves, C.R. Carnauba (Copernicia prunifera) palm tree biomass as adsorbent for Pb(II) and Cd(II) from water medium. Environ. Sci. Pollut. Res. 2021, 28, 18941–18952. [Google Scholar] [CrossRef]
- Taksitta, K.; Sujarit, P.; Ratanawimarnwong, N.; Donpudsa, S.; Songsrirote, K. Development of tannin-immobilized cellulose fiber extracted from coconut husk and the application as a biosorbent to remove heavy metal ions. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100389. [Google Scholar] [CrossRef]
- Batool, F.; Mohyuddin, A.; Amjad, A.; ul Hassan, A.; Nadeem, S.; Javed, M.; Hafiz Dzarfan Othman, M.; Wayne Chew, K.; Rauf, A.; Kurniawan, T.A. Removal of Cd(II) and Pb(II) from synthetic wastewater using Rosa damascena waste as a biosorbent: An insight into adsorption mechanisms, kinetics, and thermodynamic studies. Chem. Eng. Sci. 2023, 280, 119072. [Google Scholar] [CrossRef]
- Guyo, U.; Mhonyera, J.; Moyo, M. Pb(II) adsorption from aqueous solutions by raw and treated biomass of maize stover—A comparative study. Process Saf. Environ. Prot. 2015, 93, 192–200. [Google Scholar] [CrossRef]
- Alhogbi, B.G. Potential of coffee husk biomass waste for the adsorption of Pb(II) ion from aqueous solutions. Sustain. Chem. Pharm. 2017, 6, 21–25. [Google Scholar] [CrossRef]
- Garg, U.; Kaur, M.P.; Jawa, G.K.; Sud, D.; Garg, V.K. Removal of cadmium(II) from aqueous solutions by adsorption on agricultural waste biomass. J. Hazard. Mater. 2008, 154, 1149–1157. [Google Scholar] [CrossRef]
- Fawzy, M.A.; Darwish, H.; Alharthi, S.; Al-Zaban, M.I.; Noureldeen, A.; Hassan, S.H.A. Process optimization and modeling of Cd2+ biosorption onto the free and immobilized Turbinaria ornata using Box–Behnken experimental design. Sci. Rep. 2022, 12, 3256. [Google Scholar] [CrossRef]
- Liu, J.; Ren, S.; Zhou, Y.; Tsang, D.C.W.; Lippold, H.; Wang, J.; Yin, M.; Xiao, T.; Luo, X.; Chen, Y. High contamination risks of thallium and associated metal(loid)s in fluvial sediments from a steel-making area and implications for environmental management. J. Environ. Manag. 2019, 250, 109513. [Google Scholar] [CrossRef] [PubMed]
- Mikoda, B.; Gruszecka-Kosowska, A. Mineral and chemical characteristics, textural parameters, and the mobility of the selected elements of flotation waste, originating from the Polish copper- mining industry. Hum. Ecol. Risk Assess. 2018, 24, 1216–1232. [Google Scholar] [CrossRef]
- Bulin, C.; Zheng, R.; Guo, T.; Zhang, B. Incorporating hard-soft acid-base theory in multi-aspect analysis of the adsorption mechanism of aqueous heavy metals by graphene oxide. J. Phys. Chem. Solids 2022, 170, 110934. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and Soft Acids and Bases. J. Am. Chem. Soc. 1963, 85, 3533–3539. [Google Scholar] [CrossRef]
- Xia, Y.; Liao, G.; Wang, Z.; Wei, Y.; Liu, H.; Tang, Y.; Tang, H.; Liu, X.; Shi, J.; Liu, C. Efficient and deep adsorption of thallium(I) from complex water based on hard-soft acid-base theory. Sep. Purif. Technol. 2025, 360, 131221. [Google Scholar] [CrossRef]
- Meng, X.; Wang, X.; Ma, Y.; Wang, Y. Development of a coupled model of quantitative ion character-activity relationships-biotic ligand model (QICARs-BLM) for predicting toxicity for data poor metals. J. Hazard. Mater. 2019, 373, 620–629. [Google Scholar] [CrossRef]
- Zhou, S.; Huo, X.; Hua, M.; Luo, G.; Fan, J.; Clark, J.H.; Zhang, S. Insights on the Effect of Heavy Metals on Subcritical Hydrothermal Recycling of Heavy Metal-Contaminated Biomass and Its Derived Porous Carbon Properties Using Cu as a Case. ACS ES&T Eng. 2022, 2, 2245–2253. [Google Scholar] [CrossRef]
- Haris, M.; Usman, M.; Su, F.; Lei, W.; Saleem, A.; Hamid, Y.; Guo, J.; Li, Y. Programmable synthesis of exfoliated biochar nanosheets for selective and highly efficient adsorption of thallium. Chem. Eng. J. 2022, 434, 134842. [Google Scholar] [CrossRef]
- Cao, Y.; Xiao, W.; Shen, G.; Ji, G.; Zhang, Y.; Gao, C.; Han, L. Carbonization and ball milling on the enhancement of Pb(II) adsorption by wheat straw: Competitive effects of ion exchange and precipitation. Bioresour. Technol. 2019, 273, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Min, F.; Hu, X.; Ma, D.; Huo, Z. Biochar assists phosphate solubilizing bacteria to resist combined Pb and Cd stress by promoting acid secretion and extracellular electron transfer. J. Hazard. Mater. 2023, 452, 131176. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zeng, L.; Wang, Y.; Tian, J.; Wang, J.; Sun, W.; Han, H.; Yang, Y. Selective separation of metals from wastewater using sulfide precipitation: A critical review in agents, operational factors and particle aggregation. J. Environ. Manag. 2023, 344, 118462. [Google Scholar] [CrossRef]
- Branca, C.; D’Angelo, G.; Crupi, C.; Khouzami, K.; Rifici, S.; Ruello, G.; Wanderlingh, U. Role of the OH and NH vibrational groups in polysaccharide-nanocomposite interactions: A FTIR-ATR study on chitosan and chitosan/clay films. Polymer 2016, 99, 614–622. [Google Scholar] [CrossRef]
- Chen, X.; Jiang, S.; Wu, J.; Yi, X.; Dai, G.; Shu, Y. Three-year field experiments revealed the immobilization effect of natural aging biochar on typical heavy metals (Pb, Cu, Cd). Sci. Total Environ. 2024, 912, 169384. [Google Scholar] [CrossRef]
- Zhu, Y.; Hu, J.; Wang, J. Competitive adsorption of Pb(II), Cu(II) and Zn(II) onto xanthate-modified magnetic chitosan. J. Hazard. Mater. 2012, 221–222, 155–161. [Google Scholar] [CrossRef]
- Chen, M.; Wang, X.; Zhang, H. Comparative research on selective adsorption of Pb(II) by biosorbents prepared by two kinds of modifying waste biomass: Highly-efficient performance, application and mechanism. J. Environ. Manag. 2021, 288, 112388. [Google Scholar] [CrossRef]
- Singha, A.S.; Guleria, A. Chemical modification of cellulosic biopolymer and its use in removal of heavy metal ions from wastewater. Int. J. Biol. Macromol. 2014, 67, 409–417. [Google Scholar] [CrossRef]
- Córdova, B.M.; Infantas, G.C.; Mayta, S.; Huamani-Palomino, R.G.; Kock, F.V.C.; Montes de Oca, J.; Valderrama, A.C. Xanthate-modified alginates for the removal of Pb(II) and Ni(II) from aqueous solutions: A brief analysis of alginate xanthation. Int. J. Biol. Macromol. 2021, 179, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Herath, A.; Layne, C.A.; Perez, F.; Hassan, E.I.B.; Pittman, C.U.; Mlsna, T.E. KOH-activated high surface area Douglas Fir biochar for adsorbing aqueous Cr(VI), Pb(II) and Cd(II). Chemosphere 2021, 269, 128409. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, L.; Xu, R.; Yang, Y.; Yin, H.; Jin, S.; Jiang, T. Potentiality of phosphorus−accumulating organisms biomasses in biosorption of Cd(II), Pb(II), Cu(II) and Zn(II) from aqueous solutions: Behaviors and mechanisms. Chemosphere 2022, 303, 135095. [Google Scholar] [CrossRef] [PubMed]
- Córdova, B.M.; Venâncio, T.; Olivera, M.; Huamani-Palomino, R.G.; Valderrama, A.C. Xanthation of alginate for heavy metal ions removal. Characterization of xanthate-modified alginates and its metal derivatives. Int. J. Biol. Macromol. 2021, 169, 130–142. [Google Scholar] [CrossRef]
- Wang, N.; Wang, M.; Quan, H.; Wang, S.; Chen, D. Waste Camellia oleifera shell-derived hierarchically porous carbon modified by Fe3O4 nanoparticles for capacitive removal of heavy metal ions. Sep. Purif. Technol. 2024, 329, 125184. [Google Scholar] [CrossRef]
- Ma, R.; Feng, Y.; Li, H.; Liu, M.; Cui, Y.; Wang, J.; Shen, K.; Zhang, S.; Tong, S. Deep-sea microorganisms-driven V5+and Cd2+removal from vanadium smelting wastewater: Bacterial screening, performance and mechanism. Environ. Pollut. 2024, 360, 124599. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, Z.; Yan, B.; Zhao, L.; Chen, T.; Yang, X. Effects of active silicon amendment on Pb(II)/Cd(II) adsorption: Performance evaluation and mechanism. J. Hazard. Mater. 2024, 478, 135614. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Q.; Xia, H.; Liu, R.; Zhang, Y. Enhanced complexation and electrostatic attraction through fabrication of amino- or hydroxyl-functionalized Fe/Ni-biochar composite for the adsorption of Pb(II) and Cd(II). Sep. Purif. Technol. 2024, 328, 125074. [Google Scholar] [CrossRef]
- Yu, J.; Tong, M.; Sun, X.; Li, B. A simple method to prepare poly(amic acid)-modified biomass for enhancement of lead and cadmium adsorption. Biochem. Eng. J. 2007, 33, 126–133. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, J.; Cao, J. Combined Remediation towards Cadmium–Arsenic-Contaminated Soil via Phytoremediation and Stabilization. Resources 2023, 12, 109. [Google Scholar] [CrossRef]
- Tian, H.; Peng, S.; Zhao, L.; Chen, Y.; Cui, K. Simultaneous adsorption of Cd(II) and degradation of OTC by activated biochar with ferrate: Efficiency and mechanism. J. Hazard. Mater. 2023, 447, 130711. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Xue, Z.; Li, B.; Ou, L.; Yan, L.; Yang, L.; Yin, K.; Jouha, J.; Shao, P.; Zeng, Z.; et al. High-performance spent coffee grounds-based 3D microporous biochar for the efficient capture of Cd2+ via a multi-pathway mechanism. Chem. Eng. J. 2024, 485, 149537. [Google Scholar] [CrossRef]
- Wang, N.; Su, Z.; Deng, N.; Qiu, Y.; Ma, L.; Wang, J.; Chen, Y.; Hu, K.; Huang, C.; Xiao, T. Removal of thallium(I) from aqueous solutions using titanate nanomaterials: The performance and the influence of morphology. Sci. Total Environ. 2020, 717, 137090. [Google Scholar] [CrossRef] [PubMed]

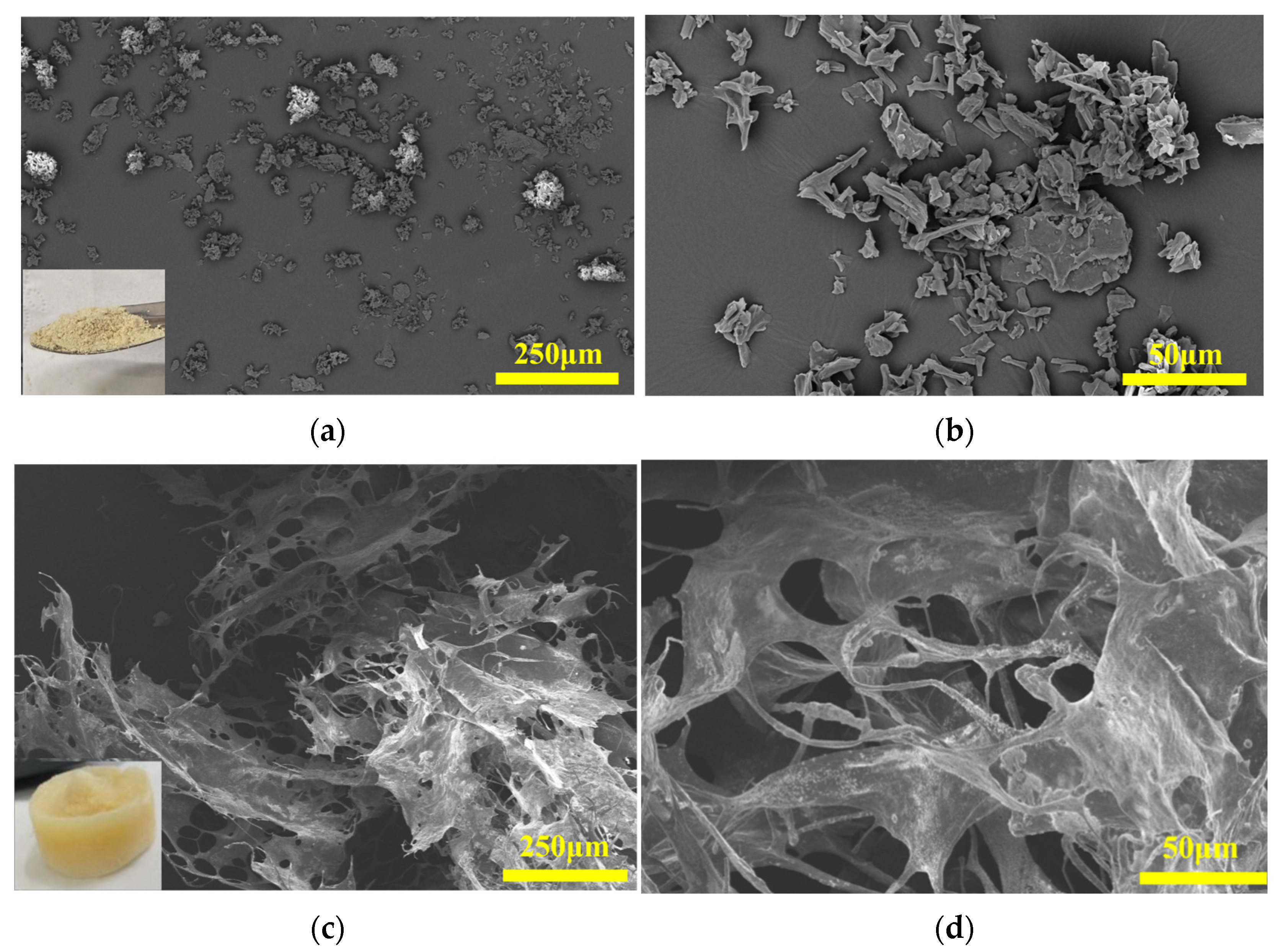
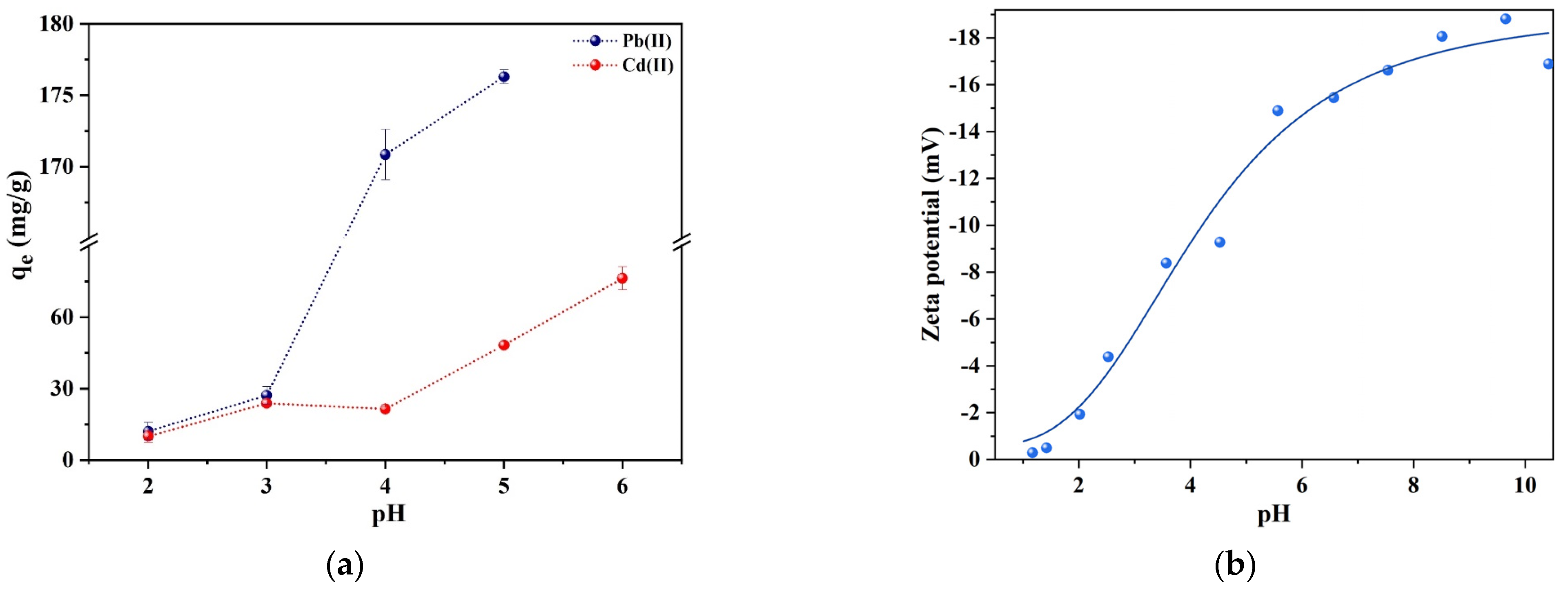
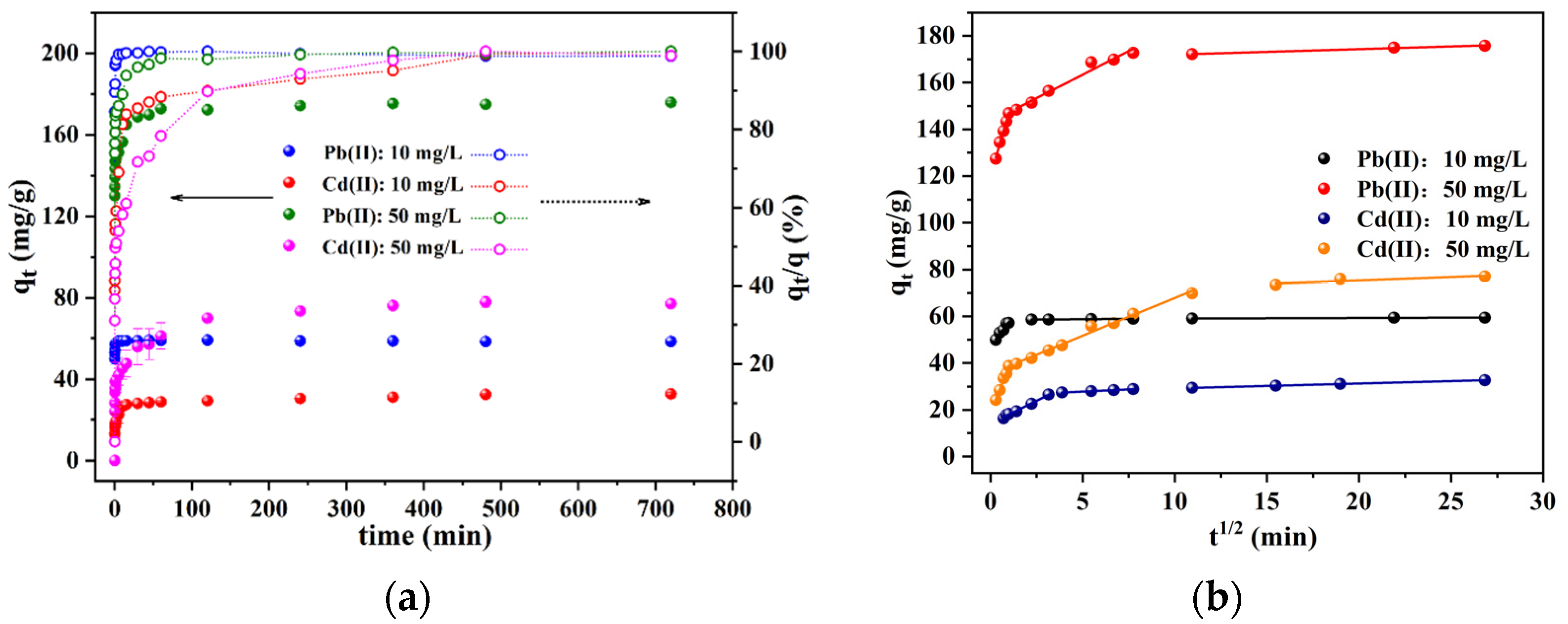
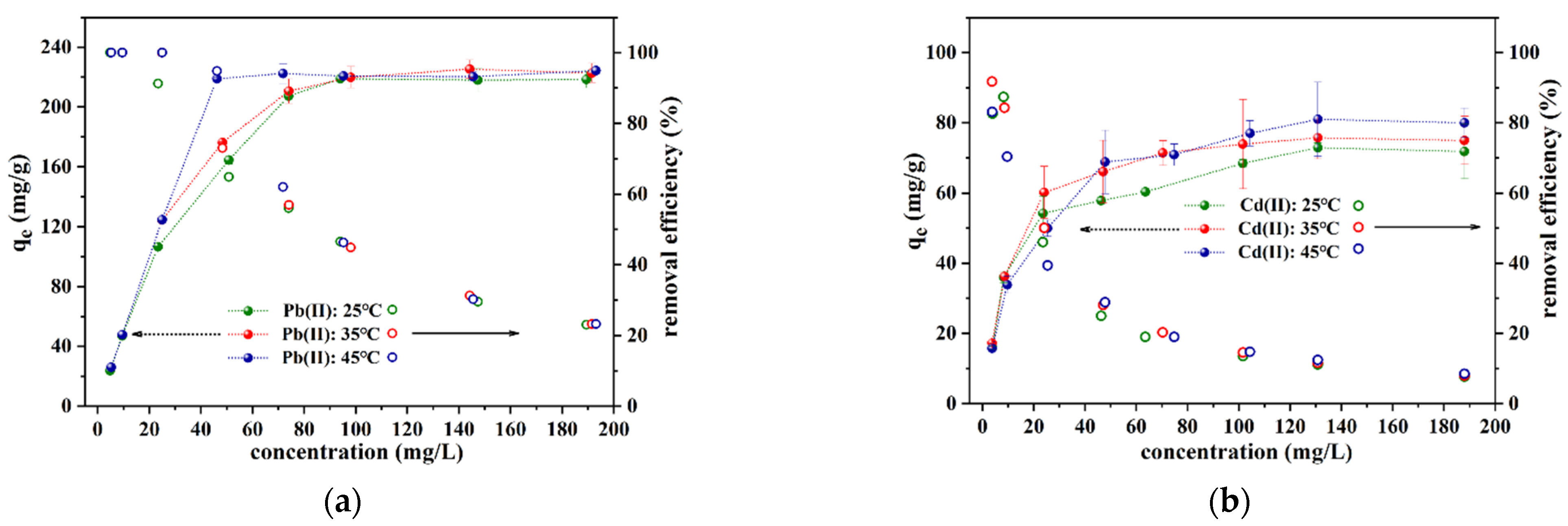
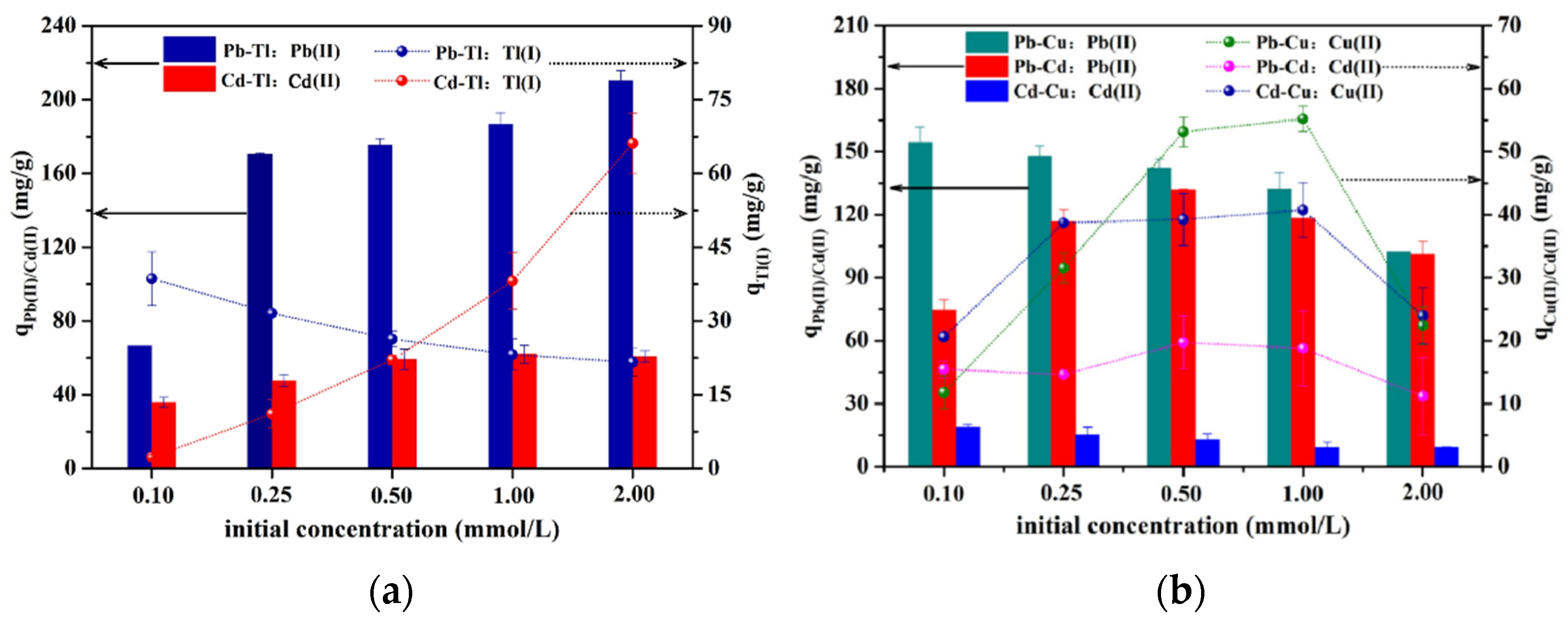
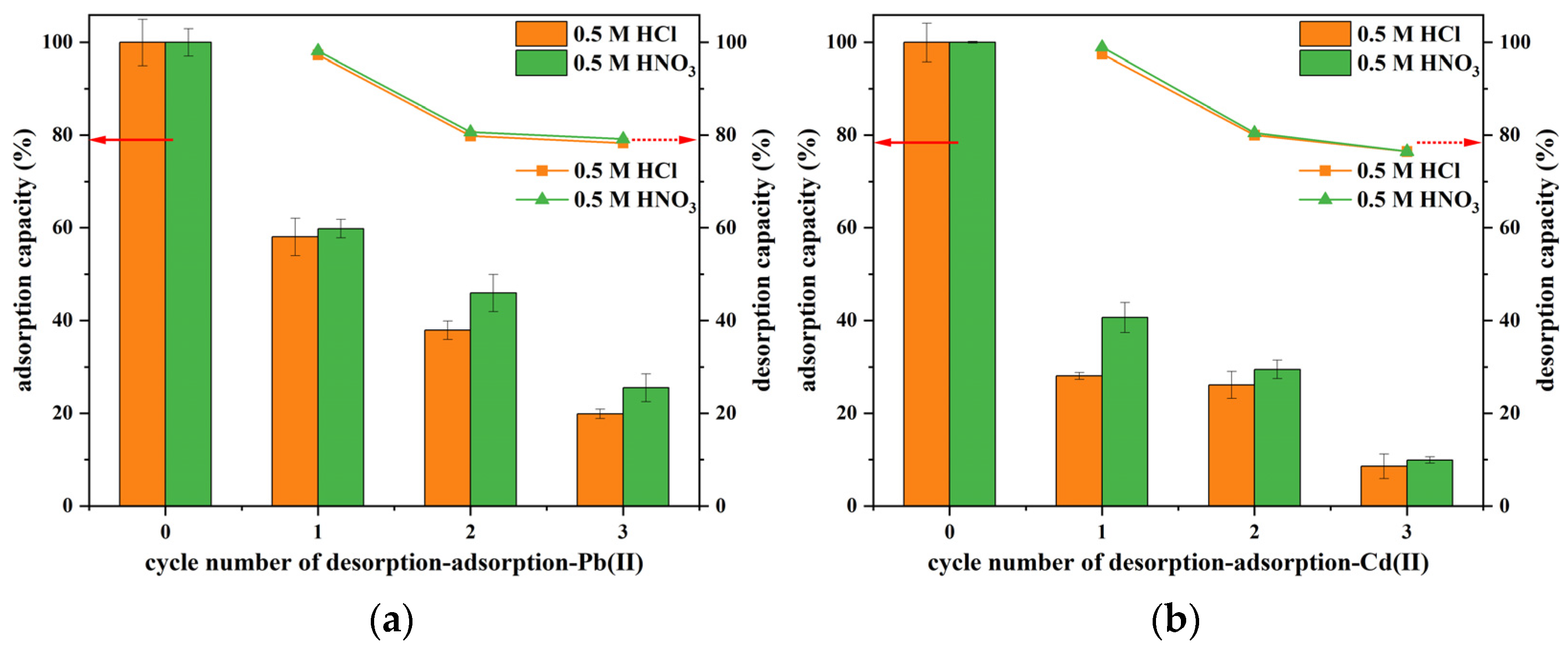
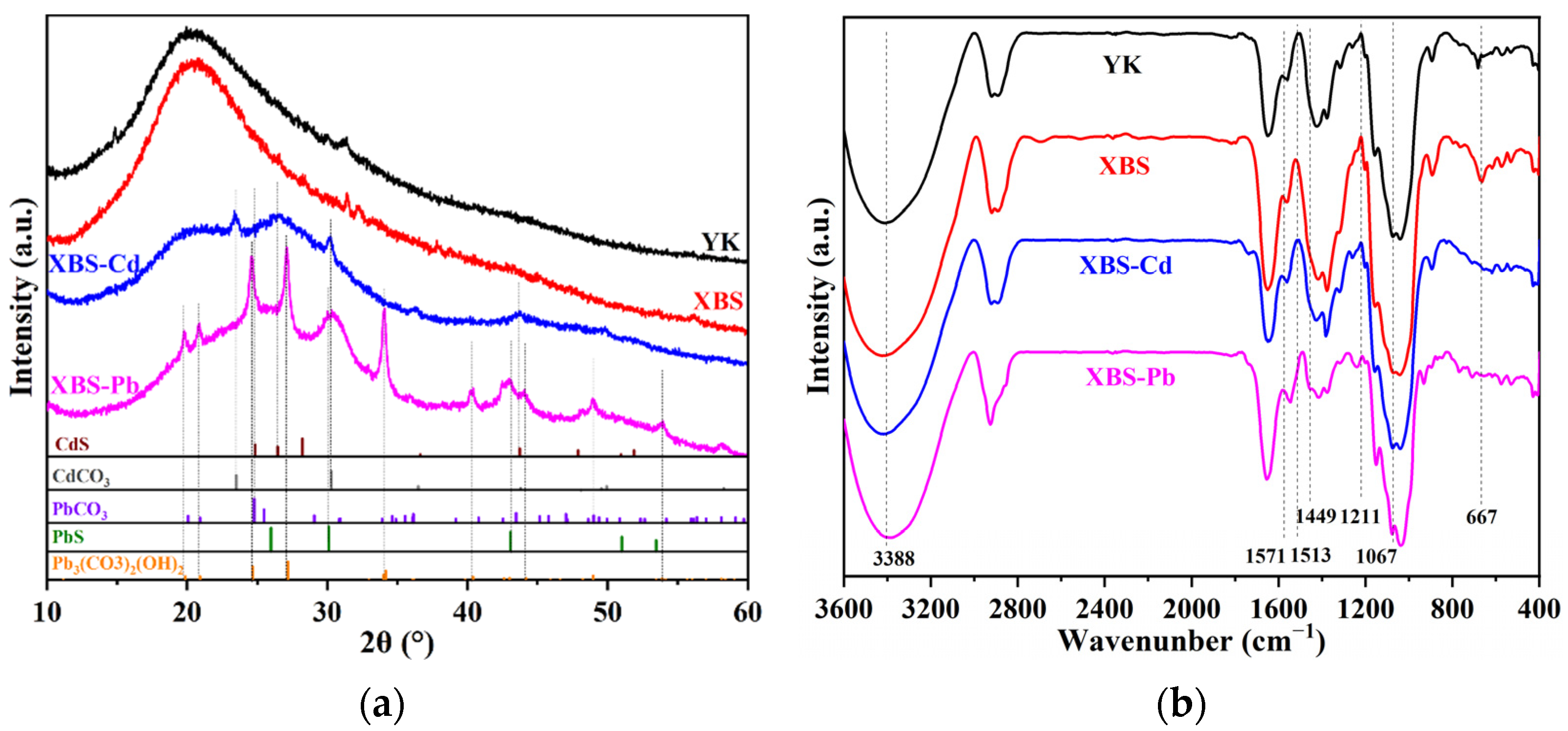
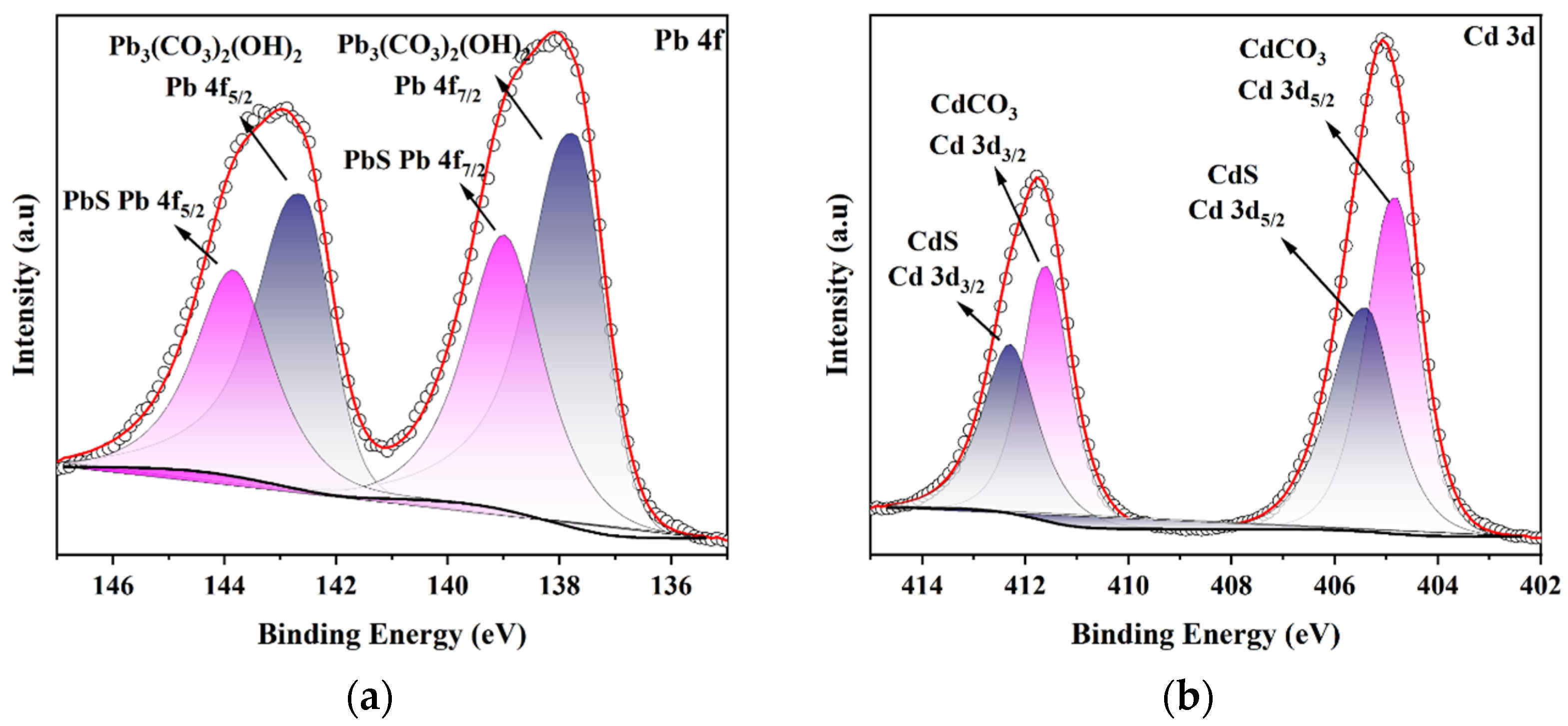

| Heavy Metal Ion | C0 (mg/L) | qe,exp (mg/g) | Pseudo-First-Order | Pseudo-Second-Order | Intra-Particle Diffusion | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| k1 (1/ min) | qe,cal (mg/g) | R2 | k2 (g/(mg min)) | qe,cal (mg/g) | R2 | kid,1 (mg/g min−0.5) | Cid,1 (mg/g) | R2 | kid,2 (mg/g min−0.5) | Cid,2 (mg/g) | R2 | kid,3 (mg/g min−0.5) | Cid,3 (mg/g) | R2 | |||
| Pb(II) | 10 | 59.04 | 0.001 | 1.89 | 0.104 | 0.049 | 59.03 | 0.999 | 10.42 | 47.19 | 0.967 | 0.064 | 58.44 | 0.999 | 0.025 | 58.78 | 0.908 |
| 50 | 175.75 | 0.004 | 3.64 | 0.749 | 0.007 | 158.59 | 1.000 | 26.87 | 120.21 | 0.996 | 4.020 | 143.29 | 0.972 | 0.230 | 169.63 | 0.993 | |
| Cd(II) | 10 | 32.75 | 0.003 | 2.78 | 0.807 | 0.008 | 32.52 | 0.999 | 3.80 | 14.13 | 0.978 | 0.460 | 25.43 | 0.954 | 0.210 | 27.20 | 1.000 |
| 50 | 77.90 | 0.003 | 4.56 | 0.880 | 0.002 | 77.70 | 0.999 | 20.21 | 18.53 | 0.994 | 3.250 | 35.44 | 0.988 | 0.290 | 69.61 | 0.800 | |
| Models | Heavy Metal Ion | Pb(II) | Cd(II) | ||||
|---|---|---|---|---|---|---|---|
| 298 K | 308 K | 318 K | 298 K | 308 K | 318 K | ||
| qe,exp (mg/g) | 218.88 | 225.30 | 224.30 | 72.90 | 75.70 | 80.98 | |
| Langmuir | qm (mg/g) | 221.24 | 224.72 | 223.21 | 73.64 | 76.28 | 82.99 |
| kL (L/min) | 0.559 | 1.014 | 6.289 | 0.194 | 0.374 | 0.158 | |
| R2 | 0.999 | 0.999 | 1.000 | 0.996 | 0.999 | 0.998 | |
| Freundlich | kF (mg/g) | 99.13 | 144.38 | 218.20 | 25.67 | 28.35 | 21.46 |
| 1/n | 0.179 | 0.096 | 0.004 | 0.221 | 0.220 | 0.287 | |
| R2 | 0.910 | 0.813 | 0.456 | 0.834 | 0.912 | 0.951 | |
| Adsorbent | Preparation Method | Heavy Metal | pH | Time (min) | qm (mg/g) | Suited Equilibrium Model Analysis | R2 Values | Kinetics Model Analysis | R2 Values | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| lignin-based porous carbon (LPC) | Mix lignin, urea, and ammonia water and stir evenly, then dry for 1 h. Heat and calcine for another 2 h. Finally, cool to room temperature. | Pb(II) | 6.0 | 60 | 250.47 | Freundlich | 0.988 | Pseudo- second- order | 0.999 | [38] |
| Cd(II) | 126.37 | 0.975 | 0.997 | |||||||
| carnauba fruit biomass (CFB) | Wash the outer skin of the white wax fruit with distilled water, dry at 60 °C for 48 h, grind and sieve. | Pb(II) | 5.0 | 120 | 27.74 | Temkin | 0.981 | Pseudo- second- order | 1.000 | [39] |
| Cd(II) | 34.16 | Freundlich | 0.993 | 1.000 | ||||||
| tannin-immobilized cellulose fiber powder (TCF) | Mix NaOH, tannins, coconut shell cellulose, and epichlorohydrin in stages and heat to stir evenly for 6 h, then wash with water and dry at 40 °C for 24 h. | Pb(II) | 5.0 | 30.00 | 38.02 | Langmuir | 0.997 | - | - | [40] |
| Cd(II) | 59.52 | 0.993 | ||||||||
| rose damascena biomass power (RWB) | Heat Damascus rose powder at 120° C for 72 h, then treat with 0.2 M H2SO4 and NaOH separately, and finally dry at 105 °C. | Pb(II) | 6.5 | 120 | 24.90 | Langmuir | 0.985 | Pseudo-second- order | 0.999 | [41] |
| Cd(II) | 24.80 | 0.989 | 0.999 | |||||||
| chemical treatment of corn stover powder | Treat corn stover with 0.1 M HNO3 for 4 h, then wash with water, dry at 105° C, and grind. | Pb(II) | 5.0 | 60 | 27.10 | Langmuir | 1.000 | Pseudo-second- order | 0.998 | [42] |
| coffee husk biomass powder (CHBW) | After washing with water, the coffee shells are dried at 25 °C for one week and then ground into powder. | Pb(II) | 5.0 | 60 | 19.02 | Freundlich | 0.980 | Pseudo-second- order | 1.000 | [43] |
| sugarcane bagasse (SCB) | Boil the sugarcane bagasse in distilled water for 30 min, dry them in a hot air stove at 120 °C for 24 h, and finally grind them into powder. | Cd(II) | 6.0 | 60 | 69.06 | Langmuir | 0.999 | - | - | [44] |
| turbinaria ornate beads | Mix T. ornata and sodium alginate solution and stir at room temperature for 5 min. Add 2% CaCl2 solution dropwise to the mixture to form microspheres, and finally immerse them in calcium chloride solution for 2 h before washing with water. | Cd(II) | 6.8 | 90 | 23.90 | Langmuir | 0.998 | Pseudo-second- order | 0.990 | [45] |
| XBS | Mix inactivated YK powder with NaOH and CS2 for yellowing for 3 h, then freeze dry to form a macroporous sponge material. | Pb(II) | 1 | 30 240 | 225.30 | Langmuir | 0.999 | Pseudo- second- order | 1.000 | this study |
| Cd(II) | 2 | 80.98 | 0.998 | 0.999 |
| Metal Ion Type | Ionic Radius | Pauling Electronegativity (PE) | Hydrated Radius (HR, Å) | Hardness Index (HI) | logkScale, M | logkH2O, M |
|---|---|---|---|---|---|---|
| Pb(II) | 1.19 | 2.33 | 4.01 | 0.131 | 2.68 | −7.60 |
| Cd(II) | 0.97 | 1.69 | 4.26 | 0.081 | 2.15 | −10.1 |
| Tl(I) | 1.50 | 1.62 | 3.30 | 0.106 | 0.46 | −13.21 |
| Cu(II) | 0.73 | 1.90 | 4.19 | 0.104 | 2.66 | −7.50 |
| Materials | Flow per Batch | Cost/USD per Ton Market Price in China (June 2025) | Cost/USD per Batch |
|---|---|---|---|
| Water for adsorbents preparation (L) | 50 | 0.59 | 0.03 |
| Water for adsorption (L) | 500 | 0.59 | 0.30 |
| White-rot fungi YK-624 contains culture medium (kg) | 5 | 2100 | 10.50 |
| NaOH (kg) | 6.1 | 471 | 2.87 |
| CS2 (L) | 2 | 694 | 1.77 |
| Electricity | 0.15/kWh | ||
| Mixing (kWh) | 3.6 | 0.15 | 0.54 |
| Washing(kWh) | 3 | 0.15 | 0.46 |
| Drying | 2 | 0.15 | 0.30 |
| Adsorption (kWh) | 20 | 0.15 | 3.00 |
| Total | 19.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Chen, Z.; Wang, N.; Wang, J.; He, R.; Chen, Y.; Nuhu, H.; Chen, H.; Lin, Z.; Fan, M.; et al. Sponge-like Modified White-Rot Fungi Adsorbent for Rapid Removal of Pb(II) and Cd(II) from Solution: Selective Performance and Mechanistic Insights. Separations 2025, 12, 172. https://doi.org/10.3390/separations12070172
Wang C, Chen Z, Wang N, Wang J, He R, Chen Y, Nuhu H, Chen H, Lin Z, Fan M, et al. Sponge-like Modified White-Rot Fungi Adsorbent for Rapid Removal of Pb(II) and Cd(II) from Solution: Selective Performance and Mechanistic Insights. Separations. 2025; 12(7):172. https://doi.org/10.3390/separations12070172
Chicago/Turabian StyleWang, Chunxiao, Zhirong Chen, Nana Wang, Jianqiao Wang, Runshen He, Yu Chen, Haerfosai Nuhu, Hang Chen, Zhixuan Lin, Minqi Fan, and et al. 2025. "Sponge-like Modified White-Rot Fungi Adsorbent for Rapid Removal of Pb(II) and Cd(II) from Solution: Selective Performance and Mechanistic Insights" Separations 12, no. 7: 172. https://doi.org/10.3390/separations12070172
APA StyleWang, C., Chen, Z., Wang, N., Wang, J., He, R., Chen, Y., Nuhu, H., Chen, H., Lin, Z., Fan, M., & Chang, M. (2025). Sponge-like Modified White-Rot Fungi Adsorbent for Rapid Removal of Pb(II) and Cd(II) from Solution: Selective Performance and Mechanistic Insights. Separations, 12(7), 172. https://doi.org/10.3390/separations12070172







