Content Determination and Impurity Profiling of Compound Glycyrrhizin Tablets by Ion-Pair High-Performance Liquid Chromatography, Coupled with Corona-Charged Aerosol Detector
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Apparatus and Software
2.3. Experimental Conditions
2.3.1. Chromatographic Conditions
2.3.2. Mass Spectrometry Conditions
2.4. Preparation of the Sample and Reference Solutions
2.4.1. Sample Preparations
2.4.2. Mixed Standard Solutions and Reference Solution
3. Results and Discussion
3.1. Method Development and Optimization
3.2. Identification of Impurity Structure
3.3. Validation of the Developed Method
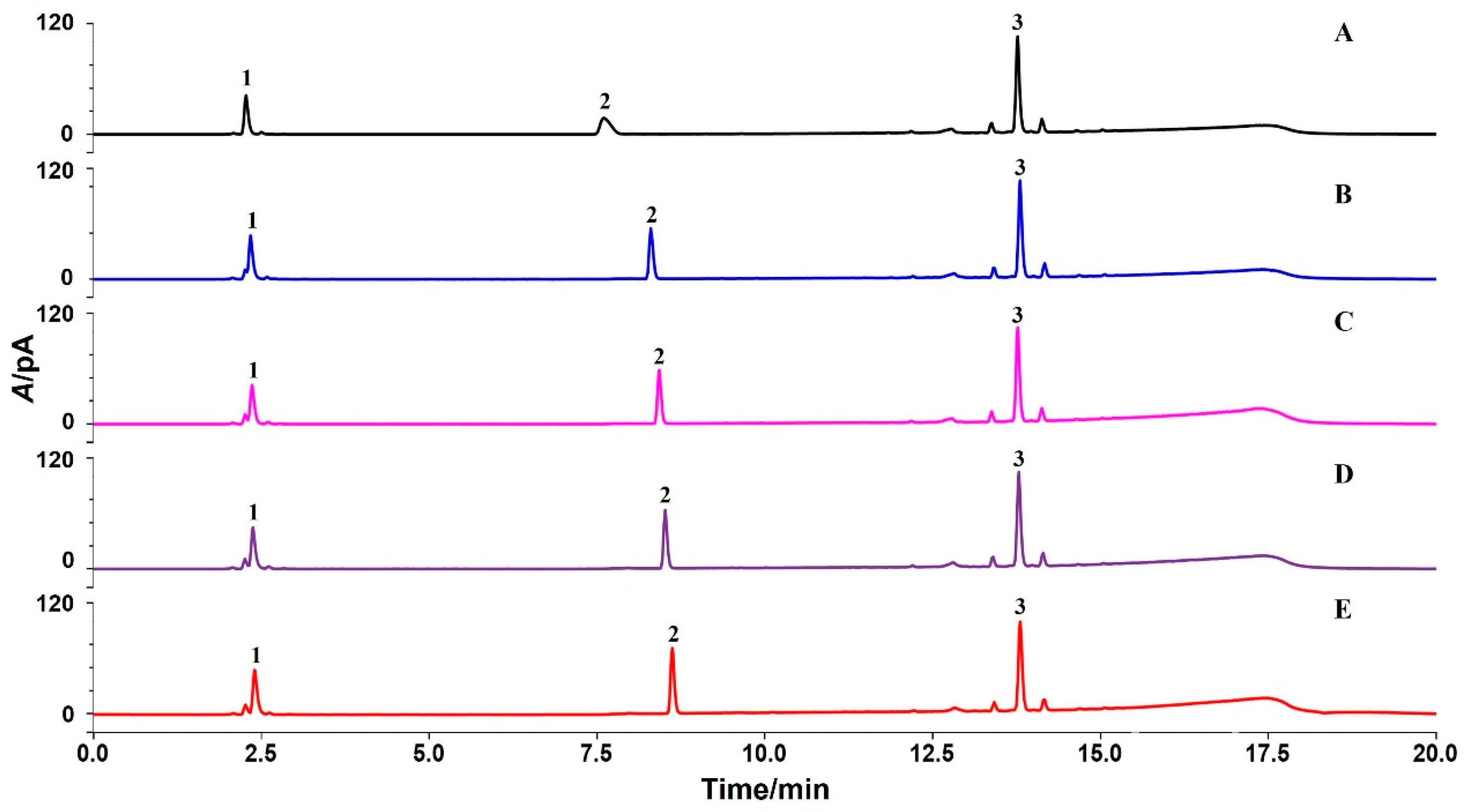
3.3.1. Specificity
3.3.2. Precision and Repeatability
3.3.3. Linearity
3.3.4. Sensitivity
3.3.5. Stability
3.3.6. Accuracy
3.3.7. Robustness
3.4. Content Determination
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Song, L.; Xie, X.; Sun, J.; Huang, S.; Li, X.; Peng, G.; Gao, X.; Zheng, Y. Structural identification and comprehensive comparison of saponin-related impurities present in the three different compound glycyrrhizin tablets. J. Pharm. Biomed. Anal. 2023, 229, 115287. [Google Scholar] [CrossRef]
- Renda, G.; Gökkaya, I.; Şöhretoğlu, D. Immunomodulatory properties of triterpenes. Phytochem. Rev. 2022, 21, 537–563. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Wang, P.H. Intravenous glycyrrhizin improved serum transaminases rapidly in a chronic hepatitis B patient with acute exacerbation. J. Formos. Med. Assoc. 2015, 114, 188–189. [Google Scholar] [CrossRef]
- Baltina, L.A. Chemical modification of glycyrrhizic acid as a route to new bioactive compounds for medicine. Curr. Med. Chem. 2003, 10, 155–171. [Google Scholar] [CrossRef]
- Shi, G.F.; Xu, J.; Zhou, B.H.; Deng, L.; Chen, X.F. Study on new process for the production and purification of monoammonium glycyrrhizinate. Sci. Technol. Food Ind. 2011, 32, 324–326. [Google Scholar]
- Shi, G.F.; Gong, J.H.; Chen, X.F.; Wang, G.Y.; Wang, H.Y. Orthogonal array design for the optimization of monoammonium glycyrrhizinate purification by activated carbon adsorption-ethanol recrystallization. Food Sci. 2013, 34, 125–128. [Google Scholar]
- Shen, H.P. Efficacy observation of compound glycyrrhizin tablets combined with traditional Chinese medicine in the treatment of chronic eczema. China Pharm. 2010, 21, 4560. [Google Scholar]
- Mingwei, Z.; Zhaojie, C.; Chunmin, L. Effect of compound glycyrrhizinon vitiligo in middle-aged and elderly patients. Chin. J. Geriatr. 2014, 34, 5715–5717. [Google Scholar]
- Huang, F.; Fu, Q.; Zhou, Z.; Tang, L.; Zhao, M.; Huang, M.; Zhou, X. Compound glycyrrhizin tablets combined with the 308 nm excimer laser in the treatment of vitiligo: A systematic review and meta-analysis. J. Cosmet. Dermatol. 2023, 22, 2930–2939. [Google Scholar] [CrossRef]
- Feng, X.Y.; Feng, Z.T.; Ma, Y.Z. Clinical trial of compound glycyrrhizin tablets in the treatment of autoimmune hepatitis-primary biliary cirrhosis overlap syndrome. Chin. J. Clin. Pharmacol. 2018, 34, 240–243. [Google Scholar]
- Luo, L.P. Clinical effect of ursodeoxycholic acid capsules combined with compound glycyrrhizin tablets in the treatment of steatohepatitis. Chin. J. Clin. Ration. Drug Use 2022, 15, 24–27. [Google Scholar]
- Jitrangsri, K.; Kamata, K.; Akiba, M.; Yajiri, Y.; Ishibashi, M.; Tatsuzaki, J. Tsutomu Ishikawa. Is 18α-Glycyrrhizin a real natural product? Improved preparation of 18α-Glycyrrhizin from 18β-Glycyrrhizin as a positive standard for HPLC analysis of licorice extracts. J. Nat. Med. 2022, 76, 367–378. [Google Scholar] [CrossRef]
- Ito, Y.; Ishizaki, K.; Sugimoto, N.; Tada, A.; Akiyama, T.; Sato, K.; Akiyama, H.; Goda, Y. Confirmation of the configuration of two glucuronic acid units in glycyrrhizic acid. Jpn. J. Food Chem. Saf. 2015, 22, 32–37. [Google Scholar]
- European Directorate for the Quality of Medicines & HealthCare. European Pharmacopoeia 11.0, Ammonium Glycyrrhizate; European Directorate for the Quality of Medicines & HealthCare: Strasbourg, France, 2023; p. 1953. [Google Scholar]
- United States Pharmacopoeia, Ammonium Glycyrrhizate. Available online: https://online.uspnf.com/uspnf/document/1_GUID-DB67280F-5E0B-413A-8153-509BC15687DD_2_en-US (accessed on 5 January 2024).
- Wahl, O.; Holzgrabe, U. Amino acid analysis for pharmacopoeial purposes. Talanta 2016, 154, 150–163. [Google Scholar] [CrossRef] [PubMed]
- Herbert, P.; Barros, P.; Ratola, N.; Alves, A. HPLC determination of amino acids in musts and port wine using OPA/FMOC derivatives. J. Food Sci. 2000, 65, 1130–1133. [Google Scholar] [CrossRef]
- Erbersdobler, H.F.; Greulich, H.G.; Trautwein, E. Determination of taurine in foods and feeds using an amino acid analyser. J. Chromatogr. A 1983, 254, 332–334. [Google Scholar] [CrossRef]
- Pawellek, R.; Holzgrabe, U. Performance of ion pairing chromatography and hydrophilic interaction liquid chromatography coupled to charged aerosol detection for the analysis of underivatized amino acids. J. Chromatogr. A 2021, 1659, 462613. [Google Scholar] [CrossRef]
- Cecchi, T. Ion pairing chromatography. Crit. Rev. Anal. Chem. 2008, 38, 161–213. [Google Scholar] [CrossRef]
- Cecchi, T. Theoretical models of ion pair chromatography: A close up of recent literature production. J. Liq. Chromatogr. Relat. Technol. 2015, 38, 404–414. [Google Scholar] [CrossRef]
- Ahmad, I.A.H.; Blasko, A.; Tam, J.; Variankaval, N.; Halsey, H.M.; Hartman, R.; Regalado, E.L. Revealing the inner workings of the power function algorithm in Charged Aerosol Detection: A simple and effective approach to optimizing power function value for quantitative analysis. J. Chromatogr. A 2019, 1601, 1–7. [Google Scholar] [CrossRef]
- Ahmad, I.A.H.; Blasko, A.; Wang, H.; Lu, T.; Mangion, I.; Regalado, E.L. Charged aerosol detection in early and late-stage pharmaceutical development: Selection of regression models at optimum power function value. J. Chromatogr. A 2021, 1641, 461997. [Google Scholar] [CrossRef]
- Schilling, K.; Holzgrabe, U. Recent applications of the Charged Aerosol Detector for liquid chromatography in drug quality control. J. Chromatogr. A 2020, 1619, 460911. [Google Scholar] [CrossRef]
- Pawellek, R.; Muellner, T.; Gamache, P.; Holzgrabe, U. Power function setting incharged aerosol detection for the linearization of detector response–optimization strategies and their application. J. Chromatogr. A 2021, 1637, 461844. [Google Scholar] [CrossRef]
- Khoomrung, S.; Chumnanpuen, P.; Jansa-Ard, S.; Stahlman, M.; Nookaew, I.; Boren, J.; Nielsen, J. Rapid quantification of yeast lipid using microwave-assisted total lipid extraction and HPLC-CAD. Anal. Chem. 2013, 85, 4912–4919. [Google Scholar] [CrossRef]
- Furota, S.; Ogawa, N.O.; Takano, Y.; Yoshimura, T.; Ohkouchi, N. Quantitative analysis of underivatized amino acids in the sub- to several-nanomolar range by ion-pair HPLC using a corona charged aerosol detector (HPLC-CAD). J. Chromatogr. B 2018, 1095, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Pawellek, R.; Schilling, K.; Holzgrabe, U. Impurity profiling of L-aspartic acid and glycine using high-performance liquid chromatography coupled with charge daerosol and ultraviolet detection. J. Pharm. Biomed. Anal. 2020, 183, 113149. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Zuo, L.; Sun, S.; Zhao, X.; Xu, S.; Zhu, Z.; Zhao, T.; Sun, Z.; Yao, J.; Shan, G. Impurity profiling of Compound Amino Acid Injection (6AA) using ion-pair high performance liquid chromatography coupled with corona-charged aerosol detection and high resolution mass spectrometry. J. Pharm. Biomed. Anal. 2021, 201, 110325. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, J.P.; Li, J.; Farrell, W.; Groeber, E.; Szucs, R.; Dicinoski, G.; Haddad, P.R. Universal response model for a corona charged aerosol detector. J. Chromatogr. A 2010, 1217, 7418–7427. [Google Scholar] [CrossRef]
- Dixon, R.W.; Peterson, D.S. Development and testing of a detection method for liquid chromatography based on aerosol charging. Anal. Chem. 2002, 74, 2930–2937. [Google Scholar] [CrossRef]
- Petritis, K.N.; Person, M.; Elfakir, C.; Dreux, M. Validation of an ion-interaction chromatography analysis of underivatized amino acids in commercial preparation using evaporative light scattering detection. Chromatographia 2004, 60, 293–298. [Google Scholar] [CrossRef]
- McCalley, D.V. Effect of buffer on peak shape of peptides in reversed-phase high performance liquid chromatography. J. Chromatogr. A 2004, 1038, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.J.; Zhu, B.Q.; Ren, X.J.; Wang, J. Universal response method for the quantitative analysis of multi-components in josamycin and midecamycin using liquid chromatography coupled with charged aerosol detector. J. Pharm. Biomed. Anal. 2021, 192, 113679. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.T.; Xu, K.H.; Lu, Y.T.; Ma, L.M.; Zhou, C.L.; Hang, T.J.; Song, M. Characterization of structurally related peptide impurities using HPLC-QTOF-MS/MS: Application to Cbf-14, a novel antimicrobial peptide. Anal. Bioanal. Chem. 2022, 414, 6485–6495. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, R. New polar constituents of the pupae of the silkworm Bombyx mori L. I. Isolation and identification of methionine sulfoxide, methionine sulfone, and gamma-cyclic di-L-glutamate. Biosci. Biotechnol. Biochem. 2007, 71, 3055–3062. [Google Scholar] [CrossRef][Green Version]
- Xu, G.; Chance, M.R. Radiolytic Modification of Sulfur-Containing Amino Acid Residues in Model Peptides: Fundamental Studies for Protein Foot printing. Anal. Chem. 2005, 77, 2437–2449. [Google Scholar] [CrossRef]
- Mu, Q.E.; Liu, Y.P.; Li, M.; Wang, Y.; Xin, D. Analysis of related substances of monoammonium glycyrrhizinate by LC-MSn. Chin. J. Pharm. 2009, 40, 844–847. [Google Scholar]
- Zhao, Y.Y.; Liu, L.Y.; Han, Y.Y.; Wang, Y.; Shi, M.J. Spectral Characteristics and Structure Identification of Principal Components and Related Substances in Bulk Drug of Ammonium Glycyrrhizate. Chin. J. Pharm. 2014, 45, 663–670. [Google Scholar]
- Song, W.; Si, L.L.; Ji, S.; Wang, H.; Fang, X.M.; Yu, L.Y.; Li, R.Y.; Liang, L.N.; Zhou, D.M.; Ye, M. Uralsaponins M–Y, antiviral triterpenoid saponins from the roots of Glycyrrhiza uralensis. J. Nat. Prod. 2014, 77, 1632–1643. [Google Scholar] [CrossRef]
- Zheng, Y.F.; Qi, L.W.; Zhou, J.L.; Li, P. Structural characterization and identification of oleanane-type triterpene saponins in Glycyrrhiza uralensis Fischer by rapid resolution liquid chromatography coupled with time-of-flight mass spectrometry. Rapid Commun. Mass. Spectrom. 2010, 24, 3261–3270. [Google Scholar] [CrossRef]
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (2022) ICH Harmonized Tripartite Guidelines Q2(R2), Geneva. Available online: https://database.ich.org/sites/default/files/Q2%28R1%29%20Guideline.pdf (accessed on 24 March 2022).
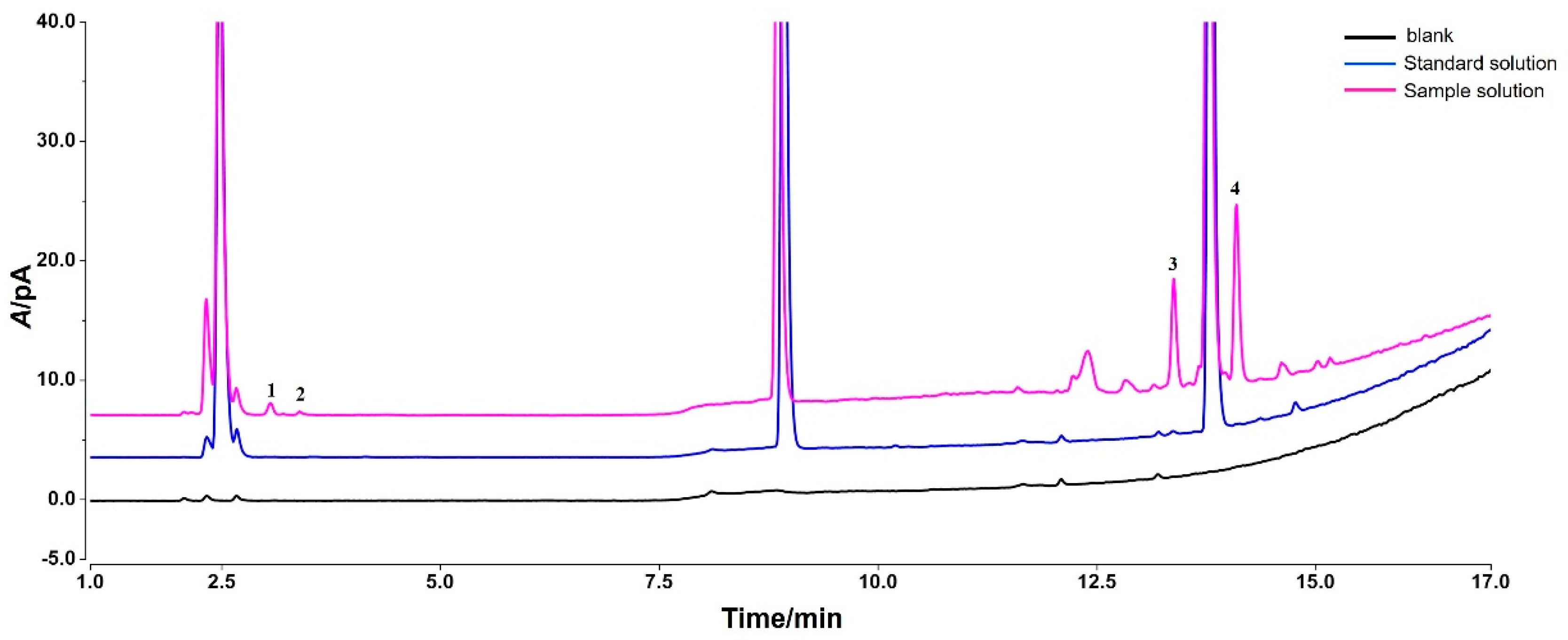
| Impurity | RT/min | [M + H]+ | Deviation | MS/MS Data | Formulas | Identification | Structure |
|---|---|---|---|---|---|---|---|
| 1 | 3.06 | 166.05316 | −0.47 | 148.95, 131.00, 95.85, 74.97 | C5H11NO3S | Methionine sulfoxide | 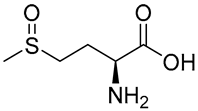 |
| 2 | 3.39 | 182.04802 | −0.72 | 165.06, 155.01, 123.04, 104.88 | C5H11NO4S | Methionine sulfone | 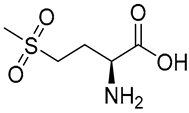 |
| 3 | 13.38 | 839.40598 | −0.26 | 821.06, 663.45, 627.31, 487.42, 469.32 | C42H62O17 | Glycyrrhizin G2 | 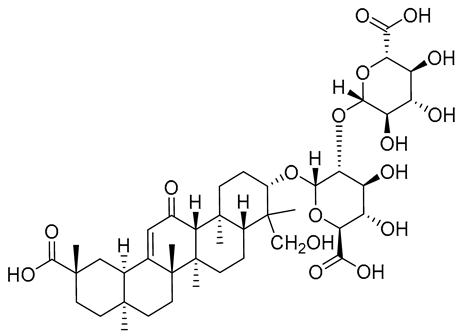 |
| 4 | 14.09 | 823.41199 | 1.12 | 777.49, 647.52, 471.62, 453.30, 435.50, 407.08 | C42H62O16 | 18α-glycyrhizin | 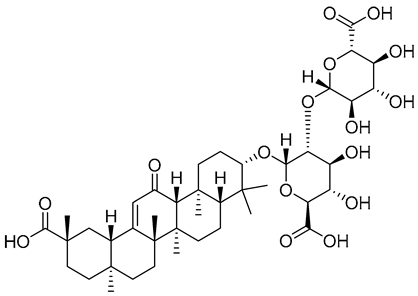 |
| Components | Standard Range (mg mL−1) | Calibration Curve | Correlation Coefficient (r) |
|---|---|---|---|
| Gly | 0.1325–0.5300 | y = 9.1203x + 1.3847 | 0.9990 |
| Met | 0.1249–0.4996 | y = 13.517x + 1.7408 | 0.9991 |
| GR | 0.1151–0.4604 | y = 16.659x + 1.4282 | 0.9992 |
| Reference solution of GR | 0.5–10.0% | y = 0.1534x + 0.0483 | 0.9994 |
| Component | Initial Amount (mg) | Amount Added (mg) | Average Amount Found (mg) | Recovery (%) | Average Recovery (%, n = 6) | RSD/% |
|---|---|---|---|---|---|---|
| Gly | 1.149 | 1.325 | 2.535 | 104.6 | 100.6 | 2.25 |
| 1.149 | 1.325 | 2.461 | 99.04 | |||
| 1.149 | 1.325 | 2.501 | 102.0 | |||
| 1.149 | 1.325 | 2.465 | 99.29 | |||
| 1.149 | 1.325 | 2.460 | 98.93 | |||
| 1.149 | 1.325 | 2.473 | 99.92 | |||
| Met | 1.150 | 1.249 | 2.379 | 98.40 | 99.70 | 2.09 |
| 1.150 | 1.249 | 2.415 | 101.3 | |||
| 1.150 | 1.249 | 2.432 | 102.6 | |||
| 1.150 | 1.249 | 2.392 | 99.45 | |||
| 1.150 | 1.249 | 2.396 | 99.71 | |||
| 1.150 | 1.249 | 2.358 | 96.73 | |||
| GR | 1.109 | 1.151 | 2.200 | 94.76 | 97.62 | 2.01 |
| 1.109 | 1.151 | 2.225 | 96.94 | |||
| 1.109 | 1.151 | 2.228 | 97.24 | |||
| 1.109 | 1.151 | 2.244 | 98.58 | |||
| 1.109 | 1.151 | 2.233 | 97.60 | |||
| 1.109 | 1.151 | 2.268 | 100.6 |
| Factor | Conditions | Gly (μg ml−1) | Met (μg ml−1) | GR (μg ml−1) | Impurity 3 (%) | Impurity 4 (%) |
|---|---|---|---|---|---|---|
| Standard conditions | / | 247.7 | 234.4 | 228.5 | 4.40 | 7.43 |
| Column temperature (°C) | 25 | 265.1 | 232.7 | 230.8 | 4.51 | 8.02 |
| 35 | 255.0 | 231.1 | 238.9 | 3.88 | 6.89 | |
| Flow rate (mL min−1) | 0.75 | 250.4 | 233.5 | 222.6 | 4.85 | 8.47 |
| 0.85 | 270.1 | 235.9 | 234.8 | 4.74 | 8.21 | |
| Nebulizer temperature (°C) | 30 | 257.2 | 233.7 | 231.2 | 4.63 | 8.04 |
| 40 | 254.5 | 239.7 | 242.5 | 4.69 | 8.27 | |
| RSD% | 3.09 | 1.16 | 2.95 | 7.07 | 6.98 |
| Batch | Gly | Met | GR | Impurity 1 Methionine Sulfoxide | Impurity 2 Methionine Sulfone | Impurity 3 Glycyrrhizin G2 | Impurity 4 18α-glycyrhizin | Total Unknown Impurities |
|---|---|---|---|---|---|---|---|---|
| 1 | 95.80 | 98.20 | 99.90 | 0.12% | <LOD | 4.92% | 6.60% | 7.03% |
| 2 | 98.43 | 100.3 | 102.5 | 0.08% | <LOD | 3.80% | 6.07% | 4.35% |
| 3 | 96.40 | 96.27 | 97.91 | 0.06% | <LOD | 4.70% | 7.54% | 6.32% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuo, L.; Su, W.; Gu, Y.; Qiu, X.; Zhao, T.; Lian, X.; Liu, H.; Jia, Q.; Zheng, R.; Shan, G. Content Determination and Impurity Profiling of Compound Glycyrrhizin Tablets by Ion-Pair High-Performance Liquid Chromatography, Coupled with Corona-Charged Aerosol Detector. Separations 2025, 12, 168. https://doi.org/10.3390/separations12070168
Zuo L, Su W, Gu Y, Qiu X, Zhao T, Lian X, Liu H, Jia Q, Zheng R, Shan G. Content Determination and Impurity Profiling of Compound Glycyrrhizin Tablets by Ion-Pair High-Performance Liquid Chromatography, Coupled with Corona-Charged Aerosol Detector. Separations. 2025; 12(7):168. https://doi.org/10.3390/separations12070168
Chicago/Turabian StyleZuo, Limin, Wenling Su, Yongsheng Gu, Xiaodan Qiu, Ting Zhao, Xiaofang Lian, Huiyi Liu, Qingying Jia, Ruifang Zheng, and Guangzhi Shan. 2025. "Content Determination and Impurity Profiling of Compound Glycyrrhizin Tablets by Ion-Pair High-Performance Liquid Chromatography, Coupled with Corona-Charged Aerosol Detector" Separations 12, no. 7: 168. https://doi.org/10.3390/separations12070168
APA StyleZuo, L., Su, W., Gu, Y., Qiu, X., Zhao, T., Lian, X., Liu, H., Jia, Q., Zheng, R., & Shan, G. (2025). Content Determination and Impurity Profiling of Compound Glycyrrhizin Tablets by Ion-Pair High-Performance Liquid Chromatography, Coupled with Corona-Charged Aerosol Detector. Separations, 12(7), 168. https://doi.org/10.3390/separations12070168







