MEMS-Based Micropacked Thermal Desorption GC/PID for In-Field Volatile Organic Compound Profiling from Hot Mix Asphalt
Abstract
1. Introduction
2. Materials and Methods
2.1. Analytical Instruments: Micro GC, Preconcentration, MEMS Packed Column
2.2. Training and Calibration: Dynamic Atmosphere Standard by ATIS
2.3. Experimental Plan on MEMS Preconcentrators
2.4. Greenness Evaluation
2.5. Model Specification and Fitting
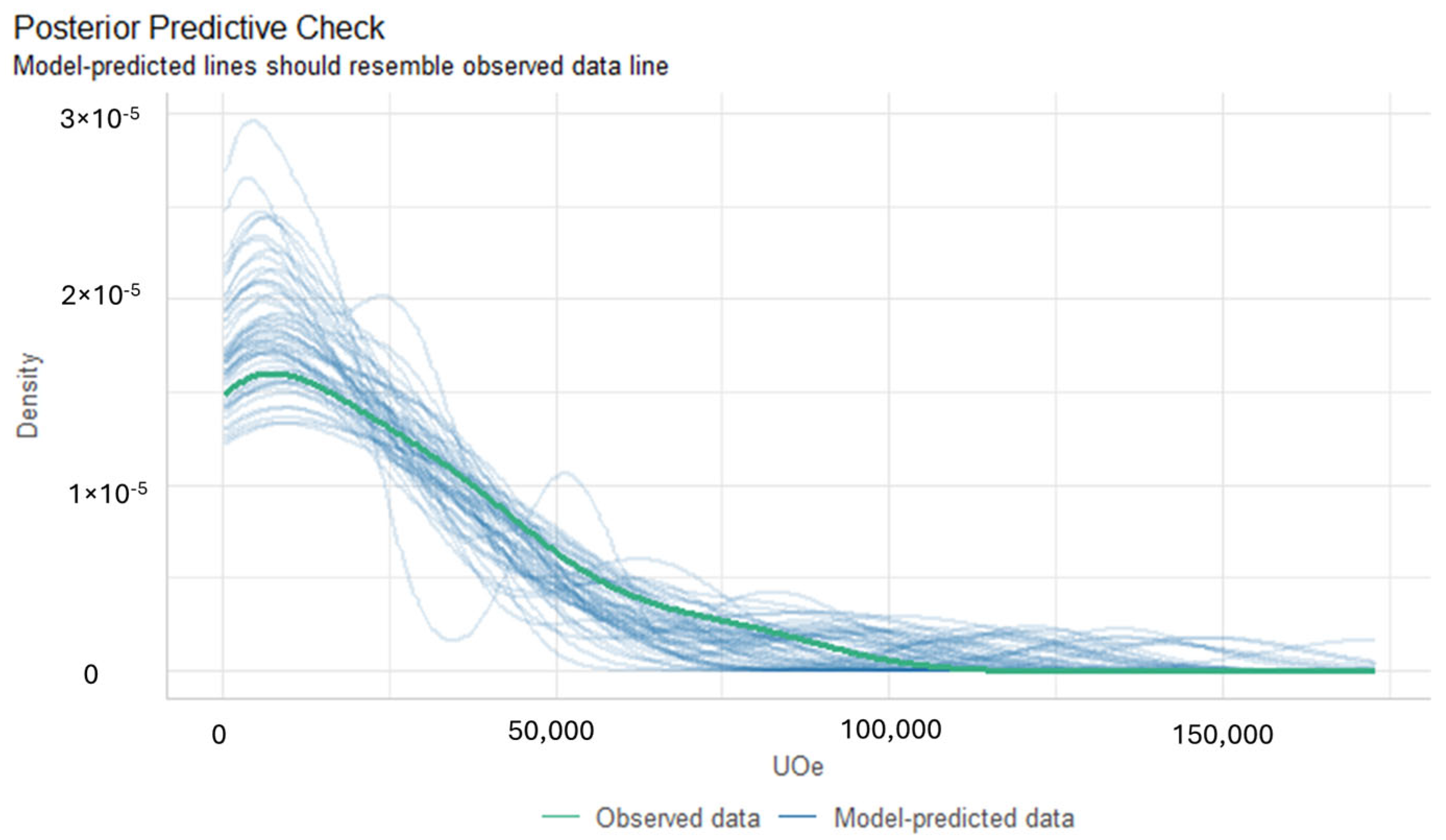
Diagnostic Evaluation
3. Results and Discussion
3.1. Analytical Set up Optimizatiom: Sorptive Material Evaluation and Method Calibration
3.2. Chemometric Analysis
3.3. Greenness Evaluation
3.4. Method of Field Application
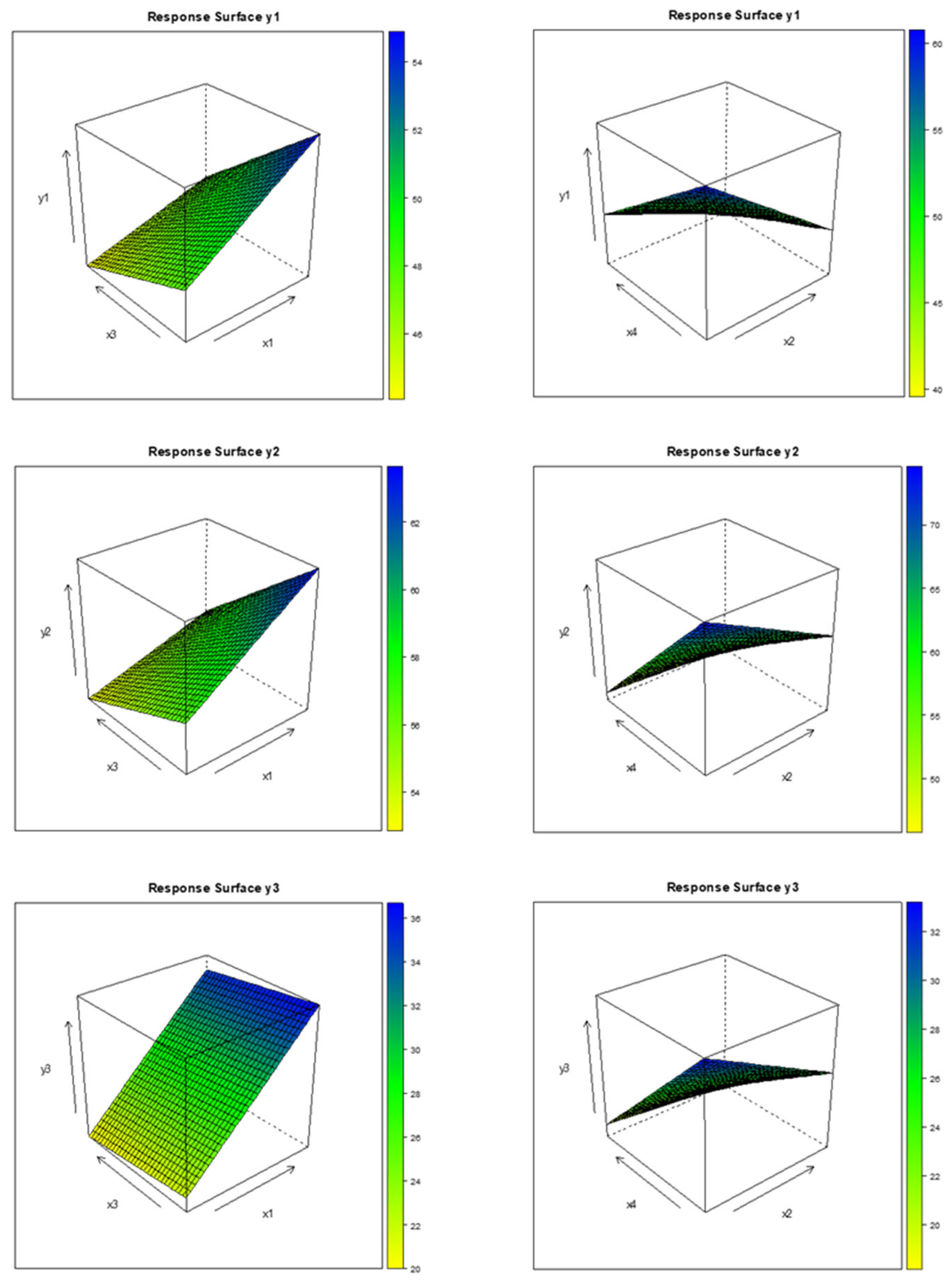
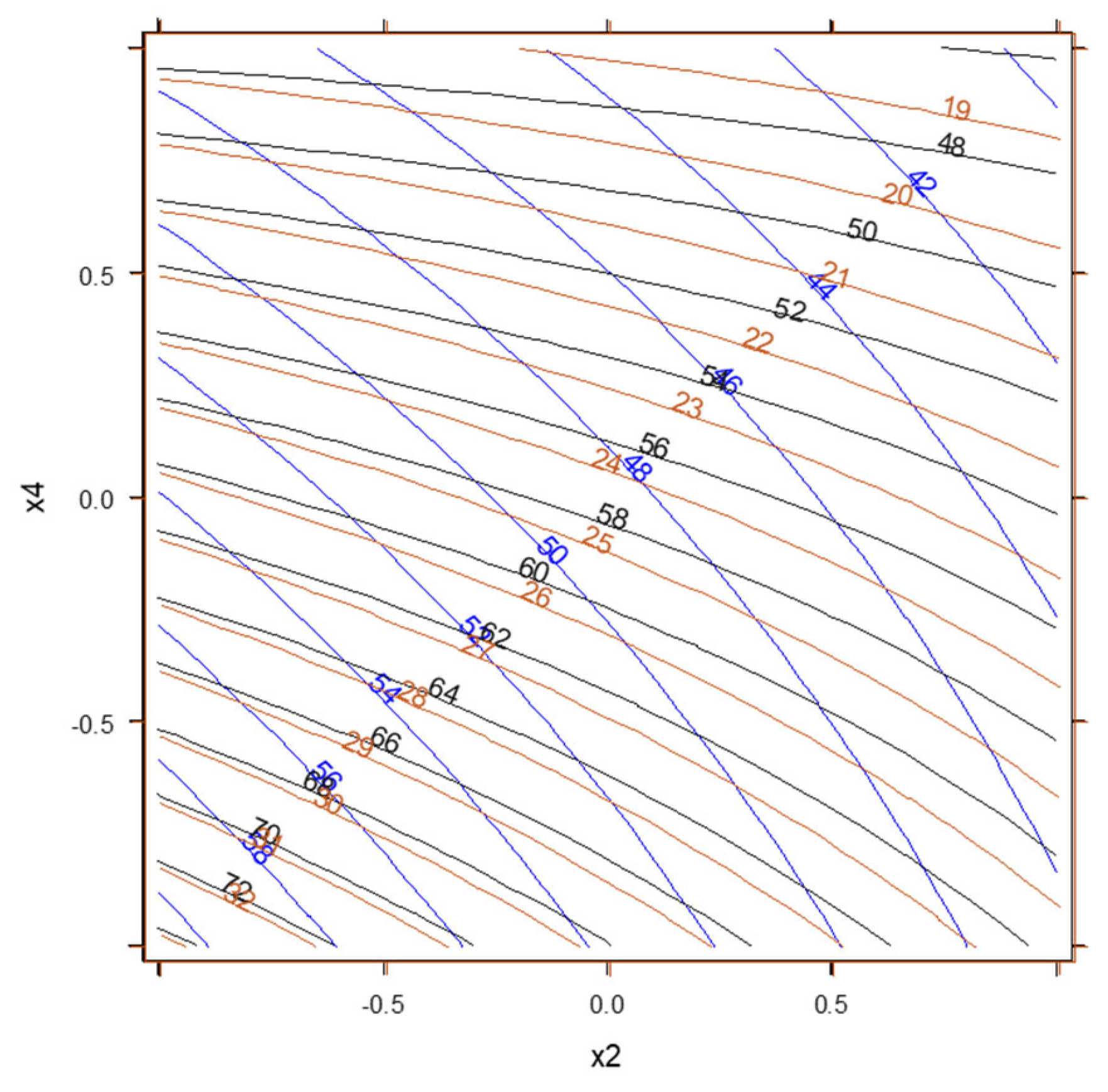
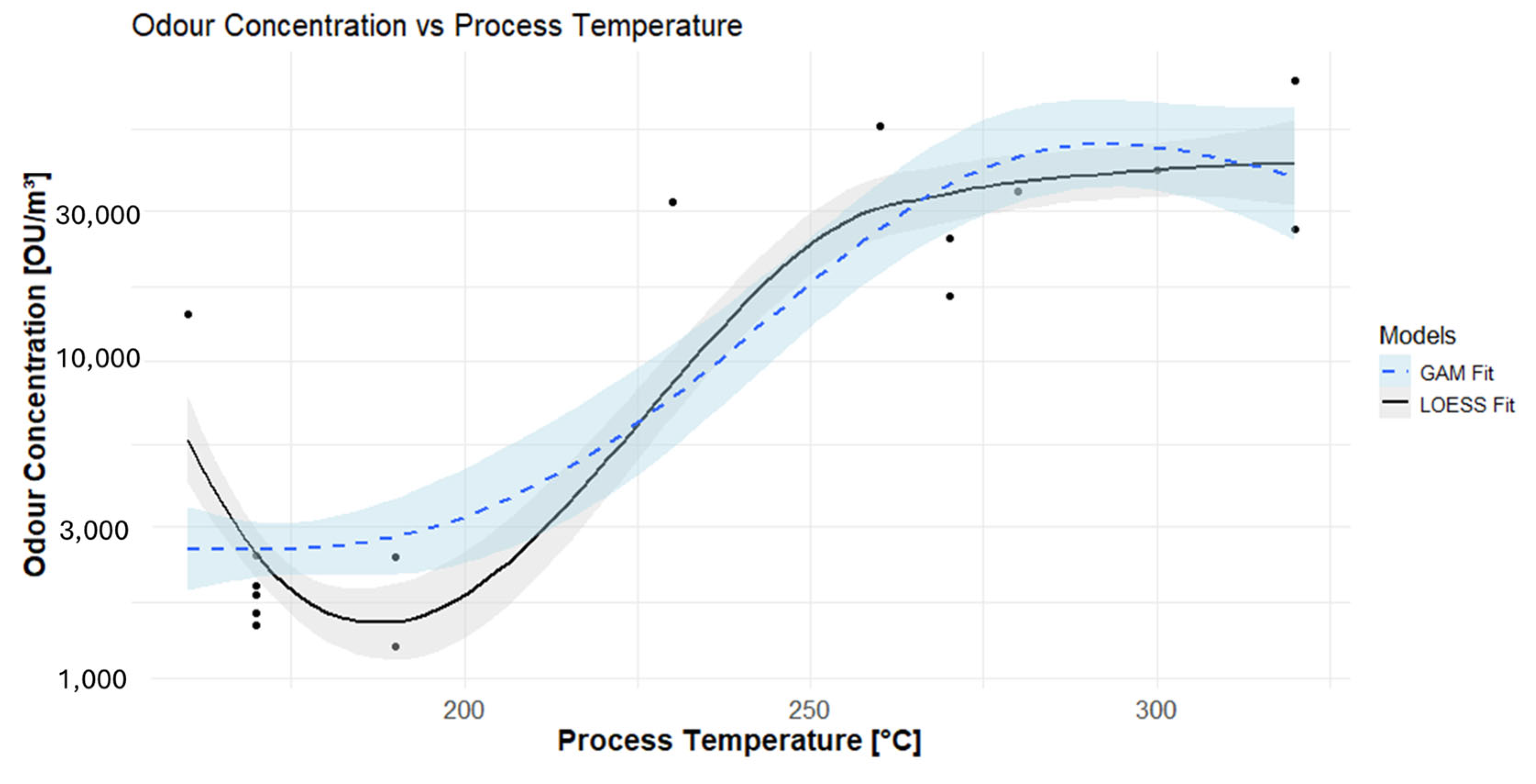
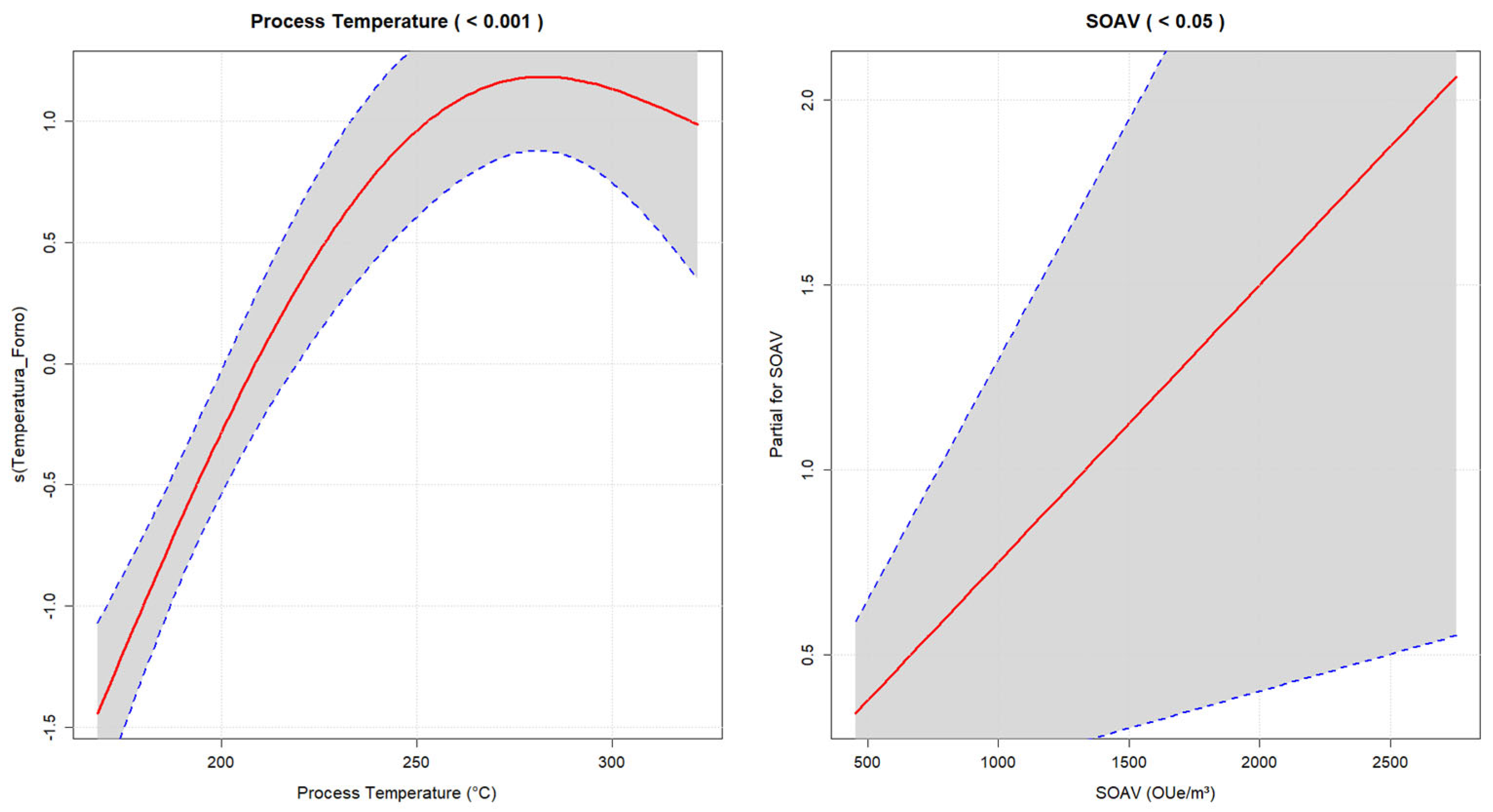
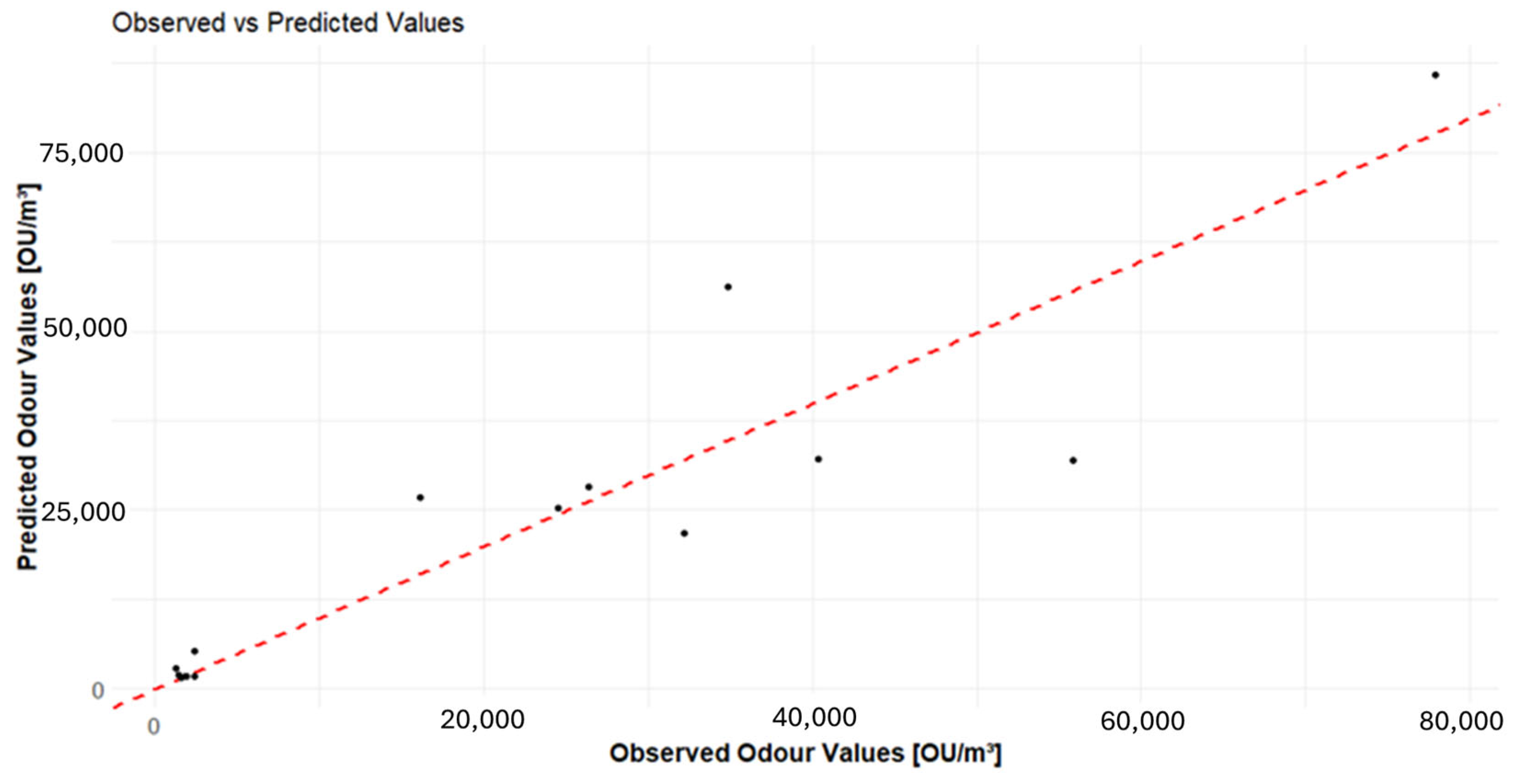
3.5. GAM Approach
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HMA | Hot Mix Asphalt |
| VOCs | Volatile Organic Compounds |
| GAPI | Green Analytical Procedure Index |
| GAM | Generalized Additive Model |
| GC | Gas Chromatography |
| MEMSs | Micro-Electro-Mechanical Systems |
| GAC | Green Analytical Chemistry |
| TCD | Thermal Conductivity Detector |
| FID | Flame Ionization Detector |
| SOAV | Sum of Odour Activity Values |
References
- Brancher, M.; Griffiths, K.D.; Franco, D.; De Melo Lisboa, H. A review of odour impact criteria in selected countries around the world. Chemosphere 2017, 168, 1531–1570. [Google Scholar] [CrossRef] [PubMed]
- Milad, A.; Babalghaith, A.M.; Al-Sabaeei, A.M.; Dulaimi, A.; Ali, A.; Reddy, S.S.; Bilema, M.; Yusoff, N.I.M. A Comparative Review of Hot and Warm Mix Asphalt Technologies from Environmental and Economic Perspectives: Towards a Sustainable Asphalt Pavement. Int. J. Environ. Res. Public Health 2022, 19, 14863. [Google Scholar] [CrossRef]
- EN 13725:2003; UNI. Air Quality-Determination of Odour Concentration by Dynamic Olfactometry. Ente Italiano di Normazione, UNI: Minato City, Tokyo, 2003.
- Płotka-Wasylka, J.; Namieśnik, J. (Eds.) Green Analytical Chemistry: Past, Present and Perspectives. In Green Chemistry and Sustainable Technology; Springer: Singapore, 2019. [Google Scholar] [CrossRef]
- Pacenti, M.; Dugheri, S.; Villanelli, F.; Bartolucci, G.; Calamai, L.; Boccalon, P.; Arcangeli, G.; Vecchione, F.; Alessi, P.; Kikic, I.; et al. Determination of organic acids in urine by solid-phase microextraction and gas chromatography–ion trap tandem mass spectrometry previous “in sample” derivatization with trimethyloxonium tetrafluoroborate. Biomed. Chromatogr. 2008, 22, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Terry, S.C.; Jerman, J.H.; Angell, J.B. A gas chromatographic air analyzer fabricated on a silicon wafer. IEEE Trans. Electron Devices 1979, 26, 1880–1886. [Google Scholar] [CrossRef]
- Wang, J.; Nuñovero, N.; Lin, Z.; Nidetz, R.; Buggaveeti, S.; Zhan, C.; Kurabayashi, K.; Steinecker, W.; Zellers, E. A Wearable MEMS Gas Chromatograph for Multi-Vapor Determinations. Procedia Eng. 2016, 168, 1398–1401. [Google Scholar] [CrossRef]
- Mason, F.; Charles, G. Microfabricated Gas Phase Chemical Analysis Systems. 1999. Available online: https://www.osti.gov/biblio/14040 (accessed on 28 March 2025).
- Lahlou, H.; Vilanova, X.; Correig, X. Gas phase micro-preconcentrators for benzene monitoring: A review. Sens. Actuators B Chem. 2013, 176, 198–210. [Google Scholar] [CrossRef]
- Bryant-Genevier, J.; Zellers, E.T. Toward a microfabricated preconcentrator-focuser for a wearable micro-scale gas chromatograph. J. Chromatogr. A 2015, 1422, 299–309. [Google Scholar] [CrossRef]
- Yeom, J.; Field, C.R.; Bae, B.; I Masel, R.; A Shannon, M. The design, fabrication and characterization of a silicon microheater for an integrated MEMS gas preconcentrator. J. Micromech. Microeng. 2008, 18, 125001. [Google Scholar] [CrossRef]
- Azzouz, I.; Bachari, K. MEMS Devices for Miniaturized Gas Chromatography. In MEMS Sensors—Design and Application; Yellampalli, S., Ed.; InTech: Haryana, India, 2018. [Google Scholar] [CrossRef]
- Brancaleoni, E.; Scovaventi, M.; Frattoni, M.; Mabilia, R.; Ciccioli, P. Novel family of multi-layer cartridges filled with a new carbon adsorbent for the quantitative determination of volatile organic compounds in the atmosphere. J. Chromatogr. A 1999, 845, 317–328. [Google Scholar] [CrossRef]
- Pollack, A.J.; Gordon, S.M.; Moschandreas, D.J. Evaluation of Portable Multisorbent Air Samplers for Use with an Automated Multitube Analyzer; US Environmental Protection Agency: Washington, DC, USA, 1993. [Google Scholar]
- Matisová, E.; Škrabáková, S. Carbon sorbents and their utilization for the preconcentration of organic pollutants in environmental samples. J. Chromatogr. A 1995, 707, 145–179. [Google Scholar] [CrossRef]
- Hewitt, C.N. (Ed.) Reactive Hydrocarbons in the Atmosphere; Academic Press: San Diego, CA, USA, 1999. [Google Scholar]
- Larsen, B.; Bomboi-Mingarro, T.; Brancaleoni, E.; Calogirou, A.; Cecinato, A.; Coeur, C.; Chatzinestis, I.; Duane, M.; Frattoni, M.; Fugit, J.-L.; et al. Sampling and analysis of terpenes in air. An interlaboratory comparison. Atmos. Environ. 1997, 31, 35–49. [Google Scholar] [CrossRef]
- Sun, J.; Guan, F.; Zhu, X.; Ning, Z.; Ma, T.; Liu, J.; Deng, T. Micro-fabricated packed gas chromatography column based on laser etching technology. J. Chromatogr. A 2016, 1429, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, S.; Agah, M. Fabrication and Characterization of a Suspended TCD Integrated with a Gas Separation Column. J. Microelectromech. Syst. 2013, 22, 1166–1173. [Google Scholar] [CrossRef]
- Yang, J.-C.; Dutta, P.K. High temperature amperometric total NOx sensors with platinum-loaded zeolite Y electrodes. Sens. Actuators B Chem. 2006, 123, 929–936. [Google Scholar] [CrossRef]
- Lorenzelli, L.; Benvenuto, A.; Adami, A.; Guarnieri, V.; Margesin, B.; Mulloni, V.; Vincenzi, D. Development of a gas chromatography silicon-based microsystem in clinical diagnostics. Biosens. Bioelectron. 2005, 20, 1968–1976. [Google Scholar] [CrossRef]
- Li, M.W.-H.; Ghosh, A.; Venkatasubramanian, A.; Sharma, R.; Huang, X.; Fan, X. High-Sensitivity Micro-Gas Chromatograph–Photoionization Detector for Trace Vapor Detection. ACS Sens. 2021, 6, 2348–2355. [Google Scholar] [CrossRef]
- Rezende, G.C.; Le Calvé, S.; Brandner, J.J.; Newport, D. Micro photoionization detectors. Sens. Actuators B Chem. 2019, 287, 86–94. [Google Scholar] [CrossRef]
- Sun, J.; Xue, N.; Wang, W.; Wang, H.; Liu, C.; Ma, T.; Li, T.; Tan, T. Compact prototype GC-PID system integrated with micro PC and micro GC column. J. Micromech. Microeng. 2018, 29, 035008. [Google Scholar] [CrossRef]
- Sun, J.; Cui, D.; Guan, F.; Zhang, L.; Chen, X.; Li, H. Detection biomarkers of lung cancer using mini-GC-PID system integrated with micro GC column and micro pre-concentrator. Nanoscale Res. Lett. 2014, 9, 576. [Google Scholar] [CrossRef]
- Sun, J.H.; Guan, F.Y.; Zhu, X.F.; Ning, Z.W.; Ma, T.J. The Integrated Mini GC-PID System for Monitoring Air Pollution. In Current Air Quality Issues; Nejadkoorki, F., Ed.; InTech: Haryana, India, 2015. [Google Scholar] [CrossRef]
- Pang, X.; Li, W.; Wang, S.; Wu, Z.; Sun, S.; Lyu, Y.; Chen, D.; Li, H. Application of homemade portable gas chromatography coupled to photoionization detector for the detection of volatile organic compounds in an industrial park. J. Chromatogr. A 2023, 1704, 464089. [Google Scholar] [CrossRef]
- Chen, F.-Y.; Chang, W.-C.; Jian, R.-S.; Lu, C.-J. Novel Gas Chromatographic Detector Utilizing the Localized Surface Plasmon Resonance of a Gold Nanoparticle Monolayer inside a Glass Capillary. Anal. Chem. 2014, 86, 5257–5264. [Google Scholar] [CrossRef] [PubMed]
- Shopova, S.I.; White, I.M.; Sun, Y.; Zhu, H.; Fan, X.; Frye-Mason, G.; Thompson, A.; Ja, S.-J. On-Column Micro Gas Chromatography Detection with Capillary-Based Optical Ring Resonators. Anal. Chem. 2008, 80, 2232–2238. [Google Scholar] [CrossRef]
- Reddy, K.; Guo, Y.; Liu, J.; Lee, W.; Oo, M.K.K.; Fan, X. Rapid, sensitive, and multiplexed on-chip optical sensors for micro-gas chromatography. Lab A Chip 2012, 12, 901–905. [Google Scholar] [CrossRef]
- Moriaux, A.-L.; Vallon, R.; Parvitte, B.; Zeninari, V.; Liger-Belair, G.; Cilindre, C. Monitoring gas-phase CO2 in the headspace of champagne glasses through combined diode laser spectrometry and micro-gas chromatography analysis. Food Chem. 2018, 264, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Venkatasubramanian, A.; Sauer, V.T.K.; Roy, S.K.; Xia, M.; Wishart, D.S.; Hiebert, W.K. Nano-Optomechanical Systems for Gas Chromatography. Ano Lett. 2016, 16, 6975–6981. [Google Scholar] [CrossRef]
- Biswas, P.; Zhang, C.; Chen, Y.; Liu, Z.; Vaziri, S.; Zhou, W.; Sun, Y. A Portable Micro-Gas Chromatography with Integrated Photonic Crystal Slab Sensors on Chip. Biosensors 2021, 11, 326. [Google Scholar] [CrossRef] [PubMed]
- Zampolli, S.; Mengali, S.; Liberatore, N.; Elmi, I.; Masini, L.; Sanmartin, M.; Viola, R. A MEMS-Enabled Deployable Trace Chemical Sensor Based on Fast Gas-Chromatography and Quartz Enhanced Photoacousic Spectoscopy. Sensors 2019, 20, 120. [Google Scholar] [CrossRef]
- HSE. Manual Handling at Work—A Brief Guide. TSO London. 2020. Available online: https://www.hse.gov.uk/pubns/indg143.pdf (accessed on 16 March 2025).
- UNI ISO. MMC (Movimentazione Manuale Dei Carichi)—UNI ISO 11228-1:2022. 2022. Available online: https://www.certifico.com/sicurezza-lavoro/documenti-sicurezza/67-documenti-riservati-sicurezza/16255-mmc-iso-11228-1-2022 (accessed on 10 March 2025).
- Gałuszka, A.; Migaszewski, Z.M.; Namieśnik, J. Moving your laboratories to the field—Advantages and limitations of the use of field portable instruments in environmental sample analysis. Environ. Res. 2015, 140, 593–603. [Google Scholar] [CrossRef]
- Thermo Fisher Scientific. Thermo Scientific C2V-200 Micro GC. Available online: https://photos.labwrench.com/equipmentManuals/13023-5086.pdf (accessed on 10 March 2025).
- Regmi, B.P.; Agah, M. Micro Gas Chromatography: An Overview of Critical Components and Their Integration. Anal. Chem. 2018, 90, 13133–13150. [Google Scholar] [CrossRef]
- Paris, E.; Avino, P.; Guerriero, E.; Vincenti, B.; Palma, A.; Carnevale, M.; Benedetti, P.; Torre, M.; Gallucci, F. Activated Porous Carbon Fiber: New Adsorbent for Sampling and Analysis by Thermal Desorption of Siloxanes in Biogas and Biomethane. Int. J. Environ. Res. Public Health 2022, 19, 10890. [Google Scholar] [CrossRef]
- Voiculescu, I.; Zaghloul, M.; Narasimhan, N. Microfabricated chemical preconcentrators for gas-phase microanalytical detection systems. TrAC Trends Anal. Chem. 2008, 27, 327–343. [Google Scholar] [CrossRef]
- Abraham, J. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. In Handbook of Transnational Economic Governance Regimes; Tietje, C., Brouder, A., Eds.; Brill|Nijhoff: Leiden, The Netherlands, 2010; pp. 1041–1053. ISBN 978-90-04-18156-4. [Google Scholar]
- Leardi, R.; Melzi, C.; Polotti, G. CAT (Chemometric Agile Tool). Available online: http://gruppochemiometria.it/index.php/software (accessed on 10 March 2025).
- Płotka-Wasylka, J. A new tool for the evaluation of the analytical procedure: Green Analytical Procedure Index. Talanta 2018, 181, 204–209. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J. GAPI—Green Analytical Procedure Index. Available online: https://mostwiedzy.pl/en/justyna-plotka-wasylka,647762-1/gapi (accessed on 10 March 2025).
- Rothe, M.; Thomas, B. Aromastoffe des Brotes: Versuch einer Auswertung chemischer Geschmacksanalysen mit Hilfe des Schwellenwertes. Z Lebensm Unters Forch. 1963, 119, 302–310. [Google Scholar] [CrossRef]
- Nagata, Y. Measurement of odor threshold value Review of “Measurement of threshold value by tiriangle odor bag method Bull. Jap. Env. Sanit. Cent. No. 17. 1990” paper. J. Jpn. Assoc. Odor Environ. 2012, 43, 401–407. [Google Scholar] [CrossRef]
- Package “Mgcv”. 2023. Available online: https://cran.r-project.org/web/packages/mgcv/mgcv.pdf (accessed on 10 March 2025).
- Dugheri, S.; Cappelli, G.; Fanfani, N.; Squillaci, D.; Rapi, I.; Venturini, L.; Vita, C.; Gori, R.; Sirini, P.; Cipriano, D.; et al. Vacuum-Assisted MonoTrapTM Extraction for Volatile Organic Compounds (VOCs) Profiling from Hot Mix Asphalt. Molecules 2024, 29, 4943. [Google Scholar] [CrossRef] [PubMed]
- Zampolli, S.; Betti, P.; Elmi, I.; Dalcanale, E. A supramolecular approach to sub-ppb aromatic VOC detection in air. Chem. Commun. 2007, 2790–2792. [Google Scholar] [CrossRef]
- Rozzi, A.; Pedrini, A.; Pinalli, R.; Massera, C.; Elmi, I.; Zampolli, S.; Dalcanale, E. Tuning the conformational flexibility of quinoxaline cavitands for complexation at the gas–solid interface. Chem. Commun. 2022, 58, 7554–7557. [Google Scholar] [CrossRef]
- Zampolli, S.; Elmi, I.; Mancarella, F.; Betti, P.; Dalcanale, E.; Cardinali, G.; Severi, M. Real-time monitoring of sub-ppb concentrations of aromatic volatiles with a MEMS-enabled miniaturized gas-chromatograph. Sens. Actuators B Chem. 2009, 141, 322–328. [Google Scholar] [CrossRef]
- Bertani, F.; Riboni, N.; Bianchi, F.; Brancatelli, G.; Sterner, E.S.; Pinalli, R.; Geremia, S.; Swager, T.M.; Dalcanale, E. Triptycene-Roofed Quinoxaline Cavitands for the Supramolecular Detection of BTEX in Air. Chem.—A Eur. J. 2016, 22, 3312–3319. [Google Scholar] [CrossRef]
- Pacenti, M.; Dugheri, S.; Traldi, P.; Degli Esposti, F.; Perchiazzi, N.; Franchi, E.; Calamante, M.; Kikic, I.; Alessi, P.; Bonacchi, A.; et al. New Automated and High-Throughput Quantitative Analysis of Urinary Ketones by Multifiber Exchange-Solid Phase Microextraction Coupled to Fast Gas Chromatography/Negative Chemical-Electron Ionization/Mass Spectrometry. J. Anal. Methods Chem. 2010, 2010, 1–13. [Google Scholar] [CrossRef]
- Pacenti, M.; Dugheri, S.; Gagliano-Candela, R.; Strisciullo, G.; Franchi, E.; Degli Esposti, F.; Perchiazzi, N.; Boccalon, P.; Arcangeli, G.; Cupelli, V. Analysis of 2-Chloroacetophenone in air by multi-fiber solid-phase microextraction and fast gas chromatography-mass spectrometry. Acta Chromatogr. 2009, 21, 379–397. [Google Scholar] [CrossRef]
- Cimorelli, A.J.; Perry, S.G.; Venkatram, A.; Weil, J.C.; Paine, R.J.; Wilson, R.B.; Lee, R.F.; Peters, W.D.; Brode, R.W. AERMOD: A Dispersion Model for Industrial Source Applications. Part I: General Model Formulation and Boundary Layer Characterization. J. Appl. Meteorol. Clim. 2005, 44, 682–693. [Google Scholar] [CrossRef]
- Scire, J.S.; Stimaitis, D.G.; Yamartino, R.J. A User’s Guide for the CALPUFF Dispersion Model (Version 5). Earth Tech, Inc. 2000. Available online: https://www.calpuff.org/calpuff/download/CALPUFF_UsersGuide.pdf (accessed on 10 March 2025).
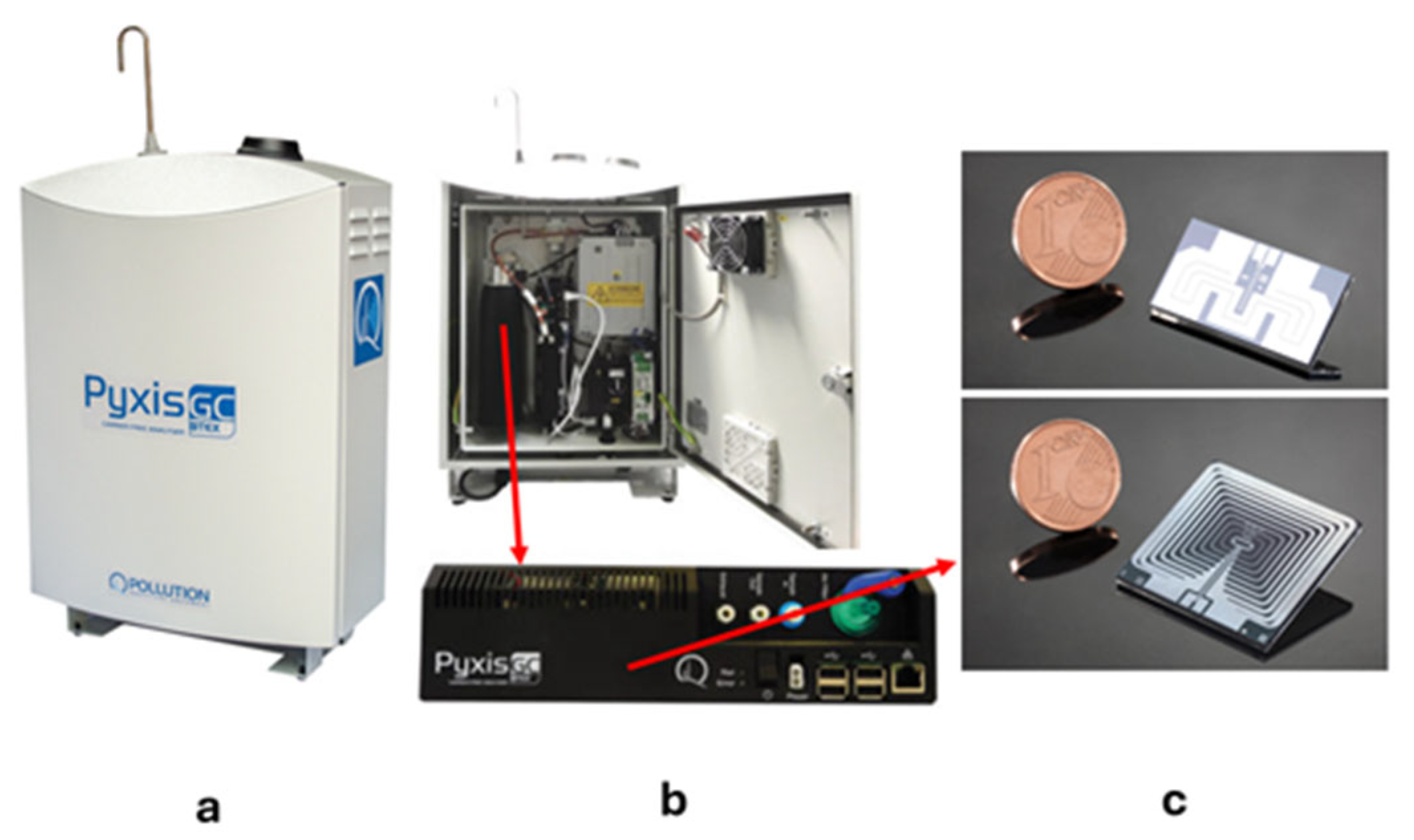
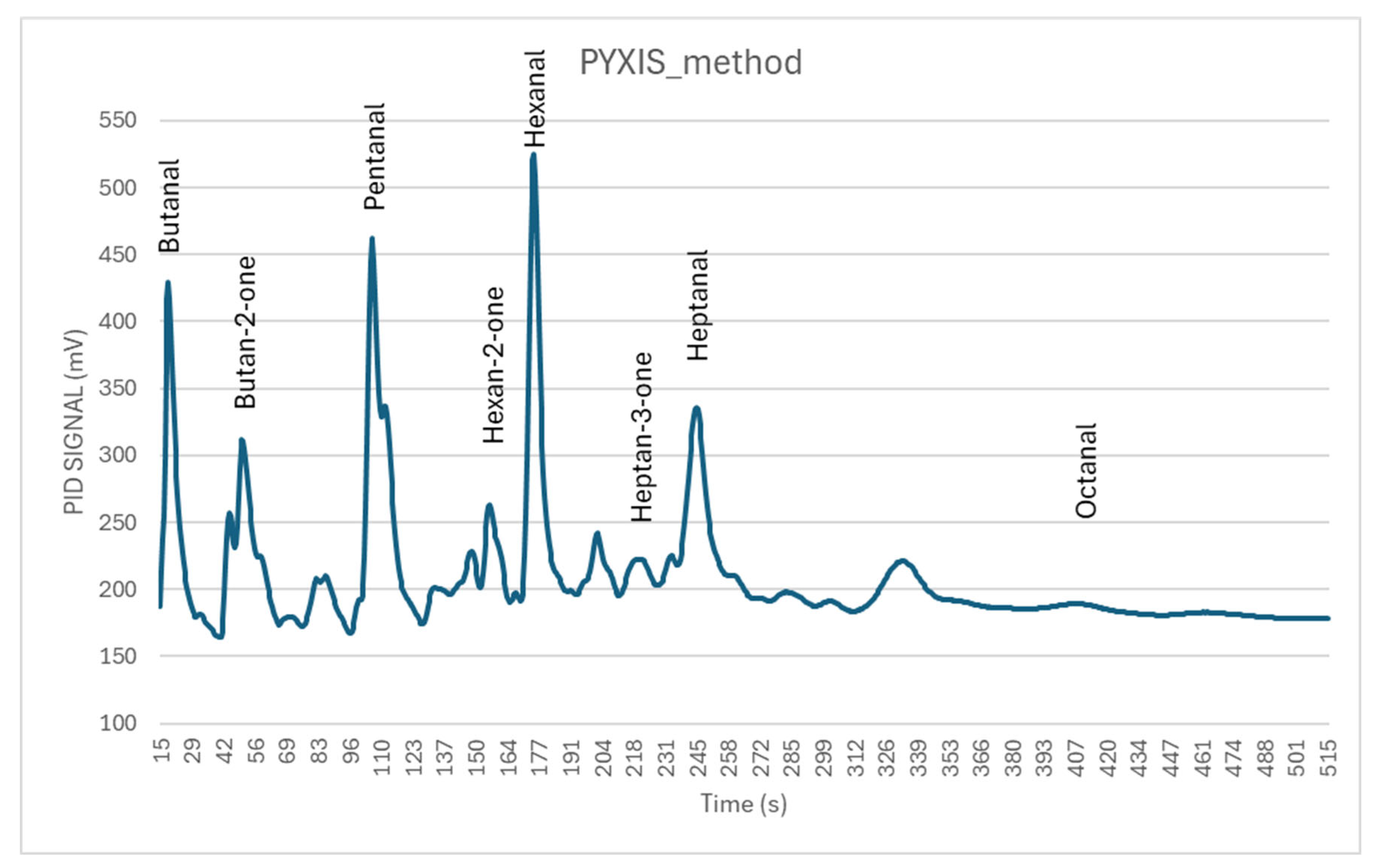
| Instrument | Pre-Concentrator | GC Column | Rugged | LOQ | Dimensions (cm) | Weight (kg) | Producer | |
|---|---|---|---|---|---|---|---|---|
| GC/MS | Torion T-9 | None | Capillary | Rugged | ppb-ppt | 38.1 × 39.4 × 22.9 | 14.5 | PerkinElmer Inc. (Hopkinton, MA, USA) Link: PerkinElmer Inc. https://content.perkinelmer.com/library/prd-torion-t-9-portable-gcms.html (28 March 2025) |
| HAPSITE ER | None | Capillary (15 M, Rtx-1 MS, 0.25 mm × 0.1 µm) | Rugged | ppm to-ppt | 46 × 43 × 18 | 18 | INFICON (Bad Ragaz, Switzerland) Link: https://www.inficon.com/en (28 March 2025) | |
| Griffin G510 | On-board | Capillary | Rugged, classified asIP65 | ppb or less | 33.7 × 33.7 × 40 | 16.3 | Teledyne FLIR LLC (Wilsonville, OR, USA) Link: https://www.flir.it/ (28 March 2025) | |
| Enviroprobe | None | Capillary (es. DB-1, 5 m, 0.5 mm, 0.25 µm) | Rugged | ppb | 15 × 12.7 × 1.7 | 1.3 | FemtoScan Corporation (Bountiful, UT, USA) Link: http://www.femtoscan.com/index.htm (28 March 2025) | |
| GC/FID | NCMS 6300 | On-board (low-temperature preconcentration) | Capillary | Rugged | 0.5 ppb | Data not available | Data not available | Nutech Instruments (Richardson, TX, USA) Link: https://www.nutechinst.com/ (28 March 2025) |
| PetroAlert® 9100 | None | Packed, micro-packed, or capillary columns; Specific to application | Rugged | ppm | 13 × 48.26 × 41 | 13.6 | AMETEK MOCON (Brooklyn Park, MN, USA) Link: https://www.ametekmocon.com/ (28 March 2025) | |
| GC/TCD | Micro GC 990 | None | Capillary (WCOT, PLOT, micropacked) | Rugged | WCOT 05 ppm; PLOT 2 ppm Micropacked 10 ppm | 28.28 × 14.5 × 32.9 | 7.3 (15.6 cabined) | Agilent (Santa Clara, CA, USA) Link: https://www.agilent.com/ (28 March 2025) |
| Micro GC Fusion | None | Capillary | Rugged | 1 ppm | 46.2 × 19.6 × 25.4 | 6.2 | INFICON (Bad Ragaz, Switzerland) Link: https://www.inficon.com/en/products/chemical-detection-and-utility-monitoring/micro-gc-fusion-gas-analyzer (28 March 2025) | |
| DynamiQ | None | MEMS | Rugged, classified as IP65 | 0.5 ppm | 28.9 × 12.2 × 25.8 | 15 | QMicro-Sensirion (Enschede, NL, USA) Link: https://www.qmicro.com/about/ (28 March 2025) | |
| I-Graph X | None | MEMS | Rugged | 1 ppm | 31 × 12 × 29 | 4.7 | I-GRAPHX GmbH (Reinbek, DE, USA) Link: https://www.i-graphx.com/ (28 March 2025) | |
| DPS Micro-TCD GC | None | Capillary | Rugged | ppm | 20 × 15 × 10 | 8 | DPS Instruments Europe GmbH (Bad Honnef, DE, USA) Link: https://www.dps-instruments.com/ (28 March 2025) | |
| GC/PID | FROG-4000 VOCAM | On-board | MEMS | Rugged | sub-ppb | 25.4 × 19.1 × 37 | 2.2 | Defiant Technologies Inc (Albuquerque, NM, USA) Link: https://www.defiant-tech.com/ (28 March 2025) |
| eGC | Selective-trap | Capillary (e.g., 0.53 mm × 10 m) | Rugged | 0.5 ppb | 44.5 × 49.5 × 21.3 | 19.9 | ENMET (Ann Arbor, MI, USA) Link: https://enmet.com/ (28 March 2025) | |
| GC PID | Selective-trap | Capillary | Rugged | <1 ppb | 36 × 30 × 15 | 7 | PAS Technology Deutschland GmbH (Magdala, DE, USA) Link: http://www.pas-technologies.com/(11 Feburary 2025) | |
| PyxisGC | Selective-trap | MEMS | Rugged | 0.01 ppb | 42 × 62 × 21 | 19 | Pollution (Budrio, IT, USA) Link: https://www.pollution.it/it/ (28 March 2025) | |
| Baseline® 9200 | Selective-trap | Capillary | Rugged | sub-ppb | 13 × 48.26 × 56.9 | 13.6 | AMETEK MOCON (Brooklyn Park, MN, USA) Link: https://www.ametekmocon.it/products/gaschromatographs/9200dualdetectorgc (28 March 2025) | |
| PID-Analyzer | None | Capillary | Data not available | ppb | Data not available | Data not available | IUT Technologies GmbH (Berlin, DE, USA) Link: https://iut-technologies.de/ (28 March 2025) | |
| NovaTest P300 | Selective-trap | Capillary | Rugged | sub-ppb | 47 × 35.7 × 17.6 | 10 | Nanova Environmental, Inc. (Columbia, MO, USA) Link: https://nanovaenv.com/ (28 March 2025) | |
| 312 Portable GC | Selective-trap | Capillary | Rugged | ppb | 47.5 × 35 × 19 | 12.2 | PID ANALYZERS, LLC (Sandwich, MA, USA) Link: https://hnu.com/ (28 March 2025) | |
| GC 866 microVOC-Trap | Selective-trap | Capillary | Rugged | ppt | 48.2 × 60.0 × 18.0 | 17 | Chromatotec® (Saint-André de Cubzac, France) Link: https://chromatotec.com/ (28 March 2025) |
| Compound | Level 1 | Level 2 | Level 3 | Level 4 | Level 5 |
|---|---|---|---|---|---|
| Butanal | 0 | 200 | 500 | 700 | 1000 |
| Pentanal | 0 | 200 | 500 | 700 | 1000 |
| Hexanal | 0 | 200 | 500 | 700 | 1000 |
| Heptanal | 0 | 200 | 500 | 700 | 1000 |
| Octanal | 0 | 200 | 500 | 700 | 1000 |
| Butan-2-one | 0 | 100 | 300 | 400 | 600 |
| Hexan-2-one | 0 | 100 | 300 | 400 | 700 |
| Heptan-3-one | 0 | 100 | 300 | 400 | 700 |
| Experimental Matrix | Experimental Plan | |||||||
|---|---|---|---|---|---|---|---|---|
| Exp# | x1 | x2 | x3 | x4 | Sampling Time (x1) | Flow Rate Sampling (x2) | Desorption Time (x3) | Desorption Temperature (x4) |
| 1 | 0 | −1 | −1 | −1 | 50 s | 250 mL/min | 10 s | 120 °C |
| 2 | 1 | 1 | 0 | −1 | 100 s | 450 mL/min | 40 s | 120 °C |
| 3 | −1 | 0 | 1 | −1 | 25 s | 350 mL/min | 80 s | 120 °C |
| 4 | 1 | 0 | −1 | 0 | 100 s | 350 mL/min | 10 s | 140 °C |
| 5 | −1 | −1 | 0 | 0 | 25 s | 250 mL/min | 40 s | 140 °C |
| 6 | 0 | 1 | 1 | 0 | 50 s | 450 mL/min | 80 s | 140 °C |
| 7 | −1 | 1 | −1 | 1 | 25 s | 450 mL/min | 10 s | 180 °C |
| 8 | 0 | 0 | 0 | 1 | 50 s | 350 mL/min | 40 s | 180 °C |
| 9 | 1 | −1 | 1 | 1 | 100 s | 250 mL/min | 80 s | 180 °C |
| Chemical Name | CAS | IE, eV | Lamp Type (RF) | LOQ |
|---|---|---|---|---|
| 10.6 eV | µg/m3 | |||
| Butanal | 123-72-8 | 9.86 | 1.7 | 5.0 |
| Pentanal | 110-62-3 | 9.74 | 1.5 | 5.3 |
| Hexanal | 66-25-1 | 9.72 | 1.2 | 4.9 |
| Heptanal | 111-71-7 | N.D. | N.D. | 5.4 |
| Octanal | 124-13-0 | N.D. | 1.10 | 5.8 |
| Butan-2-one | 78-93-3 | 9.51 | 0.96 | 2.8 |
| Hexan-2-one | 591-78-6 | 9.34 | 0.8 | 3.3 |
| Heptan-3-one | 106-35-4 | 9.02 | 0.73 | 3.4 |
| Butanal | Butan-2-One | Pentanal | Hexanal | |
| μg/m3 | ||||
| Average | 227.62 | 117.39 | 129.97 | 153.01 |
| Dev. Std | 113.91 | 58.58 | 69.28 | 85.23 |
| Median | 224.28 | 115.91 | 123.99 | 140.73 |
| Hexan-2-one | Heptanal | Heptan-3-one | Octanal | |
| μg/m3 | ||||
| Average | 62.98 | 105.7 | 52.79 | 46.27 |
| Dev. Std | 41.94 | 74.01 | 57.15 | 34.88 |
| Median | 45.08 | 73.04 | 19.46 | 42.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dugheri, S.; Cappelli, G.; Gori, R.; Zampolli, S.; Fanfani, N.; Guerriero, E.; Squillaci, D.; Rapi, I.; Venturini, L.; Pittella, A.; et al. MEMS-Based Micropacked Thermal Desorption GC/PID for In-Field Volatile Organic Compound Profiling from Hot Mix Asphalt. Separations 2025, 12, 133. https://doi.org/10.3390/separations12050133
Dugheri S, Cappelli G, Gori R, Zampolli S, Fanfani N, Guerriero E, Squillaci D, Rapi I, Venturini L, Pittella A, et al. MEMS-Based Micropacked Thermal Desorption GC/PID for In-Field Volatile Organic Compound Profiling from Hot Mix Asphalt. Separations. 2025; 12(5):133. https://doi.org/10.3390/separations12050133
Chicago/Turabian StyleDugheri, Stefano, Giovanni Cappelli, Riccardo Gori, Stefano Zampolli, Niccolò Fanfani, Ettore Guerriero, Donato Squillaci, Ilaria Rapi, Lorenzo Venturini, Alexander Pittella, and et al. 2025. "MEMS-Based Micropacked Thermal Desorption GC/PID for In-Field Volatile Organic Compound Profiling from Hot Mix Asphalt" Separations 12, no. 5: 133. https://doi.org/10.3390/separations12050133
APA StyleDugheri, S., Cappelli, G., Gori, R., Zampolli, S., Fanfani, N., Guerriero, E., Squillaci, D., Rapi, I., Venturini, L., Pittella, A., Vita, C., Cioni, F., Cipriano, D., Sajewicz, M., Elmi, I., Masini, L., Sio, S. D., Baldassarre, A., Traversini, V., & Mucci, N. (2025). MEMS-Based Micropacked Thermal Desorption GC/PID for In-Field Volatile Organic Compound Profiling from Hot Mix Asphalt. Separations, 12(5), 133. https://doi.org/10.3390/separations12050133















