Sunflower Seed Oil Enriched with Compounds from the Turmeric Rhizome: Extraction, Characterization and Cell Viability
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Raw Materials
2.3. Extraction Procedure
2.4. Analytical Methods
2.4.1. Curcuminoids Content
2.4.2. Total Phenolic Compound Content, Antioxidant Potential and Phenolic Compound Profile
2.4.3. Fatty-Acid Profile
2.4.4. Induction Time
2.4.5. Cell Viability
2.5. Statistical Analysis
3. Results
3.1. Curcuminoids Content in Enriched Oils
3.2. Characterization of the Oils Obtained
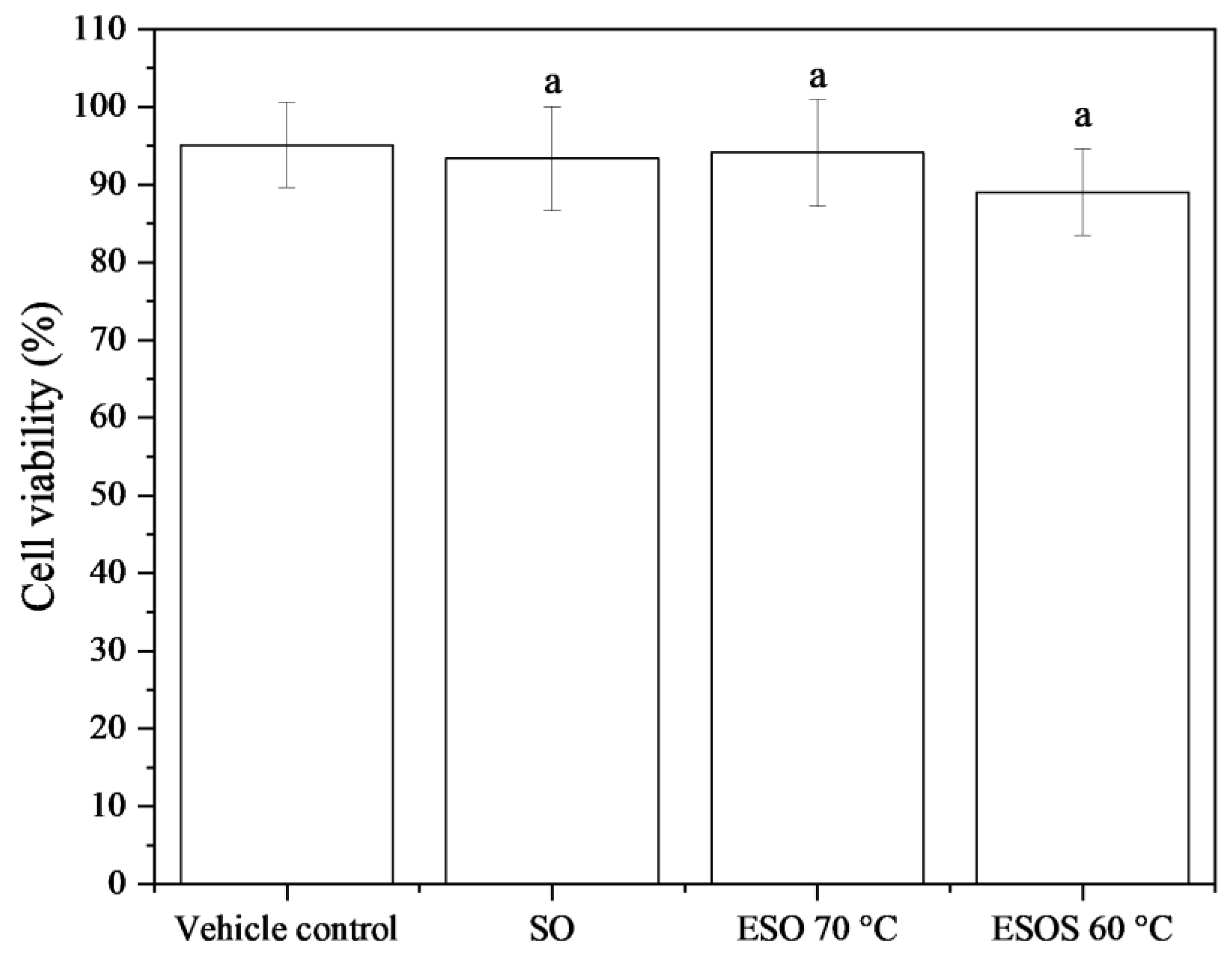
3.3. Cell Viability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SO | sunflower seed oil |
| TR | turmeric rhizome |
| ESO | enriched oil obtained using sunflower seed oil as solvent |
| ESOS | enriched oil obtained from the simultaneous extraction of sunflower seed oil and compounds from turmeric rhizome |
| TPC | total phenolic compounds |
| AP | antioxidant potential |
| HaCaT | human immortalized keratinocyte |
References
- Moore, E.M.; Wagner, C.; Komarnytsky, S. The Enigma of Bioactivity and Toxicity of Botanical Oils for Skin Care. Front. Pharmacol. 2020, 11, 785. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.S.; Conto, J.C.K.; Silva, C.M.; Scheneider, R.C.S.; Müller, C.R. Análisis de nanoformulaciones de aceite de aguacate para aplicacióncutánea. Ars. Pharm. 2021, 62, 66–74. [Google Scholar] [CrossRef]
- Wang, D.; Fan, W.; Guan, Y.; Huang, H.; Yi, T.; Ji, J. Oxidative stability of sunflower oil flavored by essential oil from Coriandrumsativum L. during accelerated storage. Food Sci. Technol. 2018, 98, 268–275. [Google Scholar] [CrossRef]
- Poljšak, N.; Kreft, S.; KočevarGlavač, N. Vegetable butters and oils in skin wound healing: Scientific evidence for new opportunities in dermatology. Phytother. Res. 2020, 34, 254–269. [Google Scholar] [CrossRef]
- Lira, A.L.; Vesoloski, J.F.; Peruzzolo, M.; Flôres, D.Z.; Cansian, R.L.; Paroul, N. Atividades antioxidante, antimicrobiana e compostos fenólicos de extratos comercial e in natura de Curcuma longa. Rev. Perspec. 2021, 45, 107–114. [Google Scholar] [CrossRef]
- Petraru, A.; Ursachi, F.; Amariei, S. Nutritional Characteristics Assessment of Sunflower Seeds, Oil and Cake. Perspective of Using Sunflower Oilcakes as a Functional Ingredient. Plants 2021, 10, 2487. [Google Scholar] [CrossRef]
- Keihanian, F.; Saeidinia, A.; Bagheri, R.K.; Johnston, T.P.; Sahebkar, A. Curcumin, hemostasis, thrombosis, and coagulation. J. Cell. Physiol. 2018, 233, 4497–4511. [Google Scholar] [CrossRef] [PubMed]
- Eli, S.; Orluwene, C.G.; Oku, I.Y.; Bob-Manuel, M.; Enyinnaya, S.O.; Iyama, A.C.; Nnoka, V.N.; Emeghara, G.I. Proposed treatment of COVID-19 infection in poor resource setting—The use of medicinal extract from sunflower seed (Helianthus annuus). Int. J. Sci. Res. Publ. 2022, 5, 9–15. [Google Scholar] [CrossRef]
- Hung, C.-T.; Huang, S.-M.; Cheng, H.-C.; Liu, S.-T.; Chang, Y.-L.; Liu, Y.-C.; Wang, W.-M. The inhibitory mechanism by curcumin on the Zac1-enhanced cyclin D1 expression in human keratinocytes. J. Dermatol. Sci. 2015, 79, 262–267. [Google Scholar] [CrossRef]
- Torres, S.B.; Queiroz, A.L.F.G.; Santos, A.N.A.; Alves, G.Q.; Silva, I.A.; Brito, J.K.C.; Sultanun, R.F.S.; Monteiro, A.C.S. Sunflower oil (Helianthus annus L.) As a wound healer in diabetic elderly people. Braz. J. Rev. 2021, 4, 4692–4703. [Google Scholar] [CrossRef]
- Lateh, L.; Yuenyongsawad, S.; Chen, H.; Panichayupakaranant, P. A green method for preparation of curcuminoid-rich Curcuma longa extract and evaluation of its anticancer activity. Pharmacogn. Mag. 2019, 15, 730. [Google Scholar] [CrossRef]
- Silva, Á.D.F.; Ferreira, G.L.; Dias, A.J.A.; Barros, N.B.; Silva, Z.P.N. Uso e eficácia de plantasmedicinais com açõesemdoençascardiovasculares e em Diabetes Tipo 2: Panax ginseng, Curcuma longa, Adonis vernalis. Braz. J. Dev. 2021, 7, 86526–86549. [Google Scholar] [CrossRef]
- Kanjana, S.; Piya, K.; Kanitta, J.; Wannee, J. Antithrombotic activity of turmeric (Curcuma longa): A review. Indian J. Agric. Res. 2016, 50, 101–106. [Google Scholar] [CrossRef]
- Lee, G.; Chung, H.-S.; Lee, K.; Lee, H.; Kim, M.; Bae, H. Curcumin attenuates the scurfy-induced immune disorder, a model of IPEX syndrome, with inhibiting Th1/Th2/Th17 responses in mice. Phytomedicine 2017, 33, 1–6. [Google Scholar] [CrossRef]
- Razavi, B.M.; GhasemzadehRahbardar, M.; Hosseinzadeh, H. A review of therapeutic potentials of turmeric (Curcuma longa) and its active constituent, curcumin, on inflammatory disorders, pain, and their related patents. Phytother. Res. 2021, 35, 6489–6513. [Google Scholar] [CrossRef] [PubMed]
- Draelos, Z.D. The Efficacy and Tolerability of Turmeric and Salicylic Acid in Psoriasis Treatment. Psoriasis 2022, 12, 63–71. [Google Scholar] [CrossRef]
- Atef, B.; Ishak, R.A.H.; Badawy, S.S.; Osman, R. Exploring the potential of oleic acid in nanotechnology-mediated dermal drug delivery: An up-to-date review. J. Drug Deliv. Sci. Technol. 2022, 67, 103032. [Google Scholar] [CrossRef]
- Pelikh, O.; Pinnapireddy, S.R.; Keck, C.M. Dermal Penetration Analysis of Curcumin in an ex vivo Porcine Ear Model Using Epifluorescence Microscopy and Digital Image Processing. Skin Pharmacol. Physiol. 2021, 34, 281–299. [Google Scholar] [CrossRef] [PubMed]
- Scomoroscenco, C.; Teodorescu, M.; Raducan, A.; Stan, M.; Voicu, S.N.; Trica, B.; Ninciuleanu, C.M.; Nistor, C.L.; Mihaescu, C.I.; Petcu, C.; et al. Novel Gel Microemulsion as Topical Drug Delivery System for Curcumin in Dermatocosmetics. Pharmaceutics 2021, 13, 505. [Google Scholar] [CrossRef]
- Almoshari, Y. Novel Hydrogels for Topical Applications: An Updated Comprehensive Review Based on Source. Gels 2022, 8, 174. [Google Scholar] [CrossRef]
- Malik, D.S.; Mital, N.; Kaur, G. Topical drug delivery systems: A patent review. Expert Opin. Ther. Pat. 2016, 26, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.-W.; Jee, S.-H. Strategies to Develop a Suitable Formulation for Inflammatory Skin Disease Treatment. Int. J. Mol. Sci. 2021, 22, 6078. [Google Scholar] [CrossRef] [PubMed]
- Castejón, N.; Luna, P.; Señoráns, F.J. Alternative oil extraction methods from Echiumplantagineum L. seeds using advanced techniques and green solvents. Food Chem. 2018, 244, 75–82. [Google Scholar] [CrossRef]
- Yusuff, A.S. Parametric optimization of solvent extraction of Jatropha curcas seed oil using design of experiment and its quality characterization. S. Afr. J. Chem. Eng. 2021, 35, 60–68. [Google Scholar] [CrossRef]
- Silva-Buzanello, R.A.; Ferro, A.C.; Bona, E.; Cardozo-Filho, L.; Araújo, P.H.H.; Leimann, F.V.; Gonçalves, O.H. Validation of an Ultraviolet–visible (UV–Vis) technique for the quantitative determination of curcumin in poly (l-lactic acid) nanoparticles. Food Chem. 2015, 172, 99–104. [Google Scholar] [CrossRef]
- Algahtani, M.S.; Ahmad, M.Z.; Ahmad, J. Nanoemulsion loaded polymeric hydrogel for topical delivery of curcumin in psoriasis. J. Drug Deliv. Sci. Technol. 2020, 59, 101847. [Google Scholar] [CrossRef]
- Haiyan, Z.; Bedgood, D.R.; Bishop, A.G.; Prenzler, P.D.; Robards, K. Endogenous biophenol, fatty acid and volatile profiles of selected oils. Food Chem. 2007, 100, 1544–1551. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Gu, C.; Howell, K.; Dunshea, F.R.; Suleria, H.A.R. LC-ESI-QTOF/MS Characterisation of Phenolic Acids and Flavonoids in Polyphenol-Rich Fruits and Vegetables and Their Potential Antioxidant Activities. Antioxidants 2019, 8, 405. [Google Scholar] [CrossRef]
- Pellegrini, N.; Colombi, B.; Del Rio, D.; Salvatore, S.; Bianchi, M.; Brighenti, F.; Serafini, M. Total Antioxidant Capacity of Plant Foods, Beverages and Oils Consumed in Italy Assessed by Three Different In Vitro Assays. J. Nutr. 2003, 133, 2812–2819. [Google Scholar] [CrossRef]
- Stevanato, N.; Silva, C. Radish seed oil: Ultrasound-assisted extraction using ethanol as solvent and assessment of its potential for ester production. Ind. Crops Prod. 2019, 132, 283–291. [Google Scholar] [CrossRef]
- Pattnaik, M.; Mishra, H.N. Oxidative stability of ternary blends of vegetable oils: A chemometric approach. LWT 2021, 142, 111018. [Google Scholar] [CrossRef]
- Malich, G.; Markovic, B.; Winder, C. The sensitivity and specificity of the MTS tetrazolium assay for detecting the in vitro cytotoxicity of 20 chemicals using human cell lines. Toxicology 1997, 124, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Park, C.Y.; Lee, K.Y.; Gul, K.; Rahman, M.S.; Kim, A.N.; Chun, J.; Choi, S.G. Phenolics and antioxidant activity of aqueous turmeric extracts as affected by heating temperature and time. LWT 2019, 105, 149–155. [Google Scholar] [CrossRef]
- Adnanet, N.A.; Kassim, A.S.M.; Razak, A.H.A. Evaluation of Antioxidant Property of Curcumin-loaded Nanoemulsion. PEAT 2022, 3, 70–79. Available online: https://publisher.uthm.edu.my/periodicals/index.php/peat/article/view/6397 (accessed on 10 March 2025).
- Burapan, S.; Kim, M.; Paisooksantivatana, Y.; Eser, B.E.; Han, J. Thai Curcuma Species: Antioxidant and Bioactive Compounds. Foods 2020, 9, 1219. [Google Scholar] [CrossRef]
- Paul, B.; Munshi, M.; Ahmed, M.; Saha, G.; Roy, S. The Fatty Acid Composition and Properties of Oil Extracted from Fresh Rhizomes of Turmeric (Curcuma longa Linn.) Cultivars of Bangladesh. Bangladesh J. Sci. Ind. Res. 2011, 46, 127–132. [Google Scholar] [CrossRef]
- Yang, Y.; Song, X.; Sui, X.; Qi, B.; Wang, Z.; Li, Y.; Jiang, L. Rosemary extract can be used as a synthetic antioxidant to improve vegetable oil oxidative stability. Ind. Crop Prod. 2016, 80, 141–147. [Google Scholar] [CrossRef]
- Franco, D.; Rodriguez-Amado, I.; Agregán, R.; Munekata, P.E.; Vázquez, J.A.; Barba, F.J.; Lorenzo, J.M. Optimization of antioxidants extraction from peanut skin to prevent oxidative processes during soybean oil storage. LWT 2018, 88, 1–8. [Google Scholar] [CrossRef]
- Pedro, A.C.; Maurer, J.B.B.; Zawadzki-Baggio, S.F.; Ávila, S.; Maciel, G.M.; Haminiuk, C.W.I. Bioactive compounds of organic goji berry (Lyciumbarbarum L.) prevents oxidative deterioration of soybean oil. Ind. Crop Prod. 2018, 112, 90–97. [Google Scholar] [CrossRef]
- Almoselhy, R.I.M. Comparative Study of Vegetable Oils Oxidative Stability using DSC and Rancimat Methods. Egypt. J. Chem. 2021, 64, 1. [Google Scholar] [CrossRef]
- Symoniuk, E.; Ksibi, N.; Wroniak, M.; Lefek, M.; Ratusz, K. Oxidative Stability Analysis of Selected Oils from Unconventional Raw Materials Using Rancimat Apparatus. Appl. Sci. 2022, 12, 10355. [Google Scholar] [CrossRef]
- Chiou, A.; Kalogeropoulos, N. Virgin Olive Oil as Frying Oil. Compr. Rev. Food Sci. Food Saf. 2017, 16, 632–646. [Google Scholar] [CrossRef]
- Trajkovska, M.; Derwiche, F.; Grigorakis, S.; Makris, D.P. Natural Phenolic Acids as Effective Bulk Oil Antioxidants: Oxidative Stability Modeling Using Olive Kernel Oil as a Case Study. Appl. Sci. 2024, 14, 6508. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Mechanisms of antioxidants in the oxidation of foods. Compr. Rev. Food Sci. Food Saf. 2009, 8, 345–358. [Google Scholar] [CrossRef]
- Eshghi, N.; Asnaashari, M.; Khodaparast, M.H.H.; Hosseini, F. Evaluating the potential of natural curcumin for oxidative stability of soybean oil. Nat. Prod. Res. 2014, 28, 1375–1378. [Google Scholar] [CrossRef]
- Liczbiński, P.; Michałowicz, J.; Bukowska, B. Molecular mechanism of curcumin action in signaling pathways: Review of the latest research. Phytother. Res. 2020, 34, 1992–2005. [Google Scholar] [CrossRef]
- Hussein, Y.M.; El-Masry, R.A.; Al-Gaby, A.M.; Osman, A. Antioxidant activity of curcumin as a natural antioxidant on the oxidative stability of soybean oil under thermoxidation. Zagazig J. Agric. Res. 2023, 50, 529–537. [Google Scholar] [CrossRef]
- Lee, K.-Y.; Gul, K.; Kim, A.-N.; Rahman, M.S.; Lee, M.H.; Kim, J.I.; Kwak, D.; Shin, E.-C.; Kim, H.-J.; Kerr, W.L.; et al. Impact of supercritical carbon dioxide turmeric extract on the oxidative stability of perilla oil. Int. J. Food Sci. Technol. 2020, 55, 183–191. [Google Scholar] [CrossRef]
- Jaski, J.M.; Cruz, R.M.S.; Pimentel, T.C.; Stevanato, N.; Silva, C.; Barão, C.E.; Cardozo-Filho, L. Simultaneous Extraction of Bioactive Compounds from Olea europaea L. Leaves and Healthy Seed Oils Using Pressurized Propane. Foods 2023, 12, 948. [Google Scholar] [CrossRef] [PubMed]
- Rosa, A.C.S.; Costa, A.J.N.; Santos Júnior, O.O.; Silva, C. Ultrasound-Assisted Extraction of Sunflower Seed Oil Enriched with Active Compounds from Jambolan Leaf. J. Braz. Chem. Soc. 2025, 36, e-20240116. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- Lundvig, D.M.S.; Pennings, S.W.C.; Brouwer, K.M.; Mtaya-Mlangwa, M.M.; Mugonzibwa, E.; Kuijpers-Jagtman, A.M.; Wagener, F.A.D.T.G.; Von den Hoff, J.W. Cytoprotective responses in HaCaT keratinocytes exposed to high doses of curcumin. Exp. Cell Res. 2015, 336, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.N.P.; Leite, M.N.; Paula, N.A.P.; Martins, A.; Figueiredo, S.A.; Frade, M.A.C.; Lopez, R.F.V. Nanoemulsions Based on Sunflower and Rosehip Oils: The Impact of Natural and Synthetic Stabilizers on Skin Penetration and an Ex Vivo Wound Healing Model. Pharmaceutics 2023, 15, 999. [Google Scholar] [CrossRef]
- Puxeddu, S.; Scano, A.; Scorciapino, M.A.; Delogu, I.; Vascellari, S.; Ennas, G.; Manzin, A.; Angius, F. Physico-Chemical Investigation and Antimicrobial Efficacy of Ozonated Oils: The Case Study of Commercial Ozonated Olive and Sunflower Seed Refined Oils. Molecules 2024, 29, 679. [Google Scholar] [CrossRef]
 ), ESO 70 °C (
), ESO 70 °C ( ) and ESOS 60 °C (
) and ESOS 60 °C ( ) samples, which was 2.19 h ± 0.05, 5.81 h ± 0.13 and 8.25 h ± 0.11, respectively.
) samples, which was 2.19 h ± 0.05, 5.81 h ± 0.13 and 8.25 h ± 0.11, respectively.
 ), ESO 70 °C (
), ESO 70 °C ( ) and ESOS 60 °C (
) and ESOS 60 °C ( ) samples, which was 2.19 h ± 0.05, 5.81 h ± 0.13 and 8.25 h ± 0.11, respectively.
) samples, which was 2.19 h ± 0.05, 5.81 h ± 0.13 and 8.25 h ± 0.11, respectively.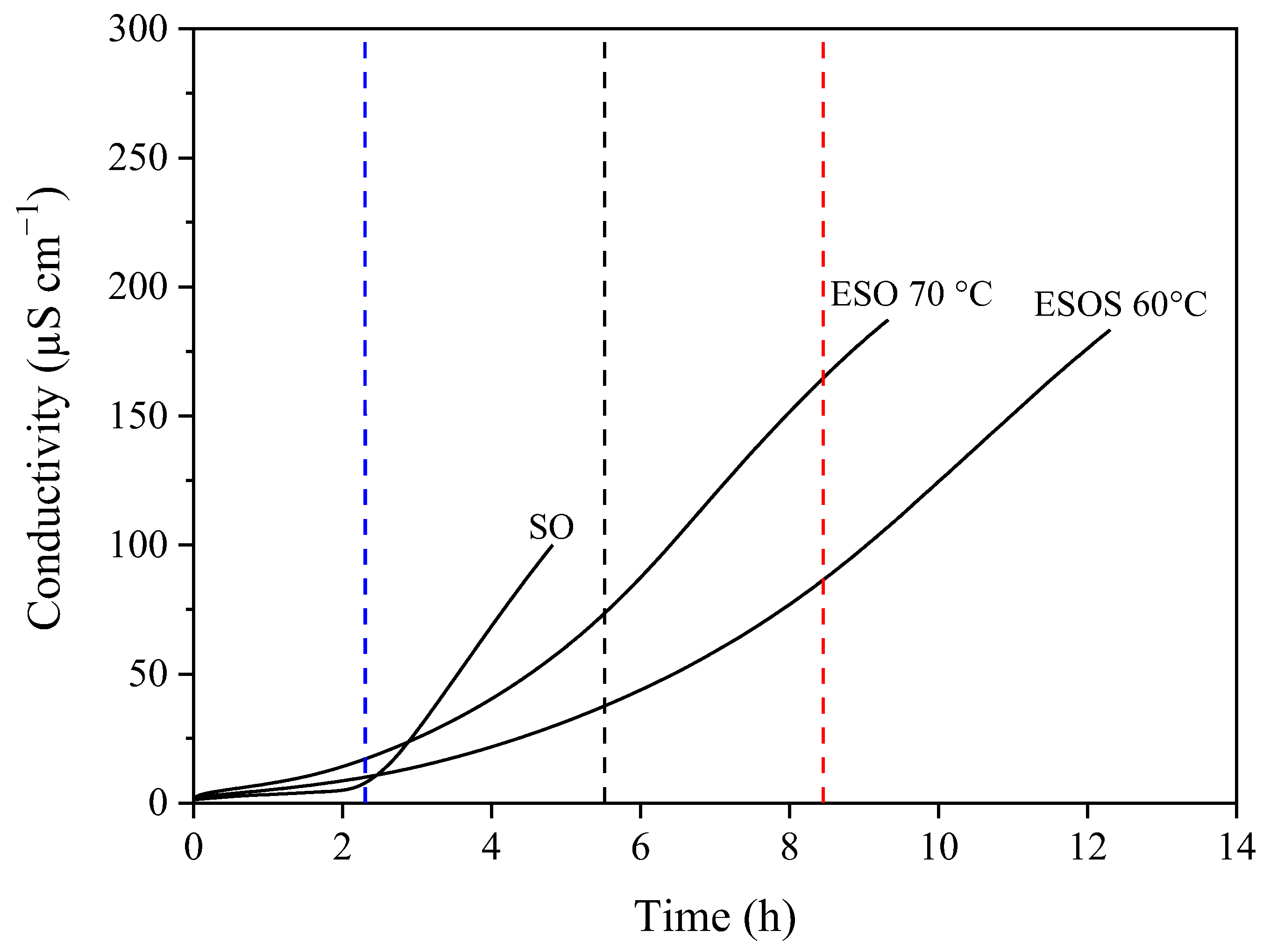
| Product/Acronym | Materials | Experimental Conditions | ||
|---|---|---|---|---|
| Temperature (°C) | Proportion | Time (min) | ||
| Sunflower seed oil/SO | Sunflower seeds + ethyl acetate | 60 | 1:8 (g/mL) | 60 |
| Enriched oil/ESO | Turmeric rhizome + sunflower seed oil | 60 and 70 | 1:2 (g/g) | 15 and 30 |
| Enriched oil/ESOS | Turmeric rhizome + sunflower seeds + ethyl acetate | 1:4.5:8 (g/g/mL) | 15 and 30 | |
| Sample 1 | Temperature (°C) | |||
|---|---|---|---|---|
| 60 | 70 | |||
| 15 min | 30 min | 15 min | 30 min | |
| ESO (mg/100 g oil) | 352.8 aA ± 0.1 | 355.6 aA ± 0.6 | 431.8 aB ± 0.3 | 447.7 aB ± 2.8 |
| ESOS (mg/100 g oil) | 512.5 aA ± 1.2 | 497.3 bA ± 4.7 | 509.6 aA ± 6.2 | 511.5 aA ± 7.3 |
| Property | Sample 1 | ||||
|---|---|---|---|---|---|
| SO | ESO | ESOS | |||
| 60 °C/15 min | 70 °C/15 min | 60 °C/15 min | |||
| Total phenolic compound content (mg GAE/100 g oil) | 13.8 a ± 0.1 | 437.7 b ± 5.4 | 449.5 c ± 8.0 | 643.4 d ± 18.4 | |
| Antioxidant potential (µmolTrolox/100 g oil) | FRAP | 5.8 a ± 0.2 | 1098.5 b ± 118.5 | 1763.9 c ± 3.8 | 3522.2 d ± 163.2 |
| DPPH• | 178.0 a ± 8.5 | 1016.7 b ± 15.0 | 1361.6 c ± 259.9 | 2427.1 d ± 81.2 | |
| ABTS•+ | 79.6 a ± 3.4 | 991.8 b ± 112.8 | 1150.7 c ± 114.0 | 1657.6 d ± 71.7 | |
| Class | Identified Compound | Precursor Ion (m/z) | Fragment (m/z) | Ionization Mode | Sample 1 (Peak Intensity/1.00 × 109) | ||
|---|---|---|---|---|---|---|---|
| SO | ESO | ESOS | |||||
| 70 °C/15 min | 60 °C/15 min | ||||||
| Phenolic acids | Cinnamic | 147 | 103.0 | Negative | 0.35 | 0.27 | 0.57 |
| Gallic | 169 | 125.0 | 4.39 | 2.34 | 1.46 | ||
| Ferulic | 193 | 134.9 | 0.28 | 2.82 | 15.33 | ||
| Caffeic | 178 | 135.0 | 4.01 | 1.65 | 4.18 | ||
| Curcuminoids | Curcumin | 367 | 177.1 | Negative | nd | 50.11 | 134.00 |
| Demethoxycurcumin | 337 | 217.1 | nd | 20.83 | 134.00 | ||
| Bisdemethoxycurcumin | 307 | 179.1 | nd | 134.00 | 134.00 | ||
| Dihydroxycurcumin | 369 | 191.1 | nd | 2.37 | 21.39 | ||
| Tetrahydrocurcumin | 371 | 137.1 | nd | 0.07 | 1.96 | ||
| Terpenes | Bisaccumol | 229 | 137.1 | Positive | nd | 0.09 | 7.46 |
| Dehydrocurdione | 235 | 191.2 | nd | nd | 1.31 | ||
| Linalool | 137 | 95.1 | nd | 6.13 | 1.95 | ||
| α-turmerone | 216 | 161.1 | nd | 1.15 | 0.29 | ||
| β-turmerone | 218 | 161.1 | nd | 1.32 | 5.63 | ||
| Fatty Acid (%) 1 | SO | ESO | ESOS |
|---|---|---|---|
| 70 °C/15 min | 60 °C/15 min | ||
| Palmitic | 6.76 a±0.1 | 7.75 b±0.1 | 5.72 c±0.04 |
| Palmitoleic | 0.11 a±0.01 | 0.10 a±0.01 | 0.07 a±0.01 |
| Stearic | 4.06 a±0.1 | 4.25 a ± 0.07 | 3.77 c±0.01 |
| Oleic | 34.89 a±0.3 | 32.07 b ± 0.01 | 41.80 c±0.3 |
| Linoleic | 54.17 a±0.3 | 55.90 b ± 0.1 | 48.64 c±0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Oliveira Segantini, K.C.; de Oliveira Santos Junior, O.; Garcia, V.A.D.S.; Raspe, D.T.; da Silva, C. Sunflower Seed Oil Enriched with Compounds from the Turmeric Rhizome: Extraction, Characterization and Cell Viability. Separations 2025, 12, 121. https://doi.org/10.3390/separations12050121
de Oliveira Segantini KC, de Oliveira Santos Junior O, Garcia VADS, Raspe DT, da Silva C. Sunflower Seed Oil Enriched with Compounds from the Turmeric Rhizome: Extraction, Characterization and Cell Viability. Separations. 2025; 12(5):121. https://doi.org/10.3390/separations12050121
Chicago/Turabian Stylede Oliveira Segantini, Késia Corsato, Oscar de Oliveira Santos Junior, Vitor Augusto Dos Santos Garcia, Djéssica Tatiane Raspe, and Camila da Silva. 2025. "Sunflower Seed Oil Enriched with Compounds from the Turmeric Rhizome: Extraction, Characterization and Cell Viability" Separations 12, no. 5: 121. https://doi.org/10.3390/separations12050121
APA Stylede Oliveira Segantini, K. C., de Oliveira Santos Junior, O., Garcia, V. A. D. S., Raspe, D. T., & da Silva, C. (2025). Sunflower Seed Oil Enriched with Compounds from the Turmeric Rhizome: Extraction, Characterization and Cell Viability. Separations, 12(5), 121. https://doi.org/10.3390/separations12050121








