Abstract
The benzodiazepines are essential drugs used in medicine for anxiolytic, sedative, and hypnotic effects. According to the World Health Organization, the benzodiazepines are the most prescribed hypnotic drugs in the last decade (2010 at time), and their inappropriate use can damage the environment and human health. The availability of efficient analytical methods is crucial for the determination of these drugs in a complex matrix such as biological samples in clinical settings. In the last decade, several methods have been developed and have been applied to the detection and determination of benzodiazepines or their derivates. The present manuscript reviews selective and sensitive methodologies based on chromatographic, electrophoretic, and electrochemical systems for the determination of benzodiazepines in biological samples, covering the time of the last years and providing detailed information on sample pretreatment and instrumental conditions.
1. Introduction
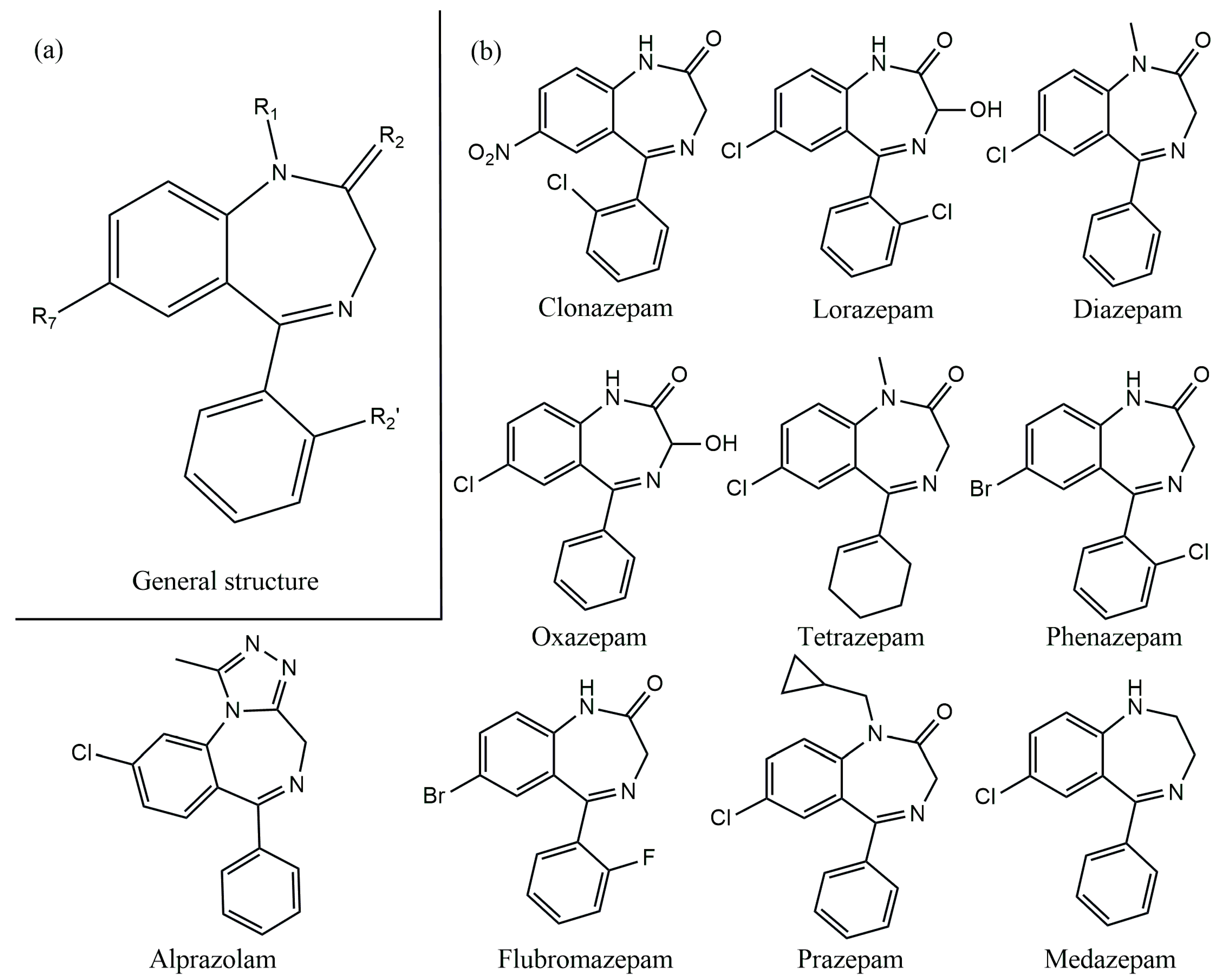
Benzodiazepines (BDZs) are a group of psychoactive substances introduced in the 1960s. Benzodiazepines derive their chemical structure (Figure 1a) from the combination of a benzene ring with a diazepine ring [1,2,3]. A wide range of drugs belong to this group. In the human body, the BDZs act upon the central nervous system via the GABAA receptor (an ion channel comprised of five different subunits two α, two β, and one γ) [4]. The BDZ utilizes the α and γ subunits of the GABAA receptor and induces some effects through the central nervous system, including anxiolytic, antiseizure, hypnotic, amnestic, and muscle relaxant effects [4].
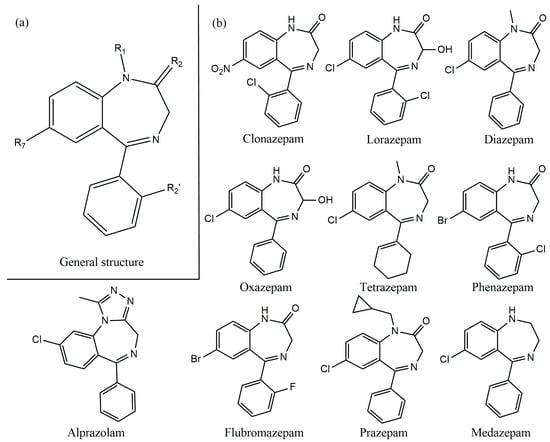
Figure 1.
(a) General structure of BDZs; (b) chemical structures of several BDZs.
The type of BDZ depends on the components present as substituents in the diazepam ring, for example, Figure 1b shows several BDZ structures and the difference in substituents are categorized as sedative, anxiolytic, anticonvulsant, and hypnotic drugs [4,5,6]. According to the World Health Organization (WHO), BDZs are essential drugs used in the treatment of many central nervous system diseases such as depression, phobias, panic, aggressiveness, anxiety, insomnia, and epileptic attacks [1,3,6,7,8,9,10]. The advantages of using these drugs include efficiency, a fast start to action, a minor number of collateral effects, and minimum toxicity. These characteristics have allowed BDZs to be the psychotropic drugs most currently prescribed in the world, particularly in Western countries [8].
However, despite its advantages, BDZs can lead to negative health effects. Some studies attributed the drugs’ abuse to cognitive and sensory impairment, impaired psychomotor skills, and development of ataxia and hypotonia. Additionally, studies have reported that aggression and anxiety episodes can occur, non-melanoma cancer can develop, or even death [11,12]. For these reasons, in recent years, it has been necessary to apply rapid and accurate methodologies for the determination of benzodiazepines. The present work focuses on reviewing the applications of methodologies based on chromatographic, electrochemical, and electrophoretic systems in biological samples over the last few decades.
2. Methodology
The present review was conducted using literature from scientific databases in the last decades (2010–present), focusing on “Determination of benzodiazepines” as the primary keyword. The references were selected considering analytical techniques (liquid chromatography, gas chromatography, electrophoresis, and electrochemical techniques were included in the present work) to determine benzodiazepines in biological samples.
3. Pretreatment Samples
Sample pretreatment is a fundamental part of analysis in biological samples; the appropriate procedure influences the reproducibility and accuracy of analysis. The principal methodologies by pretreatment samples are solid phase extraction (SPE), liquid–liquid extraction (LLE), dispersive solid phase extraction (DSPE), and dispersive liquid–liquid extraction (DLLE) with their miniaturized versions (solid phase microextraction (SPME), dispersive solid phase microextraction (DSPME), liquid–liquid microextraction (LLME), and dispersive liquid–liquid microextraction (DLLME) [13,14,15,16].
SPE is based on the distribution of analytes between two phases (a liquid or donator phase and a solid or acceptor phase), where the sample (donator phase) is passed through adsorbent material (acceptor phase) to which the analytes have more affinity than the donator phase; later, the analytes are reextracted by elution with a specific solvent [13,14]. The principal advantage of SPE is the several sorbent materials that can be used such as fused-silica and carbonaceous or polymeric materials. Pretreatment sample methodologies based on SPE have been employed to extract several analytes in biological samples such as plasma, hair, blood, tissue, urine, and seminal fluid [13,14].
On the other hand, LLE is a common technique for pretreatment samples. LLE involves the use of two immiscible solvents: water (donator phase) and an organic solvent (acceptor phase), where the analytes partition between two liquid phases. Subsequently, the acceptor phase is evaporated and reconstituted in an appropriate solvent. In recent years, the methodologies based on LLE have been developed using ionic liquids and eco-friendly solvents, achieving acceptable results in the pretreatment of several samples for the determination of drugs, pesticides, metals, and other analytes [15,16].
4. Methods of Chromatography
Chromatographic methods are separation techniques that rely on the distribution of one or more analytes between a stationary phase and a mobile phase. The separation process occurs through interactions according to the different stationary phases and the analytes’ chemical properties. The procedure allows for the separation of one or more compounds [17]. Complex matrices are processed using coupled sample pretreatments [18,19,20,21,22,23,24,25,26,27,28,29,30].
4.1. Liquid Chromatography
Liquid chromatographic techniques, including rapid resolution liquid chromatography (RRLC), ultra performance liquid chromatography (UPLC), and high-performance liquid chromatography (HPLC), have been developed for the analysis of BDZs, metabolites, abused drugs, and psychoactive substances. Table 1 presents an overview of the application of liquid chromatography techniques for the determination of BDZs residues in biological samples [18,19,20,21,22,23,24,25,26,27,28,29,30].

Table 1.
Liquid chromatography applications for the determination of BDZs in biological samples.
RRLC has been used to analyze 34 substances in diluted urine samples, including BDZs, their metabolites, and analogous compounds such as zopiclone, zolpidem, and zaleplon. The RRLC was compared to LC-MS/MS with satisfactory results in terms of accuracy, precision, limits of detection (LODs) ranging from 0.01 ng mL−1 to 0.5 ng mL−1, and % recovery ranging from 80.2% to 98.5% [18].
In 2017, Dunlop et al. utilized an atmospheric pressure chemical ionization liquid chromatography/mass spectrometry (APCI-LC/MS/MS) to analyze seven BDZs (diazepam, oxazepam, temazepam, nordiazepam, desalkylflurazepam, alprazolam, and α-hydroxyalprazolam) in drivers who might have been under the influence of drugs for law enforcement purposes. The analysis entails the breakdown of conjugated BDZs using β-glucuronidase in an acetate electrolyte solution for a duration of 2 h at a temperature of 60 °C. The method was developed and validated in terms of matrix effect, accuracy (ranging from 90.82 to 108.65%), and precision with %RSD (Relative Standard Deviation) values of 10% for one day and 15% for different days. The linear range of the methodology was from 20 to 500 ng mL−1. The approach was utilized to analyze 480 cases with positive samples for alprazolam (35%), oxazepam, nordiazepam, or temazepam (70%) [19].
Microextraction techniques, such as hollow fiber solid–liquid phase microextraction, have been used to determine alprazolam, clonazepam, diazepam, and lorazepam in complex matrices such as hair, urine, and wastewater by HPLC [20,21,22]. Eshaghi et al. utilized a membrane extraction technique using 1-pentyl-3-methylimidazolium bromide-coated titanium dioxide ([PMIM]Br@TiO2) for sample treatment. This approach demonstrated simplicity and effectiveness in comparison with traditional methodologies. According to the reports from the author, the fiber is employed once in order to minimize the possibility of cross-contamination, hence guaranteeing LODs ranging from 0.08 to 0.5 ng mL−1. Patients undergoing therapy with BDZs were found to have positive samples of clonazepam, lorazepam, alprazolam, and diazepam in hair samples; the concentrations of these substances were measured to be 6.05, 7.34, 7.23, and 6.59, respectively, with a %RSD of less than 10.0 [22].
In 2019, a new type of Molecularly Imprinted Polymer (MIP) designate as Restricted Access Molecularly Imprinted Polymers (RAMIPs) was described. The RAMIPs are designed, synthesized, characterized, and applied as a fiber in SPME. The fiber was synthesized employing diazepam as a template molecule, methacrylic acid (MAA) as a functional monomer, and bovine serum albumin (BSA) as a cross-linking agent [23]. Carvalho et al. describe the use of BSA as a protective barrier against proteins in the analytical matrix. This improved the selectivity of the approach, resulting in a 98% exclusion capacity. The method’s validation was determined in a plasma sample, with LODs from 5.0 to 30 µg L−1 [23].
Du, L. et al., describe the use of SPE coupled with HPLC for the analysis of three benzodiazepines (triazolam, midazolam, and diazepam) in urine samples. The adsorbent consists of diatomite-supported zeolite imidazolate framework-8 (ZIF-8@Dt-COOH). The contact modes between the analytes and the adsorbent are hydrophobic and π-π, based on their chemical composition. The development approach ensures LODs ranging from 0.3 to 0.4 ng ml−1, with %recoveries ranging from 80.0 to 98.7% in spiked samples. The analysis of samples with values ranging from 10.9 to 21.7 ng mL−1 for midazolam proved its feasibility [24].
Traditional sample preparation methods, such as LLE, have been successfully used to treat human plasma samples, providing a straightforward and dependable approach. The protein removal is carried out by utilizing 1 mL of methanol (MeOH). Afterwards, 50 µL of the supernatant is mixed with 100 µL of mobile phase and analyzed using RPLC/DAD under optimal conditions. The approach ensures minimum detectable amounts ranging from 1.78 to 5.59 ng mL−1 (standard samples), and 4.16 to 6.34 ng mL−1 (plasma). The recoveries range from 96.5% to 107.5%, and the precision, expressed as the %RSD, is less than 4.0% [26].
In liquid chromatography, the principal difference in methodologies was the pretreatment of the sample, highlighting the use of SPE with materials such as MIP, RAMIPs, and zeolites in the separation of BDZs, with apolar stationary phases, and the mobile phase consisted of mixtures of formic acid, ammonium formate, acetonitrile (ACN), phosphate buffer (PB), water, and MeOH in different proportions. In contrast, the spectroscopy detectors (UV–Vis, DAD) provide a LODS of 0.01 ng mL−1 at 30.00 μg L−1; however, the mass detectors (MS, MS/MS) provide a LODS of 0.01 ng mL−1 at 400.00 ng mL−1 in biological samples [18,19,20,21,22,23,24,25,26,27,28,29,30].
4.2. Gas Chromatography
Other chromatographic methodologies used in the analysis of BDZs include gas chromatography (GC). This technique requires that the analytes be transformed into the gaseous phase or volatile derivatives that can be separated and analyzed in the gaseous phase [17]. Table 2 shows an overview of the methodologies used for the determination of BDZs by GC [31,32,33,34,35,36,37,38,39].

Table 2.
Gas chromatography applications for the determination of BDZs in biological samples.
Álvarez-Freire L et al. published a study in 2018 describing the implementation of an SPE method employing Bond Elut Certify cartridges for sample extraction. An investigation was conducted on blood and pericardial fluid samples to analyze nine benzodiazepines, namely diazepam, midazolam, nordiazepam, oxazepam, bromazepam, temazepam, lorazepam, alprazolam, and clonazepam. The LOD of the SPE-GC/MS approach was verified at a concentration of µg mL−1. The precision and accuracy were determined by measuring inter-day and intra-day variations at three different concentration levels (0.05, 0.15, and 0.30 µg mL−1). The %RSD was found to be less than 11.63%, with %recoveries ranging from 93.76 to 106.15%. The approach was utilized on 12 positive samples obtained from cases of both natural and suicide deaths. The results indicate considerable variations between the two samples for nordiazepam, with concentrations of 0.3 µg mL−1 in pericardial fluid and 0.02 µg mL−1 in blood. Similarly, there are significant variances for oxazepam, with concentrations of 0.24 µg mL−1 in pericardial fluid and 0.18 µg mL−1 in blood [31].
Supramolecular solvents and the LLE method were used to analyze nine benzodiazepines and a benzodiazepine analogue (zolpidem) in biological samples, specifically human urine and blood. The technique uses a supramolecular solvent (tetrahydrofuran/1-hexanol) to efficiently extract low-molecular-weight molecules, followed by their detection using GC-MS/MS. The SUPRASs-GC-MS/MS methodology provides LODs ranging from 0.30 to 1.50 ng mL−1 [32]. The precision and accuracy were assessed in terms of inter- and intra-day repeatability of each analyte. In all cases, a %RSD of less than 10% was achieved, indicating high precision. Furthermore, the analysis of real blood samples yielded recovery rates ranging from 80.74% to 95.84%. The procedure was effectively utilized in two participants, from whose samples were obtained 24 h following the oral administration of diazepam and zolpidem. The collected samples were then examined, and the analytes were identified based on their retention times. The concentrations of diazepam in urine ranged from 1.98 to 49.19 ng mL−1, whereas the quantities of zolpidem in blood ranged from 8.37 to 10.95 ng mL−1 [32]. The supramolecular solvent that is created has the ability to efficiently extract low-molecular-weight chemicals from samples that contain water.
Hollow fibers have been applied in liquid phase microextraction (LPME) coupled with GC to analyze BDZs and their primary metabolites in urine samples. The LPME-GC method involves enzymatic hydrolysis, followed by extracting the analytes using a hollow fiber. The acceptor phase in the fiber is then removed, dried, and the residue is derivatized for subsequent GC analysis. The approach yields LODs ranging from 0.1 to 15 ng mL−1. The intra-day precision was consistently below 111.5% in all cases, while the inter-day precision was below 20.5%. To verify the precision, spiked samples were analyzed providing %recovery ranging from 89.1% to 111.6%. The suggested method was used to test samples from volunteers who reported using benzodiazepines for medical purposes. The analysis revealed the presence of nordiazepam and oxazepam in quantities of 69.1 and 109.6 ng mL−1, respectively [33].
In 2016, Perez et al. conducted a thorough comparison of GC-MS/MS and LC-MS/MS techniques for analyzing five benzodiazepines: alpha-hydroxyalprazolam, oxazepam, lorazepam, nordiazepam, and temazepam [34]. The LOD values obtained for GC-MS/MS ranged from 5.53 to 19.31 ng mL−1, while for LC-MS/MS, they ranged from 1.96 to 15.83 ng mL−1. The accuracy and precision of both methods were assessed at eight different levels (20, 40, 75, 100, 125, 200, 500, and 1000 ng mL−1). A t-test was used to confirm the absence of significant differences in the findings obtained from both approaches. The efficacy of LC-MS/MS in the analysis of BDZs has been proven through statistical demonstration. This technique provides accurate and precise results while minimizing the need for sample preparation [34].
The technique of DLLME coupled to GC-QQQ-MS has been used to analyze four benzodiazepines (phenazepam, etizolam, flubromazepam, and diclazepam) in urine, using low-density solvents. The extraction process parameters were optimized using a Box–Behnken design. The best conditions for the pH of the sample solution, volume of the extracting solvent, and extraction time were determined to be pH 11.3, 165 μL of ethyl acetate, and a mixing duration of 5.5 min. The technique provides LODs ranging from 1 to 3 ng mL−1. The approach demonstrates a %RSD (precision and accuracy.) < 5.9%. The method was employed to quantify the concentration of phenazepam, which was reported to be 158 ng mL−1 [35].
Cation-exchange polymeric sorbents have been utilized in SPE, in conjunction with GC and negative-ion chemical ionization mass spectrometry, for the detection and quantification of fifteen benzodiazepines in human blood. The methodology involves collecting the blood serum, which is then passed through an SPE cartridge to clean up the sample and remove any unwanted substances. The analytes are then treated with a derivative and injected into the GC/NICI-MS system. The efficiency of the sample preparation was assessed based on the relative response factor [39]. The LODs provided by the method range from 0.24–0.62 ng mL−1. The accuracy of the method was assessed by comparing the measured concentration in blood, obtained through calibration curves, with spiked samples of benzodiazepines in blood samples. The obtained values for accuracy range from 89.5% to 110.5% [39].
According to the literature, gas chromatography techniques for the determination of BDZs are used, the principal stationary phase is an HP-5 column (30 m × 320 μm i.d. 250 μm), and helium gas is used as a carrier gas at a flow rate of 1 mL min−1 at 3 mL min−1. DLLME is one of the most used sample pretreatments, highlighting the low solvent consumption that provides an eco-friendly analysis. In addition, the main detector system is mass detectors (MS, MS/MS) with LODs of 0.06–0.1 ng mL−1 at 0.1 µg mL−1 in biological samples such as serum, urine, blood, and pericardial fluid [31,32,33,34,35,36,37,38,39].
5. Capillary Electrophoresis
Capillary electrophoresis has been employed routinely in the determination of benzodiazepines such as clonazepam, tetrazepam, midazolam, and diazepam [17,40] due to the property of electromigration of these molecules. This technique allows the separation of ionic, polar, and nonpolar molecules, molecules with different chirality, and different molar masses. There are different electrophoretic techniques to separate benzodiazepines, such as capillary electrophoresis (CE), capillary zone electrophoresis (CZE), capillary isoelectric focusing (CIEF), non-aqueous capillary (NACE), capillary gel electrophoresis (CGE), capillary isotachophoresis (CITP), micellar electrokinetic capillary chromatography (MECC), and capillary electrochromatography (CEC) [40]. Table 3 shows the main electrophoresis methods used and the conditions of separation and determination [41,42,43,44,45,46]. CZE is limited in the study of benzodiazepines due to poor separation of these compounds caused by their similar hydrophobicity. To identify the behavior of benzodiazepines in CZE, Shiung et al. studied the separation of eight benzophenones with a phosphate-borate buffer in a pH range from 7.5 to 11.5 with an optimum separation of pH = 9.2 (25 °C and 20 kV). They describe the effect of the amount of OH groups in the benzophenone structure on the electrophoretic mobility that was observed. They concluded that with a higher amount of OH groups, the electrophoretic mobility increases due to the conjugation of the OH groups to the aromatic rings. However, the use of CZE in biological samples has been limited in recent years due to the properties [40].

Table 3.
Determination of BDZs by capillary electrophoresis in biological samples.
On the other hand, Švidrnoch et al. performed the separation of nine benzodiazepines (bentazepam, pyrazolam, deschloroetizolam, flubromazepam, flubromazolam, nimetazepam, diclazepam, phenazepam, and etizolam) by NACE. Table 3 displays the separation conditions. In this investigation, a volatile nonaqueous electrolyte is based on 20 mM of ammonium acetate in ACN and 100 mM of trifluoroacetic acid. The separation and selectivity of this investigation was satisfactory with an LOD suitable for biological samples where diazepines can be found in the order of tens to hundreds of ng L−1 [41].
Woźniakiewicz et al. studied the simultaneous determination by capillary electrophoresis of eight benzodiazepines (lorazepam, 7-aminoclonazepam, alprazolam, clonazepam, diazepam, 1-benzylpiperazine, estazolam, and tetrazepam) in human serum and hair samples. To perform each analysis, the samples were previously extracted with 1 mL of borate buffer (pH = 9.5) and 3 mL of ethyl acetate by microwave-assisted extraction, with a recovery of 88.6–113.4% for serum and 86.1–107.4% for hair. The separation was carried out using a mixture of 100 mM of formic acid and ACN for 20 min with a voltage over 30 kV in a polyamide-coated fused silica capillary. With these conditions, the separation had a good resolution, an LOD of 0.4–1.2 ng mL−1 for serum, 6.0–23.0 pg mg−1 for hair, and a limit of quantification (LOQ) of 1.3–4.1 ng mL−1 for serum and 20.0–77.0 pg mg−1 for hair [42].
Cui et al. studied the separation of five benzodiazepines (diazepam, midazolam, nordiazepam, flurazepam, and diazepam) in a mixture of forty-six drugs in human blood samples by CE. They performed an SPE to determine the drugs in blood. The separation was carried out at 25 °C with a running electrolyte of 150 mM phosphate (pH 2.4) with 20% MeOH with a UV detector (200 and 210 nm). The LODs were in the range of 8–30 ng mL−1 being lower for nordiazepam and flurazepam. This determination allowed for the analysis of forensic samples and to determine the cause of death by different drugs [43].
Ole et al. proposed a method to adequately determine eight benzodiazepines (chlordiazepoxide, estazolam, temazepam, midazolam, clonazepam, medazepam, lorazepam, and lormetazepam) using electrokinetic capillary chromatography in urine samples. The analytes were previously extracted by D LLME. The best separation of BZDs was obtained with a running buffer composed of 30 mM SDS, 10 mM sodium tetraborate, and 15% MeOH (pH 8.8); a sample buffer composed of 10 mM SDS and 2 mM sodium tetraborate and a voltage of 23 kV were applied. The application of field-amplified sample stacking improves the analysis time and sensitivity of the method, as well as the performance the analysis on urine samples [44].
Świądro et al. developed a method to determine different types of drugs in blood samples collected in vivo or post-mortem by capillary mass-coupled electrophoresis combined with the dried blood spot (DBS/CE-MS) method. To perform the analysis, the samples were prepared using microwave-assisted extraction (MAE). Separation of the analytes was performed for 25 min using a voltage of +30 kV in a silica capillary. The temperature was set to 25 °C. The detection was carried out in the positive ion mode, and profile spectra were acquired in the mass range of 100–1450 m/z using a mass spectrometer. The analysis of the benzodiazepine tetrazepam showed an LOD of 14.7 ng mL−1 with an LOQ of 49 ng mL−1 with a matrix effect of 99.8% (n = 6) and a recovery of 99.5%. In addition to presenting a good LOD, LOQ, and recovery, this method has the advantage of using the DBS technique to guarantee the stability of the analytes for 14 days after sample collection (storage at 15 °C) [45].
On the other hand, Seyfinejad et al. [46] developed another method to determine chlordiazepoxide in human plasma using ultrasound-assisted electromembrane extraction coupled with capillary electrophoresis (UA-EME-CE) with photodiode array detection. Utilizing the optimal conditions (100 mM PB adjusted to pH 2.0, 25 °C and +18 kV) the UA-EME provided an extraction recovery of 58% in 13 min. Furthermore, this technique achieved a pre-concentration factor of 203 and a LOD of 3 ng mL−1 with good repeatability.
Electrophoretic techniques for determination of BDZs are diverse (NACE, CE, and MECC); therefore, the pretreatment of sample and experimental conditions as background electrolyte and separation voltage are specific to each technique; however, by these techniques, the lower LODs were of 0.4 ng mL−1 and 6 pg mg−1 reported by Woźniakiewicz et al., and provided with a mass detector in serum and hair samples, respectively. This represents an important difference compared to spectroscopic detectors with LODs around a range from 3 to 20 ng mL−1 in samples such as blood, plasma, and urine samples [41,42,43,44,45,46].
6. Electrochemical Methods
Electrochemical methods such as voltammetry or potentiometry have been recently studied in drugs such as diazepam, clonazepam, tetrazepam, and oxazepam. Recent potentiometric and voltammetric studies are reported in Table 4 [47,48,49,50,51,52,53,54,55,56].

Table 4.
Determination of BDZs by electrochemical methods.
Ashrafi et al. designed a polydopamine-polyfolic acid nanocomposite modified glassy carbon electrode for the determination of five benzodiazepines (alprazolam, diazepam, clonazepam, oxazepam, chlordiazepoxide) in human plasma [47]. Cyclicvoltammetry differential pulse voltammetry and square wave voltammetry methods were used to study the electrochemical behavior of the benzodiazepines. The most sensitive detection was obtained with the differential-pulse voltammetry (measurements step potential = 0.2 Epulse = 0.05 V, tpulse = 0.2 s and scan rate = 50 mV/s) with an electrolytic solution of 0.1 M NaOH. Under these conditions, the LOQ obtained was 0.040, 0.033, 0.50, 0.025, and 0.04 μM for alprazolam, diazepam, clonazepam, oxazepam, and chlordiazepoxide, respectively. The results indicate that polydopamine-polyfolic acid has an essential role in signal amplification. The same method was employed by modifying a gold electrode with silver nanoparticle-nitrogen-doped graphene quantum dots [48]. In this case, the LOQs were increased to 3.8, 61.8, 3.8, 7.7, and 61.8 μM in plasma for diazepam, clonazepam, oxazepam, chlordiazepoxide, respectively.
On the other hand, Ma et al. developed a modified carbon paste electrode with nitrogen-doped carbon nanoparticles for electrochemical detection of tetrazepam in human blood serum [49]. The differential-pulse voltammetry analysis shows that the electrode is sensitive and selective with an LOD of 5 ng mL−1 and a broad linear range of 0–650 µg mL−1. These results were compared with an ELISA technique. Khoshroo et al. [50] developed an electrochemical sensing platform based on nanostructured silver fibers and ionic liquid composites for an electrochemical sensor for the detection of clonazepam. The electrochemical analysis was carried out by differential pulse voltammetry in urine and human serum. This method offers a wide linear range from 0.1 to 250 μM with an LOD of 66 nM, and acceptable reproducibility and stability compared with the HPLC method.
For their part, Lofti et al. [51] carried out an electrochemical determination of the clonazepam drug based on a glassy carbon electrode modified with Fe3O4/R-SH/Pd nanocomposite by differential-pulse voltammetry. The synthesized nanocomposite consists of Fe3O4, 3-aminopropyltriethoxysilane, cyanuricchloride, 2-mercaptoethanol, and Pd. The optimal conditions were a pulse amplitude of 55 mV, a pulse width of 40 ms, and the scan rate of 80 mV/s with a PB solution (pH 7.0). With these conditions, the designed sensor has a linear range from 10 nM to1 μM with an LOD of 3.02 nM.
Asiabar et al. have determined flunitrazepam in human plasma with a differential-pulse voltammetry method. This investigation was carried out with a carbon paste electrode modified with MnFe2O4 and gold nanoparticles. At the optimal experimental conditions, the anodic current measured during the oxidation of the flunitrazepam at 0.41 V showed a linear response to the concentrations of the flunitrazepam in a range of 0.1–100 μM. This method has an LOD of 0.33 μM. The authors report that this method permits measuring flunitrazepam in human plasma samples in the presence of some organic compounds and mineral ions with an economic advantage.
Potentiometric methods were studied in human samples [53,54,55,56]. Rouhani and Soleymanpour [53] developed a potentiometric sensor fabricated for the analysis of olanzapine in real samples. The electrode design consists of a carbon paste electrode modified with an olanzapine–tungstophosphate ion pair. The modified electrode showed an LOD of 5 × 10−7 M with high selectivity. The electrode has excellent thermal stability in a temperature range of 15–55 °C. This method permits studying the interaction between olanzapine drug and β-cyclodextrin and determining olanzapine in human blood serum. Wassel and Abdullatif developed a potentiometric sensor with a PVC-membrane type to determinate olanzapine, oxazepam, and lorazepam in urine [54]. The membranes incorporate the ion associates of the drug cations with ammonium reineckate and later dispersed into 2-nitrophenyl-octylether or dibutyl sebacate. The inorganic or organic compounds studied did not present any interference in this study. The proposed method was applied to the determination of BDZs in urine, with results comparable to HPLC measurements.
Allahnouri et al. carried out a voltammetry determination of clonazepam with a screen-printed carbon electrode modified with copper nanoparticles anchored on porous silicon in spike human blood serum samples [55]. Under the optimum conditions (tpulse = modulation time: 100 ms and Epulse = modulation amplitude: 70 mV), the calibration is linear in the 0.05–7.6 μM clonazepam concentration range, and the LOD is 15 nM. The authors discuss that the method is reproducible, repeatable, highly selective, and sensitive. Chen et al. developed a screen-printed carbon electrode modified with WS2 and nanorods and WS2 nanoballs to determine clonazepam in biological samples (human serum and urine) [56]. The modifications made to the electrode increased the peak current and improved its ability to facilitate reactions compared to the unmodified electrode. These properties permit a linear response at 10–551 µM range, a lower LOD (2.37 nM), and high sensitivity. This electrode has excellent selectivity, including co-interfering compounds. Moreover, the electrode has good recovery (96.5% to 98.6%) for serum and urine samples.
7. Conclusions
Benzodiazepines are an important group of drugs prescribed for different health conditions. However, the misuse or excessive use of these drugs is a potential public health and environmental problem. For these reasons, in the last decades, the development of techniques and methodologies to determine these molecules has attracted attention. Therefore, this review covers different methods using chromatography, electrophoresis, and electrochemistry techniques for the determination of BDZs in biological samples. The different methodologies show acceptable LODs for the analytes, highlighting the use of mass detectors in chromatography and electrophoretic systems. Looking at the sample preparation techniques, electrochemical methodologies make use only of dilution of the original sample in an electrolyte solution, while the chromatographic and electrophoretic methodologies make use of SPE, SPME, among others, with different, innovative, and modern sorbent materials or modification of materials preexisting. On the other hand, other preparation techniques, such as LLME and LPME, employ environmentally friendly solvents. With respect to methodologies analyzed in the present work, the different techniques have different advantages and advantages such as minimal pretreatment (electrochemical techniques), multianalyte determination with lower LODs in samples (electrophoretic and chromatographic systems), demonstrating significant progress in the development of efficient and effective eco-friendly methodologies for the determination of BDZs and their derivatives in complex matrices.
Author Contributions
Conceptualization, I.S.I. and J.F.F.-A.; investigation, I.V.-G. and G.I. writing—original draft preparation, I.S.I.; writing—review and editing, J.F.F.-A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sanabria, E.; Cuenca, R.E.; Esteso, M.Á.; Maldonado, M. Benzodiazepines: Their use either as essential medicines or as toxics substances. Toxics 2021, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Arora, N.; Dhiman, P.; Kumar, S.; Singh, G.; Monga, V. Recent advances in synthesis and medicinal chemistry of benzodiazepines. Bioorg. Chem. 2020, 97, 103668. [Google Scholar] [CrossRef]
- Dinis-Oliveira, R.J. Metabolic profile of oxazepam and related benzodiazepines: Clinical and forensic aspects. Drug Metab. Rev. 2017, 49, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Edinoff, A.N.; Nix, C.A.; Odisho, A.S.; Babin, C.P.; Derouen, A.G.; Lutfallah, S.C.; Cornett, E.M.; Murnane, K.S.; Kaye, A.M.; Kaye, A.D. Novel designer benzodiazepines: Comprehensive review of evolving clinical and adverse effects. Neurol. Int. 2022, 14, 648–663. [Google Scholar] [CrossRef] [PubMed]
- Ettcheto, M.; Olloquequi, J.; Sanchez-Lopez, E.; Busquets, O.; Cano, A.; Manzine, P.R.; Beas-Zarate, C.; Castro-Torres, R.D.; García, M.L.; Bulló, M.; et al. Benzodiazepines and related drugs as a risk factor in Alzheimer’s disease dementia. Front. Aging Neurosci. 2020, 11, 344. [Google Scholar] [CrossRef]
- Guina, J.; Merrill, B. Benzodiazepines I: Upping the care on downers: The evidence of risks, benefits and alternatives. J. Clin. Med. 2018, 7, 17. [Google Scholar] [CrossRef]
- Ochoa, J.G.; Kilgo, W.A. The role of benzodiazepines in the treatment of epilepsy. Curr. Treat. Options Neurol. 2016, 18, 18. [Google Scholar] [CrossRef]
- Markota, M.; Rummans, T.A.; Bostwick, J.M.; Lapid, M.I. Benzodiazepine use in older adults: Dangers, management, and alternative therapies. Mayo Clin. Proc. 2016, 91, 1632–1639. [Google Scholar] [CrossRef]
- Atkin, T.; Comai, S.; Gobbi, G. Drugs for insomnia beyond benzodiazepines: Pharmacology, clinical applications, and discovery. Pharmacol. Rev. 2018, 70, 197–245. [Google Scholar] [CrossRef]
- Dubovsky, S.L.; Marshall, D. Benzodiazepines remain important therapeutic options in psychiatric practice. Psychother. Psychosom. 2022, 91, 307–334. [Google Scholar] [CrossRef]
- Howard, P.; Twycross, R.; Shuster, J.; Mihalyo, M.; Wilcock, A. Benzodiazepines. J. Pain Symptom Manag. 2014, 47, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Edinoff, A.N.; Nix, C.A.; Hollier, J.; Sagrera, C.E.; Delacroix, B.M.; Abubakar, T.; Cornett, E.M.; Kaye, A.M.; Kaye, A.D. Benzodiazepines: Uses, dangers, and clinical considerations. Neurol. Int. 2021, 13, 594–607. [Google Scholar] [CrossRef] [PubMed]
- Badawy, M.E.; El-Nouby, M.A.; Kimani, P.K.; Lim, L.W.; Rabea, E.I. A review of the modern principles and applications of solid-phase extraction techniques in chromatographic analysis. Anal. Sci. 2022, 38, 1457–1487. [Google Scholar] [CrossRef] [PubMed]
- Dugheri, S.; Mucci, N.; Cappelli, G.; Trevisani, L.; Bonari, A.; Bucaletti, E.; Squillaci, D.; Arcangeli, G. Advanced Solid-Phase Microextraction Techniques and Related Automation: A Review of Commercially Available Technologies. J. Anal. Methods Chem. 2022, 2022, 8690569. [Google Scholar] [CrossRef]
- Hammad, S.F.; Abdallah, I.A.; Bedair, A.; Mansour, F.R. Homogeneous liquid–liquid extraction as an alternative sample preparation technique for biomedical analysis. J. Sep. Sci. 2022, 45, 185–209. [Google Scholar] [CrossRef]
- Grau, J.; Azorín, C.; Benedé, J.L.; Chisvert, A.; Salvador, A. Use of green alternative solvents in dispersive liquid-liquid microextraction: A review. J. Sep. Sci. 2022, 45, 210–222. [Google Scholar] [CrossRef]
- Szatkowska, P.; Koba, M.; Kośliński, P.; Wandas, J.; Bączek, T. Analytical methods for determination of benzodiazepines. A short review. Open Chem. 2014, 12, 994–1007. [Google Scholar] [CrossRef]
- Hsu, R.Y.; Chan, S.A.; Lin, S.L.; Lin, T.Y.; Chu, W.L.; Fuh, M.R. Direct quantitative analysis of benzodiazepines, metabolites, and analogs in diluted human urine by rapid resolution liquid chromatography–tandem mass spectrometry. J. Food Drug Anal. 2013, 21, 376–383. [Google Scholar] [CrossRef]
- Dunlop, S.; Hayes, K.; Leavy, P.; Cusack, D.; Maguire, R. An atmospheric pressure chemical ionisation liquid chromatographic–tandem mass spectrometry method for the analysis of benzodiazepines in urine. J. Chromatogr. B 2017, 1064, 22–27. [Google Scholar] [CrossRef]
- Ghadi, M.; Hadjmohammadi, M.R. Extraction and determination of three benzodiazepines in aqueous and biological samples by air-assisted liquid–liquid microextraction and high-performance liquid chromatography. J. Iran. Chem. Soc. 2019, 16, 1147–1155. [Google Scholar] [CrossRef]
- Al-Hawasli, H.; Al-Khayat, M.A.; Al-Mardini, M.A. Development of a validated HPLC method for the separation and analysis of a Bromazepam, Medazepam and Midazolam mixture. J. Pharm. Anal. 2012, 2, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Es’haghi, Z.; Nezhadali, A.; Bahar, S.; Bohlooli, S.; Banaei, A. [PMIM] Br@ TiO2 nanocomposite reinforced hollow fiber solid/liquid phase microextraction: An effective extraction technique for measurement of benzodiazepines in hair, urine and wastewater samples combined with high-performance liquid chromatography. J. Chromatogr. B 2015, 980, 55–64. [Google Scholar] [CrossRef]
- de Carvalho Abrão, L.C.; Figueiredo, E.C. A new restricted access molecularly imprinted fiber for direct solid phase microextraction of benzodiazepines from plasma samples. Anal. 2019, 144, 4320–4330. [Google Scholar] [CrossRef]
- Du, L.; Xu, S.; Wu, H.; Zhao, T.; Wang, X.; Wang, M. Facile fabrication of diatomite-supported ZIF-8 composite for solid-phase extraction of benzodiazepines in urine samples prior to high-performance liquid chromatography. Molecules 2021, 26, 5209. [Google Scholar] [CrossRef]
- Dziadosz, M.; Teske, J.; Henning, K.; Klintschar, M.; Nordmeier, F. LC–MS/MS screening strategy for cannabinoids, opiates, amphetamines, cocaine, benzodiazepines and methadone in human serum, urine and post-mortem blood as an effective alternative to immunoassay based methods applied in forensic toxicology for preliminary examination. Forensic Chem. 2018, 7, 33–37. [Google Scholar] [CrossRef]
- Albishri, H.M.; Aldawsari, N.A.; Abd El-Hady, D. A Simple and Reliable Liquid Chromatographic Method for Simultaneous Determination of Five Benzodiazepine Drugs in Human Plasma. Analytica 2022, 3, 251–265. [Google Scholar] [CrossRef]
- Jeong, Y.D.; Kim, M.K.; Suh, S.I.; In, M.K.; Kim, J.Y.; Paeng, K.J. Rapid determination of benzodiazepines, zolpidem and their metabolites in urine using direct injection liquid chromatography–tandem mass spectrometry. Forensic Sci. Int. 2015, 257, 84–92. [Google Scholar] [CrossRef]
- Bergstrand, M.P.; Helander, A.; Beck, O. Development and application of a multi-component LC–MS/MS method for determination of designer benzodiazepines in urine. J. Chromatogr. B 2016, 1035, 104–110. [Google Scholar] [CrossRef]
- Lee, H.H.; Lee, J.F.; Lin, S.Y.; Chen, B.H. Simultaneous identification of abused drugs, benzodiazepines, and new psychoactive substances in urine by liquid chromatography tandem mass spectrometry. Kaohsiung J. Med. Sci. 2016, 32, 118–127. [Google Scholar] [CrossRef]
- Tomková, J.; Švidrnoch, M.; Maier, V.; Ondra, P. Analysis of selected designer benzodiazepines by ultra high performance liquid chromatography with high-resolution time-of-flight mass spectrometry and the estimation of their partition coefficients by micellar electrokinetic chromatography. J. Sep. Sci. 2017, 40, 2037–2044. [Google Scholar] [CrossRef]
- Álvarez-Freire, I.; Brunetti, P.; Cabarcos-Fernández, P.; Fernández-Liste, A.; Tabernero-Duque, M.J.; Bermejo-Barrera, A.M. Determination of benzodiazepines in pericardial fluid by gas chromatography–mass spectrometry. J. Pharm. Biomed. Anal. 2018, 159, 45–52. [Google Scholar] [CrossRef]
- Jinlei, L.; Wurita, A.; Xuejun, W.; Hongkun, Y.; Jie, G.; Liqin, C. Supramolecular solvent (SUPRASs) extraction method for detecting benzodiazepines and zolpidem in human urine and blood using gas chromatography tandem mass spectrometry. Leg. Med. 2021, 48, 101822. [Google Scholar] [CrossRef] [PubMed]
- de Bairros, A.V.; de Almeida, R.M.; Pantaleão, L.; Barcellos, T.; e Silva, S.M.; Yonamine, M. Determination of low levels of benzodiazepines and their metabolites in urine by hollow-fiber liquid-phase microextraction (LPME) and gas chromatography–mass spectrometry (GC–MS). J. Chromatogr. B 2015, 975, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Perez, E.R.; Knapp, J.A.; Horn, C.K.; Stillman, S.L.; Evans, J.E.; Arfsten, D.P. Comparison of LC–MS-MS and GC–MS analysis of benzodiazepine compounds included in the drug demand reduction urinalysis program. J. Anal. Toxicol. 2016, 40, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Ghambarian, M.; Tajabadi, F.; Yamini, Y.; Esrafili, A. Dispersive liquid–liquid microextraction with back extraction using an immiscible organic solvent for determination of benzodiazepines in water, urine, and plasma samples. RSC Adv. 2016, 6, 114198–114207. [Google Scholar] [CrossRef]
- Ghobadi, M.; Yamini, Y.; Ebrahimpour, B. SPE coupled with dispersive liquid–liquid microextraction followed by GC with flame ionization detection for the determination of ultra-trace amounts of benzodiazepines. J. Sep. Sci. 2014, 37, 287–294. [Google Scholar] [CrossRef]
- Meng, L.; Zhu, B.; Zheng, K.; Fu, S. Ultrasound-assisted low-density solvent dispersive liquid–liquid microextraction for the determination of 4 designer benzodiazepines in urine samples by gas chromatography–triple quadrupole mass spectrometry. J. Chromatogr. B 2017, 1053, 9–15. [Google Scholar] [CrossRef]
- Cabarcos-Fernández, P.; Tabernero-Duque, M.; Álvarez-Freire, I.; Bermejo-Barrera, A.M. Determination of seven antidepressants in Pericardial fluid by means of dispersive liquid–Liquid Microextraction and gas Chromatography–Mass Spectrometry. J. Anal. Toxicol. 2022, 46, 146–156. [Google Scholar] [CrossRef]
- Karlonas, N.; Padarauskas, A.; Ramanavicius, A.; Ramanaviciene, A. Mixed-mode SPE for a multi-residue analysis of benzodiazepines in whole blood using rapid GC with negative-ion chemical ionization MS. J. Sep. Sci. 2013, 36, 1437–1445. [Google Scholar] [CrossRef]
- Shiung, Y.C.; Chang, S.W.; Chen, C.Y.; Lin, C.H. Separation and Migration Behavior of Benzophenones in Capillary Zone Electrophoresis. J. Chin. Chem. Soc. 2015, 62, 456–460. [Google Scholar] [CrossRef]
- Švidrnoch, M.; Boráňová, B.; Tomková, J.; Ondra, P.; Maier, V. Simultaneous determination of designer benzodiazepines in human serum using non-aqueous capillary electrophoresis–tandem mass spectrometry with successive multiple ionic–polymer layer coated capillary. Talanta 2018, 176, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Woźniakiewicz, A.; Wietecha-Posłuszny, R.; Woźniakiewicz, M.; Bryczek, E.; Kościelniak, P. A quick method for determination of psychoactive agents in serum and hair by using capillary electrophoresis and mass spectrometry. J. Pharm. Biomed. Anal. 2015, 111, 177–185. [Google Scholar] [CrossRef]
- Cui, X.; Ni, C.; Liang, C.; Gong, F.; Wang, R.; Chen, G.; Zhang, Y. Screening and quantitation of forty-six drugs of abuse and toxic compounds in human whole blood by capillary electrophoresis: Application to forensic cases. Microchem. J. 2019, 144, 403–410. [Google Scholar] [CrossRef]
- Ole¸, D.I.; Kulińska, Z.; Prahl, A.; Ba¸, C.T. Simultaneous separation of eight benzodiazepines in human urine using field-amplified sample stacking micellar electrokinetic chromatography. J. Anal. Toxicol. 2015, 39, 436–443. [Google Scholar] [CrossRef][Green Version]
- Świądro, M.; Stelmaszczyk, P.; Wietecha-Posłuszny, R.; Dudek, D. Development of a new method for drug detection based on a combination of the dried blood spot method and capillary electrophoresis. J. Chromatogr. B 2020, 1157, 122339. [Google Scholar] [CrossRef]
- Seyfinejad, B.; Ozkan, S.A.; Jouyban, A. Ultrasound-assisted electromembrane extraction of clonazepam from plasma and determination using capillary electrophoresis. J. Chromatogr. B 2021, 1181, 122928. [Google Scholar] [CrossRef]
- Ashrafi, H.; Mobed, A.; Hasanzadeh, M.; Babaie, P.; Ansarin, K.; Jouyban, A. Monitoring of five benzodiazepines using a novel polymeric interface prepared by layer by layer strategy. Microchem. J. 2019, 146, 121–125. [Google Scholar] [CrossRef]
- Ashrafi, H.; Hassanpour, S.; Saadati, A.; Hasanzadeh, M.; Ansarin, K.; Ozkan, S.A.; Shadjou, N.; Jouyban, A. Sensitive detection and determination of benzodiazepines using silver nanoparticles-N-GQDs ink modified electrode: A new platform for modern pharmaceutical analysis. Microchem. J. 2019, 145, 1050–1057. [Google Scholar] [CrossRef]
- Ma, H.; Tian, Q. Application of nitrogen-doped carbon particles modified electrode for electrochemical determination of tetrazepam as muscle relaxant drug. Int. J. Electrochem. Sci. 2023, 18, 100084. [Google Scholar] [CrossRef]
- Khoshroo, A.; Hosseinzadeh, L.; Sobhani-Nasab, A.; Rahimi-Nasrabadi, M.; Ahmadi, F. Silver nanofibers/ionic liquid nanocomposite based electrochemical sensor for detection of clonazepam via electrochemically amplified detection. Microchem. J. 2019, 145, 1185–1190. [Google Scholar] [CrossRef]
- Lotfi, S.; Veisi, H. Electrochemical determination of clonazepam drug based on glassy carbon electrode modified with Fe3O4/R-SH/Pd nanocomposite. Mater. Sci. Eng. C 2019, 103, 109754. [Google Scholar] [CrossRef]
- Asiabar, B.M.; Karimi, M.A.; Tavallali, H.; Rahimi-Nasrabadi, M. Application of MnFe2O4 and AuNPs modified CPE as a sensitive flunitrazepam electrochemical sensor. Microchem. J. 2021, 161, 105745. [Google Scholar] [CrossRef]
- Rouhani, M.; Soleymanpour, A. A new selective carbon paste electrode for potentiometric analysis of olanzapine. Measurement 2019, 140, 472–478. [Google Scholar] [CrossRef]
- Wassel, A.A.; Abdullatif, S.A. Characteristics and electrochemical membrane sensors for selective study and determination of some antidepressant drugs in their pharmaceutical preparations. WJARR 2023, 20, 804–819. [Google Scholar] [CrossRef]
- Allahnouri, F.; Farhadi, K.; Eskandari, H.; Molaei, R. Screen printed carbon electrode modified with a copper@ porous silicon nanocomposite for voltammetric sensing of clonazepam. Microchim. Acta 2019, 186, 676. [Google Scholar] [CrossRef]
- Chen, T.W.; Rajaji, U.; Chen, S.M.; Ramalingam, R.J. A relative study on sonochemically synthesized mesoporous WS2 nanorods & hydrothermally synthesized WS2 nanoballs towards electrochemical sensing of psychoactive drug (Clonazepam). Ultrason. Sonochem. 2019, 54, 79–89. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).