Porous Composite Polymers Composed of Polyethyleneimine and Cyclodextrins: Synthesis and Application as Adsorbents for an Organic Compound
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
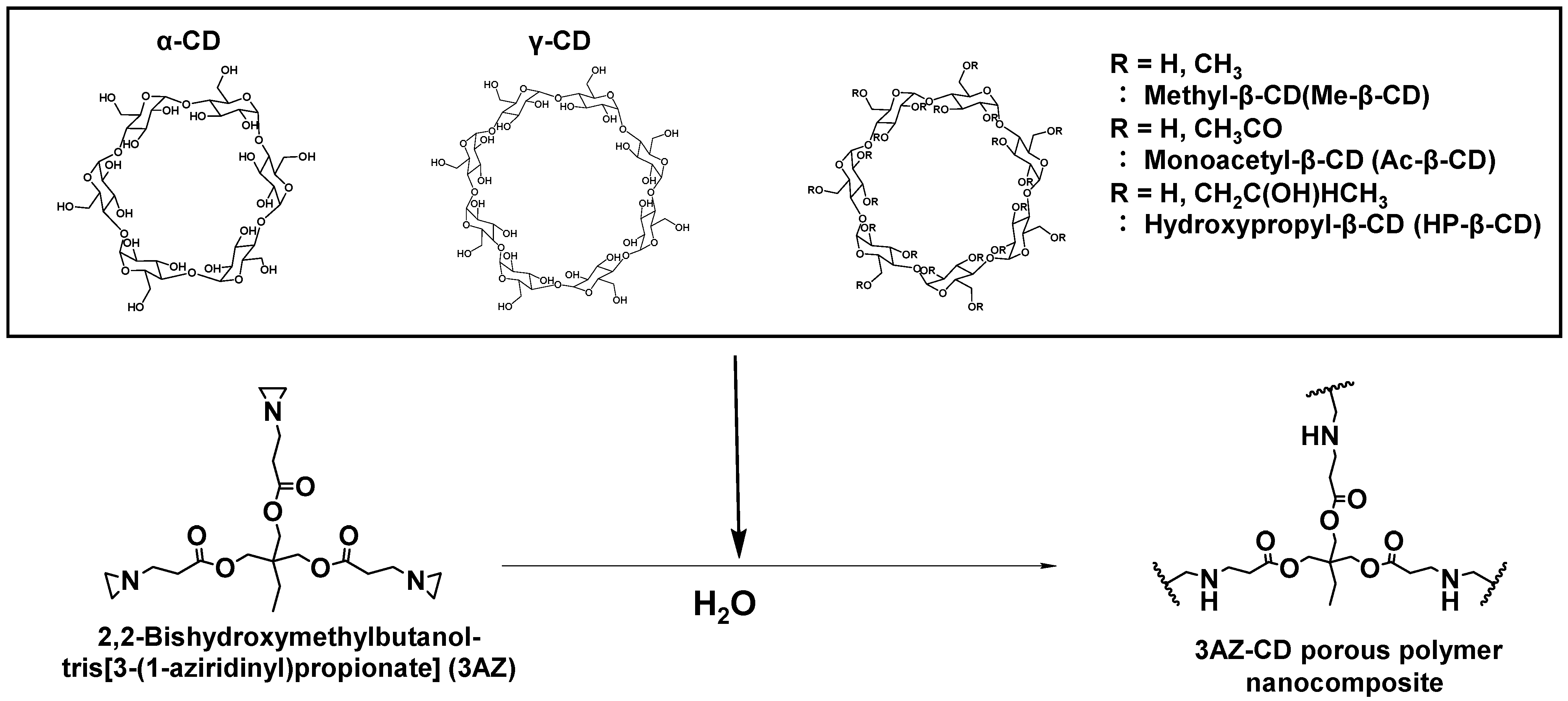
2.2. Synthesis of Porous Polymer Composites
2.3. Adsorption Test of Phph
Co: phph concentration before adsorption
Ce: phph concentration after adsorption
V: volume of phph solution used (=10 mL)
m: weight of porous polymer composite used
2.4. Analytical Procedures
Vp = {bulk density (g/cm3) × 3AZ polymer in composite (wt%)/100}/{true density of 3AZ polymer (g/cm3)}
Vcd = {bulk density (g/cm3) × CD in composite (wt%)/100}/{true density of CD (g/cm3)}
3. Results and Discussion
3.1. Synthesis of 3AZ-CD Porous Polymer Composites
3.2. Structure and Properties of 3AZ-CD Porous Polymer Composites
3.2.1. Composition and Morphology
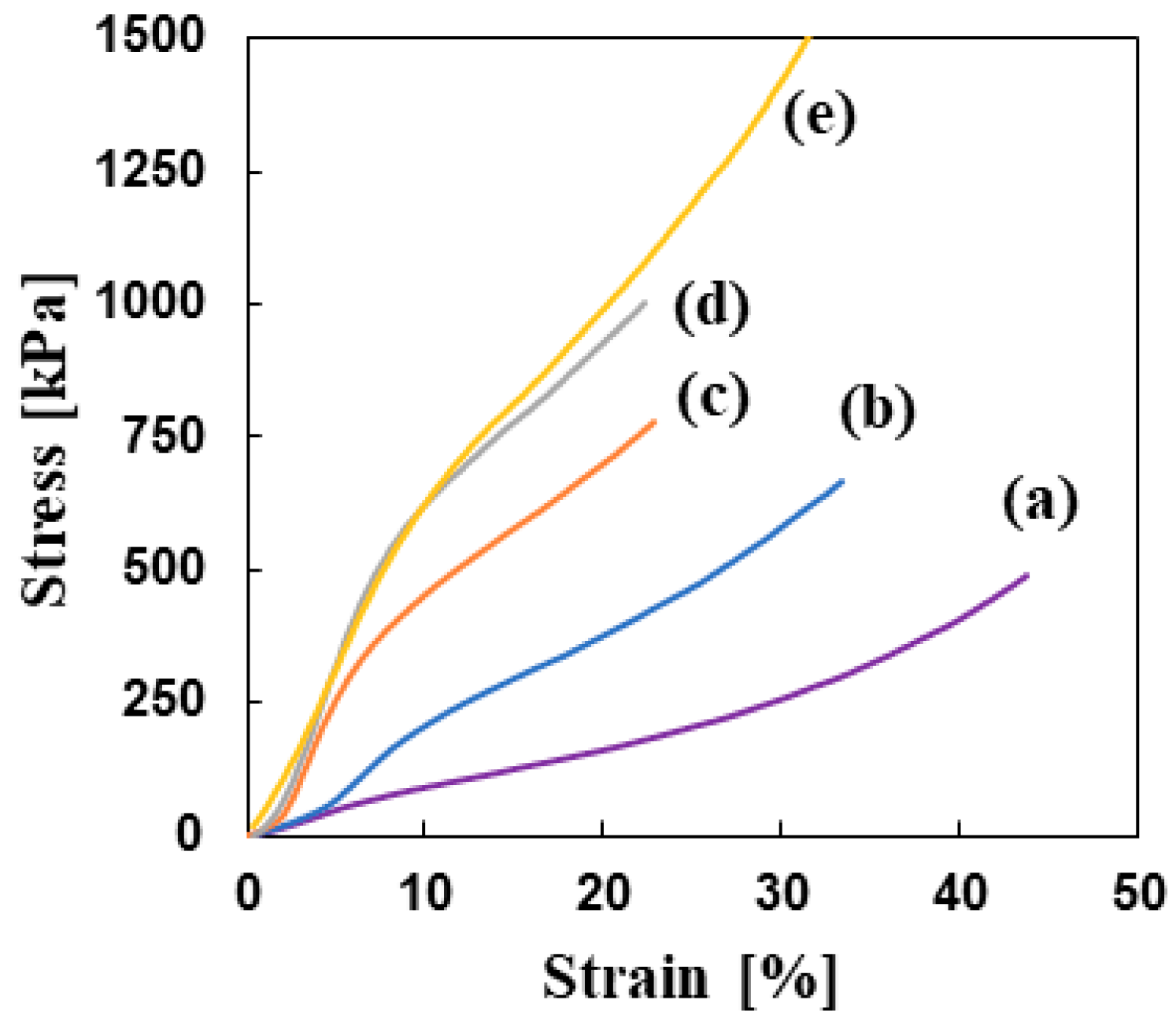
3.2.2. Mechanical Properties
3.3. Adsorption Test of Phph
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- BelBruno, J.J. Molecular Imprinted Polymers. Chem. Rev. 2019, 119, 94–119. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, A.M.; Barton, S.J.; Wren, S.P.; Barker, J. Review on molecular imprinted polymers with a focus on their application to the analysis of protein biomarkers. TrAC Trend. Anal. Chem. 2021, 144, 116431. [Google Scholar] [CrossRef]
- Sajini, T.; Mathew, B. A brief overview of molecularly imprinted polymers: Highlighting computational design, nano and photo-responsive imprinting. Talanta Open 2021, 4, 100072. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, Z.; Wang, D.; Yang, Y.; Duan, Y.; Ma, L.; Lin, T.; Liu, H. A Review on Molecularly Imprinted Polymers Preparation by Computational Simulation-Aided Methods. Polymers 2021, 13, 2657. [Google Scholar] [CrossRef]
- Önal Acet, B.; İnanan, T.; Salieva, K.; Borkoev, B.; Odabasi, M.; Acet, Ö. Molecular imprinted polymers: Important advances in biochemistry, biomedical and biotechnology. Polym. Bull. 2024, 81, 10439–10459. [Google Scholar] [CrossRef]
- Gkika, D.A.; Tolkou, A.K.; Lambropoulou, D.A.; Bikiaris, D.N.; Kokkinos, P.; Kalavrouziotis, K.; Kyzas, Z. Application of molecularly imprinted polymers (MIPs) as environmental separation tools. RSC Appl. Polym. 2024, 2, 127–148. [Google Scholar] [CrossRef]
- Amarh, F.A.; Kangmennaa, A.; Agorku, E.S.; Voegborlo, R.B. A state-of-the-art review of trends in molecularly imprinted polymers in the clean-up of pesticides in environmental samples. Sustain. Environ. 2024, 10, 2298067. [Google Scholar] [CrossRef]
- Yaacob, S.F.F.S.; Uwaibatu, M.; Jamil, R.Z.R.; Zain, N.N.M.; Raoov, M.; Suah, F.B.M. Revie of molecular imprinting polymer: Basic characteristics and removal of phenolic contaminants based on the functionalized cyclodextrin monomer. J. Chem. Technol. Biotechnol. 2022, 98, 312–330. [Google Scholar] [CrossRef]
- Fresco-Cala, B.; Batista, A.D.; Cárdenas, S. Molecularly Imprinted Polymer Micro- and Nano-Particles: A Review. Molecules 2020, 25, 4740. [Google Scholar] [CrossRef]
- Chen, L.; Wang, X.; Lu, W.; Wu, X.; Li, J. Molecular imprinting: Perspectives and applications. Chem. Soc. Rev. 2016, 45, 2137–2211. [Google Scholar] [CrossRef]
- Haupt, K. Peer Reviewed: Molecularly Imprinted Polymers: The Next Generation. Anal. Chem. 2003, 75, 376A–383A. [Google Scholar] [CrossRef] [PubMed]
- Cheong, W.J.; Yang, S.H.; Ali, F. Molecular imprinted polymers for separation science: A review or reviews. J. Sep. Sci. 2013, 36, 609–628. [Google Scholar] [CrossRef]
- Wan, Q.; Liu, H.; Deng, Z.; Bu, J.; Li, T.; Yang, Y.; Zhong, S. A critical review of molecularly imprinted solid phase extraction technology. J. Polym. Res. 2021, 28, 401. [Google Scholar] [CrossRef]
- Crini, G. Review: A History of Cyclodextrins. Chem. Rev. 2014, 114, 10940–10975. [Google Scholar] [CrossRef] [PubMed]
- Esteso, M.E.; Romero, C.M. Cyclodextrins: Properties and Applications. Int. J. Mol. Sci. 2024, 25, 4547. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhang, Y.; Niu, Y.; Ke, Q.; Kou, X. Cyclodextrins as carriers for volatile aroma compounds: A review. Carbohydr. Polym. 2021, 268, 118292. [Google Scholar] [CrossRef] [PubMed]
- Del Valle, E.M.M. Cyclodextrins and their uses: A review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Arrua, R.D.; Strumia, M.C.; Igarzable, C.I.A. Macroporous Monolithic Polymers: Preparation and Applications. Materials 2009, 2, 2429–2466. [Google Scholar] [CrossRef]
- Wu, D.; Xu, F.; Sun, B.; Fu, R.; He, H.; Matyjaszewski, K. Design and Preparation of Porous Polymers. Chem. Rev. 2012, 112, 3959–4015. [Google Scholar] [CrossRef]
- Liu, Q.; Xiong, J.; Lin, W.; Liu, J.; Wan, Y.; Guo, C.; Wang, Q.; Liu, Z. Porous polymers: Structure, fabrication and application. Matr. Horiz. 2025. [Google Scholar] [CrossRef]
- Bohr, S.J.; Wang, F.; Metze, M.; Vukusic, J.L.; Sapalidis, A.; Ulbricht, M.; Nestier, B.; Barbe, S. State-of-the art review of porous polymer membrane formation characterization-How numerical and experimental approaches dovetail to drive innovation. Front. Sustain. 2023, 4, 1093911. [Google Scholar] [CrossRef]
- Mohamed, M.G.; El-Mahdy, A.F.M.; Kotp, M.G.; Kuo, S.W. Advances in porous organic polymers: Syntheses, structures, and diverse applications. Mater. Adv. 2022, 3, 707–733. [Google Scholar] [CrossRef]
- Song, W.; Zhang, Y.; Tran, C.H.; Choi, H.K.; Yu, D.G.; Kim, I. Porous organic polymers with defined morphologies: Synthesis, assembly, and emerging applications. Prog. Polym. Sci. 2023, 142, 101691. [Google Scholar] [CrossRef]
- Ahmed, D.S.; El-Hiti, G.; Yousif, E.; Ali, A.A.; Hameed, A.S. Design and synthesis of porous polymeric materials and their applications in gas capture and storage: A review. J. Polym. Res. 2018, 25, 75. [Google Scholar] [CrossRef]
- Liu, Z.; Ou, J.; Zou, H. Click polymerization for preparation of monolithic columns for liquid chromatography. TrAC-Trend Anal. Chem. 2016, 82, 89–99. [Google Scholar] [CrossRef]
- Eeltink, S.; Meston, D.; Svec, F. Recent developments and applications of polymer monolithic stationary phases. Anal. Sci. Adv. 2021, 2, 250–260. [Google Scholar] [CrossRef]
- Mansour, F.R.; Waheed, S.; Paull, B.; Maya, F. Porogens and porogen selection in the preparation of porous polymer monoliths. J. Sep. Sci. 2019, 43, 56–69. [Google Scholar] [CrossRef]
- Svec, F. Porous polymer monoliths: Amazingly wide variety of techniques enabling their preparation. J. Chromatogr. A 2010, 1217, 902–924. [Google Scholar] [CrossRef]
- Cheng, X.; Bae, J. Recent Advancements in Fabrication, Separation, and Purification of Hierarchically Porous Polymer Membranes and Their Applications in Next-Generation Electrochemical Energy Storage Devices. Polymers 2024, 16, 3269. [Google Scholar] [CrossRef]
- Liu, W.; Yang, Y.; Guo, L.; Di, J.; Lau, C.H.; Bermeshev, M.V.; Shao, L. Recent advances in porous organic polymers for sustainable gas separation. Chem. Eng. J. 2024, 498, 155569. [Google Scholar] [CrossRef]
- Denter, U.; Schollmeyer, E. Surface modification of synthetic and natural fibres by fixation of cyclodextrin derivatives. J. Incl. Phenom. Macrocycl. Chem. 1996, 25, 197–202. [Google Scholar] [CrossRef]
- Naga, N.; Takenouchi, T.; Nakano, T. Ring-Opening Polymerization of Triaziridine Compounds in Water: An Extremely Facile Method to Synthesize a Porous Polymer through Polymerization-Induced Phase Separation. ACS Macro. Lett. 2022, 11, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Abe, I.; Hayashi, K.; Hirashima, T.; Kitagawa, M. Relationship between the Freundlich adsorption constants K and 1/M for hydrophobic adsorption. J. Am. Chem. Soc. 1982, 104, 6452–6453. [Google Scholar] [CrossRef]
- Abe, I.; Hayashi, K.; Hirashima, T.; Kitagawa, M. Adsorption of saccharides from aqueous so1ution onto activated carbon. Carbon. 1983, 21, 189–191. [Google Scholar] [CrossRef]
- Kano, F.; Abe, I.; Kayama, H.; Ueda, I. Derivation of Langmuir and Freundlich Adsorption Isotherms. Proc. Instuitte Stat. Math. 1991, 39, 53–61. [Google Scholar]




| Entry | CD | CD in Feed wt% | CD in Composite 1wt% | Average Diameter μm | Bulk Density g/cm3 | Porosity % | Young’s Modulus kPa |
|---|---|---|---|---|---|---|---|
| 0 | non | 0 | 4.58 | 0.267 | 75.7 | 1150 | |
| 1 | α-CD | 5.0 | 12.7 | 3.70 | 0.329 | 70.8 | 1657 |
| 2 | α-CD | 10.0 | 18.2 | 3.34 | 0.330 | 70.9 | 1861 |
| 3 | α-CD | 15.0 | 20.0 | n.d. 2 | 0.359 | 68.4 | 1974 |
| 4 | α-CD | 20.0 | 21.8 | n.d. 2 | 0.432 | 62.1 | 3809 |
| 5 | γ-CD | 5.0 | 9.7 | 4.26 | 0.334 | 70.1 | 2790 |
| 6 | γ-CD | 10.0 | 12.0 | 3.88 | 0.345 | 69.2 | 3284 |
| 7 | γ-CD | 15.0 | 20.5 | 3.28 | 0.366 | 67.6 | 5192 |
| 8 | γ-CD | 20.0 | 30.0 | 2.88 | 0.385 | 66.1 | 8800 |
| 9 | Me-β-CD | 5.0 | 9.7 | 3.80 | 0.311 | 72.4 | 2560 |
| 10 | Me-β-CD | 10.0 | 15.7 | 3.43 | 0.311 | 72.6 | 3250 |
| 11 | Me-β-CD | 15.0 | 23.2 | 3.24 | 0.344 | 70.1 | 3902 |
| 12 | HP-β-CD | 5.0 | 11.4 | 4.00 | 0.340 | 70.1 | 1342 |
| 13 | HP-β-CD | 10.0 | 14.7 | 3.71 | 0.344 | 70.0 | 2160 |
| 14 | HP-β-CD | 15.0 | 19.3 | 3.10 | 0.347 | 70.1 | 5679 |
| 15 | HP-β-CD | 20.0 | 26.7 | 2.81 | 0.368 | 68.7 | 5950 |
| Run | CD | CD Content wt% | qe 1 mg/g |
|---|---|---|---|
| 9 | Me-β-CD | 9.7 | 104.9 |
| 10 | Me-β-CD | 15.7 | 483.5 |
| 11 | Me-β-CD | 23.2 | 624.1 |
| 12 | HP-β-CD | 11.4 | 247.3 |
| 13 | HP-β-CD | 14.7 | 542.5 |
| 14 | HP-β-CD | 19.3 | 624.5 |
| 15 | HP-β-CD | 26.7 | 579.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naga, N.; Miyazaki, Y.; Nakano, T. Porous Composite Polymers Composed of Polyethyleneimine and Cyclodextrins: Synthesis and Application as Adsorbents for an Organic Compound. Separations 2025, 12, 94. https://doi.org/10.3390/separations12040094
Naga N, Miyazaki Y, Nakano T. Porous Composite Polymers Composed of Polyethyleneimine and Cyclodextrins: Synthesis and Application as Adsorbents for an Organic Compound. Separations. 2025; 12(4):94. https://doi.org/10.3390/separations12040094
Chicago/Turabian StyleNaga, Naofumi, Yuma Miyazaki, and Tamaki Nakano. 2025. "Porous Composite Polymers Composed of Polyethyleneimine and Cyclodextrins: Synthesis and Application as Adsorbents for an Organic Compound" Separations 12, no. 4: 94. https://doi.org/10.3390/separations12040094
APA StyleNaga, N., Miyazaki, Y., & Nakano, T. (2025). Porous Composite Polymers Composed of Polyethyleneimine and Cyclodextrins: Synthesis and Application as Adsorbents for an Organic Compound. Separations, 12(4), 94. https://doi.org/10.3390/separations12040094







