Investigation of Impurities in Peptide Pools
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Sample Preparation
2.2.1. Impurity Identification
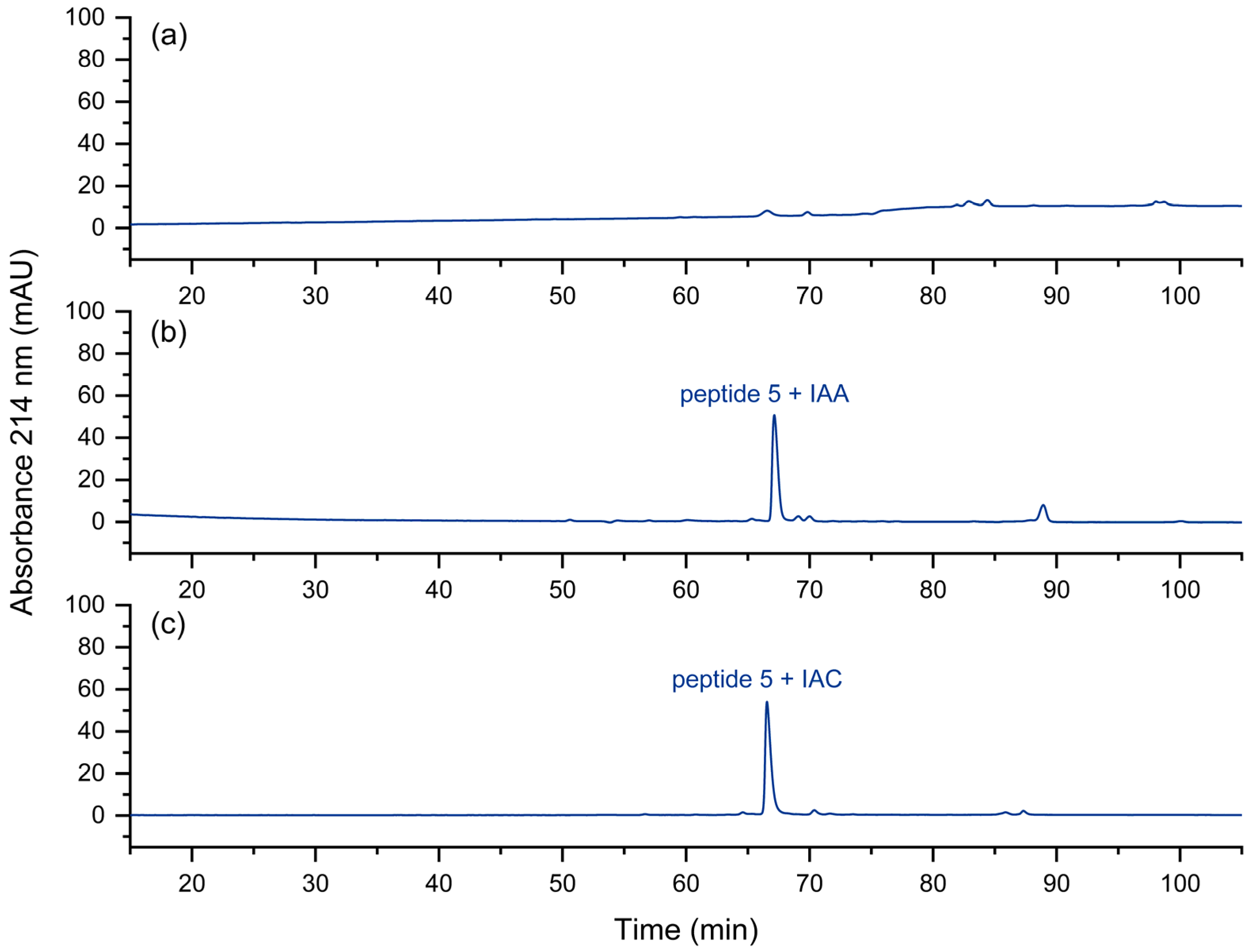
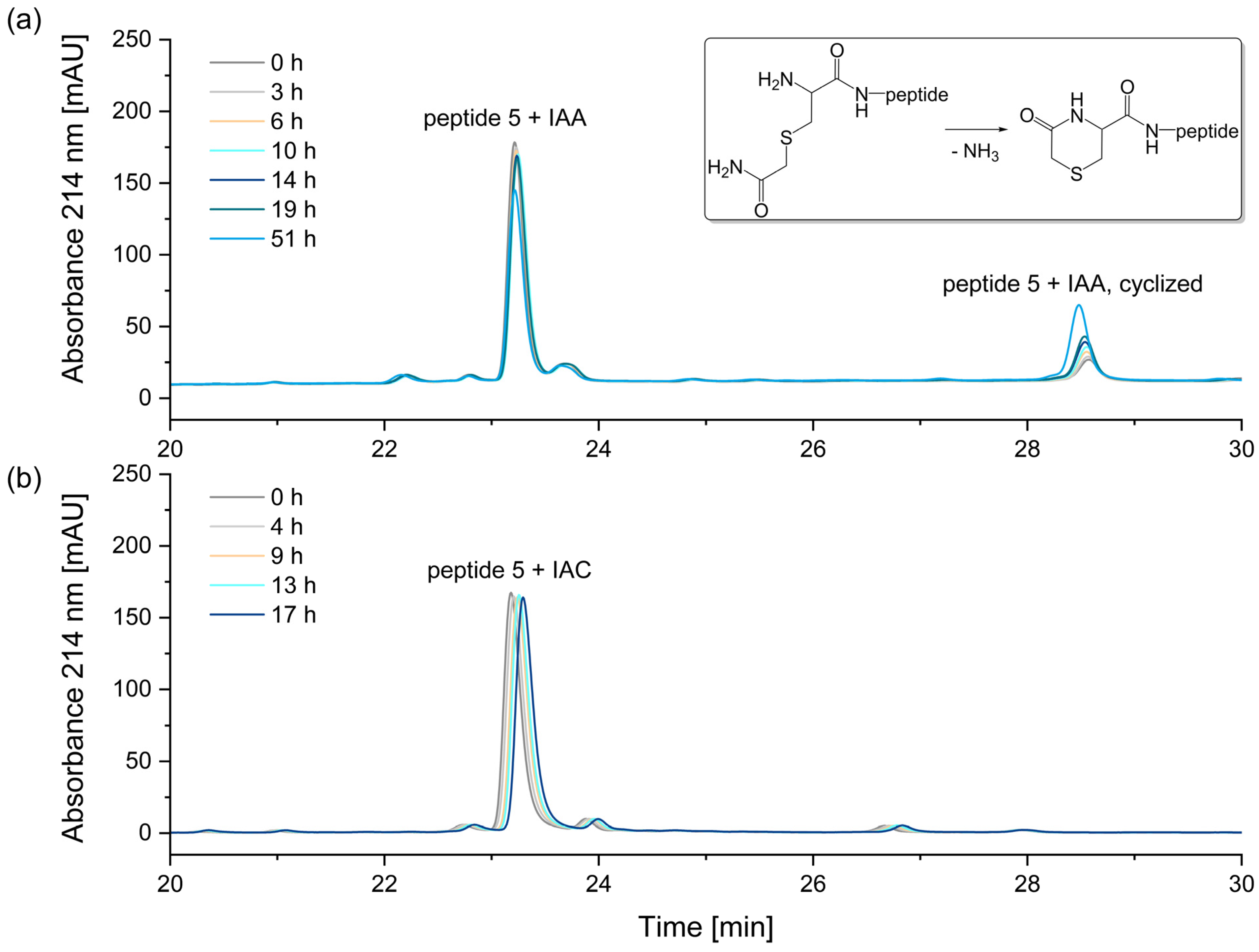
2.2.2. Alkylation of Cysteine-Residues
2.3. UHPLC-HRMS Analysis
2.4. Data Processing
3. Results
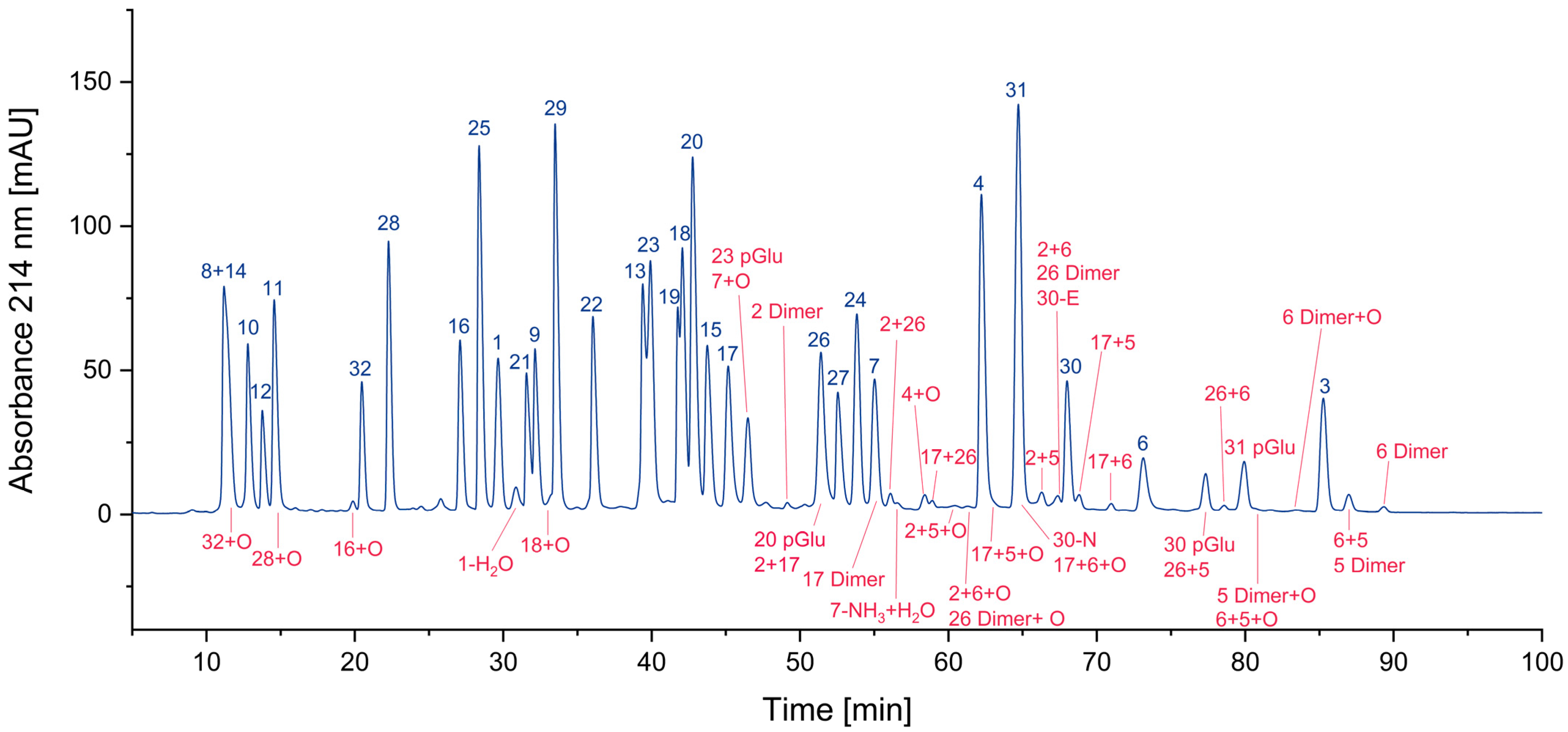
3.1. Impurity Identification
3.1.1. Deletion Peptides
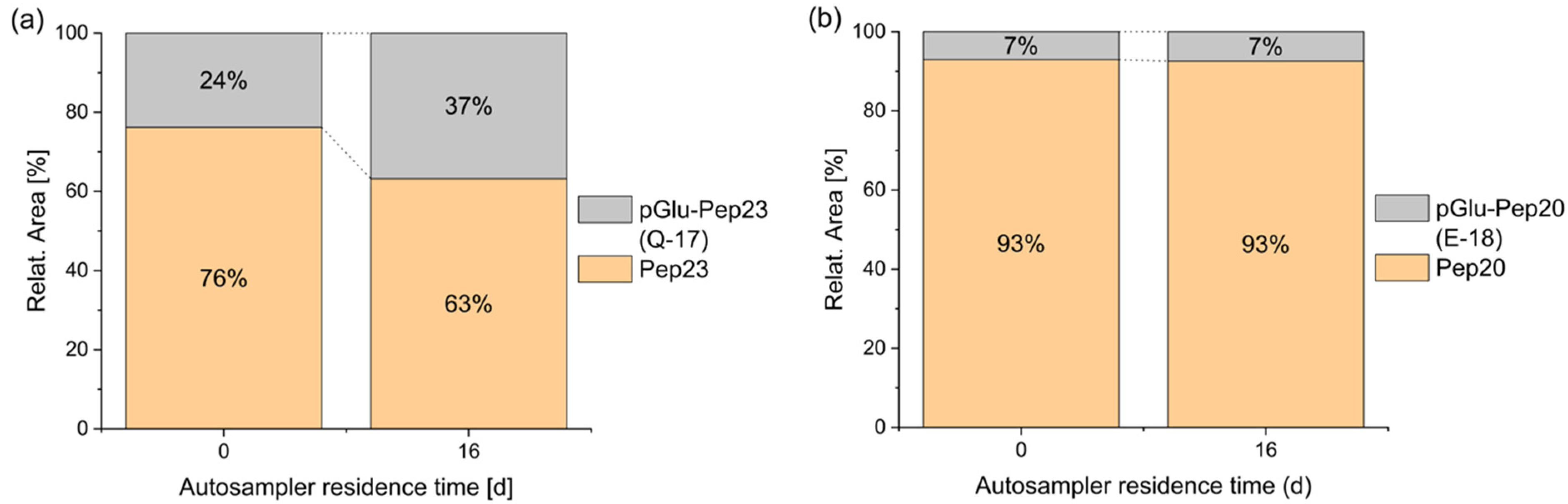
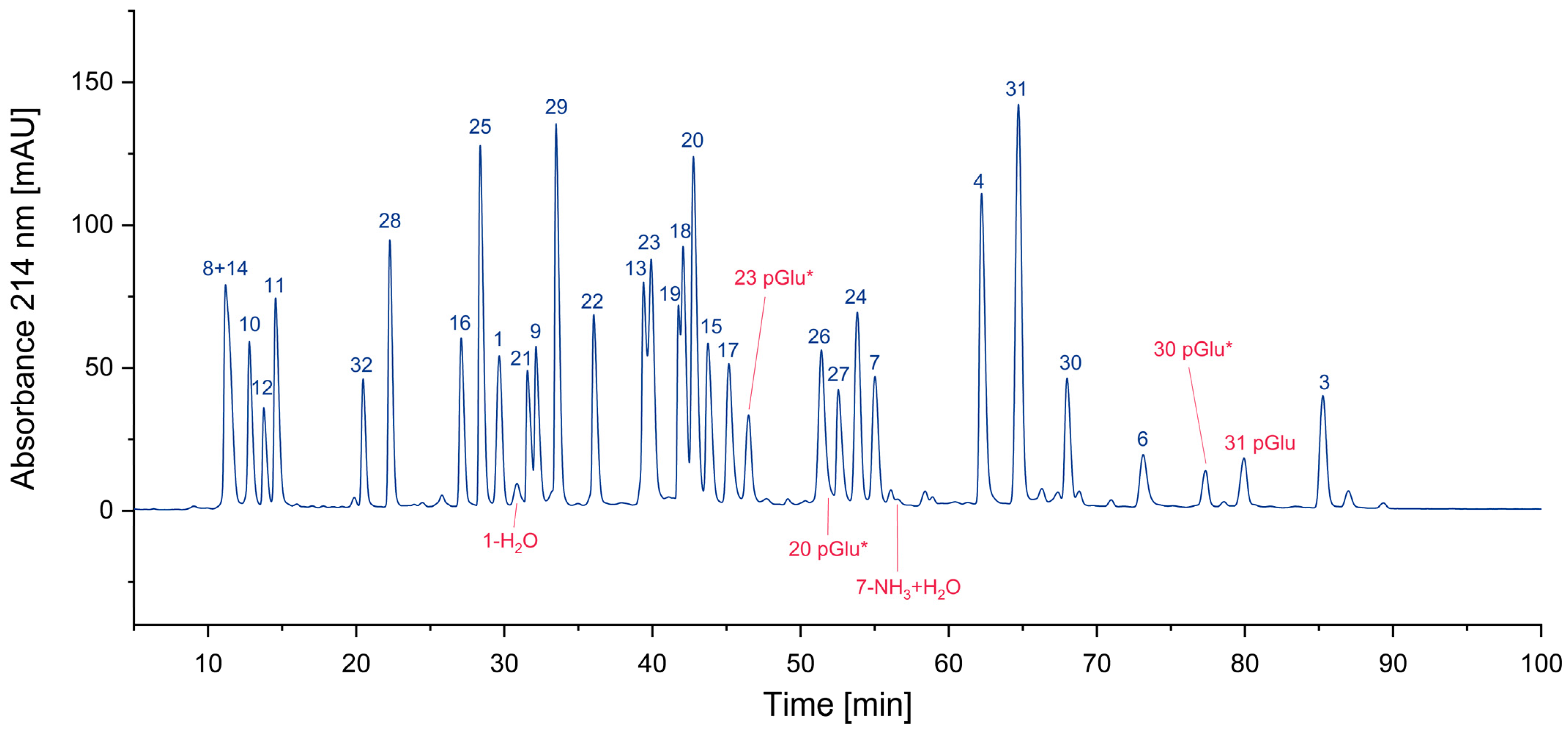
3.1.2. Cyclized Amino Acid Residues and Their Derivatives
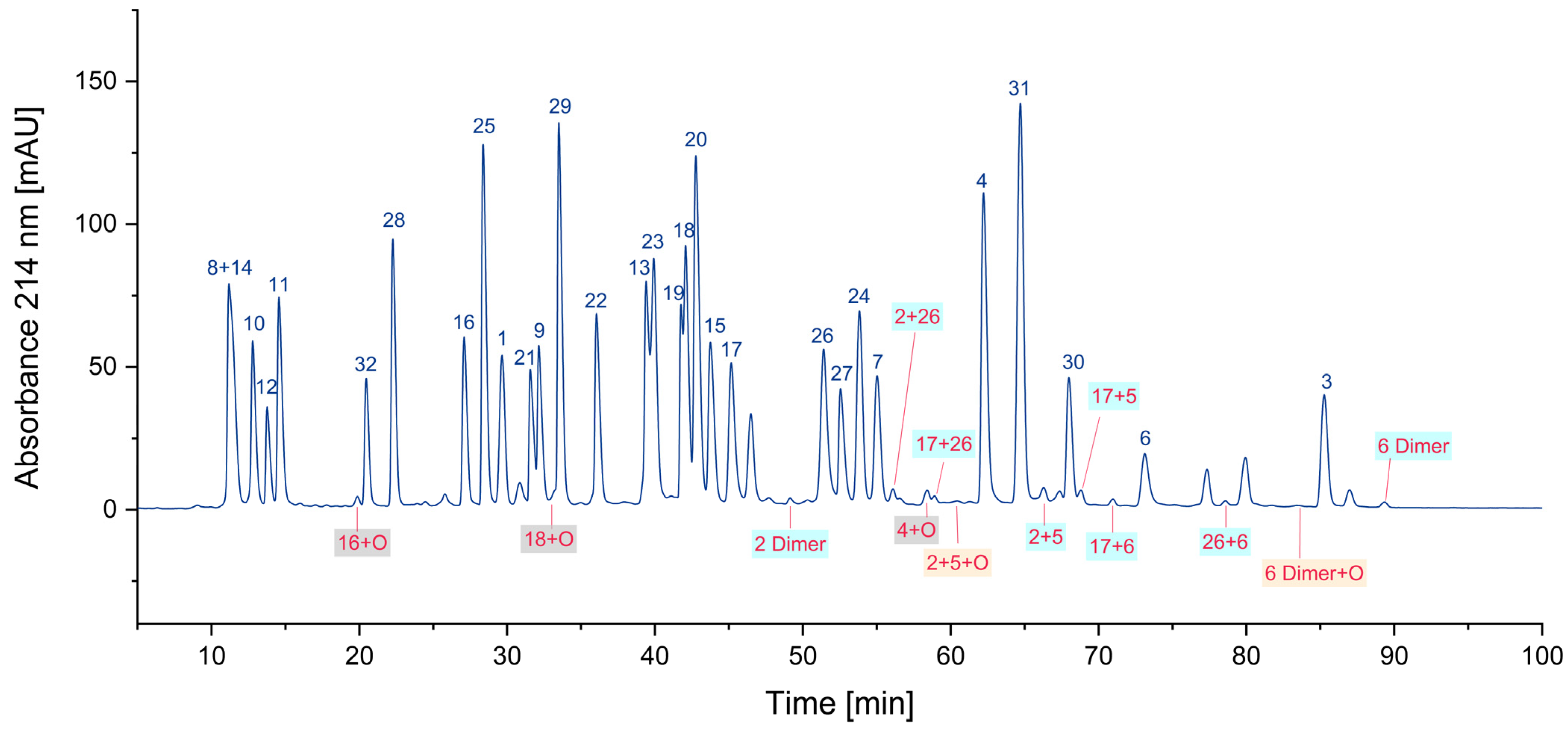
3.1.3. Oxidized Peptides
3.1.4. Overview
3.2. Alkylation of Cysteine Residues
4. Discussion
5. Conclusions
- Choice of different peptide sequences, avoiding any cysteines
- Replacement of cysteine by a more stable amino acid, such as alanine, valine [41], or methylcysteine
- Alkylation of the cysteines by iodoacetamide, iodoacetic acid or other known reagents. This seems to be particularly relevant in the case of analytical studies.
- Addition of reductants, such as DTT, TCEP, cysteine, glutathione, and others
- Strict avoidance of any oxidant, such as oxygen by use of protecting gases, such as helium, argon or nitrogen during synthesis, purification, storage, transportation, and use
- The use of dimethyl sulfoxide (DMSO), which is often recommended as a universal solvent for peptides and peptide pools [42,43,44], should be used with caution, as DMSO can act as an effective reagent for cysteine dimerization under certain conditions [45,46]. Similarly, methionine can be oxidized to methionine sulfoxide under acidic conditions with DMSO [47,48]. The occurrence of oxidized methionine could also be avoided by some of the previously mentioned approaches, particularly points 1, 2 and 5.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tobery, T.W.; Wang, S.; Wang, X.M.; Neeper, M.P.; Jansen, K.U.; McClements, W.L.; Caulfield, M.J. A simple and efficient method for the monitoring of antigen-specific T cell responses using peptide pool arrays in a modified ELISpot assay. J. Immunol. Methods 2001, 254, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.; Bussat, M.C.; Klinguer-Hamour, C.; Goetsch, L.; Aubry, J.P.; Champion, T.; Julien, E.; Haeuw, J.F.; Bonnefoy, J.Y.; Corvaia, N. Stability and CTL activity of N-terminal glutamic acid containing peptides. J. Pept. Res. 2001, 57, 528–538. [Google Scholar] [CrossRef]
- de Beukelaar, J.W.; Gratama, J.W.; Smitt, P.A.S.; Verjans, G.M.; Kraan, J.; Luider, T.M.; Burgers, P.C. The impact of impurities in synthetic peptides on the outcome of T-cell stimulation assays. Rapid Commun. Mass Spectrom. 2007, 21, 1282–1288. [Google Scholar] [CrossRef] [PubMed]
- Verbeken, M.; Wynendaele, E.; Lefebvre, R.A.; Goossens, E.; De Spiegeleer, B. The influence of peptide impurity profiles on functional tissue-organ bath response: The 11-mer peptide INSL6[151-161] case. Anal. Biochem. 2012, 421, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Currier, J.R.; Galley, L.M.; Wenschuh, H.; Morafo, V.; Ratto-Kim, S.; Gray, C.M.; Maboko, L.; Hoelscher, M.; Marovich, M.A.; Cox, J.H. Peptide impurities in commercial synthetic peptides and their implications for vaccine trial assessment. Clin. Vaccine Immunol. 2008, 15, 267–276. [Google Scholar] [CrossRef]
- Finder, V.H.; Vodopivec, I.; Nitsch, R.M.; Glockshuber, R. The Recombinant Amyloid-β Peptide Aβ1-42 Aggregates Faster and Is More Neurotoxic than Synthetic Aβ1-42. J. Mol. Biol. 2010, 396, 9–18. [Google Scholar] [CrossRef]
- Weller, M.G. The Protocol Gap. Methods Protoc. 2021, 4, 12. [Google Scholar] [CrossRef]
- Bosc-Bierne, G.; Ewald, S.; Kreuzer, O.J.; Weller, M.G. Efficient Quality Control of Peptide Pools by UHPLC and Simultaneous UV and HRMS Detection. Separations 2024, 11, 156. [Google Scholar] [CrossRef]
- Zhang, W.J.; Moldovan, I.; Targoni, O.S.; Subbramanian, R.A.; Lehmann, P.V. How much of Virus-Specific CD8 T Cell Reactivity is Detected with a Peptide Pool when Compared to Individual Peptides? Viruses 2012, 4, 2636–2649. [Google Scholar] [CrossRef]
- Castro, A.; Holenya, P.; Eckey, M.; Schulz, M.; Wenschuh, H.; Reimer, U.; Tech, T.; Chan, K.; Janani, R.; Broaten, B.; et al. A novel peptide pool with broad infectious antigen and MHC coverage for use as a positive stimulation control or as a means to elicit general T-cell responsiveness. J. Immunol. 2018, 200, 120.15. [Google Scholar] [CrossRef]
- Patiny, L.; Borel, A. ChemCalc: A Building Block for Tomorrow’s Chemical Infrastructure. J. Chem. Inf. Model. 2013, 53, 1223–1228. [Google Scholar] [CrossRef] [PubMed]
- Currier, J.R.; Kuta, E.G.; Turk, E.; Earhart, L.B.; Loomis-Price, L.; Janetzki, S.; Ferrari, G.; Birx, D.L.; Cox, J.H. A panel of MHC class I restricted viral peptides for use as a quality control for vaccine trial ELISPOT assays. J. Immunol. Methods 2002, 260, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa-Kumagaye, K.; Nishiuchi, Y.; Nishio, H.; Kimura, T. Amino acid deletion products resulting from incomplete deprotection of the Boc group from benzyloxy-methylhistidine residues during solid-phase peptide synthesis. J. Pept. Sci. 2005, 11, 512–515. [Google Scholar] [CrossRef]
- D’Hondt, M.; Bracke, N.; Taevernier, L.; Gevaert, B.; Verbeke, F.; Wynendaele, E.; De Spiegeleer, B. Related impurities in peptide medicines. J. Pharm. Biomed. 2014, 101, 2–30. [Google Scholar] [CrossRef]
- Jad, Y.E.; Govender, T.; Kruger, H.G.; El-Faham, A.; de la Torre, B.G.; Albericio, F. Green Solid-Phase Peptide Synthesis (GSPPS) 3. Green Solvents for Fmoc Removal in Peptide Chemistry. Org. Process Res. Dev. 2017, 21, 365–369. [Google Scholar] [CrossRef]
- Mthethwa, N.; Nandhini, K.P.; Kumar, A.; Sharma, A.; de la Torre, B.G.; Albericio, F. Toward sustainable solid-phase peptide synthesis strategy—Fmoc removal. Green Chem. Lett. Rev. 2024, 17, 2325993. [Google Scholar] [CrossRef]
- Dimarchi, R.D.; Tam, J.P.; Kent, S.B.H.; Merrifield, R.B. Weak Acid-Catalyzed Pyrrolidone Carboxylic-Acid Formation from Glutamine during Solid-Phase Peptide-Synthesis—Minimization by Rapid Coupling. Int. J. Pept. Prot. Res. 1982, 19, 88–93. [Google Scholar] [CrossRef]
- Viau, M.; Létourneau, M.; Sirois-Deslongchamps, A.; Boulanger, Y.; Fournier, A. Study of solid-phase synthesis and purification strategies for the preparation of polyglutamine peptides. Biopolymers 2007, 88, 754–763. [Google Scholar] [CrossRef]
- Khandke, K.M.; Fairwell, T.; Chait, B.T.; Manjula, B.N. Influence of Ions on Cyclization of the Amino Terminal Glutamine Residues of Tryptic Peptides of Streptococcal Pepm49 Protein—Resolution of Cyclized Peptides by Hplc and Characterization by Mass-Spectrometry. Int. J. Pept. Prot. Res. 1989, 34, 118–123. [Google Scholar] [CrossRef]
- Fernandez Garcia, A.; Butz, P.; Trierweiler, B.; Zöller, H.; Stärke, J.; Pfaff, E.; Tauscher, B. Pressure/temperature combined treatments of precursors yield hormone-like peptides with pyroglutamate at the N terminus. J. Agr. Food Chem. 2003, 51, 8093–8097. [Google Scholar] [CrossRef]
- Gazme, B.; Boachie, R.T.; Tsopmo, A.; Udenigwe, C.C. Occurrence, properties and biological significance of pyroglutamyl peptides derived from different food sources. Food Sci. Hum. Well. 2019, 8, 268–274. [Google Scholar] [CrossRef]
- Behrendt, R.; White, P.; Offer, J. Advances in Fmoc solid-phase peptide synthesis. J. Pept. Sci. 2016, 22, 4–27. [Google Scholar] [CrossRef] [PubMed]
- Bodanszky, M.; Tolle, J.C.; Deshmane, S.S.; Bodanszky, A. Side reactions in peptide synthesis. VI. A reexamination of the benzyl group in the protection of the side chains of tyrosine and aspartic acid. Int. J. Pept. Prot. Res. 1978, 12, 57–68. [Google Scholar] [CrossRef]
- Yang, Y.; Sweeney, W.V.; Schneider, K.; Thornqvist, S.; Chait, B.T.; Tam, J.P. Aspartimide Formation in Base-Driven 9-Fluorenylmethoxycarbonyl Chemistry. Tetrahedron Lett. 1994, 35, 9689–9692. [Google Scholar] [CrossRef]
- Nicolas, E.; Pedroso, E.; Giralt, E. Formation of Aspartimide Peptides in Asp-Gly Sequences. Tetrahedron Lett. 1989, 30, 497–500. [Google Scholar] [CrossRef]
- Tam, J.P.; Wong, T.W.; Riemen, M.W.; Tjoeng, F.S.; Merrifield, R.B. Cyclohexyl Ester as a New Protecting Group for Aspartyl Peptides to Minimize Aspartimide Formation in Acidic and Basic Treatments. Tetrahedron Lett. 1979, 20, 4033–4036. [Google Scholar] [CrossRef]
- Lauer, J.L.; Fields, C.G.; Fields, G.B. Sequence dependence of aspartimide formation during 9-fluorenylmethoxycarbonyl solid-phase peptide synthesis. Lett. Pept. Sci. 1995, 1, 197–205. [Google Scholar] [CrossRef]
- Mergler, M.; Dick, F.; Sax, B.; Weiler, P.; Vorherr, T. The aspartimide problem in Fmoc-based SPPS. Part I. J. Pept. Sci. 2003, 9, 36–46. [Google Scholar] [CrossRef]
- Mergler, M.; Dick, F.; Sax, B.; Stähelin, C.; Vorherr, T. The aspartimide problem in Fmoc-based SPPS.: Part II. J. Pept. Sci. 2003, 9, 518–526. [Google Scholar] [CrossRef]
- Mergler, M.; Dick, F. The aspartimide problem in Fmoc-based SPPS. Part III. J. Pept. Sci. 2005, 11, 650–657. [Google Scholar] [CrossRef]
- Liu, S.; Moulton, K.R.; Auclair, J.R.; Zhou, Z.S. Mildly acidic conditions eliminate deamidation artifact during proteolysis: Digestion with endoprotease Glu-C at pH 4.5. Amino Acids 2016, 48, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Borchardt, R.T. Chemical Pathways of Peptide Degradation. III. Effect of Primary Sequence on the Pathways of Deamidation of Asparaginyl Residues in Hexapeptides. Pharm. Res. 1990, 7, 787–793. [Google Scholar] [CrossRef] [PubMed]
- McKerrow, J.H.; Robinson, A.B. Deamidation of Asparaginyl Residues as a Hazard in Experimental Protein and Peptide Procedures. Anal. Biochem. 1971, 42, 565–568. [Google Scholar] [CrossRef] [PubMed]
- Kreuzer, O.J.; Weller, M.G.; Bosc-Bierne, G. Stabilization of N-terminal cysteines in HPLC-HRMS quality control of peptide pools. J. Immunol. 2023, 210, 159.09. [Google Scholar] [CrossRef]
- Müller, T.; Winter, D. Systematic Evaluation of Protein Reduction and Alkylation Reveals Massive Unspecific Side Effects by Iodine-containing Reagents. Mol. Cell Proteom. 2017, 16, 1173–1187. [Google Scholar] [CrossRef]
- Geoghegan, K.F.; Hoth, L.R.; Tan, D.H.; Borzillerl, K.A.; Withka, J.M.; Boyd, J.G. Cyclization of N-terminal S-carbamoylmethylcysteine causing loss of 17 Da from peptides and extra peaks in peptide maps. J. Proteome Res. 2002, 1, 181–187. [Google Scholar] [CrossRef]
- Krokhin, O.V.; Ens, W.; Standing, K.G. Characterizing degradation products of peptides containing N-terminal Cys residues by (off-line high-performance liquid chromatography)/matrix-assisted laser desorption/ionization quadrupole time-of-flight measurements. Rapid Commun. Mass Spectrom. 2003, 17, 2528–2534. [Google Scholar] [CrossRef]
- Reimer, J.; Shamshurin, D.; Harder, M.; Yamchuk, A.; Spicer, V.; Krokhin, O.V. Effect of cyclization of N-terminal glutamine and carbamidomethyl-cysteine (residues) on the chromatographic behavior of peptides in reversed-phase chromatography. J. Chromatogr. A 2011, 1218, 5101–5107. [Google Scholar] [CrossRef]
- Sachs, A.; Moore, E.; Kosaloglu-Yalcin, Z.; Peters, B.; Sidney, J.; Rosenberg, S.A.; Robbins, P.F.; Sette, A. Impact of Cysteine Residues on MHC Binding Predictions and Recognition by Tumor-Reactive T Cells. J. Immunol. 2020, 205, 539–549. [Google Scholar] [CrossRef]
- Bruno, P.M.; Timms, R.T.; Abdelfattah, N.S.; Leng, Y.M.; Lelis, F.J.N.; Wesemann, D.R.; Yu, X.G.; Elledge, S.J. High-throughput, targeted MHC class I immunopeptidomics using a functional genetics screening platform. Nat. Biotechnol. 2023, 41, 980–992. [Google Scholar] [CrossRef]
- Karapetyan, A.R.; Chaipan, C.; Winkelbach, K.; Wimberger, S.; Jeong, J.S.; Joshi, B.; Stein, R.B.; Underwood, D.; Castle, J.C.; Van Dijk, M.A.; et al. TCR Fingerprinting and Off-Target Peptide Identification. Front. Immunol. 2019, 10, 2501. [Google Scholar] [CrossRef] [PubMed]
- Suneetha, P.V.; Schlaphoff, V.; Wang, C.; Stegmann, K.A.; Fytili, P.; Sarin, S.K.; Manns, M.P.; Cornberg, M.; Wedemeyer, H. Effect of peptide pools on effector functions of antigen-specific CD8+T cells. J. Immunol. Methods 2009, 342, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Ay, B.; Streitz, M.; Boisguerin, P.; Schlosser, A.; Mahrenholz, C.C.; Schuck, S.D.; Kern, F.; Volkmer, R. Sorting and pooling strategy: A novel tool to map a virus proteome for CD8 T-cell epitopes. Biopolymers 2007, 88, 64–75. [Google Scholar] [CrossRef]
- Al-kolla, R.; Grifoni, A.; Crotty, S.; Sette, A.; Gianella, S.; Dan, J. Design and validation of HIV peptide pools for detection of HIV-specific CD4 and CD8 T cells. PLoS ONE 2022, 17, e0268370. [Google Scholar] [CrossRef]
- Tam, J.P.; Wu, C.R.; Liu, W.; Zhang, J.W. Disulfide Bond Formation in Peptides by Dimethyl-Sulfoxide—Scope and Applications. J. Am. Chem. Soc. 1991, 113, 6657–6662. [Google Scholar] [CrossRef]
- Otaka, A.; Koide, T.; Shide, A.; Fujii, N. Application of Dimethylsulphoxide (DMSO)/Trifluoroacetic Acid (TFA) Oxidation to the Synthesis of Cystine-Containing Peptide. Tetrahedron Lett. 1991, 32, 1223–1226. [Google Scholar] [CrossRef]
- Shechter, Y. Selective Oxidation and Reduction of Methionine Residues in Peptides and Proteins by Oxygen-Exchange between Sulfoxide and Sulfide. J. Biol. Chem. 1986, 261, 66–70. [Google Scholar] [CrossRef]
- Lipton, S.H.; Bodwell, C.E. Specific Oxidation of Methionine to Methionine Sulfoxide by Dimethyl-Sulfoxide. J. Agr. Food Chem. 1976, 24, 26–31. [Google Scholar] [CrossRef]
- Yang, Y. Side Reactions in Peptide Synthesis; Academic Press: Cambridge, MA, USA, 2016; p. 146. [Google Scholar]
- Behrendt, R.; Huber, S.; White, P. Preventing aspartimide formation in Fmoc SPPS of Asp-Gly containing peptides practical aspects of new trialkylcarbinol based protecting groups. J. Pept. Sci. 2016, 22, 92–97. [Google Scholar] [CrossRef]
- Wade, J.D.; Mathieu, M.N.; Macris, M.; Tregear, G.W. Base-induced side reactions in Fmoc-solid phase peptide synthesis: Minimization by use of piperazine as N-deprotection reagent. Lett. Pept. Sci. 2000, 7, 107–112. [Google Scholar] [CrossRef]
- Quibell, M.; Owen, D.; Packman, L.C.; Johnson, T. Suppression of Piperidine-Mediated Side Product Formation for Asp(Obu(T))-Containing Peptides by the Use of N-(2-Hydroxy-4-methoxybenzyl) (Hmb) Backbone Amide Protection. J. Chem. Soc. Chem. Comm. 1994, 7, 2343–2344. [Google Scholar] [CrossRef]
- Neumann, K.; Farnung, J.; Baldauf, S.; Bode, J.W. Prevention of aspartimide formation during peptide synthesis using cyanosulfurylides as carboxylic acid-protecting groups. Nat. Commun. 2020, 11, 982. [Google Scholar] [CrossRef]
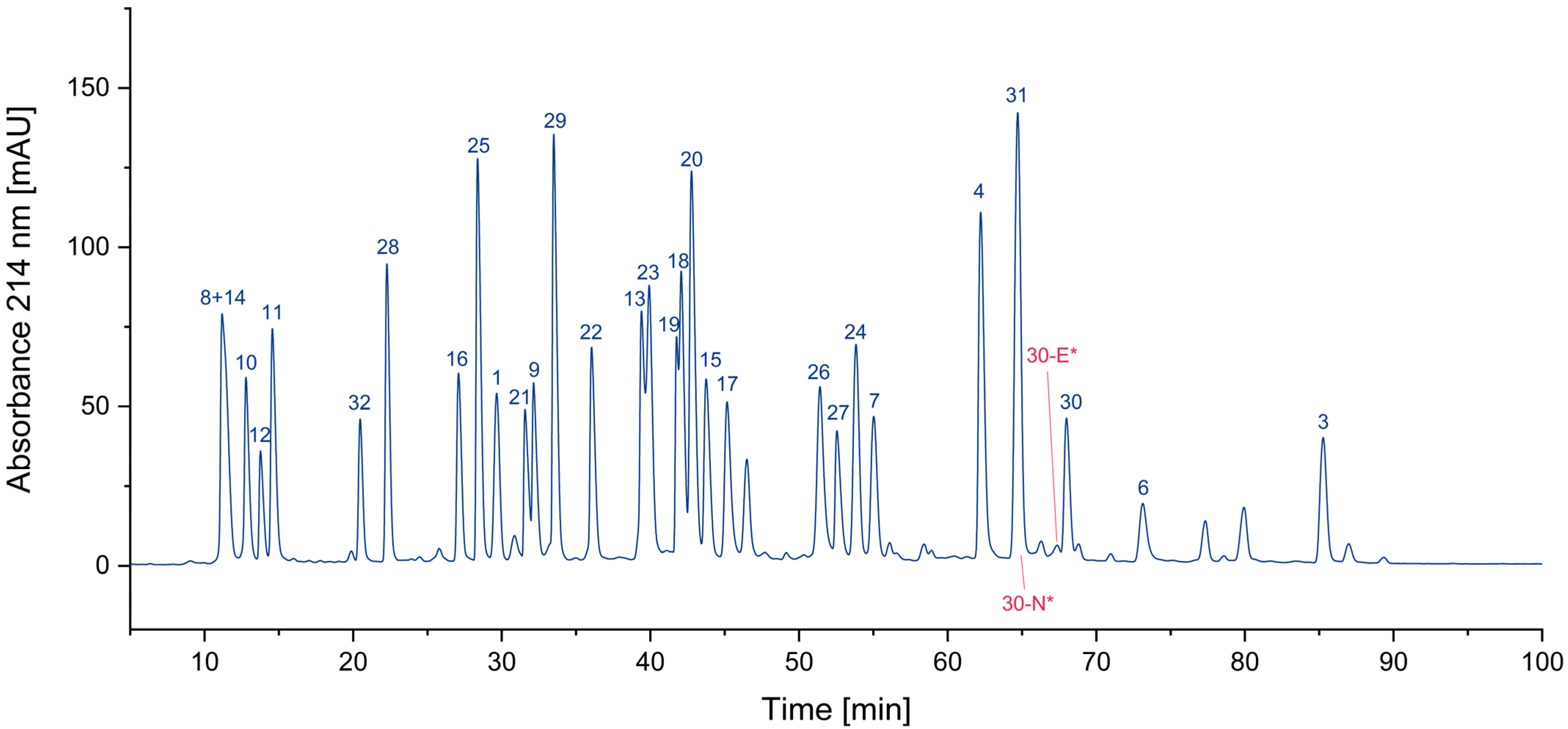


| Peptide | Sequence | Exact Mass [11,12] |
|---|---|---|
| 1 | VSDGGPNLY | 920.4240 |
| 2 | CTELKLSDY | 1070.4954 |
| 3 | GILGFVFTL | 965.5586 |
| 4 | FMYSDFHFI | 1205.5216 |
| 5 | CLGGLLTMV | 905.4715 |
| 6 | GLCTLVAML | 919.4871 |
| 7 | NLVPMVATV | 942.5208 |
| 8 | KTGGPIYKR | 1018.5924 |
| 9 | RVLSFIKGTK | 1147.7077 |
| 10 | ILRGSVAHK | 979.5927 |
| 11 | RVRAYTYSK | 1142.6196 |
| 12 | RLRAEAQVK | 1069.6356 |
| 13 | SIIPSGPLK | 910.5488 |
| 14 | AVFDRKSDAK | 1135.5986 |
| 15 | IVTDFSVIK | 1020.5856 |
| 16 | ATIGTAMYK | 954.4845 |
| 17 | DYCNVLNKEF | 1243.5543 |
| 18 | LPFDKTTVM | 1050.5420 |
| 19 | RPPIFIRRL | 1166.7400 |
| 20 | ELRSRYWAI | 1192.6353 |
| 21 | RAKFKQLL | 1002.6338 |
| 22 | FLRGRAYGL | 1051.5927 |
| 23 | QAKWRLQTL | 1142.6560 |
| 24 | SDEEEAIVAYTL | 1338.6191 |
| 25 | SRYWAIRTR | 1207.6574 |
| 26 | ASCMGLIY | 856.3823 |
| 27 | RRIYDLIEL | 1189.6819 |
| 28 | YPLHEQHGM | 1110.4917 |
| 29 | IPSINVHHY | 1078.5560 |
| 30 | EENLLDFVRF | 1280.6401 |
| 31 | EFFWDANDIY | 1318.5506 |
| 32 | TPRVTGGGAM | 945.4702 |
| Peptide | Sequence | ∆ mass [Da] | Impurity |
|---|---|---|---|
| 1 | VSDGGPNLY | −18 | aspartimide |
| 2 | CTELKLSDY | - | |
| 4 | FMYSDFHFI | - | |
| 7 | NLVPMVATV | +1 | deamidation |
| 12 | RLRAEAQVK | - | |
| 14 | AVFDRKSDAK | - | |
| 15 | IVTDFSVIK | - | |
| 17 | DYCNVLNKEF | - | |
| 18 | LPFDKTTVM | - | |
| 20 | ELRSRYWAI | −18 | pyroglutamate |
| 21 | RAKFKQLL | - | |
| 23 | QAKWRLQTL | −17 | pyroglutamate |
| 24 | SDEEEAIVAYTL | - | |
| 27 | RRIYDLIEL | - | |
| 28 | YPLHEQHGM | - | |
| 29 | IPSINVHHY | - | |
| 30 | EENLLDFVRF | −18 | pyroglutamate |
| 31 | EFFWDANDIY | −18 | pyroglutamate |
| Peptide No. | Relative Area (UV) [%] |
|---|---|
| 1 | 82 |
| 1-H2O | 18 |
| 31 | 87 |
| 31 pGlu | 13 |
| 16 | 92 |
| 16+O | 8 |
| 4 | 94 |
| 4+O | 6 |
| 6 | 71 |
| 6 Dimer | 6 |
| 6 Dimer+O | 5 |
| 17+6 | 9 |
| 26+6 | n.a. |
| 2+6+O | n.a. |
| 2+6 | n.a. |
| 17+6+O | n.a. |
| 6+5 | n.a. |
| 6+5+O | n.a. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bosc-Bierne, G.; Weller, M.G. Investigation of Impurities in Peptide Pools. Separations 2025, 12, 36. https://doi.org/10.3390/separations12020036
Bosc-Bierne G, Weller MG. Investigation of Impurities in Peptide Pools. Separations. 2025; 12(2):36. https://doi.org/10.3390/separations12020036
Chicago/Turabian StyleBosc-Bierne, Gaby, and Michael G. Weller. 2025. "Investigation of Impurities in Peptide Pools" Separations 12, no. 2: 36. https://doi.org/10.3390/separations12020036
APA StyleBosc-Bierne, G., & Weller, M. G. (2025). Investigation of Impurities in Peptide Pools. Separations, 12(2), 36. https://doi.org/10.3390/separations12020036







