1. Introduction
Chlorination is one of the most widely applied disinfection methods to ensure the microbiological safety of drinking water. However, this process generates disinfection by-products (DBPs) through reactions between chlorine and natural organic matter (NOM) [
1,
2,
3,
4,
5]. Among these, halobenzoquinones—particularly chlorinated benzoquinones (CBQs)—have recently attracted increasing attention due to their persistence, high reactivity, and potential mutagenic and cytotoxic effects [
6,
7]. One of the most toxic DBPs identified to date is 2,6-dichlorobenzoquinone (DCBQ), which is associated with oxidative stress, DNA damage, and cellular dysfunction in both aquatic organisms and human cells [
8,
9]. DCBQ formation has been linked to the chlorination of aromatic compounds, including phenolic derivatives and substituted antioxidants [
10], and its occurrence has been confirmed in drinking water distribution systems and recreational environments such as swimming pools [
11,
12,
13]. As regulatory frameworks for DBPs are still evolving, the development of efficient and sustainable remediation technologies remains a priority [
4].
Various treatment strategies have been proposed for the removal of DCBQ and related halobenzoquinones. Conventional methods such as activated carbon adsorption and ozonation [
14,
15,
16] offer partial removal but may generate secondary by-products or fail to achieve complete mineralization. Advanced oxidation processes (AOPs) encompass a group of chemical treatment technologies designed to remove organic pollutants from water through the generation of highly reactive species, primarily hydroxyl radicals (HO
•). These radicals exhibit non-selective and powerful oxidative capacity, enabling the breakdown of a wide range of persistent contaminants into less harmful or mineralized forms. AOPs include methods such as UV/H
2O
2, ozonation, Fenton and photo-Fenton reactions, and combinations thereof. Their effectiveness depends on the continuous generation of reactive oxygen species and the ability to sustain radical-mediated reactions under controlled conditions. Due to their versatility and efficiency, AOPs have gained prominence in environmental engineering as promising tools for the remediation of emerging pollutants, including disinfection by-products, pharmaceuticals, and endocrine disruptors [
17].
In recent years, the field of photo-Fenton and Fenton-like reactions has evolved significantly, with the development of novel photo-induced systems that operate under milder conditions and offer enhanced degradation performance. These advanced approaches include heterogeneous catalysts, visible-light activation, and hybrid systems that integrate photocatalysis with Fenton chemistry. Such innovations aim to overcome traditional limitations related to pH sensitivity, reagent consumption, and sludge formation, while improving the selectivity and sustainability of the process. For instance, recent studies have demonstrated the use of iron-based composites and solar-driven Fenton-like reactions for the efficient degradation of emerging contaminants [
18,
19]. These developments highlight the dynamic progress in the field and reinforce the relevance of optimizing iron dosage and reaction conditions to achieve scalable and eco-efficient water treatment solutions.
Among these, the photo-Fenton process—combining UV irradiation, hydrogen peroxide, and ferrous ions—has demonstrated high efficiency in water treatment [
20]. However, its large-scale application faces limitations related to pH control, reagent consumption, and iron sludge management [
20,
21]. Elevated Fe
2+ dosages enhance hydroxyl radical production and pollutant degradation but may also cause turbidity, sludge generation, and accumulation of toxic intermediates [
22,
23,
24]. It could be attributed to several physicochemical phenomena. First, excess ferrous ions can undergo rapid oxidation to ferric species under UV irradiation, especially in the presence of hydrogen peroxide. These ferric ions tend to hydrolyze and precipitate as iron hydroxides, which contribute to colloidal turbidity and sediment formation. Second, the accumulation of intermediate organic compounds—such as hydroxylated quinones and aromatic fragments—can complex with iron species, forming stable organometallic aggregates that resist further oxidation and settle as sludge. Third, the high ionic strength and radical flux in the system may promote coagulation and flocculation of suspended matter, further increasing turbidity. These mechanisms highlight the dual role of iron: while essential for hydroxyl radical generation, its excess can lead to secondary pollution through particulate formation. Therefore, understanding the speciation and reactivity of iron under photo-Fenton conditions is crucial to minimize undesired by-products and optimize water clarity and treatment sustainability. Conversely, insufficient iron concentrations reduce treatment efficiency and hinder mineralization. Therefore, optimizing iron dosage is essential to balance degradation performance with sustainability and minimize adverse effects.
A critical parameter in photo-Fenton treatment is ferrous ion concentration [
23,
24]. Elevated Fe
2+ dosages enhance hydroxyl radical production and pollutant degradation but may also cause turbidity, sludge generation, and accumulation of toxic intermediates. Conversely, insufficient iron concentrations reduce treatment efficiency and hinder mineralization [
25]. Although strategies have been proposed to optimize iron dosage and allow operation under milder conditions, the balance between efficiency and sustainability remains under debate. The photo-Fenton process is recognized for its high oxidative efficiency; its practical implementation is often constrained by the need for acidic conditions (typically pH ≈ 3.0) and the generation of iron-containing sludge. To address these limitations, several strategies have been proposed to optimize iron dosage and enable operation under milder, more sustainable conditions. One widely explored approach involves the use of iron-chelating agents, such as ethylenediamine-N,N’-disuccinic acid (EDDS) or citrate, which stabilize Fe
2+/Fe
3+ in solution and allow the photo-Fenton reaction to proceed effectively at near-neutral pH [
26,
27]. This not only reduces the need for pH adjustment but also minimizes iron precipitation and sludge formation. Another strategy is the application of heterogeneous iron-based catalysts (e.g., supported iron oxides or zero-valent iron), which facilitate catalyst recovery and reuse, thereby improving process sustainability and reducing secondary pollution [
28,
29]. Furthermore, solar-driven photo-Fenton systems have gained attention as cost-effective alternatives, leveraging natural sunlight to activate the process and reduce energy consumption [
30].
Despite these advancements, the balance between treatment efficiency and environmental sustainability remains a subject of ongoing research. Challenges such as the control of iron speciation, the fate of intermediate by-products, and the scalability of optimized systems continue to be critical bottlenecks. Therefore, further studies are needed to refine these strategies and develop robust, low-impact photo-Fenton technologies suitable for real-world water treatment applications.
This study investigates the photo-Fenton degradation of DCBQ under controlled laboratory conditions, with emphasis on iron dosage optimization. Kinetic modeling and monitoring of aromaticity, color, dissolved oxygen, and turbidity were performed to assess contaminant removal and water quality transformation. The results demonstrate that optimal Fe2+ dosage enables complete DCBQ removal, accelerates the decay of aromatic and chromophoric intermediates, and minimizes turbidity and sludge formation. These findings highlight the potential of optimized photo-Fenton systems as sustainable and effective technologies for the remediation of chlorinated DBPs in water.
While numerous studies have explored the application of photo-Fenton processes for the degradation of organic pollutants, this work presents a distinctive contribution by focusing on the fine-tuning of ferrous ion dosage for the efficient and sustainable removal of 2,6-dichlorobenzoquinone (DCBQ), a highly toxic and persistent disinfection by-product. Unlike previous studies that often overlook the formation and fate of aromatic and chromophoric intermediates, this research provides a comprehensive kinetic and mechanistic analysis of DCBQ degradation, aromaticity loss, color evolution, and dissolved oxygen consumption. The identification of an optimal Fe2+ dosage demonstrates a balanced strategy that maximizes pollutant removal while minimizing turbidity and secondary by-products. This level of optimization, supported by second-order kinetic modeling and multi-parameter monitoring, advances the understanding of photo-Fenton systems and reinforces their role as scalable and eco-efficient technologies for water detoxification.
This study introduces several original contributions that distinguish it from prior work. It focuses on DCBQ, a highly toxic and underexplored disinfection by-product. While photo-Fenton processes are well-documented, their application to DCBQ—particularly with detailed kinetic modeling and intermediate tracking—is not widely reported. The work identifies an optimal Fe2+ dosage that balances degradation efficiency with minimal turbidity and by-product formation. This precise optimization, supported by kinetic constants and water quality indicators, adds practical value for real-world applications. Unlike many previous studies that focus solely on pollutant removal, this research integrates aromaticity, color, turbidity, and dissolved oxygen as performance indicators, offering a comprehensive view of water quality transformation. The identification of second-order kinetics for both DCBQ and its intermediates, along with the detailed discussion of transient aromatic and chromophoric species, provides new mechanistic understanding of degradation pathways. Furthermore, the study emphasizes minimal reagent use and sludge generation, aligning with current trends in eco-efficient water treatment—a perspective not always prioritized in earlier photo-Fenton research.
Although this study was conducted under controlled laboratory conditions, the identification of an optimal iron dosage that minimizes turbidity and potential sludge formation provides valuable insights for addressing key limitations commonly encountered in large-scale photo-Fenton applications. These findings contribute to the development of more sustainable treatment strategies that can be adapted to real-world scenarios.
2. Materials and Methods
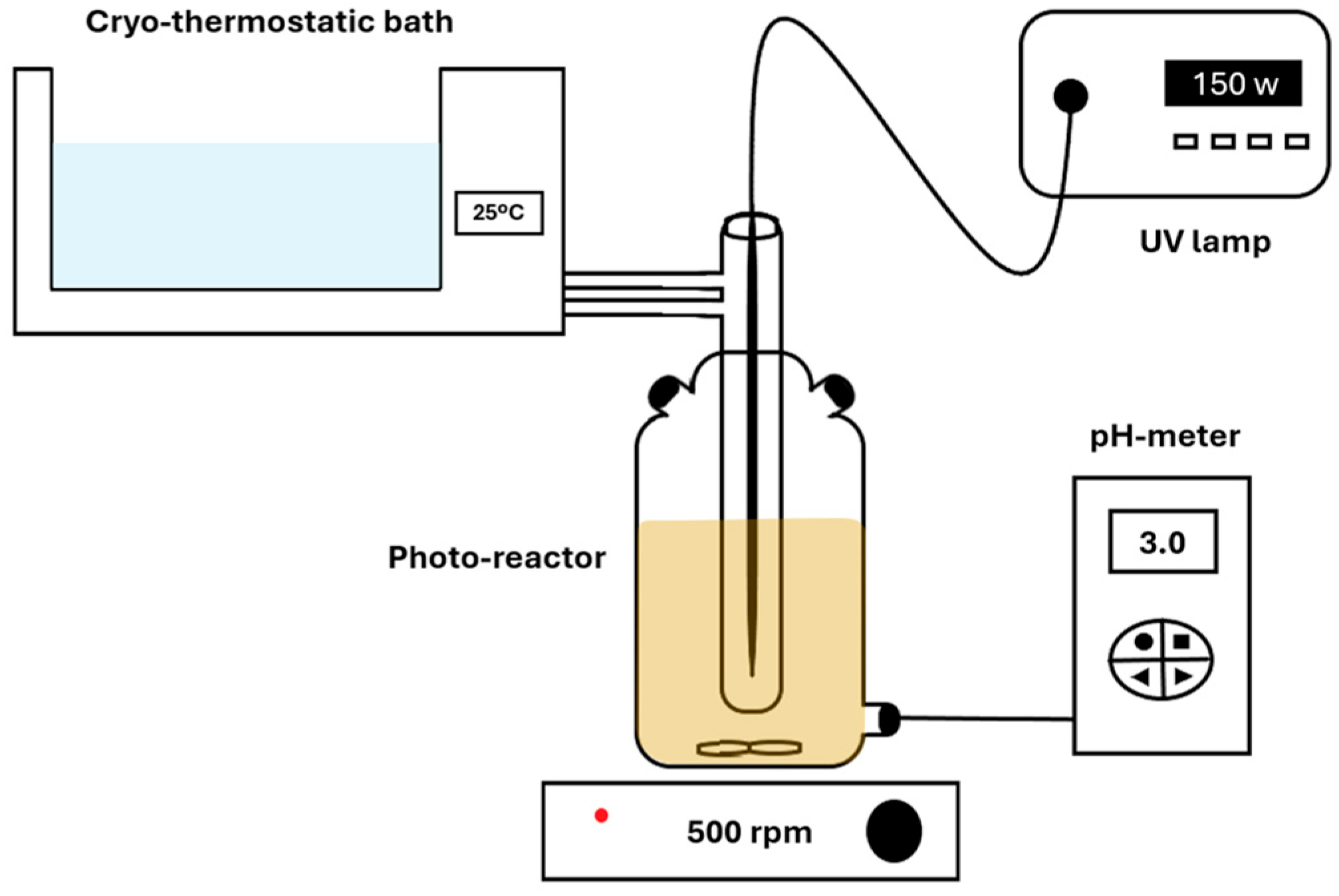
The experimental tests were carried out in a photocatalytic reactor (
Figure 1) equipped with a 150 W medium-pressure mercury lamp (Heraeus) and a magnetic stirring system [
22]. The reactor was filled with 1.0 L of an aqueous solution of 2,6-dichlorobenzoquinone (DCBQ, 98%, Sigma-Aldrich, Darmstadt, Germany; C
6H
2Cl
2O
2) at an initial concentration of 50.0 mg/L. The pH of the solution was adjusted to 3.0 using 0.1 M HCl (Panreac, Castellar del Vallès, Spain; 37%
v/v HCl) and monitored with a pH meter (Kent EIL9142, Cambridge, UK). All experiments were conducted at pH 3.0, which corresponds to the optimal acidic conditions typically required for efficient photo-Fenton reactions. The decision to maintain a constant pH was based on the need to isolate the effect of iron dosage on the degradation performance. The temperature was maintained at 25 °C using a recirculating thermostatic bath (Frigiterm-P, Selecta, Barcelona, Spain). Baseline measurements of DCBQ concentration, color, turbidity, and aromaticity were recorded before irradiation.
The photocatalytic process was initiated by turning on the UV lamp, followed by the addition of hydrogen peroxide at an initial concentration of 1.0 mM (Panreac, Castellar del Vallès, Spain; 30% w/v H2O2). Subsequently, ferrous ions were introduced as a catalyst in the range of 0.0 to 1.0 mg/L (Panreac, FeSO4·7H2O, 99%). Each experiment lasted 120 min, during which water quality parameters were periodically monitored every 4 to 5 min. Dissolved oxygen (mg/L) was determined with a portable oxygen meter (model HI 9142, Hanna Instruments S.L., Eibar, Spain). Aromaticity (254 nm) and color (455 nm) were measured using a UV/Vis spectrophotometer (model V-630, Jasco, Madrid, Spain). The color of the water samples was monitored spectrophotometrically at 455 nm, a wavelength commonly used to quantify visible coloration associated with chromophoric and conjugated aromatic intermediates. Although these species absorb broadly across the visible range, the absorbance at 455 nm provides a representative measure of the overall color intensity and was used consistently throughout the experiment to evaluate chromophore generation and degradation.
No chemical quenching agent was used to stop the reaction with hydrogen peroxide. Instead, all measurements were performed immediately after sample withdrawal from the reactor to ensure that the data accurately reflected the state of the system at the end of irradiation. This approach was adopted to avoid introducing external reagents that could interfere with the sensitive analytical parameters being monitored, such as aromaticity, color, and dissolved oxygen. The rapid handling and direct analysis minimized the risk of ongoing reactions and ensured the reliability of the results.
Quantification of DCBQ was performed by high-performance liquid chromatography (HPLC) using a Waters 2695 system equipped with a dual-wavelength absorbance detector (model 2487, Waters Cromatografía S.A., Cerdanyola del Vallès, Spain). Separation was carried out on a Zorbax Eclipse PAH column (150 mm × 4.6 mm; 5 μm) with a matching guard column (4.6 mm × 12.5 mm), both supplied by Agilent Technologies (Santa Clara, CA, USA). The mobile phase consisted of water (A) and acetonitrile (B) with the following gradient program: 95% A–5% B (0 min), 90% A–10% B (3 min), 10% A–90% B (5 min), 100% B (10 min), returning to 95% A–5% B at 12 min for re-equilibration. The flow rate was set at 0.8 mL/min, the column temperature was maintained at 25 °C, and detection was performed at 254 nm.
3. Results
3.1. Kinetic Modeling of 2,6-Dichlorobenzoquinone Oxidation
The degradation of 2,6-dichlorobenzoquinone (DCBQ) was analyzed through kinetic modeling under UV, UV/H2O2, and photo-Fenton conditions. Kinetic studies are essential for understanding the mechanisms governing the degradation of halogenated quinones in advanced oxidation processes (AOPs). Among these compounds, DCBQ is particularly relevant due to its persistence in aquatic environments and its potential toxicity. The application of UV irradiation, UV/H2O2, and photo-Fenton processes generates reactive oxygen species (ROS), with hydroxyl radicals (HO•) being the predominant oxidizing agents. Since the oxidative pathways of quinones typically involve electrophilic attack by hydroxyl radicals, kinetic modeling provides valuable insight into the relative contribution of direct photolysis and radical-mediated reactions.
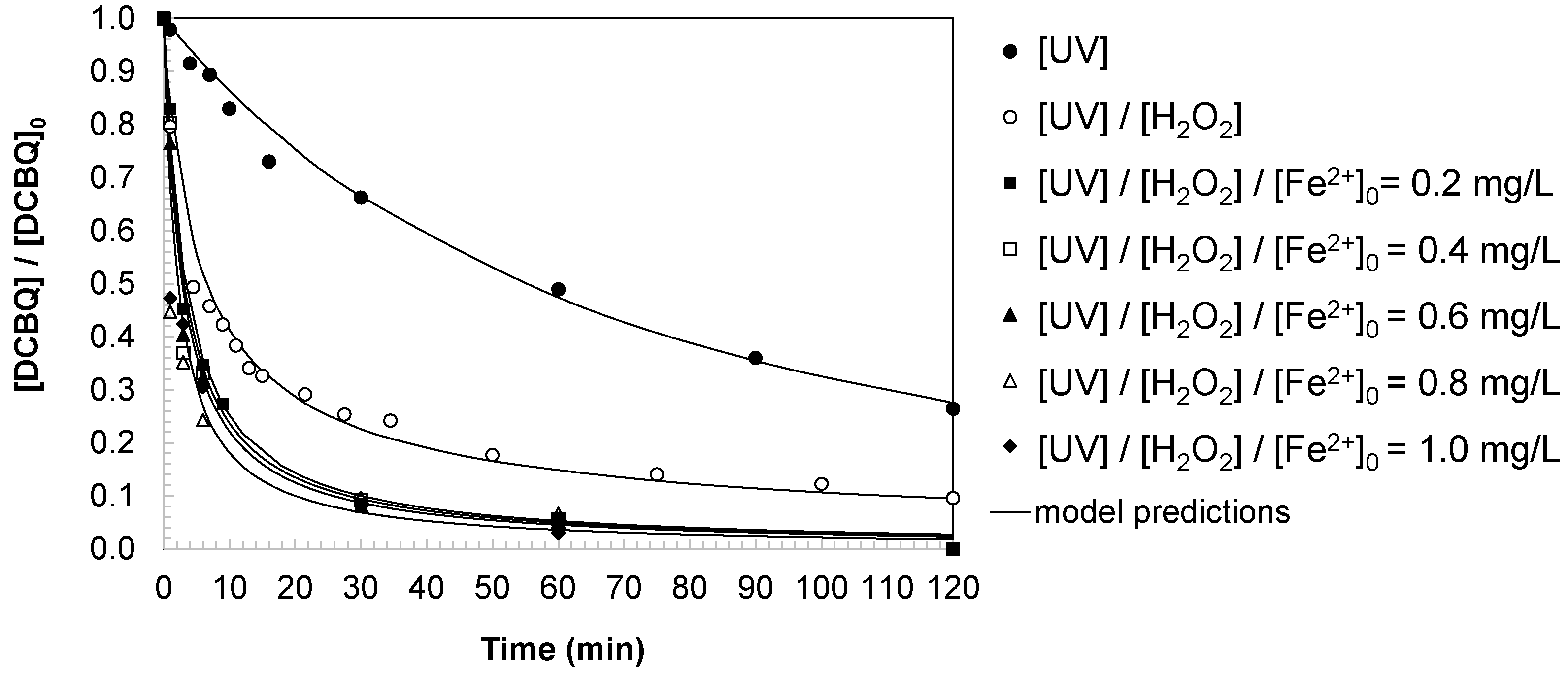
Experimental data (
Figure 2) demonstrated that the oxidation process followed a second-order rate law (Equations (1)–(3)). This behavior can be attributed to the bimolecular nature of the reaction between DCBQ and hydroxyl radicals, the latter being the primary oxidizing species generated in the system.
The general second-order kinetic expression is given by
Since hydroxyl radicals are continuously generated from H
2O
2 photolysis and from the photo-Fenton cycle, their concentration can be considered in large excess with respect to DCBQ. In the photo-Fenton system, hydroxyl radicals are continuously generated through both hydrogen peroxide photolysis and the Fe
2+/Fe
3+ catalytic cycle. Although the instantaneous steady-state concentration of HO
• is extremely low—typically in the range of 10
−12 to 10
−10 M due to their short lifetime and rapid consumption—the overall radical flux sustained during irradiation is several orders of magnitude greater than the amount of DCBQ initially present (0.28 mM). This continuous regeneration of HO
• justifies the assumption that the radical concentration remains effectively constant, or in large excess, relative to DCBQ. Consequently, the degradation kinetics can be treated under pseudo-steady-state conditions, where the apparent rate constant (k
DCBQ) integrates both the intrinsic reactivity of DCBQ toward HO
• and the steady production rate of radicals in the photo-Fenton system. Under this assumption, [HO
•] may be regarded as pseudo-constant, leading to a simplified second-order kinetic expression dependent only on the DCBQ concentration:
where k
DCBQ (L/mg min) is the apparent second-order rate constant, which incorporates the effective concentration of hydroxyl radicals under the studied conditions.
Integration of the above rate law yields:
The fitted kinetic constants for the different systems are presented in
Table 1. The results indicated that direct UV irradiation produced moderate degradation with a rate constant of 0.0022 (L/mg min), leaving a residual concentration of 12.4 mg/L. The addition of H
2O
2 under UV conditions slightly reduced the apparent rate constant, likely due to scavenging effects of excess peroxide, but improved the final removal efficiency. In contrast, the introduction of ferrous ion as a catalyst significantly enhanced the oxidation rate, with maximum values of 0.0090 (L/mg min) at [Fe]
0 > 0.8 mg/L, achieving complete DCBQ removal within 120 min.
Model predictions (
Figure 2) exhibited agreement with experimental data, validating the assumption of second-order kinetics and confirming that hydroxyl radical attack governs the degradation pathway of DCBQ under the tested advanced oxidation processes.
3.2. Loss of Water Aromaticity During DCBQ Oxidation
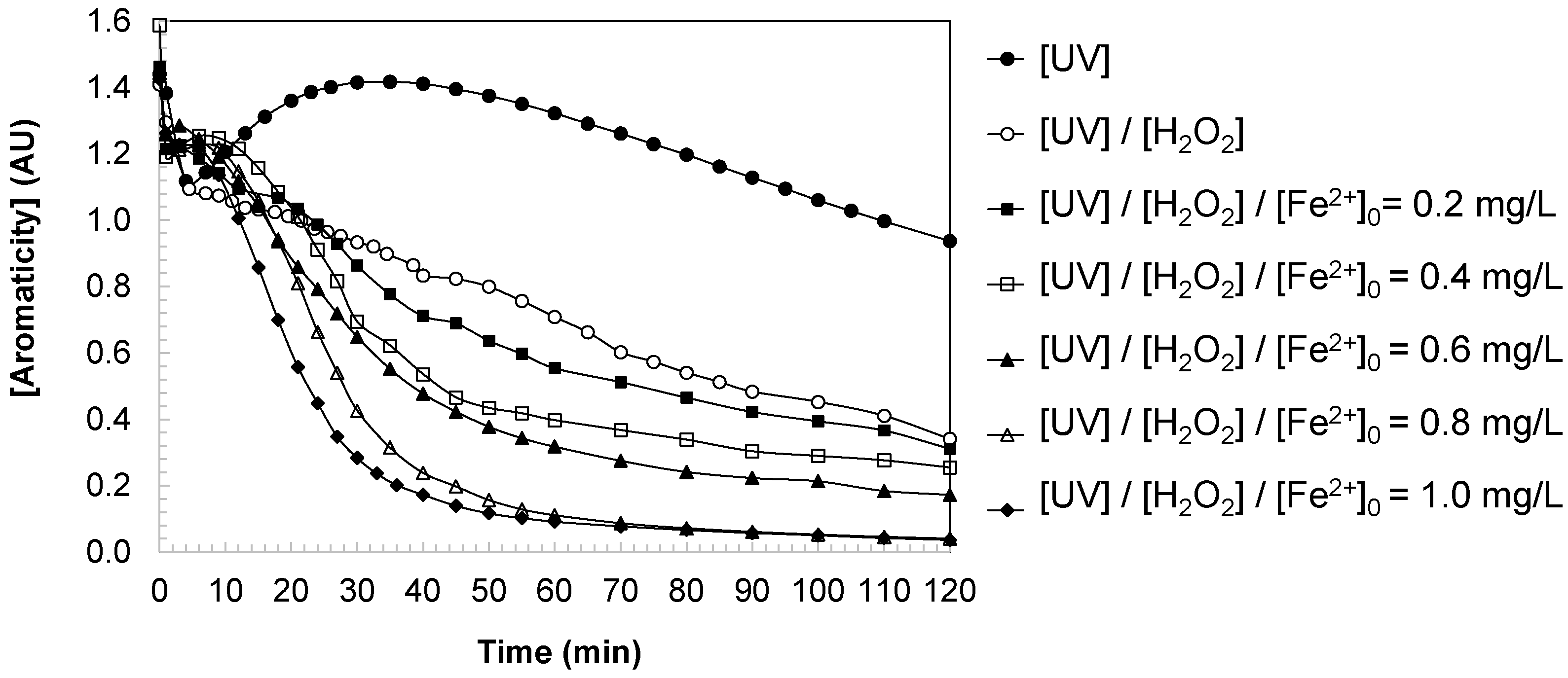
The evolution of water aromaticity (expressed as absorbance units, AU at 254 nm), provided insight into the structural transformations occurring during DCBQ oxidation under different advanced oxidation conditions (
Figure 3). The experimental dynamics of aromaticity revealed three clearly differentiated stages: (i) an initial sharp decline during the first 2–4 min of reaction, (ii) a subsequent transient increase associated with the accumulation of aromatic intermediates, and (iii) a decrease that could be described by second-order kinetics until a baseline value was reached (
Figure 4).
Stage I: Rapid initial decline (2–4 min). Immediately upon UV irradiation and the addition of H2O2/Fe2+, a significant fraction of DCBQ molecules was consumed through two parallel mechanisms: direct photolysis and electrophilic attack by hydroxyl radical. Given the high molar absorptivity of DCBQ at 254 nm, its prompt removal led to a steep decrease in aromaticity. This behavior reflects the effective disruption of the parent aromatic structure, confirming the high reactivity of HO• towards halogenated quinones in the early stages of the process.
Stage II: Transient increase in absorbance. Following the initial decline, a short-lived increase in aromaticity was observed. This phenomenon is consistent with the formation of aromatic and polyaromatic intermediate species generated during the partial oxidation of DCBQ. Likely contributors include hydroxylated quinones, conjugated ring-retaining derivatives, and low molecular weight polyaromatic intermediate species, all of which absorb strongly at 254 nm. The accumulation of these compounds temporarily elevates the absorbance, reflecting the release of by-products with conjugated structures into the solution. When the rate of intermediate generation temporarily exceeded their degradation, an increase in aromaticity was recorded. This stage highlights the complexity of the oxidative pathways, where fragmentation and substitution reactions coexist with the transient stabilization of new aromatic species.
Under UV irradiation alone, a noticeable increase in aromaticity was observed during the second stage of the reaction. This behavior can be attributed to the formation and accumulation of partially oxidized aromatic intermediates, such as hydroxylated quinones and conjugated polyaromatic derivatives, produced by the limited photolysis of DCBQ in the absence of additional oxidants. Since the UV process generates reactive oxygen species only through direct photolysis of water or DCBQ itself, the oxidative capacity is relatively low. As a result, intermediate compounds with aromatic or conjugated structures temporarily accumulate faster than they are degraded, leading to the observed rise in absorbance at 254 nm. This transient increase thus reflects an incomplete oxidation pathway, where UV light promotes structural transformation but not full mineralization of the aromatic species.
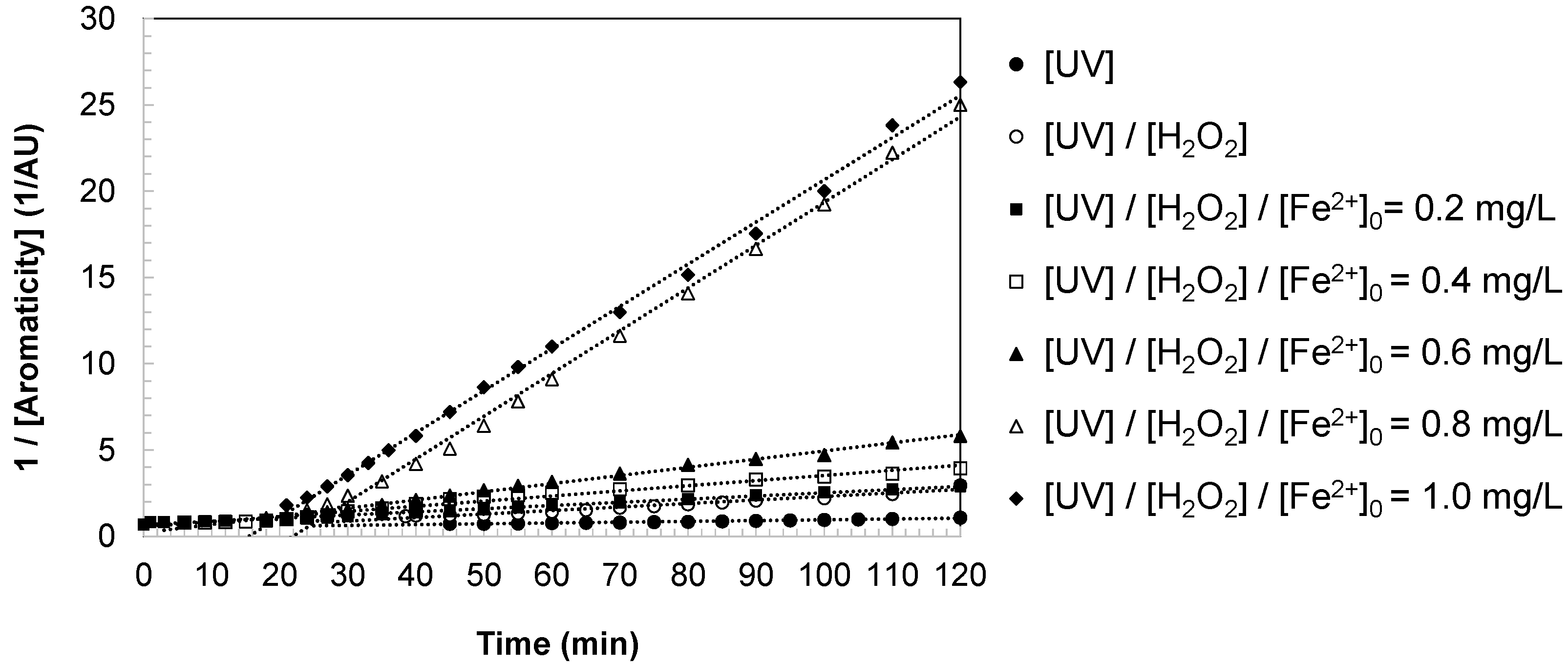
Stage III: Progressive second-order decay (
Figure 4). After the transient maximum, aromaticity underwent a sustained decrease that followed second-order kinetics. As the parent compound was depleted, the rate of formation of intermediates diminished, while hydroxyl radicals progressively fragmented existing aromatic structures. The concentration of aromatic intermediates therefore decayed until only non-aromatic or refractory residuals remained. At extended reaction times, the absorbance stabilized, yielding a steady-state baseline corresponding to small organic acids, non-aromatic residues, and final inorganic mineralization products such as CO
2.
This kinetic profile provides strong evidence that the degradation of aromaticity during DCBQ oxidation is governed by radical-mediated pathways. The initial elimination of the parent compound, the transient accumulation of polyaromatic intermediates, and the subsequent second-order decay together reflect the sequential dismantling of conjugated systems.
The kinetic constants summarized in
Table 1 confirm the strong dependence of aromaticity degradation on the oxidation system and catalyst dosage. Under direct UV irradiation, the rate constant for aromaticity degradation was low (k
Arom = 0.0047 L/mg·min), with a high final aromaticity of 0.937 AU, indicating poor removal efficiency. The addition of H
2O
2 markedly increased the degradation rate (k
Arom = 0.0203 L/mg·min) and reduced the residual aromaticity to 0.340 AU, confirming the contribution of photogenerated hydroxyl radicals. The introduction of iron in the photo-Fenton process further enhanced performance. At [Fe
2+]
0 = 0.4 mg/L, k
Arom reached 0.0298 L/mg·min with a residual aromaticity of 0.254 AU. Increasing the iron concentration to 0.6 mg/L doubled the kinetic constant (k
Arom = 0.0474 L/mg·min), with aromaticity decreasing to 0.172 AU. The highest efficiency was observed at [Fe
2+]
0 = 0.8–1.0 mg/L, where near-complete removal was achieved ([Aromaticity]
final ≈ 0.04 AU) with degradation constants approaching 0.25 L/mg·min.
These results highlight two important aspects. First, the degradation of aromaticity closely follows the kinetics of DCBQ disappearance, confirming that both the parent compound and aromatic intermediates are effectively oxidized by hydroxyl radicals. Second, the optimization of ferrous ion dosage plays a decisive role: while low iron concentrations improve aromaticity removal compared to UV/H2O2, intermediate concentrations (0.6–0.8 mg/L) provide the best balance between efficiency and minimization of excess iron. At higher iron loadings (1.0 mg/L), although the final aromaticity remained low (0.036 AU), no significant improvement was observed compared to 0.8 mg/L, indicating saturation of the catalytic cycle.
The combined mechanistic and kinetic evidence demonstrates that aromaticity loss during DCBQ oxidation proceeds through a sequential process involving: (i) rapid consumption of the parent compound, (ii) transient accumulation of aromatic intermediates, and (iii) second-order decay to a stable non-aromatic baseline. The photo-Fenton system, particularly at optimized ferrous ion dosages, proved the most effective in sustaining hydroxyl radical production, accelerating aromaticity degradation, and minimizing the persistence of harmful aromatic by-products. This finding underscores its potential as a sustainable strategy for water detoxification.
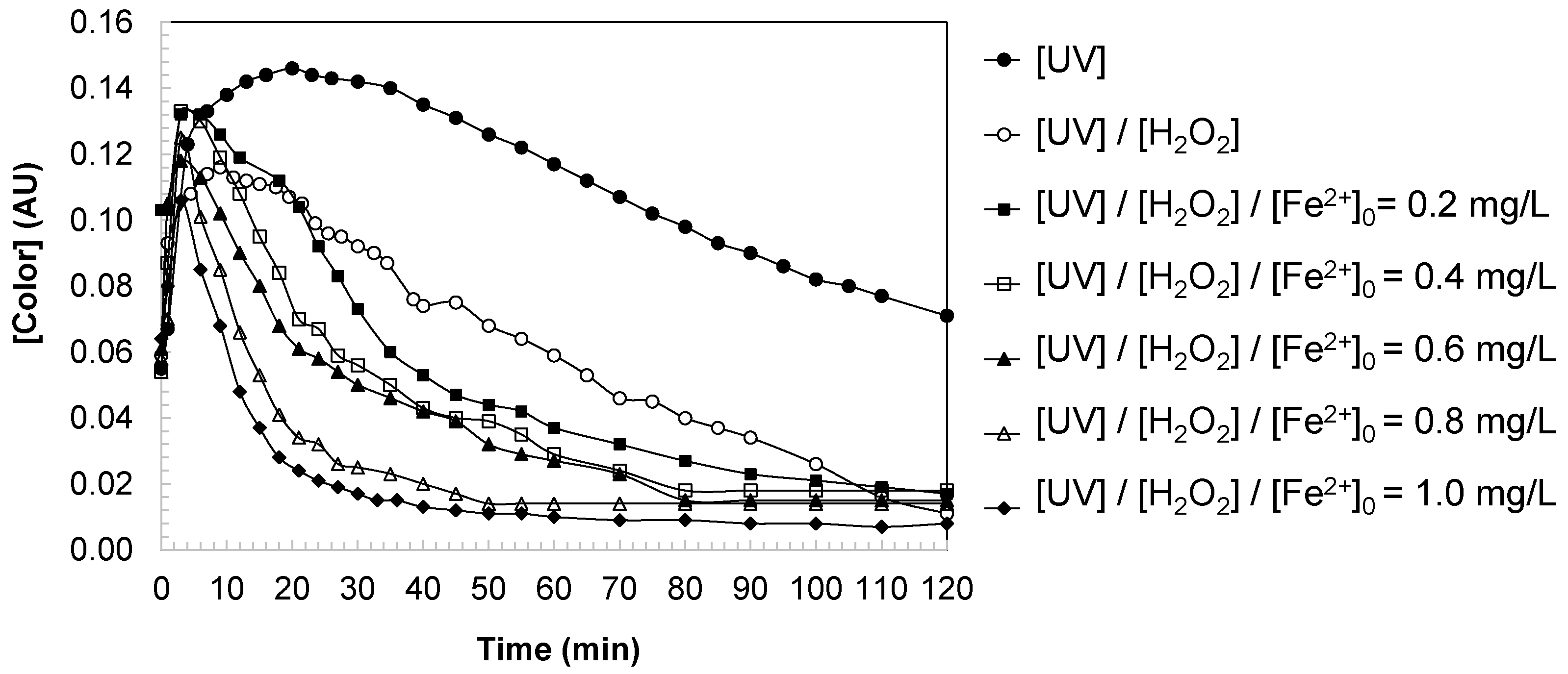
3.3. Color Changes in Water During DCBQ Oxidation
The evolution of water color revealed two distinct phases during DCBQ oxidation under UV, UV/H
2O
2, and photo-Fenton conditions (
Figure 5).
Phase I: Color generation. At the onset of the reaction, water color increased progressively, reaching a maximum within the first 5–10 min depending on the oxidation system. This phenomenon is attributed to the formation and accumulation of colored aromatic intermediates, including hydroxylated quinones and conjugated polyaromatic by-products, which exhibit strong absorption in the visible range. The extent of this initial increase was strongly dependent on the oxidative system: UV irradiation alone produced limited color development, while UV/H2O2 enhanced chromophore formation due to the generation of hydroxyl radicals. In the photo-Fenton process, the accumulation of intermediates was more pronounced at low iron dosages (<0.4 mg/L), reflecting the balance between the formation of conjugated by-products and their subsequent degradation.
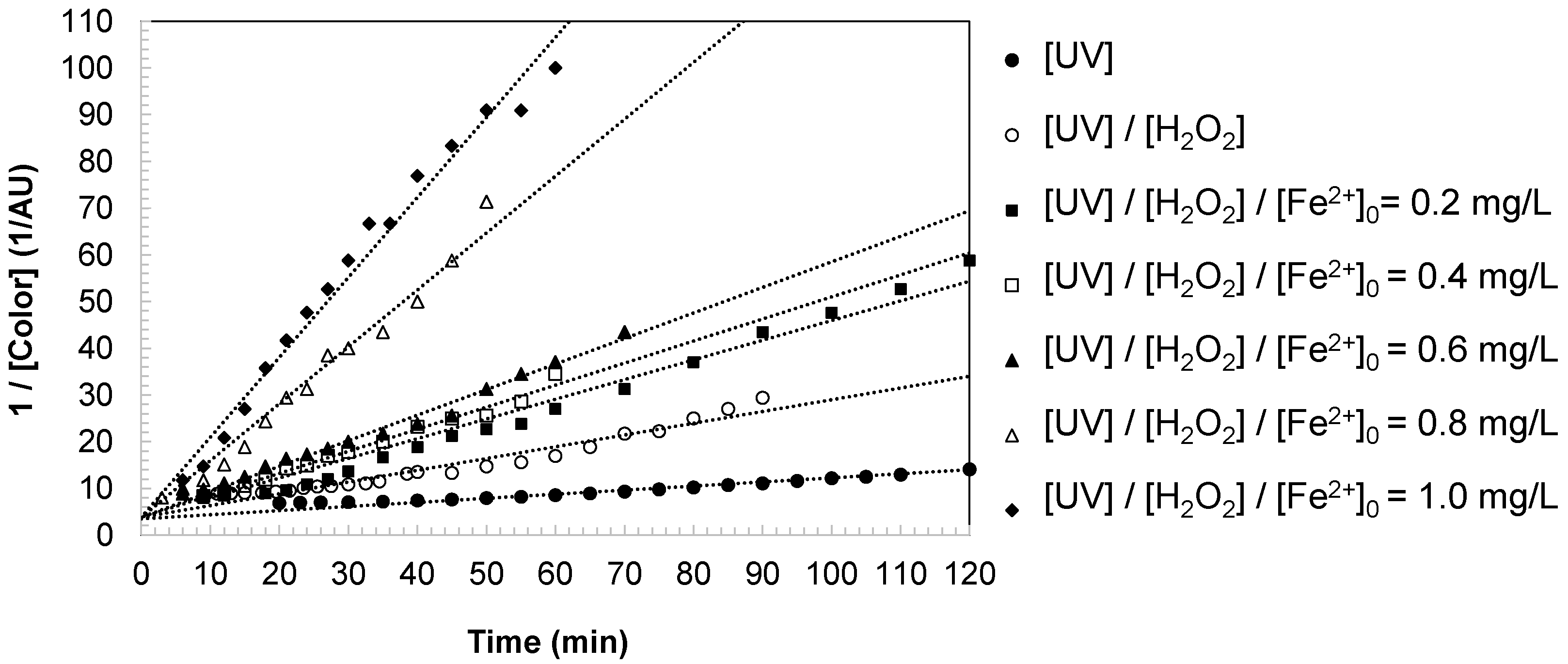
Phase II: Second-order decay. After reaching the maximum, color decreased progressively as oxidative degradation of the chromophoric intermediates dominated. Kinetic analysis confirmed that this decay followed a second-order model (
Figure 6), consistent with hydroxyl radical attack on conjugated structures and the progressive breakdown of aromatic rings into smaller, less colored molecules. Ultimately, color values stabilized at near-baseline levels, reflecting the predominance of low-molecular-weight acids and inorganic mineralization products such as CO
2.
The degradation constants summarized in
Table 1 highlight the critical role of the oxidation system and iron dosage. Under UV irradiation, color removal was limited (k
Color = 0.0874 L/mg·min). The addition of H
2O
2 accelerated the process (k
Color = 0.2522 L/mg·min), while the photo-Fenton system markedly enhanced degradation efficiency, with rate constants increasing steadily with iron dosage. At 0.8 mg/L, k
Color reached 1.2174 L/mg·min, and at 1.0 mg/L the process achieved the maximum value (k
Color = 1.7132 L/mg·min), confirming the catalytic role of iron in sustaining hydroxyl radical production and accelerating the degradation of chromophoric intermediates.
Unlike aromaticity, which displayed an initial decrease followed by transient increases, the color evolution of water during DCBQ oxidation was characterized by two stages: an initial generation of color due to the accumulation of chromophoric intermediates, followed by a second-order decay reflecting their oxidative breakdown. The photo-Fenton process demonstrated the highest efficiency in both minimizing the peak color intensity and accelerating its subsequent removal, with optimized [Fe2+]0 dosages 0.8 mg/L providing the best balance between rapid decolorization and minimization of residual by-products.
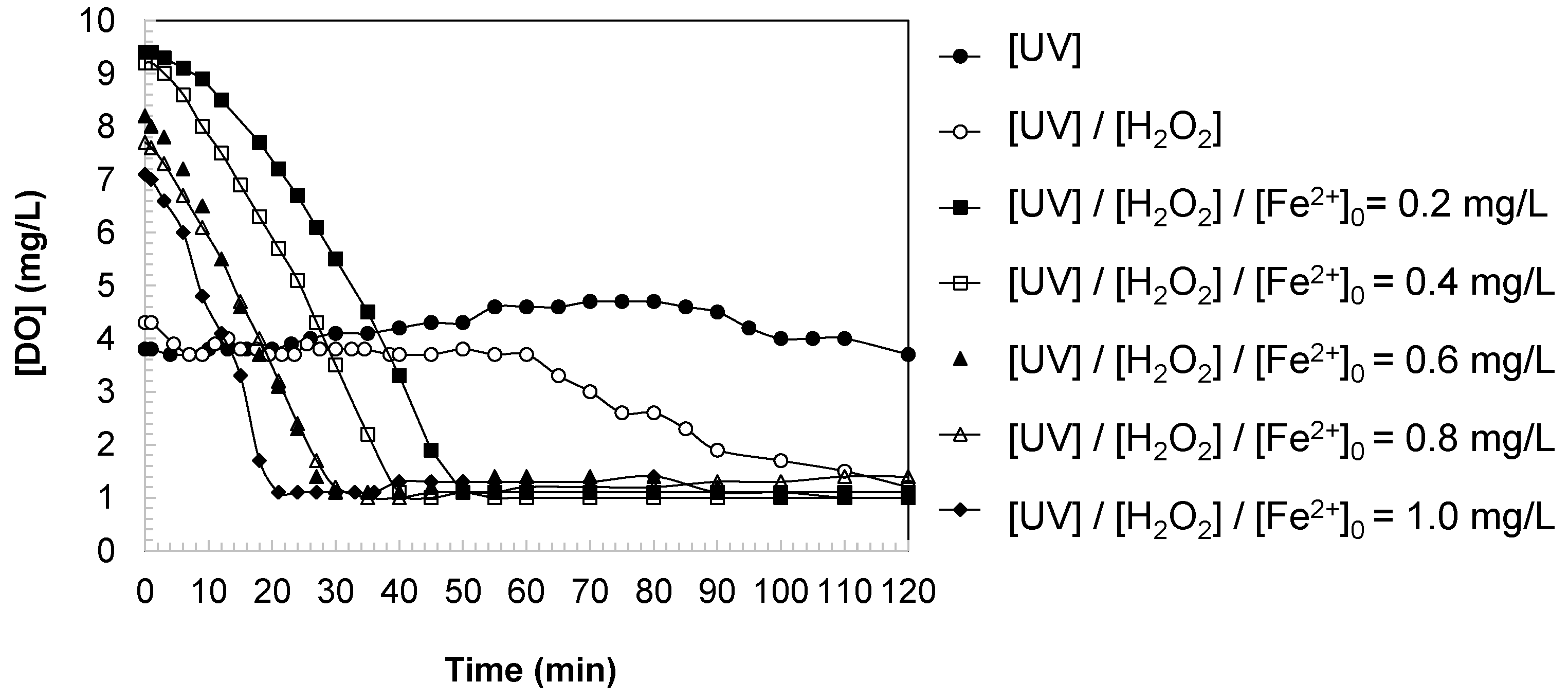
3.4. Changes in Dissolved Oxygen in Water During DCBQ Oxidation
The temporal evolution of dissolved oxygen (DO) during DCBQ oxidation provides complementary insight into the oxidative pathways and the extent of mineralization (
Figure 7).
Distinct behaviors were observed depending on the applied system:
UV irradiation. Under UV alone, the DO concentration remained nearly constant throughout the experiment, indicating that photolysis of DCBQ without additional oxidants does not significantly consume oxygen. This behavior reflects the limited reactivity of direct UV irradiation, which induces only partial transformation of the parent compound without substantial oxidative mineralization.
UV/H2O2 system. When hydrogen peroxide was introduced, a slight decrease in DO was observed over time. This trend indicates the partial activation of H2O2 under UV light, leading to the generation of hydroxyl radicals that react with DCBQ and its intermediates. The mild DO consumption reflects both the oxidative attack on aromatic structures and secondary reactions in which oxygen is consumed during radical-driven pathways.
Photo-Fenton system. In the presence of ferrous ion, the decline in DO became more pronounced and strongly dependent on the iron dosage. As [Fe2+]0 increased from 0.4 to 1.0 mg/L, the DO consumption accelerated, consistent with more efficient generation of hydroxyl radicals through the Fenton cycle. This enhanced reactivity promoted deeper oxidation of DCBQ, fragmentation of aromatic intermediates, and partial mineralization, all of which are processes that consume dissolved oxygen. At higher iron loadings, DO dropped markedly during the reaction, confirming that the photo-Fenton system achieved the most effective contaminant degradation, albeit at the cost of increased oxygen demand in the aqueous medium.
The comparative analysis demonstrates that DO concentration is a sensitive indicator of the oxidative strength of the tested systems. While UV and UV/H2O2 treatments induced minimal oxygen consumption, the photo-Fenton process significantly increased DO depletion, correlating with its superior performance in DCBQ degradation and aromaticity removal. These results underscore a trade-off intrinsic to highly efficient advanced oxidation processes: greater pollutant removal and mineralization potential are associated with higher dissolved oxygen consumption.
In the experiments conducted, notable differences were observed in the initial concentration of dissolved oxygen ([DO]0) depending on the system employed. The experimental results showed lower values in the UV (3.8 mg/L) and UV/H2O2 (4.3 mg/L) systems, whereas the photo-Fenton systems exhibited initial concentrations closer to saturation (7.1–9.2 mg/L). This variation can be explained by the immediate oxygen consumption occurring at the onset of the experiment, prior to the zero-time measurement. In the case of UV irradiation, the photolysis of water or the contaminant itself (DCBQ) may induce excitation reactions that consume dissolved oxygen through the formation of oxygenated radicals. In the UV/H2O2 system, hydrogen peroxide photolysis generates hydroxyl radicals (HO•), which trigger almost instantaneous oxidation of DCBQ and consequently lead to an early decrease in DO. In contrast, when iron is added (photo-Fenton system), the measurement of [DO]0 is typically carried out after solution preparation but before the Fe2+/Fe3+ catalytic cycle is fully activated under UV irradiation. For this reason, the initial values remain higher, reflecting conditions closer to air–water equilibrium. In summary, the lower initial dissolved oxygen concentrations in the UV and UV/H2O2 systems can be attributed to the rapid consumption of O2 in photoinduced and radical-mediated reactions occurring immediately after irradiation. In the photo-Fenton system, however, the early stage does not significantly consume oxygen, which explains the higher initial concentrations recorded.
3.5. Turbidity of Water During DCBQ Oxidation
The evolution of water turbidity during DCBQ oxidation under different treatment conditions is presented in
Figure 8. The turbidity was monitored over 120 min under UV irradiation alone, UV combined with H
2O
2, and UV/H
2O
2 with varying initial ferrous ion concentrations ([Fe
2+]
0 = 0.2–1.0 mg/L) in the photo-Fenton system.
The results indicate that the UV-only treatment maintained relatively high turbidity values throughout the reaction, fluctuating around 3–4 NTU, which suggests limited removal of suspended particles in the absence of oxidative reagents. The addition of H2O2 under UV irradiation led to a moderate decrease in turbidity, stabilizing near 2–3 NTU, indicating partial oxidation and aggregation of suspended matter.
The photo-Fenton system exhibited a more pronounced decrease in turbidity, which was dependent on the initial ferrous ion concentration. At lower iron levels (0.2–0.4 mg/L), turbidity gradually decreased to approximately 2 NTU, while higher iron concentrations (0.6–1.0 mg/L) accelerated the reduction, reaching values close to 1 NTU by the end of the experiment. This trend demonstrates that the generation of hydroxyl radicals through the photo-Fenton reaction enhances the oxidation and subsequent coagulation/settling of particulate matter in the solution, effectively clarifying the water during DCBQ degradation.
Overall, the data highlight the synergistic effect of UV, H2O2, and ferrous ion in reducing turbidity, with higher ferrous ion doses promoting faster water clarification, aligning with the expected behavior of the photo-Fenton process in water treatment applications.
The observed enhancement in DCBQ degradation with increasing Fe
2+ dosage is consistent with previous studies on the photo-Fenton process, which emphasize the critical role of iron in sustaining hydroxyl radical production [
21,
22]. The optimal Fe
2+ concentration identified in this study (0.8 mg/L) aligns with other findings [
19], which reported that moderate iron dosages maximize efficiency while minimizing sludge formation. Moreover, the second-order kinetic behavior observed for both DCBQ and its aromatic intermediates supports mechanistic insights [
17,
20] that also noted the sequential transformation of halogenated quinones under oxidative conditions. The transient accumulation of aromatic and chromophoric intermediates, followed by their progressive degradation, reflects patterns described in studies on other halobenzoquinones and phenolic compounds [
14,
16]. These correlations reinforce the robustness of the experimental approach and validate the proposed kinetic model within the broader context of advanced oxidation literature.