High-Resolution Analysis of DNA Electrophoretic Separations via Digital Image Processing
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. The Self-Build Compact Gel Electrophoresis System
2.3. Separation of DNA in the Self-Built Gel Electrophoresis System
2.4. Amplification of Periodontal Pathogens in the PCR Thermal Cycler
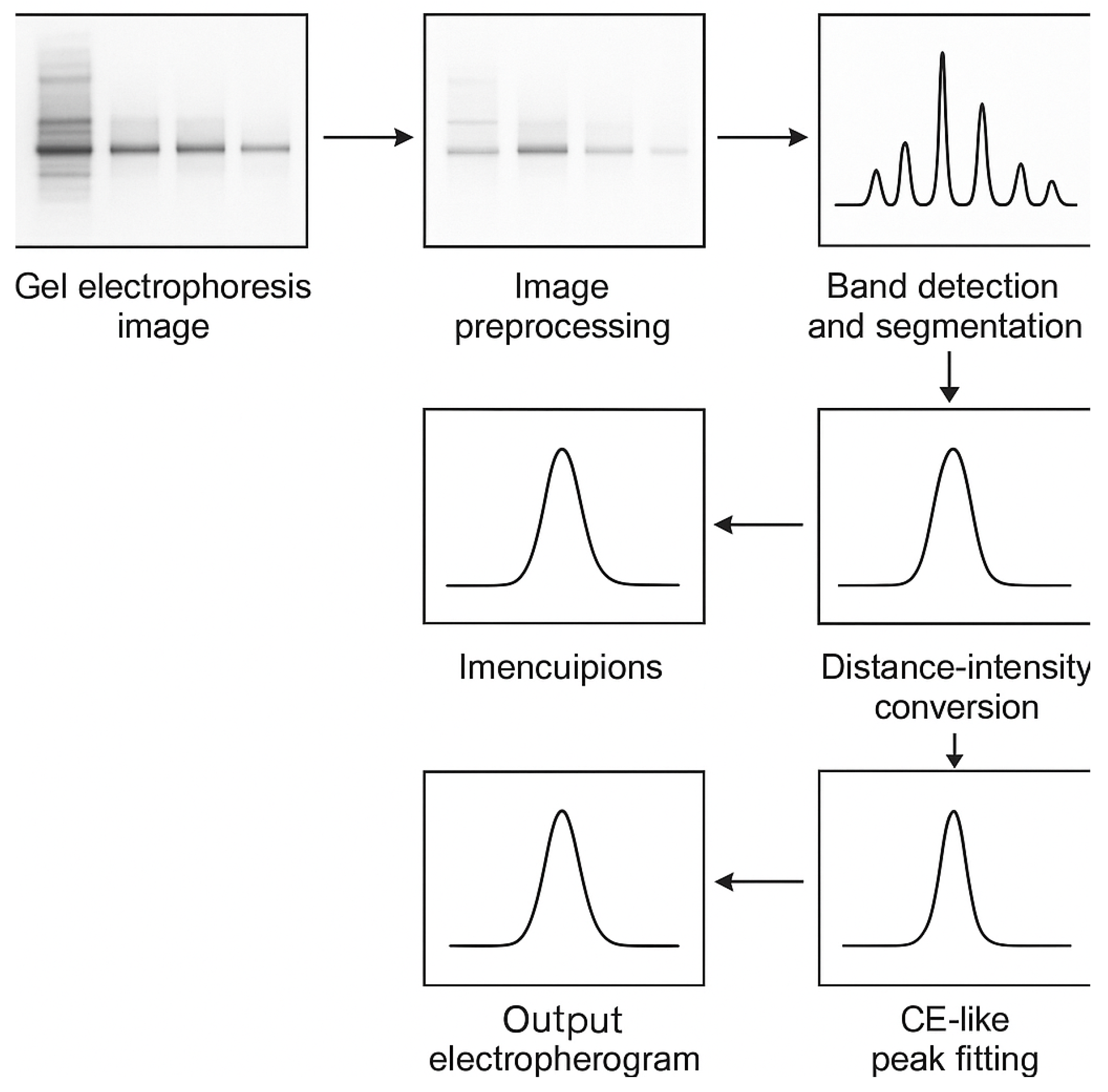
2.5. Digital Image Processing for Gel Electrophoresis Image
3. Results and Discussion
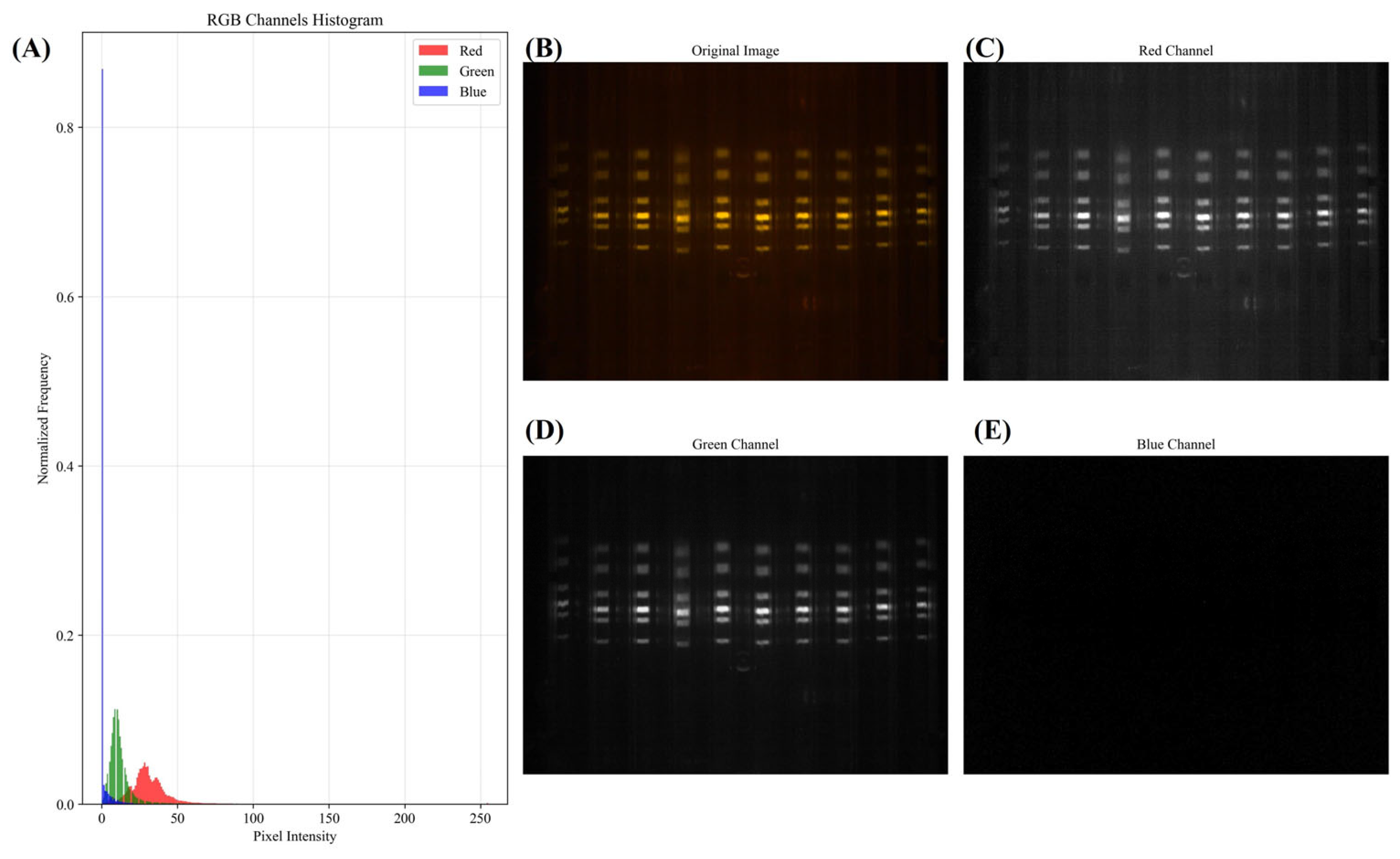
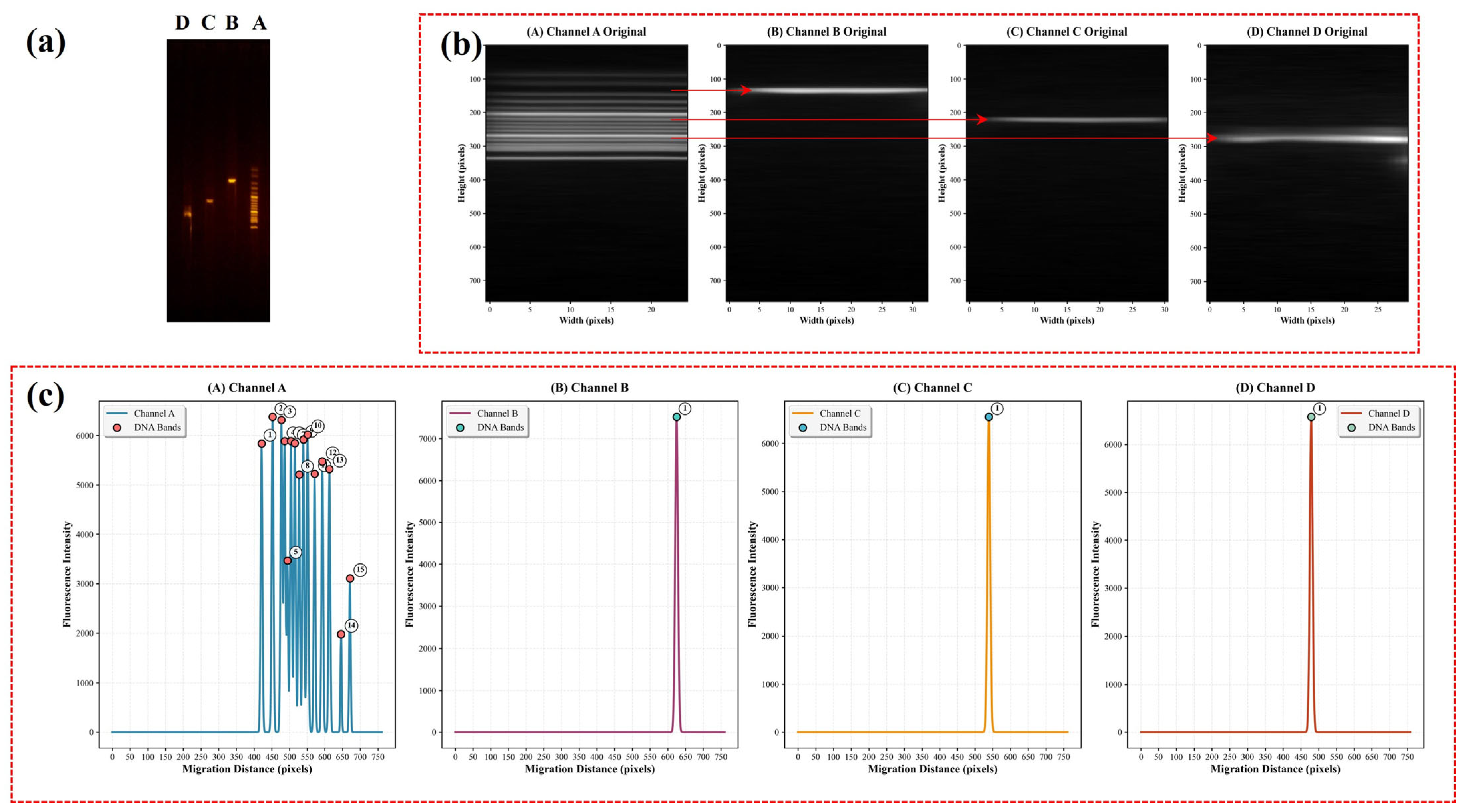
3.1. RGB Channel Analysis of SYBR Green I–Stained Gel Electrophoresis DNA Images
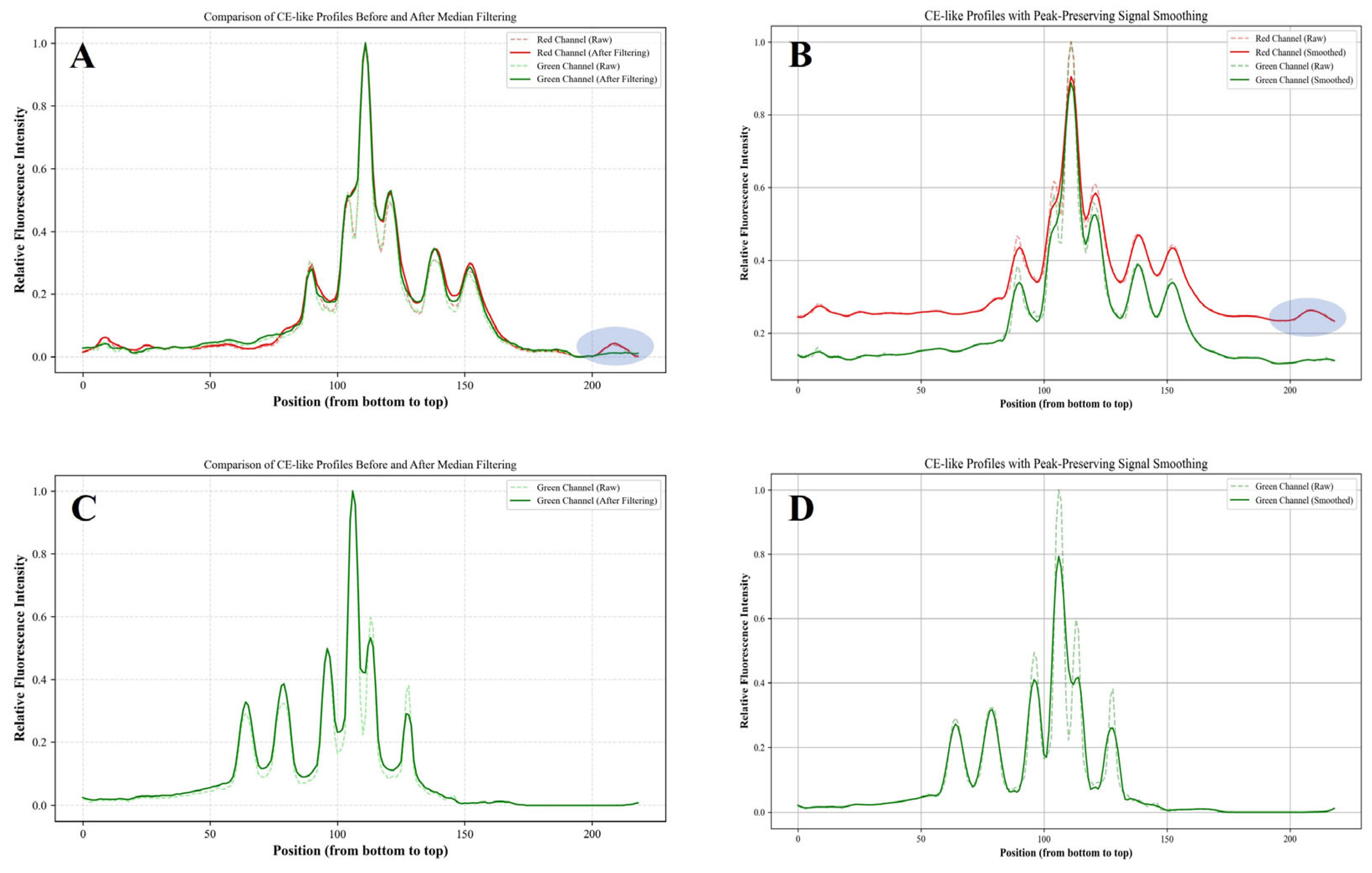
3.2. Transformation of Gel Electrophoresis Emage into CE-like Electropherogram
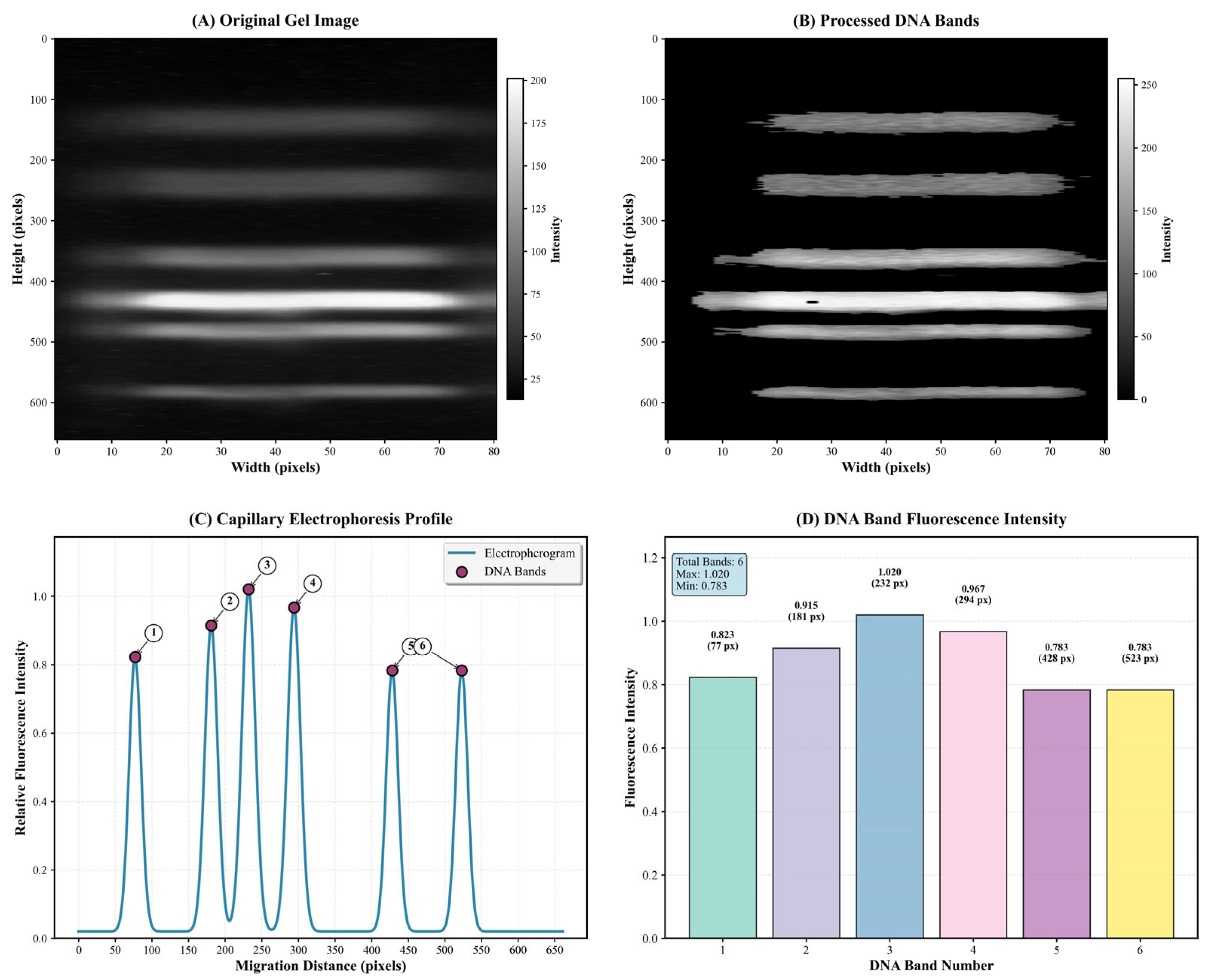
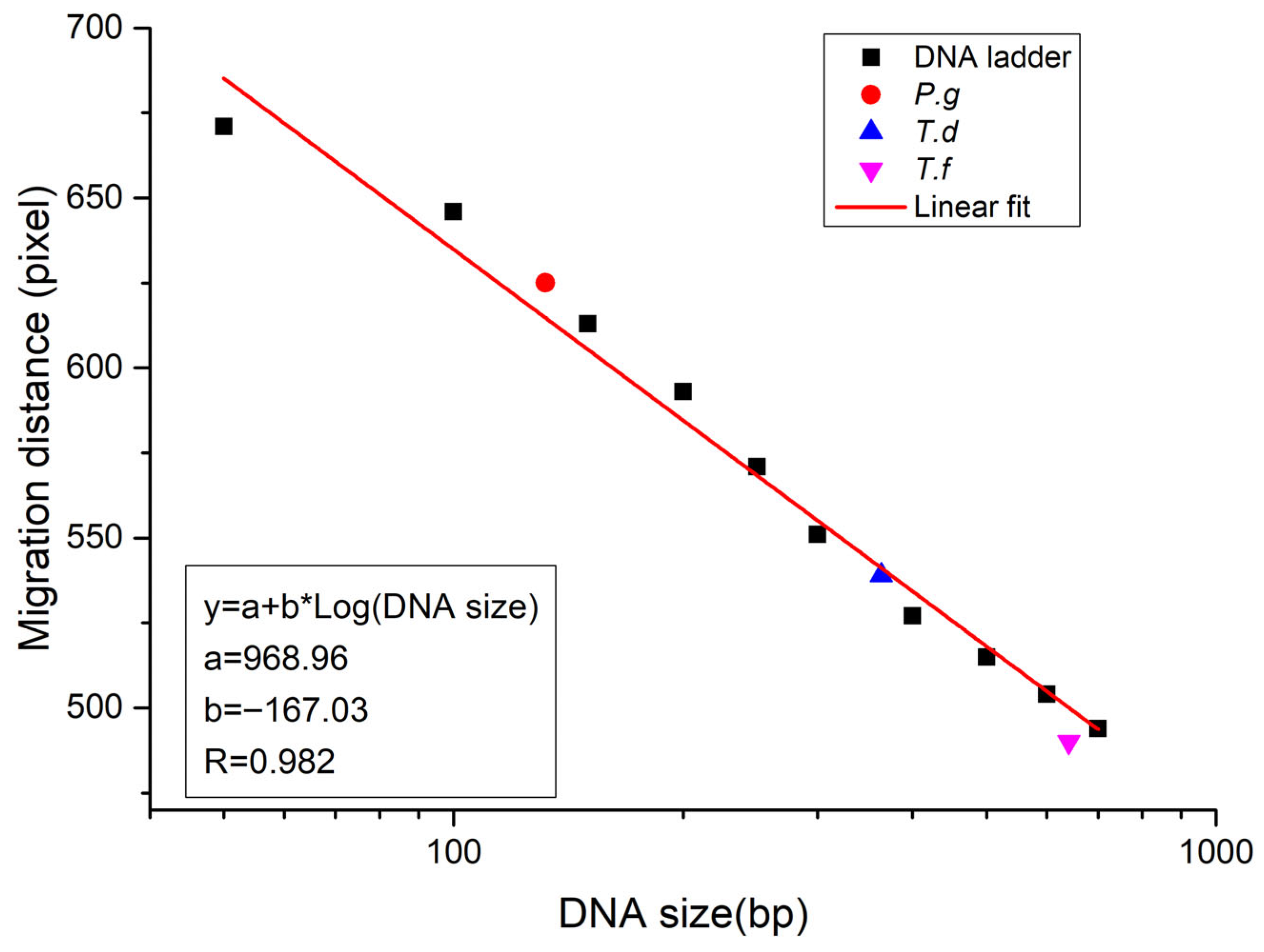
3.3. Quantitative and Qualitative Insights into DNA Gel Electrophoresis Through Advanced Imaging Techniques
3.4. Application of the Self-Built Algorithm
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kachwala, M.J.; Smith, C.W.; Nandu, N.; Yigit, M.V. Reprogrammable gel electrophoresis detection assay using CRISPR-Cas12a and hybridization chain reaction. Anal. Chem. 2021, 93, 1934–1938. [Google Scholar] [CrossRef] [PubMed]
- Al-Shuhaib, M.B.S.; Hashim, H.O. Mastering DNA chromatogram analysis in Sanger sequencing for reliable clinical analysis. J. Genet. Eng. Biotechnol. 2023, 21, 115. [Google Scholar] [CrossRef] [PubMed]
- Howarth, A.; Drummond, B.; Wasef, S.; Matheson, C.D. An assessment of DNA extraction methods from blood-stained soil in forensic science. Forensic Sci. Int. 2022, 341, 111502. [Google Scholar] [CrossRef] [PubMed]
- Gritti, F.; Sawyer, K.; Vaishnav, J.; Addepali, B.; Lauber, M.; Wyndham, K. Retention and efficiency of a novel slalom chromatography column: An alternative to agarose gel electrophoresis for DNA separation. J. Chromatogr. A 2025, 1761, 466293. [Google Scholar] [CrossRef] [PubMed]
- Dangerfield, T.L.; Huang, N.Z.; Johnson, K.A. High throughput quantification of short nucleic acid samples by capillary electrophoresis with automated data processing. Anal. Biochem. 2021, 629, 114239. [Google Scholar] [CrossRef] [PubMed]
- Bawane, H.; Kadam, K.; Mahale, V.; Kulkarni, R. Comprehensive assessment of 12 commercial DNA-binding dyes as alternatives to ethidium bromide for agarose gel electrophoresis. Electrophoresis 2024, 45, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Halder, S.; Sanchez, A.; Ranjha, L.; Reginato, G.; Ceppi, I.; Acharya, A.; Anand, R.; Cejka, P. Double-stranded DNA binding function of RAD51 in DNA protection and its regulation by BRCA2. Mol. Cell 2022, 82, 3553–3565.e5. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, S.; Liu, C.; Zhang, D.; Dou, X.; Yamaguchi, Y. Quantification of periodontal pathogens cell counts by capillary electrophoresis. J. Chromatogr. A 2014, 1361, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Huang, J.; Yang, B.; You, Q.; Sekine, S.; Zhang, D.; Yamaguchi, Y. Miniaturized Gel electrophoresis system for fast separation of nucleic acids. Sens. Actuators B Chem. 2018, 254, 153–158. [Google Scholar] [CrossRef]
- Wheelock, Å.M.; Buckpitt, A.R. Software-induced variance in two-dimensional gel electrophoresis image analysis. Electrophoresis 2005, 26, 4508–4520. [Google Scholar] [CrossRef] [PubMed]
- Khakabimamaghani, S.; Najafi, A.; Ranjbar, R.; Raam, M. GelClust: A software tool for gel electrophoresis images analysis and dendrogram generation. Comput. Methods Programs Biomed. 2013, 111, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Intarapanich, A.; Kaewkamnerd, S.; Shaw, P.J.; Ukosakit, K.; Tragoonrung, S.; Tongsima, S. Automatic DNA diagnosis for 1D gel electrophoresis images using bio-image processing technique. BMC Genom. 2015, 16, S15. [Google Scholar] [CrossRef] [PubMed]
- Dragan, A.; Pavlovic, R.; McGivney, J.; Casas-Finet, J.; Bishop, E.; Strouse, R.; Schenerman, M.; Geddes, C. SYBR Green I: Fluorescence properties and interaction with DNA. J. Fluoresc. 2012, 22, 1189–1199. [Google Scholar] [CrossRef] [PubMed]
- Van Winkle, D.H.; Beheshti, A.; Rill, R.L. DNA electrophoresis in agarose gels: A simple relation describing the length dependence of mobility. Electrophoresis 2002, 23, 15–19. [Google Scholar] [CrossRef]
- Maniatis, T.; Jeffrey, A.; Van deSande, H. Chain length determination of small double-and single-stranded DNA molecules by polyacrylamide gel electrophoresis. Biochemistry 1975, 14, 3787–3794. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, J.; Russell, D. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2001; Volume 3, pp. A2.2–A2.4. [Google Scholar]
- García-Alvarez-Coque, M.C.; Simó-Alfonso, E.F.; Sanchis-Mallols, J.M.; Baeza-Baeza, J.J. A new mathematical function for describing electrophoretic peaks. Electrophoresis 2005, 26, 2076–2085. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Xu, Q.; Liu, X.; Wang, K. Quantitative Analysis of Chondroitin Sulfate and Dermatan Sulfate Using Capillary Electrophoresis With Gaussian Distribution-Based Peak Fitting for Improved Peak Resolution. Sep. Sci. Plus 2024, 7, e202300244. [Google Scholar] [CrossRef]
- Liu, Y.; Dang, B.; Li, Y.; Lin, H.; Ma, H. Applications of Savitzky-Golay filter for seismic random noise reduction. Acta Geophys. 2016, 64, 101–124. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Zhang, T.; Yang, B.; Liu, J.; Li, Z.; Yamaguchi, Y. High-Resolution Analysis of DNA Electrophoretic Separations via Digital Image Processing. Separations 2025, 12, 296. https://doi.org/10.3390/separations12110296
Yang J, Zhang T, Yang B, Liu J, Li Z, Yamaguchi Y. High-Resolution Analysis of DNA Electrophoretic Separations via Digital Image Processing. Separations. 2025; 12(11):296. https://doi.org/10.3390/separations12110296
Chicago/Turabian StyleYang, Jing, Tengfei Zhang, Bo Yang, Jiahe Liu, Zhenqing Li, and Yoshinori Yamaguchi. 2025. "High-Resolution Analysis of DNA Electrophoretic Separations via Digital Image Processing" Separations 12, no. 11: 296. https://doi.org/10.3390/separations12110296
APA StyleYang, J., Zhang, T., Yang, B., Liu, J., Li, Z., & Yamaguchi, Y. (2025). High-Resolution Analysis of DNA Electrophoretic Separations via Digital Image Processing. Separations, 12(11), 296. https://doi.org/10.3390/separations12110296





