Study on the Emission Characteristics of Fine Particulate Matter in the White Mud Desulfurization Process
Abstract
1. Introduction
2. Materials and Methods
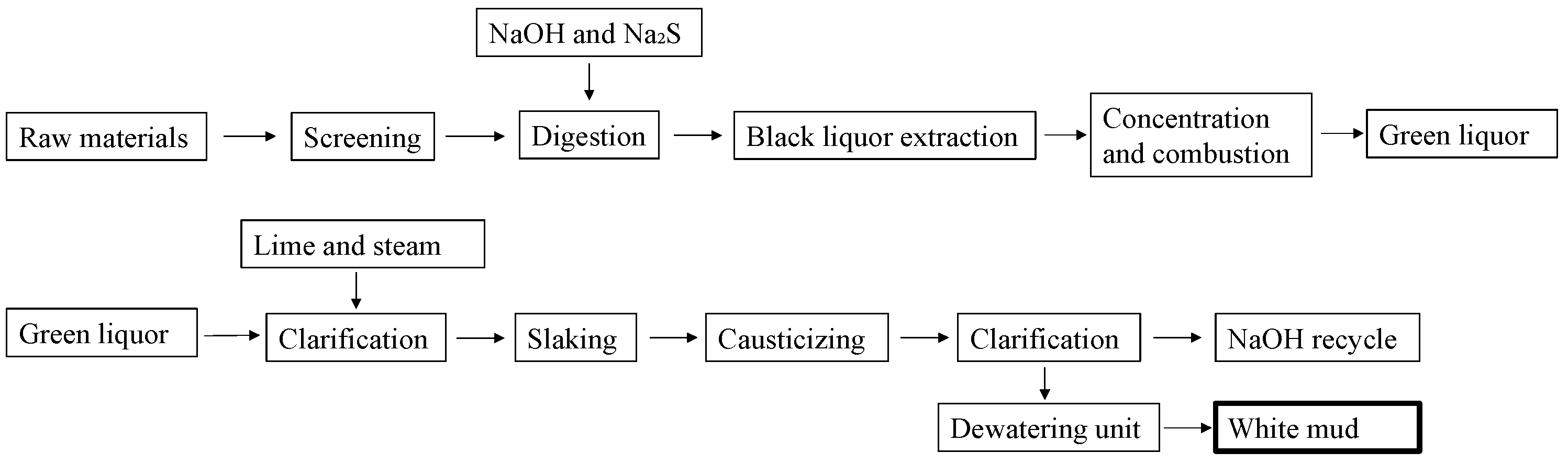
2.1. Formation of White Mud
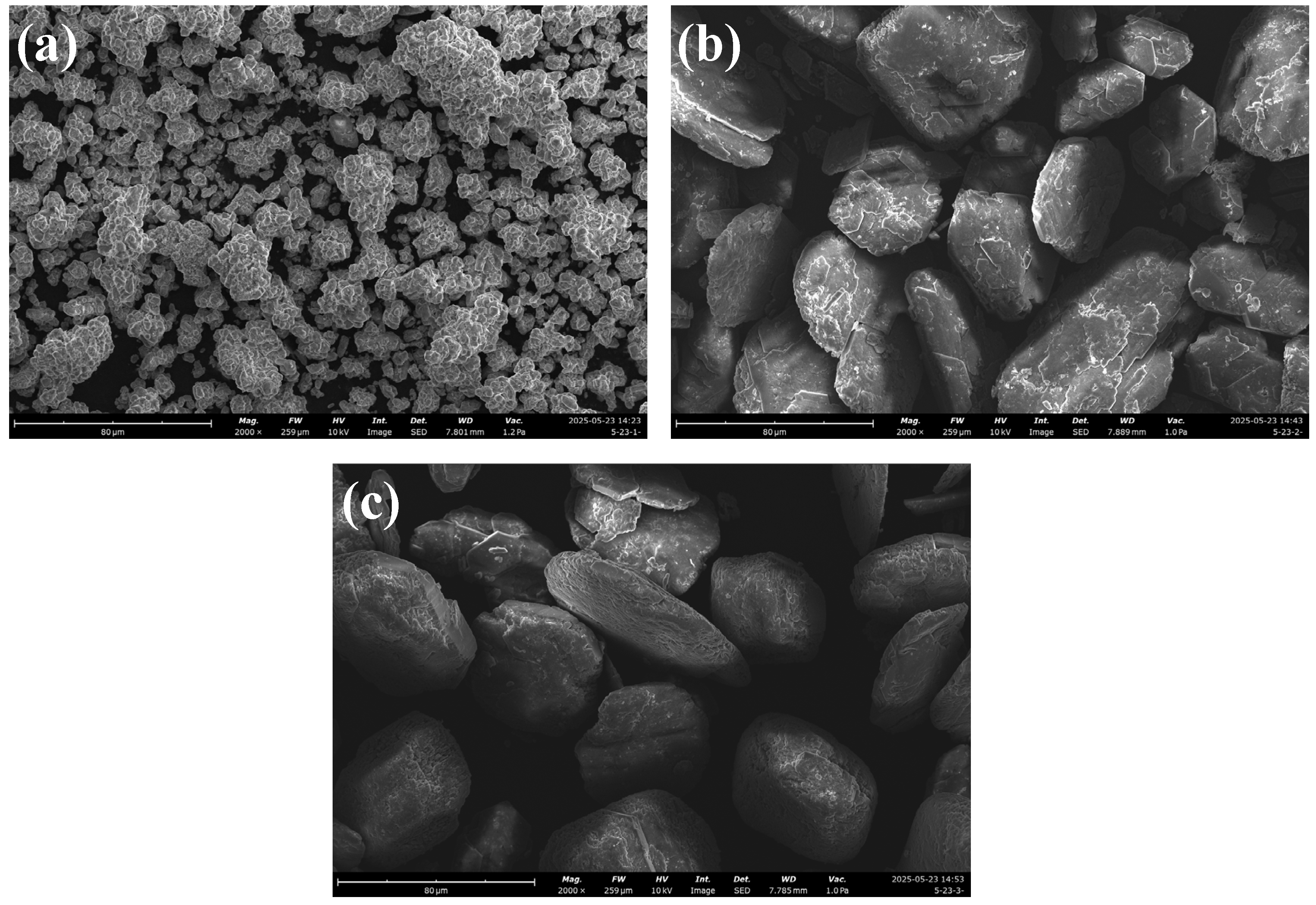
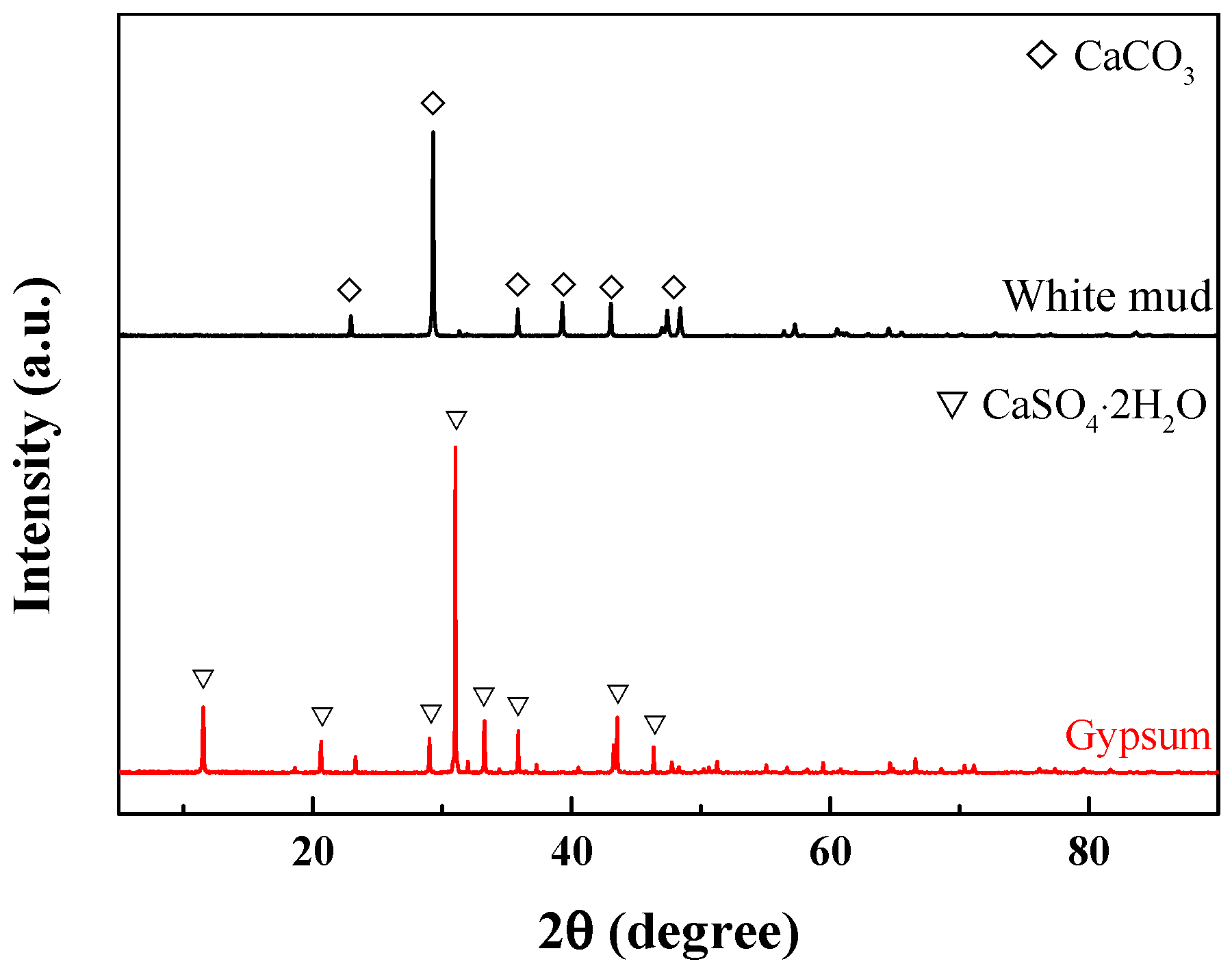
2.2. Physicochemical Characterization of White Mud
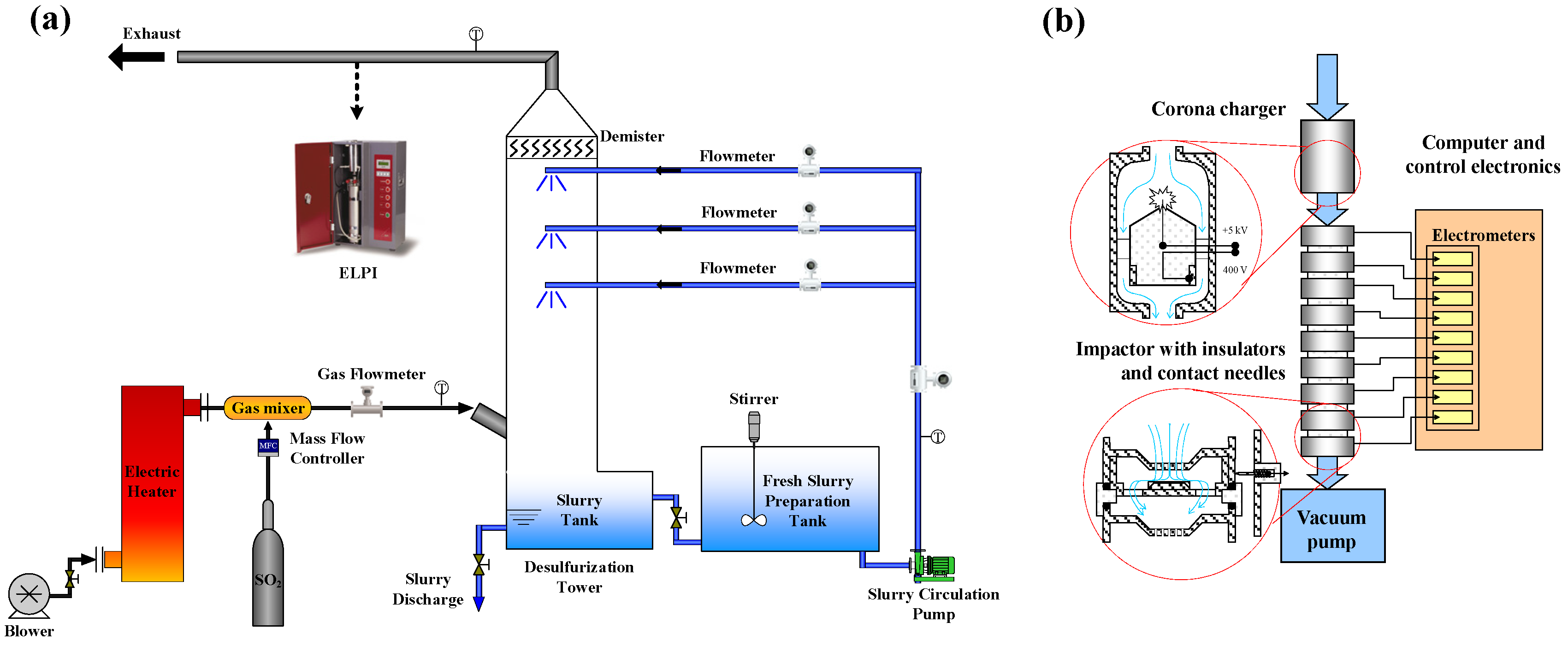
2.3. Experimental System
3. Results and Discussion
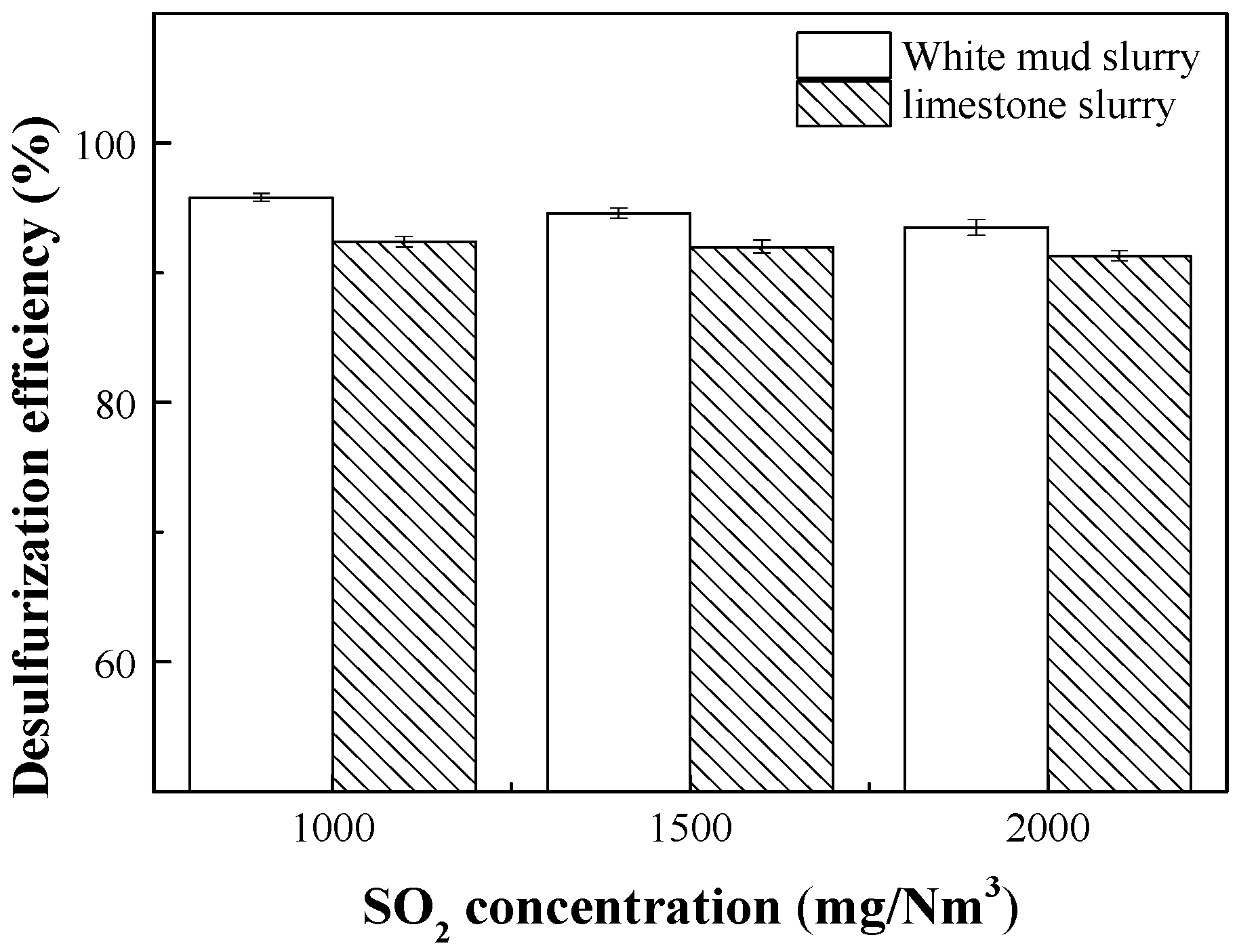
3.1. Desulfurization Performance of White Mud Slurry
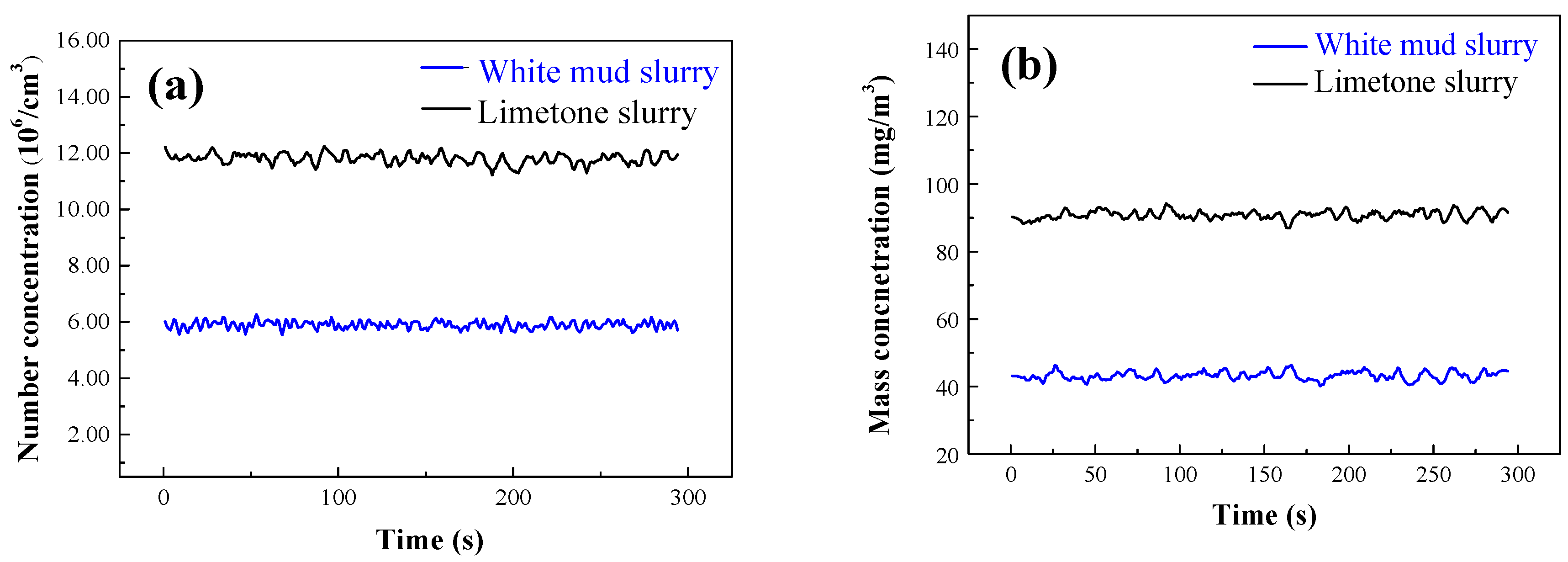
3.2. Study on Fine Particulate Emissions in the White Mud Desulfurization Process
3.3. Study on the Factors Affecting Fine Particulate Emissions
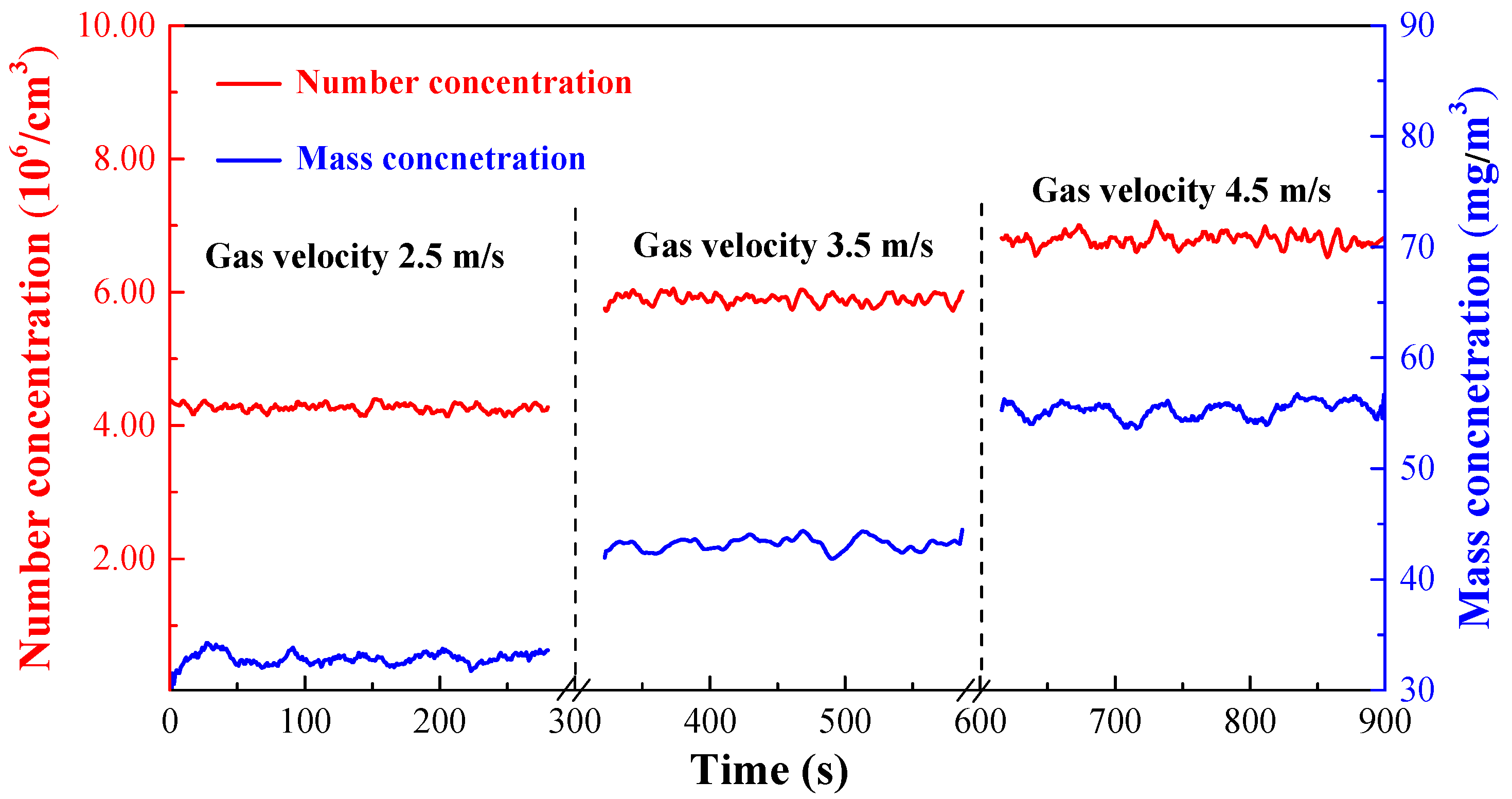
3.3.1. Effect of Empty Tower Gas Velocity
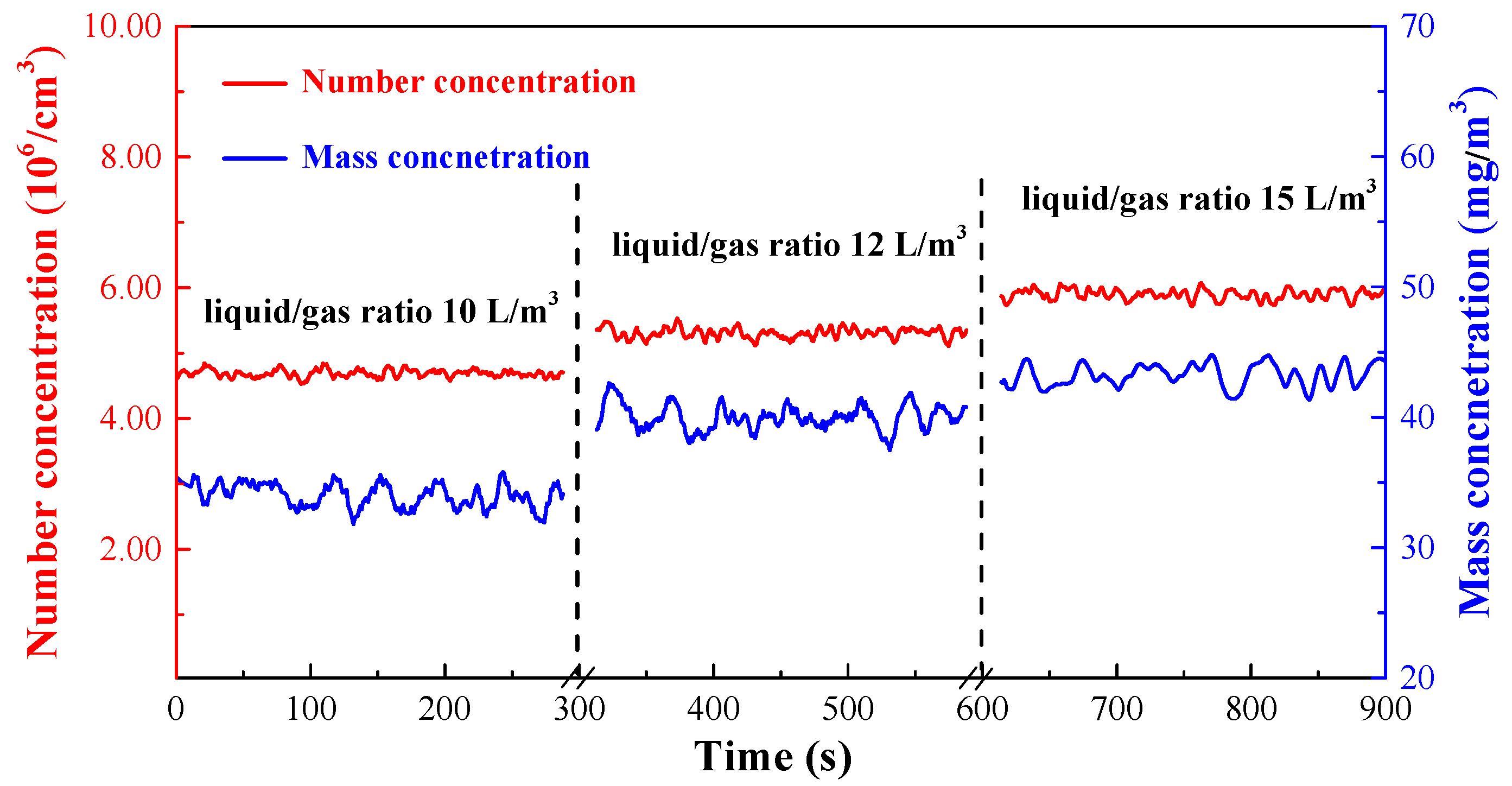
3.3.2. Effect of Gas-to-Liquid Ratio
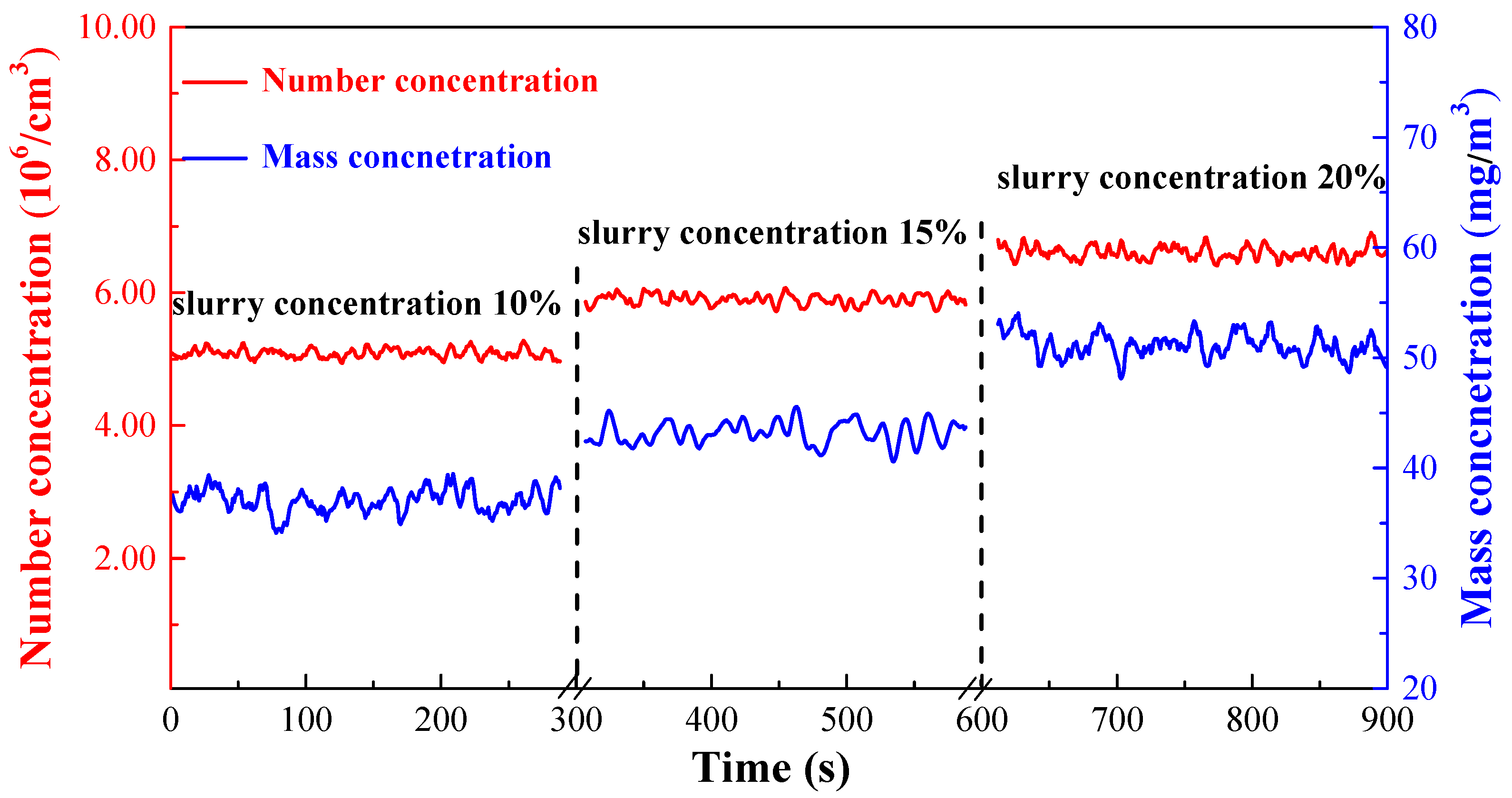
3.3.3. Effect of Slurry Concentration
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, P.; Gu, Y.; Wang, R.H.; Yang, C.; Wang, K.; Chang, M.; Wu, H. A synergy of DUT-67(Zr)@AFP with hierarchical pores and amines to remove trace SO2 from flue gas. J. Hazard. Mater. 2025, 495, 139170. [Google Scholar]
- Zhao, Y.Y.; Cheng, Y.P.; Zhang, T.T.; Wang, S.; Guo, X.; Yuan, J.; Ji, Z.; Liu, J.; Li, F.; Wang, J.; et al. Bipolar membrane electrodialysis combined with ozone oxidation wet scrubbing to remove SO2 and NOx from flue gas and achieve CO2 mineralization. Fuel 2023, 340, 127436. [Google Scholar] [CrossRef]
- Bao, J.C.; Li, K.; Ning, P.; Wang, C.; Song, X.; Luo, Y.; Sun, X. Study on the role of copper converter slag in simultaneously removing SO2 and NOx using KMnO4/copper converter slag slurry. J. Environ. Sci. 2021, 108, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.Z.; Yu, M.Y.; Luo, G.Q.; Wang, L.; Zhou, M.; Lu, X.; Li, X.; Yao, H. Research on mercury re-release model in wet flue gas desulfurization (WFGD) in coal-fired power plants. Chem. Eng. J. 2024, 500, 156819. [Google Scholar] [CrossRef]
- Łuszkiewicz, D.; Jędrusik, M.; Świerczok, A.; Kobylańska-Pawlisz, M. Effect of addition of sulphide based additive to WFGD slurry on mercury removal from flue gas. Energy 2023, 270, 126953. [Google Scholar] [CrossRef]
- Zhao, Z.; Fan, H.; Li, Q.; Liu, C.; Chen, Z.; Li, L.; Zheng, C.; Gao, X. Hybrid modeling and operating optimization method of oxidation process of wet flue gas desulfurization (WFGD) system. Chem. Eng. Res. Des. 2022, 188, 406–416. [Google Scholar] [CrossRef]
- Ma, J.X.; Chen, Y.X.; Xu, D.S.; Xu, F.; Xue, S.F.; Fan, B.B.; Liu, D.K.; Ma, S.C. Effects of Particle Size Difference Between White Mud and Limestone on Desulfurization Performance. J. Chin. Soc. Power Eng. 2021, 6, 497–504. [Google Scholar]
- Ma, S.C.; Hua, J.Z.; Gou, F.Q.; Wen, X.C.; Yang, J.; Zhang, L.N. Physical and chemical properties research of white mud desulfurization slurry and gypsum. Chem. Ind. Eng. Prog. 2016, 35, 381–388. [Google Scholar]
- Hong, R.K.; Gao, W.; Hu, P.F.; Yang, T.; Zhang, S.S.; Liao, Y.J.; Chen, Y. Experimental Study on Wet Desulphurization System of Large-Scale Thermal Power Plant with Ammonia Soda Solid Waste (White Mud). In Proceedings of the International Conference on Materials for Environmental Protection and Energy Application (MEPEA), Kuala Lumpur, Malaysia, 27–28 September 2011; pp. 227–234. [Google Scholar]
- Liao, Y.J.; Yang, Q.S.; Wang, Y.J. Application Research of White Mud in 660 MW Unit FGD System. Adv. Mater. Res. 2012, 524–527, 940–944. [Google Scholar] [CrossRef]
- Gao, X.; Su, J.X.; Pan, Q.; Cao, X.; Wu, S.; Long, X.; Song, M.; Wu, Y. Photooxidation potential of fine particles from desulfurization flue gas aerosol. Chem. Eng. J. 2023, 466, 143096. [Google Scholar] [CrossRef]
- Lu, J.W.; Geng, K.; Zhang, Q.; Yao, J.; Cui, L.; Dong, Y. Effect of charged desulfurization wastewater droplet evaporation on the agglomeration of fine particles. Sep. Purif. Technol. 2022, 283, 120158. [Google Scholar] [CrossRef]
- Wang, Q.W.; Wang, L.L.; Wu, H.; Yang, H. Promoting fine particle removal in double-tower cascade wet flue gas desulfurization system by flue gas temperature reduction. Powder Technol. 2020, 373, 581–589. [Google Scholar] [CrossRef]
- Liu, S.T.; Yang, H.K.; Zhang, Z.B.; Chen, J.; Chen, C.; Guo, T.; Cao, Y.; Jia, W. Emission Characteristics of Fine Particles from Wet Flue Gas Desulfurization System Using a Cascade of Double Towers. Aerosol Air Qual. Res. 2018, 18, 1901–1909. [Google Scholar] [CrossRef]
- Yao, S.; Cheng, S.Y.; Li, J.B.; Zhang, H.; Jia, J.; Sun, X. Effect of wet flue gas desulfurization (WFGD) on fine particle (PM2.5) emission from coal-fired boilers. J. Environ. Sci. 2019, 77, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.T.; Tao, Y.C.; Chen, W.Y.; Lei, L.; Li, S.; Yang, L. Enhancing aerosol emission reduction in an ammonia-based WFGD system with tray implementation. Fuel 2024, 355, 129522. [Google Scholar] [CrossRef]
- Wu, Q.R.; Gu, M.; Du, Y.G.; Zeng, H. Chemical composition and morphology of particles emitted from a wet flue gas desulfurization (WFGD) system. Process Saf. Environ. Prot. 2019, 124, 196–203. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, X.; Xiao, Z.H.; Cheng, M.; Zou, R.; Luo, G. Enhancement of fine particle removal through flue gas cooling in a spray tower with packing materials. J. Hazard. Mater. 2024, 478, 135390. [Google Scholar] [CrossRef]
- Wang, X.F.; Wang, F.; Lu, G.J.; Huang, J.; Zhang, F.; Feng, Y.; Liu, Y. Flue-gas desulfurization technology using solid waste white mud and engineering application in coal-fired power plant. Res. Environ. Sci. 2018, 31, 1597–1602. [Google Scholar]
- Pan, D.P.; Wu, H.; Yang, L.J. Investigation on the relationship between the fine particle emission and crystallization characteristics of gypsum during wet flue gas desulfurization process. J. Environ. Sci. 2017, 55, 303–310. [Google Scholar] [CrossRef]
- Lǖ, J.; Fu, Y.; Yu, H.Y.; Wang, H.; Wang, Z.; Chen, H. Effect of relative humidity on the desulfurization performance of calcium-based desulfurizer. J. Environ. Sci. 2024, 138, 179–188. [Google Scholar]
- Li, W.R.; Wu, H.; Tong, H.; Du, Z.; Wang, H.; Zhou, C.; Zhang, Z.; Yang, H. Formation and migration of soluble ions in condensable particulate matter in limestone-gypsum wet flue gas desulfurization system. Fuel 2024, 357, 129807. [Google Scholar] [CrossRef]
- Wang, A.; Li, S.; Zheng, Q.; Zhang, S.; Zhang, S.; Wang, Z.; Liu, Z.; Yan, K. Study on the Effects of Wet Flue Gas Desulfurization on Particulate Matter Emission from Industrial Coal-Fired Power Plants. Separations 2023, 10, 356. [Google Scholar] [CrossRef]
- Huang, Y.; Zheng, C.; Li, Q.; Zhang, J.; Guo, Y.; Zhang, Y.; Gao, X. Numerical simulation of the simultaneous removal of particulate matter in a wet flue gas desulfurization system. Environ. Sci. Pollut. Res. 2020, 27, 1598–1607. [Google Scholar]
- Bao, J.J.; Sun, L.C.; Mo, Z.Y.; Xie, G.; Tang, J.; Yang, H. Investigation on Formation Characteristics of Aerosol Particles during Wet Ammonia Desulfurization Process. Energy Fuels 2017, 31, 8374–8382. [Google Scholar] [CrossRef]
- Cheng, T.; Zhou, X.C.; Yang, L.J.; Sun, Z.; Wu, H. Emission Characteristics of Soluble Ions in Fine Particulates in Limestone–Gypsum Wet Flue Gas Desulfurization System. Energy Fuels 2020, 34, 3836–3842. [Google Scholar] [CrossRef]
- Pan, D.P.; Wu, H.; Yang, L.J. Investigation of the Relationship between Droplet and Fine Particle Emissions during the Limestone–Gypsum Wet Flue Gas Desulfurization Process. Energy Fuels 2017, 31, 6472–6477. [Google Scholar] [CrossRef]
- Huang, R.T.; Wu, H.; Yang, L.J. Investigation on condensable particulate matter emission characteristics in wet ammonia-based desulfurization system. J. Environ. Sci. 2020, 92, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.B.; Tian, H.Z.; Hao, Y.; Liu, S.; Liu, X.; Liu, W.; Bai, X.; Liang, W.; Lin, S.; Wu, Y.; et al. Effects of Wet Flue Gas Desulfurization and Wet Electrostatic Precipitators on Emission Characteristics of Particulate Matter and Its Ionic Compositions from Four 300 MW Level Ultralow Coal-Fired Power Plants. Environ. Sci. Technol. 2018, 52, 14015–14026. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Feng, Y.; Wang, X.; Xie, R.; Li, G.; Yu, L.; Zhan, L. Study on the Emission Characteristics of Fine Particulate Matter in the White Mud Desulfurization Process. Separations 2025, 12, 281. https://doi.org/10.3390/separations12100281
Wang C, Feng Y, Wang X, Xie R, Li G, Yu L, Zhan L. Study on the Emission Characteristics of Fine Particulate Matter in the White Mud Desulfurization Process. Separations. 2025; 12(10):281. https://doi.org/10.3390/separations12100281
Chicago/Turabian StyleWang, Changqing, Yongchao Feng, Xin Wang, Rongliang Xie, Guanglei Li, Li Yu, and Lingxiao Zhan. 2025. "Study on the Emission Characteristics of Fine Particulate Matter in the White Mud Desulfurization Process" Separations 12, no. 10: 281. https://doi.org/10.3390/separations12100281
APA StyleWang, C., Feng, Y., Wang, X., Xie, R., Li, G., Yu, L., & Zhan, L. (2025). Study on the Emission Characteristics of Fine Particulate Matter in the White Mud Desulfurization Process. Separations, 12(10), 281. https://doi.org/10.3390/separations12100281



