Abstract
In wastewater treatment, sludge is generated during both the primary and secondary sedimentation processes. With the growing volume of wastewater, sludge production has increased accordingly. Prior to subsequent treatment or disposal, sludge dewatering is a critical step to reduce volume and improve treatment efficiency. The primary challenge lies in the removal of bonded water within the extracellular polymeric substances (EPSs) and the microorganism cells. In this study, electrochemical pretreatment was employed to improve sludge dewatering performance. The optimal electrochemical treatment was achieved at an electrode spacing of 2 cm, a stirring speed of 500 rpm, and an electrolyte (1 M calcium chloride, CaCl2) dosage of 3 mL for 50 min. Subsequently, flocculation was conducted. Compared with the widely used polyacrylamide (PAM), polydimethyldiallylammonium chloride (PDMDAAC) achieved superior dewatering performance with less than half the dosage required. Under the combined treatment, the final moisture content of the sludge cake was reduced to 53.2%. These findings indicate that the combination of Fe/Ti-based electrochemical pretreatment and flocculation process is a promising and efficient strategy for deep sludge dewatering.
1. Introduction
With the growth of urban populations and the continuous improvement of sewage pipe networks, wastewater treatment volume has significantly increased. Consequently, as a byproduct of wastewater treatment, sludge production has also risen [1]. Sludge dewatering is a critical step in sludge management, as its efficiency directly impacts transportation costs and the load on subsequent treatment processes. Therefore, enhanced sludge dewatering has attracted increasing attention. Two critical factors influencing deep sludge dewatering are sludge pretreatment and flocculant selection [2,3].
The moisture in sludge exists in three forms: free water, interstitial water, and bound water. Free and interstitial water can be removed via gravitational settling or mechanical compression. Bound water includes water trapped within microbial cells and water bound within EPS. This necessitates sludge pretreatment to disrupt the EPS matrix (releasing extracellular bound water) and further promote cell lysis (releasing intracellular bound water).
Sludge pretreatment methods include thermal treatment, acid/alkali treatment, ultrasonic pretreatment, and many others [4]. Among them, Electrochemical pretreatment represents a novel approach for enhancing sludge dewatering. It exhibits significant advantages, including minimal chemical consumption, environmental sustainability, and high operational flexibility. Furthermore, it effectively eliminates pathogenic microorganisms and contributes to odor control [5]. Electrochemical sludge pretreatment enhances dewatering efficiency by direct and indirect oxidation. The former refers to the direct decomposition of organic substances on the electrode surface. The latter refers to the destruction of sludge flocs and cells by generating strong oxidants on the electrode surface. A study has demonstrated that pretreatment using Ti/RuO2 electrodes under optimal electrolytic conditions effectively disrupts and decomposes the EPS in sludge, resulting in a 7% improvement in dewatering efficiency compared to untreated sludge [6]. Heng et al. employed Ti/RuO2 electrodes for sludge pretreatment. They achieved optimal dewatering performance at a pH of 11.65, electrolysis time of 35 min, current density of 6 mA/cm2, and sodium chloride concentration of 1 g/L [7]. He et al. utilized electrochemical cell lysis for sludge reduction, determining the optimal operating conditions as voltage 18 V, electrode gap 1 cm, pH 6.8–7.0, and electrolysis time 90 min. This process reduced the final sludge moisture content to 58.2% [8]. The moisture content of the sludge treated by these electrochemical oxidation technologies can be generally reduced to about 55% to 60% after filtration. However, there remains considerable potential for further improvement in dewatering performance.
After electrochemical pretreatment, subsequent flocculation further enhances sludge dewaterability by introducing flocculants that improve sludge properties. Flocculants contain functional groups with specific charges. These positively or negatively charged groups interact with fine particles of opposite charge that are difficult to separate by conventional methods. This interaction reduces the zeta potential of particles, leading to destabilization and promoting inter-particle attraction. As a result, larger and more compact flocs are formed, which can be more effectively separated through physical or chemical means [9]. Organic flocculants, especially PAM, are widely used in the sludge dewatering process due to their high treatment efficiency [10]. However, concerns have been raised regarding the toxicity of residual monomers in PAM, which may pose environmental risks during subsequent sludge disposal or utilization. Dao et al. reported that acrylamide monomers are highly toxic and can induce severe neurotoxic effects [11]. Therefore, alternative flocculants with lower toxicity and better environmental compatibility are desirable. PDMDAAC, a cationic polymer derived from quaternary ammonium compounds, has been widely studied for its high flocculation efficiency and relatively low monomer toxicity. With its linear quaternary ammonium groups, PDMDAAC exhibits a high positive charge density, excellent water solubility, and wide pH applicability range [12]. Studies have shown that, PDMDAAC-treated sludge exhibits a lower specific resistance to filtration (SRF) than that PAM-treated sludge, indicating better dewaterability. Moreover, PDMDAAC requires lower dosages, achieves higher dewatering efficiency, and incurs lower treatment costs compared to PAM [13].
This study investigates the impact of electrochemical treatment and flocculant assistance on sludge dewatering efficiency. The electrochemical system was constructed by using the metal iron as the anode and the metal titanium as the cathode. Optimization was conducted by varying operational parameters, including electrode spacing, electrolysis time, electrolyte dosage, and stirring speed. Furthermore, a comparative analysis was performed to validate the applicability of PDMDAAC to facilitate the dewatering. The mechanism of cell disruption was predicted.
2. Materials and Methods
2.1. Materials
2.1.1. Wastewater Sludge
The wastewater sludge used in this study was obtained from a municipal wastewater treatment plant in the southern city of China. The moisture content of the original sludge is 98.6% with a pH of 6.63, total suspended solids concentration (TSS) of 31.4 g/L, and volatile suspended solids concentration (VSS) of 18.3 g/L.
2.1.2. Flocculants
Stock solutions of PAM and PDMDAAC were prepared at concentrations of 3% (w/v, 30 g/L) and 0.6% (w/v, 6 g/L), respectively, based on their molecular characteristics and application performance.
2.1.3. Electrolyte
It is known that Ca2+ and Mg2+ can improve the compression performance of sludge [14,15]. The market prices of anhydrous MgCl2 and anhydrous CaCl2 are 2000 RMB/ton and 1250 RMB/ton, respectively. Considering cost-effectiveness, CaCl2 (1 M CaCl2 solution) was used as the electrolyte of this study.
2.2. Electrochemical Treatment
2.2.1. Electrochemical Reactor
An iron plate and a titanium plate served as anodes and cathodes, respectively. The plates have dimensions of 40 mm × 40 mm × 2 mm. The plates were connected to a direct current-regulated power supply and placed in a reactor with a working volume of 250 mL.
2.2.2. Sludge Electrochemical Treatment
A 150 mL sludge sample was filled into the reactor and treated at a constant voltage of 15 V. Optimization experiments were carried out by varying operational parameters including electrode spacing (1, 2, 3, and 4 cm), reaction time (10, 30, 50, 70, and 90 min), CaCl2 (1 M) dosage (0, 1, 3, 5, and 7 mL), and stirring speed (0, 200, 300, 400, and 500 rpm). After the electrochemical treatment, the sludge was dewatered using a mechanical filter press at a pressure of 1 MPa for 8 min. Relevant experimental indicators were then measured. Raw sludge (RS) served as the control group.
2.2.3. Flocculation
The PAM (30 g/L) or PDMDAAC (6 g/L) was added to the sludge at a dosage volume of 2, 4, 6, 8, and 10 mL. The sludge was rapidly mixed at 100 rpm for 4 min, followed by slow stirring at 500 rpm for 2 min to ensure complete flocculation. After processing, the sludge was dewatered using a mechanical filter press at a pressure of 1 MPa for 8 min. Relevant experimental indicators were then measured. RS served as the control group.
All the experiments were conducted at room temperature (about 25 °C). The initial pH of each test corresponded to the raw sludge pH (6.63).
2.3. Analysis
2.3.1. General Indicators
TSS and VSS were determined according to the “Water Quality-Determination of Suspended Substance-Gravimetric Method” (GB 11901-89) [16]. The soluble chemical oxygen demand (SCOD) was analyzed, referring to “Water and Wastewater Monitoring and Analysis Method” (4th Edition, 2002) published by the State Environmental Protection Administration of China [17].
2.3.2. EPS Content
EPS was extracted by an improved thermal extraction method [18]. The contents of protein and polysaccharide in EPS were detected by the Lowry protein content determination kit and the phenol–sulfuric acid method [19], respectively.
2.3.3. Capillary Suction Time (CST)
CST was used to assess sludge dewaterability, with lower CST values indicating improved water release after treatment. In this study, it was measured using the 304B CST (Triton, Essex, UK) sludge capillary suction time tester.
2.3.4. Degree of Cell Lysis
Lactate dehydrogenase (LDH) is commonly used as a marker to assess the degree of cell lysis, as it is only released into the extracellular environment when the cell membrane is damaged [20]. In this study, the LDH was measured by a lactate dehydrogenase kit (Biyuntian, Shanghai, China) combined with a microplate reader, Spark10M (Tecan, Männedorf, Switzerland).
2.3.5. Free Radical
The generation of free radicals was detected by electron spin resonance spectroscopy (EPR) with 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) as the free radical trap agent.
In this study, three samples were taken in each sampling for analysis. The average values are presented in the results. The ANOVA was performed, and the p-value was smaller than 0.05.
3. Results and Discussion
3.1. Optimization of Electrochemical Treatment Conditions
The electrochemical treatment was applied to the sludge samples at a constant voltage of 15 V [21]. Key operational parameters, including electrode spacing, electrolysis time, electrolyte dosage, and stirring speed, were optimized to evaluate their effects on sludge dewatering performance and sludge cell lysis.
3.1.1. Electrode Spacing Optimization
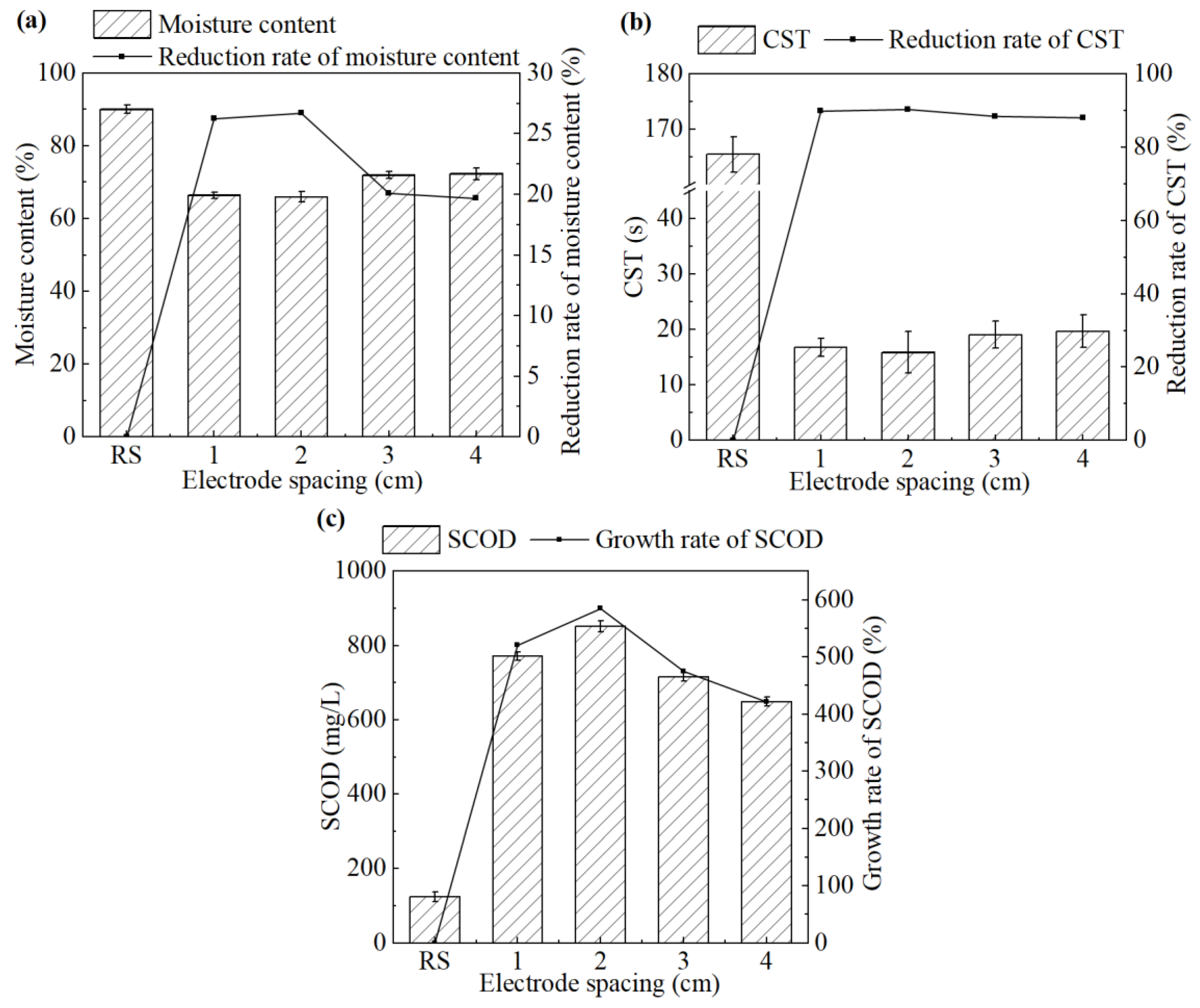
The effect of the electrode spacing on sludge dewatering performance during the electrochemical treatment is shown in Figure 1.
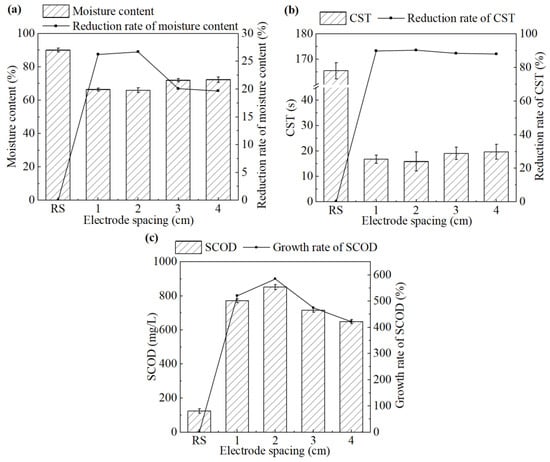
Figure 1.
(a) Moisture content and its reduction rate, (b) CST and its reduction rate, and (c) SCOD concentration in the supernatant and its growth rate after the treatment under different electrode spacings.
As shown in Figure 1a,b, when the electrode spacing increased from 1 cm to 4 cm, both the moisture content of the sludge cake and the CST of the sludge showed a trend of first decreasing and then increasing. The system achieved the best sludge dewatering performance at an electrode spacing of 2 cm. The moisture content and CST were 65.9% and 15.9 s, respectively, which have been decreased by 26.7% and 90.4%, respectively, compared with the untreated sludge.
This trend can be attributed to the formation of the electrical double layer (EDL) and electroosmotic flow [22]. Sludge flocs generally exhibit a net negative surface charge, which attracts counterions from the surrounding solution via electrostatic interactions and ionic thermal motion, leading to the formation of an EDL around each floc. Upon the application of an external electric field, positively charged ions within the diffuse layer of the EDL migrate toward the cathode. These hydrated ions drag water molecules along via electroosmosis, facilitating the separation of water from the sludge matrix [6]. However, inappropriate electrode spacing would compromise this mechanism [23]. Excessively narrow spacing may cause electrode short circuit due to the residual conductive solids in sludge, while overly wide spacing weakens the electric field strength, thus reducing ion mobility and electroosmotic efficiency.
In the process of the electrochemical treatment, intracellular substances were released due to the disruption of the sludge cells. Accordingly, the changes in SCOD of the supernatant before and after the treatment can be employed to evaluate the extent of cell lysis. As shown in Figure 1c, SCOD in the supernatant increased significantly after the treatment. At an electrode spacing of 2 cm, the SCOD reached the highest value of 851.7 mg/L, approximately seven times higher than that of the untreated sludge. Further increases in electrode spacing led to a decline in SCOD, indicating a reduced extent of cell lysis. Therefore, an electrode spacing of 2 cm was selected as the optimal condition for electrochemical conditioning and used in the subsequent study.
3.1.2. Electrolysis Time Optimization
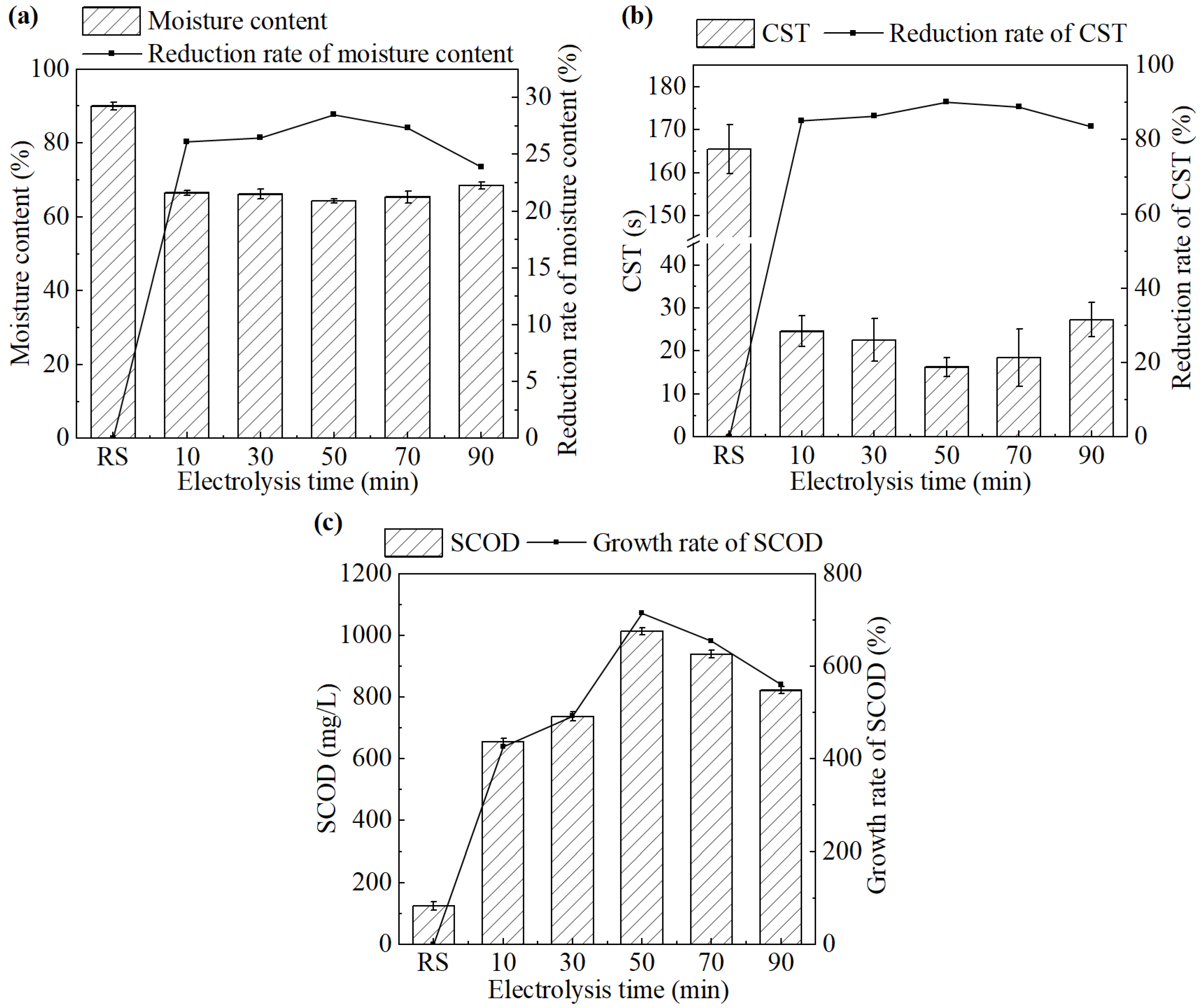
The effect of the electrolysis time on sludge dewatering performance during the electrochemical treatment is shown in Figure 2.

Figure 2.
(a) Moisture content and its reduction rate, (b) CST and its reduction rate, and (c) SCOD concentration in the supernatant and its growth rate after the treatment under different electrolysis times.
As shown in Figure 2a, the moisture content of the sludge cake exhibited minimal variation as the electrolysis time increased from 10 min to 90 min. The CST of the treated sludge showed a trend of decreasing first and then increasing with the prolonged electrolysis (Figure 2b). The system achieved the best sludge dewatering performance at an electrolysis time of 50 min. The moisture content and CST were 64.3% and 16.3 s, respectively, which have been decreased by 28.5% and 90.1%, respectively, compared with the untreated sludge.
Insufficient electrolysis duration resulted in incomplete sludge cell lysis, thereby limiting dewatering efficiency. Excessive electrolysis can lead to localized overheating near the iron electrode, causing sludge partial drying and adhesion onto the electrode surface. This phenomenon reduced electrochemical efficiency in two ways. Firstly, it hindered the diffusion of generated Fe2+ ions into the sludge matrix, thereby lowering oxidation efficiency. Secondly, it promoted electrode passivation, which obstructed current transmission. These results also suggest that the electrode plates are supposed to be periodically cleaned or replaced to avoid severe passivation.
As shown in Figure 2c, SCOD in the supernatant increased significantly after the treatment. At an electrolysis time of 50 min, the SCOD reached the highest value of 1013.5 mg/L, more than eight times higher than that of the untreated sludge. Further increases in electrolysis time led to a decline in SCOD, indicating a reduced extent of cell lysis. Therefore, an electrolysis time of 50 min was selected as the optimal condition for electrochemical conditioning and used in the subsequent study.
3.1.3. Electrolyte Dosage Optimization
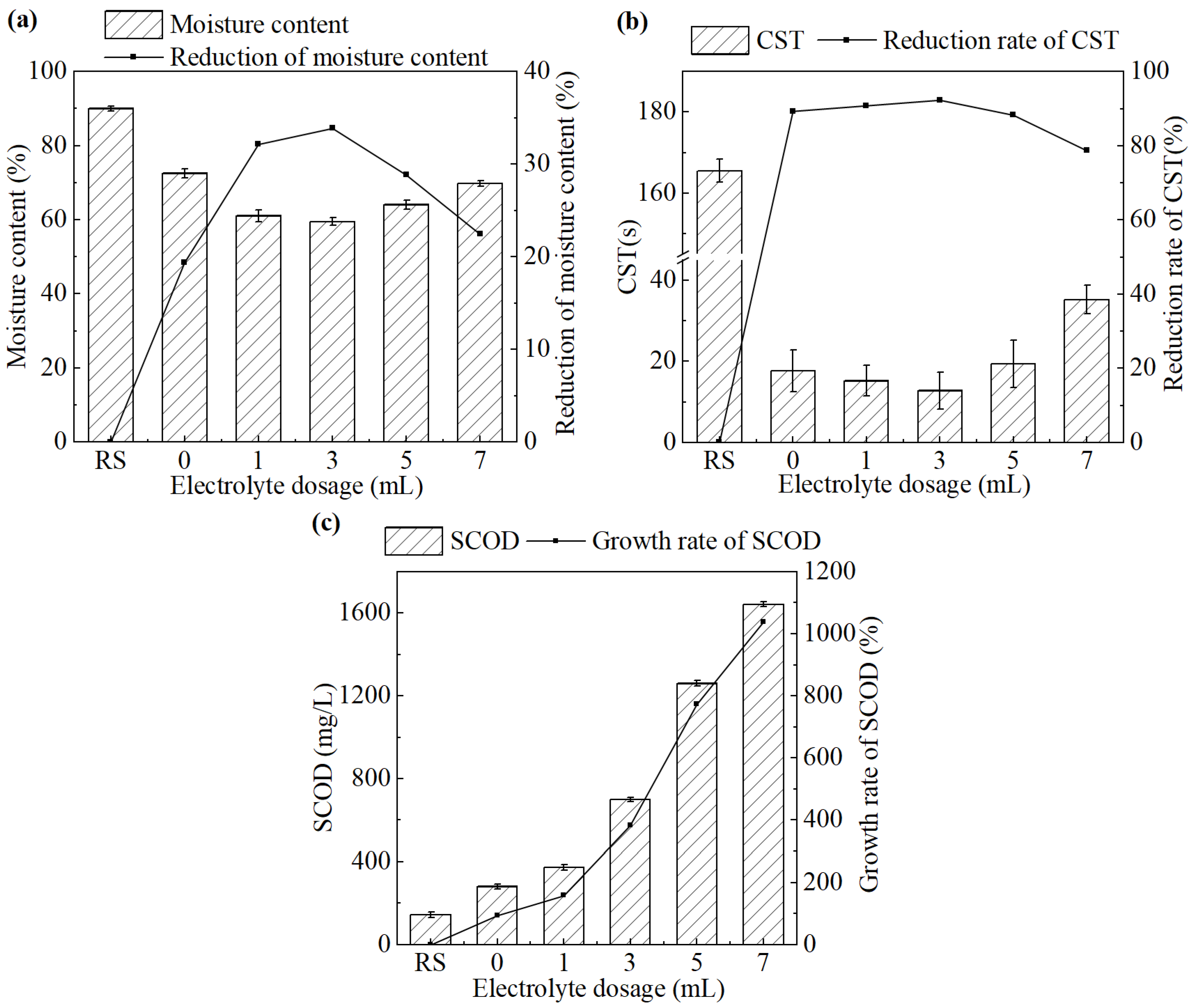
The effect of the electrolyte dosage on sludge dewatering performance during the electrochemical treatment is shown in Figure 3.
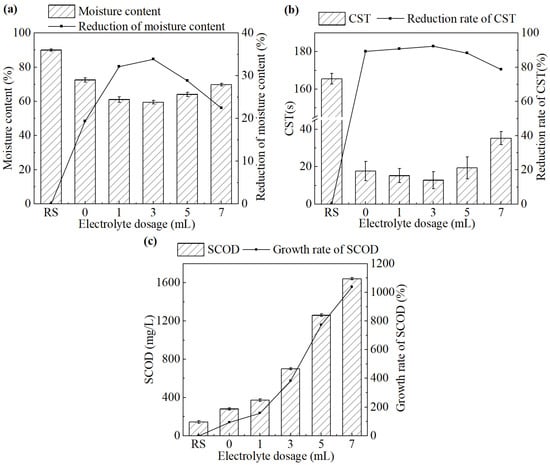
Figure 3.
(a) Moisture content and its reduction rate, (b) CST and its reduction rate, and (c) SCOD concentration in the supernatant and its growth rate after the treatment under different electrolyte dosages.
As shown in Figure 3a,b, both the moisture content of sludge cake and the CST of sludge showed a trend of decreasing first and then increasing, as the dosage of CaCl2 increased from 0 mL to 7 mL. The system achieved the best sludge dewatering performance at a CaCl2 dosage of 3 mL. The moisture content and CST were 59.5% and 12.8 s, respectively, which have been decreased by 33.9% and 92.3%, respectively, compared with the untreated sludge.
When the electrolyte concentration was too low, the current between the cathode and anode was weakened. It was not conducive to the release of Fe2+ and progress of oxidation. With a large CaCl2 dosage, the system would immediately generate a strong current at the initial stage. The rapid increase in temperature on the electrode surface would cause passivation in a short time [24].
As shown in Figure 3c, SCOD increased progressively with increasing CaCl2 dosage. This trend did not correspond with the variations observed in the moisture content or CST. It is speculated that the increase in SCOD may be due to the excessive salt concentration, which raised the osmotic pressure gradient across the cell membrane, disrupting cellular metabolism and accelerating the release of intracellular organics. Overall, an electrolyte dosage of 3 mL was selected as the optimal condition for electrochemical conditioning and used in the subsequent study.
3.1.4. Stirring Speed Optimization
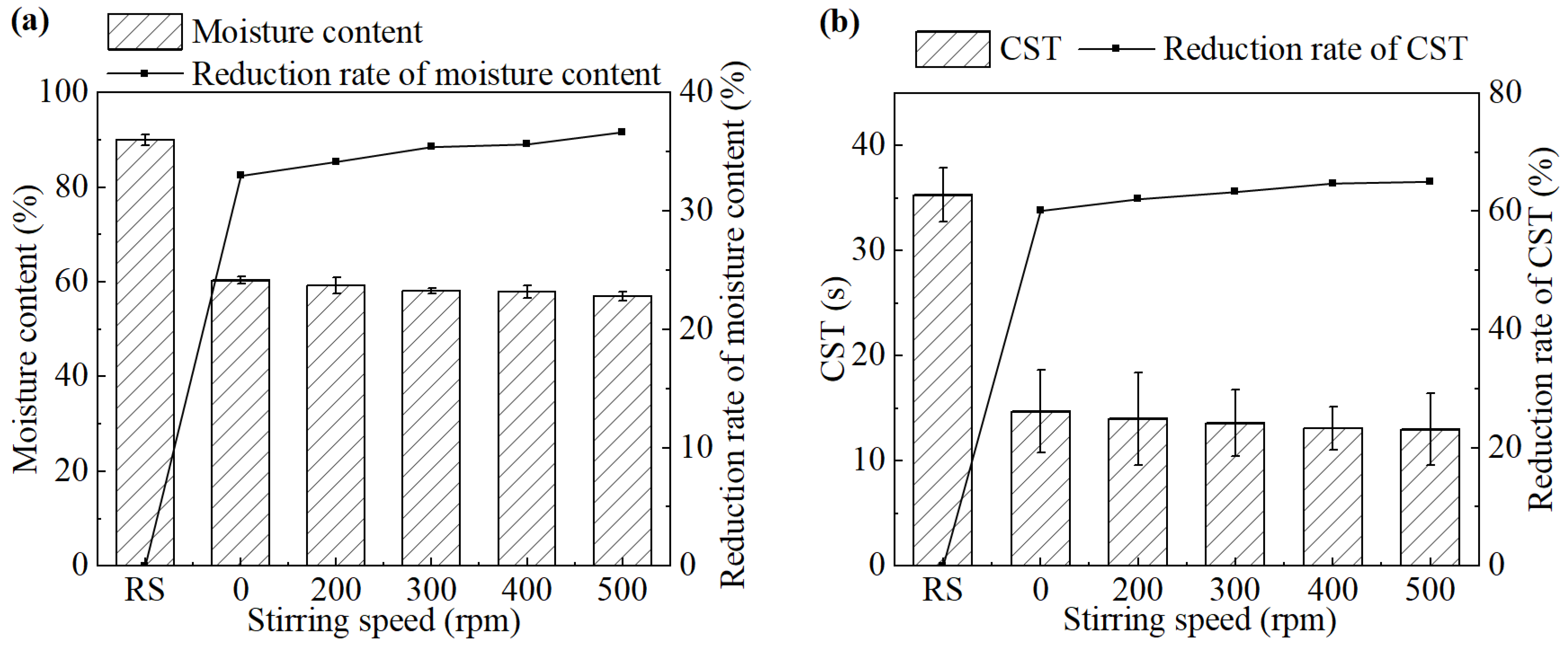
The stirring speed was controlled by the digital magnetic stirrer. Although the mode differed from the mechanical mixing employed in full-scale wastewater treatment plants, the effect of stirring intensity on sludge dewatering performance can be explored to provide a theoretical reference. The effect of the stirring speed on sludge dewatering performance during the electrochemical treatment is shown in Figure 4.

Figure 4.
(a) Moisture content and its reduction rate, (b) CST and its reduction rate after the treatment under different stirring speeds.
As the stirring speed gradually increased, both the moisture content of the sludge cake and the CST of the sludge showed a slight downward trend. The moisture content and CST were 57.0% and 13.0 s, respectively, when the stirring speed was 500 rpm.
Stirring promotes the rapid and uniform dispersion of oxidizing agents generated during electrolysis throughout the reaction system. Additionally, an appropriate stirring speed facilitates the disruption of microbial cells, thereby enhancing cell lysis. In practical applications, mechanical blade stirring or hydraulic mixing can be employed to achieve more effective mixing. In this study, no significant improvement in dewatering performance was observed beyond a stirring speed of 500 rpm. It is speculated that once the mixing intensity exceeds a certain threshold, further increases may not enhance performance but result in unnecessary energy consumption and increased maintenance costs. Therefore, an electrolyte dosage of 500 rpm was selected as the optimal condition for electrochemical conditioning.
3.2. Flocculants Selection
PDMDAAC and PAM were employed as flocculants to assist the dewatering. The effects of two flocculants on sludge dewatering performance were studied, and the optimal dosage was determined.
3.2.1. Effects of PAM on Sludge Dewatering Performance
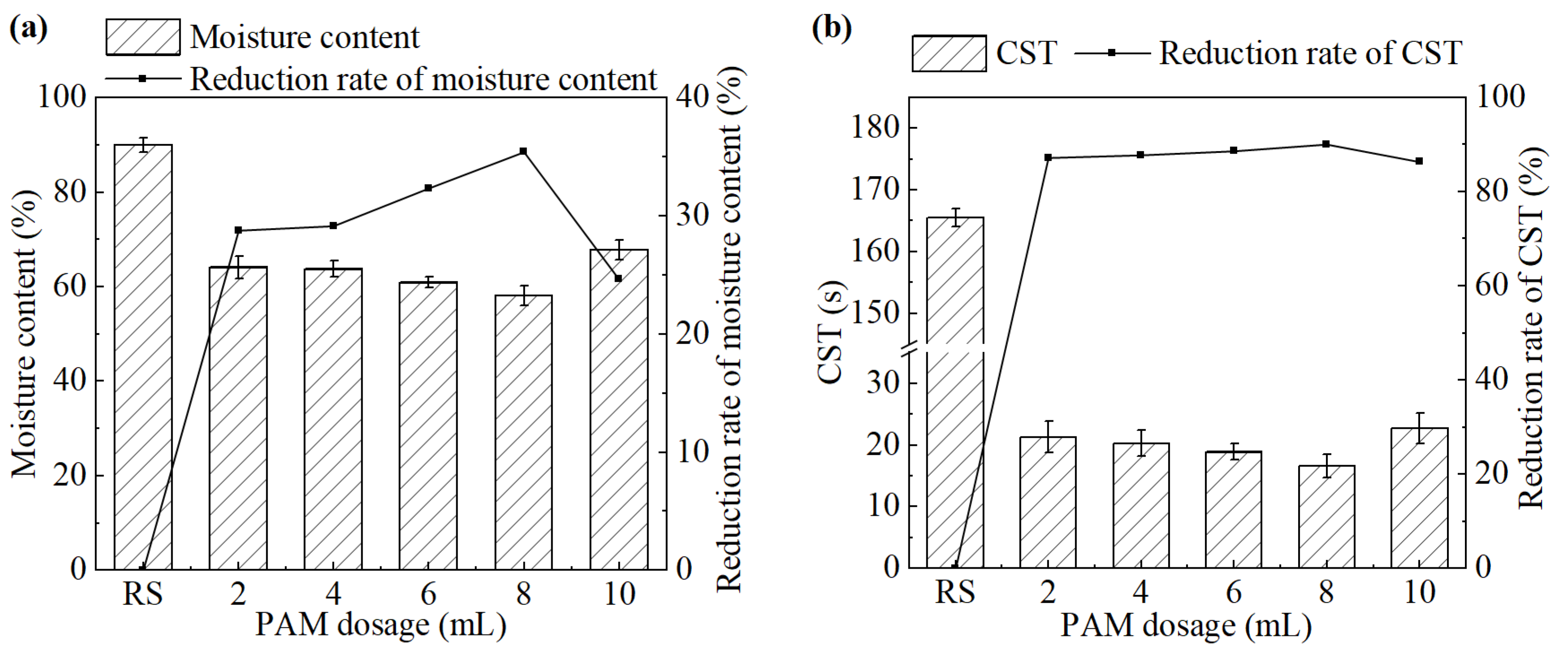
The effect of PAM dosage on sludge dewatering performance is shown in Figure 5. When the PAM dosage increased from 2 mL to 10 mL, both the moisture content of the sludge cake and the CST of the sludge significantly decreased compared to the untreated group. Both of them initially decreased and then increased. The system achieved the best sludge dewatering performance at a PAM dosage of 8 mL (1.6 g/L). The moisture content and CST were 58.1% and 16.6 s, respectively.
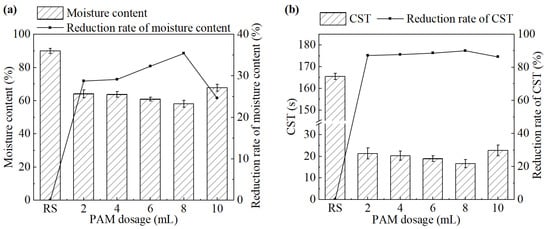
Figure 5.
(a) Moisture content and its reduction rate, (b) CST and its reduction rate after the treatment under different PAM dosages.
PAM can rapidly aggregate destabilized sludge particles into large flocs through its strong adsorption and bridging capacity. However, excessive PAM can deteriorate sludge dewatering performance. It is speculated that excessive PAM chains cannot fully extend and interact with sludge particles, which greatly weakens or even eliminates their function, resulting in deterioration of dewatering performance [25]. Additionally, PAM also has a dehydrant effect, which can convert hydrophilic colloids into hydrophobic ones to further improve the dewatering performance of sludge [26]. Sludge treated with PAM exhibited good filterability, with better dispersion during the filtration process and less adhesion to filter cloth. However, its practical application is limited by the relatively high dosage requirements and the potential toxicity associated with residual monomers.
3.2.2. Effects of PDMPAAC on Sludge Dewatering Performance
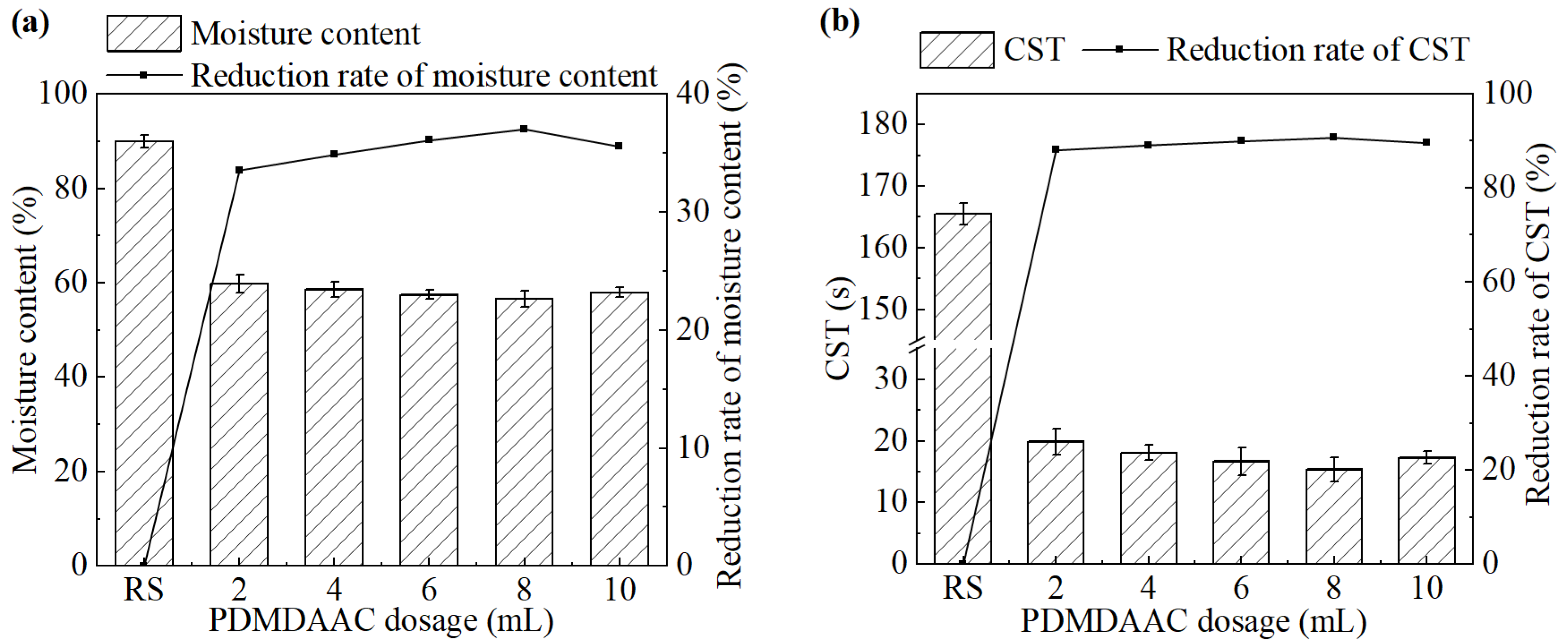
The effect of PDMPAAC dosage on sludge dewatering performance is shown in Figure 6. As the dosage of PDMDAAC increased from 2 mL to 10 mL, both the moisture content of the sludge cake and the CST of the sludge significantly decreased compared with the untreated group. Both of them initially decreased and then increased. The system achieved the best sludge dewatering performance at a PDMDAAC dosage of 8 mL (0.32 g/L). The moisture content and CST were 56.6% and 15.4 s, respectively.
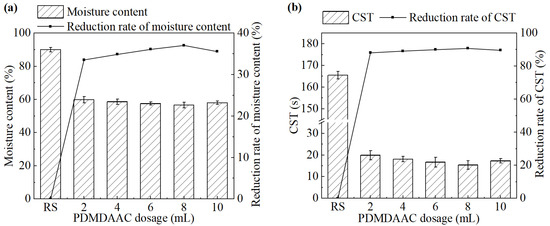
Figure 6.
(a) Moisture content and its reduction rate, (b) CST and its reduction rate after the treatment under different PDMDAAC dosages.
PDMDAAC enhances sludge dewatering performance primarily by neutralizing the negative surface charges of sludge particles and promoting their aggregation through strong electrostatic attraction [27]. However, excessive PDMDAAC dosage has been found to lower dewatering efficiency. This may be attributed to excessive charge neutralization interactions between the negatively charged sludge particles and the positively charged PDMDAAC during the conditioning process. Excessive charge reversal can induce electrostatic repulsion between particles, disrupting floc formation and weakening the flocculation efficiency of PDMDAAC [28].
3.2.3. Comparison of Effects of Two Flocculants
The comparative performance of PDMDAAC and PAM in sludge dewatering treatment is summarized in Table 1. Under significantly lower dosage conditions, PDMDAAC achieved both lower moisture content and CST than PAM, suggesting its superior flocculation efficiency in sludge dewatering. Moreover, the estimated treatment cost per ton of municipal sludge (initial moisture content of 97%) was 2.24 RMB for PDMDAAC, compared to 6.95 RMB for PAM. These findings indicate that PDMDAAC not only outperforms PAM in dewatering efficiency but also offers greater cost-effectiveness. Therefore, PDMDAAC was selected as the flocculant for subsequent experiments.

Table 1.
Comparison of sludge dewatering performance and economic cost of two flocculants.
To comprehensively evaluate the enhancement in sludge dewatering performance, electrochemical conditioning was conducted under the optimized parameters (electrode spacing of 2 cm, electrolysis time of 50 min, CaCl2 (1 M) dosage of 3 mL, and stirring speed of 500 rpm). This was followed by flocculation using PDMDAAC at a concentration of 0.32 g/L. Under these conditions, the final moisture content of the sludge cake was reduced to 53.2%, demonstrating a significant improvement compared to the 82.4% observed in the untreated group.
3.3. Electrochemical Pretreatment Mechanism
3.3.1. Generation of Free Radical
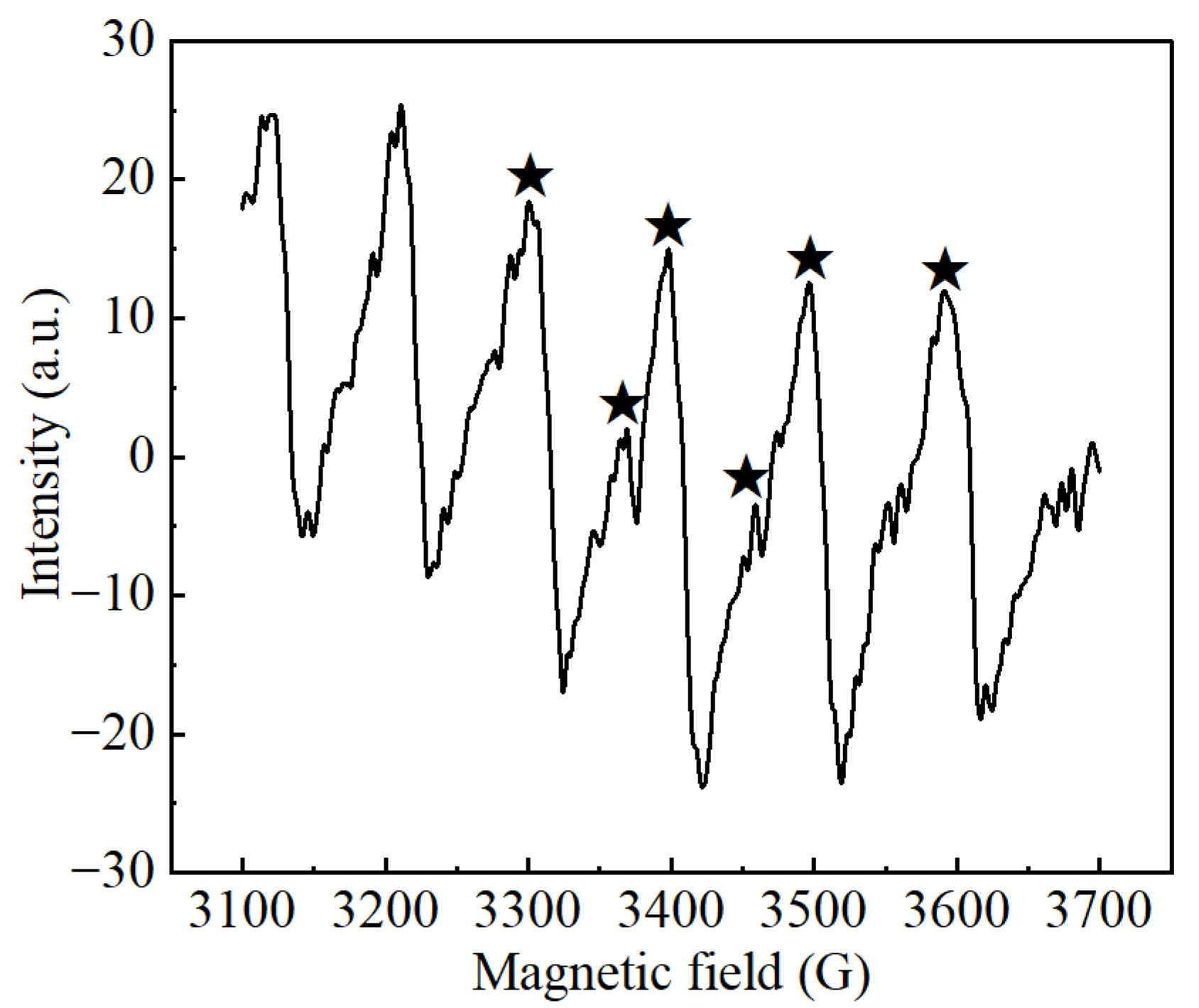
EPR analysis using DMPO as a free radical trapping agent revealed the generation of reactive oxygen species (ROS) under the optimal conditions of the electrochemical system. As shown in Figure 7, the characteristic spectrum consisted of six peaks, four major and two minor peaks from left to right, which corresponds to the spectral signature of the superoxide radical adduct (DMPO–·O2−). Similar findings were reported by Xu et al., who indicated that the activation of molecular oxygen can lead to the formation of ·O2− [29]. Ding et al. found that in the Fe@Fe2O3-based electrochemical system, ·O2− produced by electrochemical reaction can react with the Fe@Fe2O3 core–shell nanowire structure to form hydroxyl radicals (·OH) [30]. Similarly, Liu et al. verified the formation of ·OH in the electrochemical system with iron as the anode through the same reaction pathway [31].

Figure 7.
EPR spectra of DMPO-trapped radicals in the electrochemical sludge treatment system.
However, the presence of ·OH was not directly confirmed by EPR observation in this study. It is speculated that the generated ·OH may be rapidly consumed to oxidize the EPS and sludge cell. Zhao et al. indicated that organic substances or biological components (such as EPS) in electrochemical systems can rapidly quench ·OH, making it difficult to capture signals [32]. Additionally, previous studies have shown that the capture of ·OH by DMPO may be influenced by several factors, including solution pH, coexisting ions (such as Cl−), and competitive reactions (such as the preferential capture of ·O2−), all of which may contribute to signal suppression [33]. In this study, the inherent complexity of the sludge system likely interfered with the EPR measurements, further complicating the direct identification of ·OH.
3.3.2. Change in EPS Content
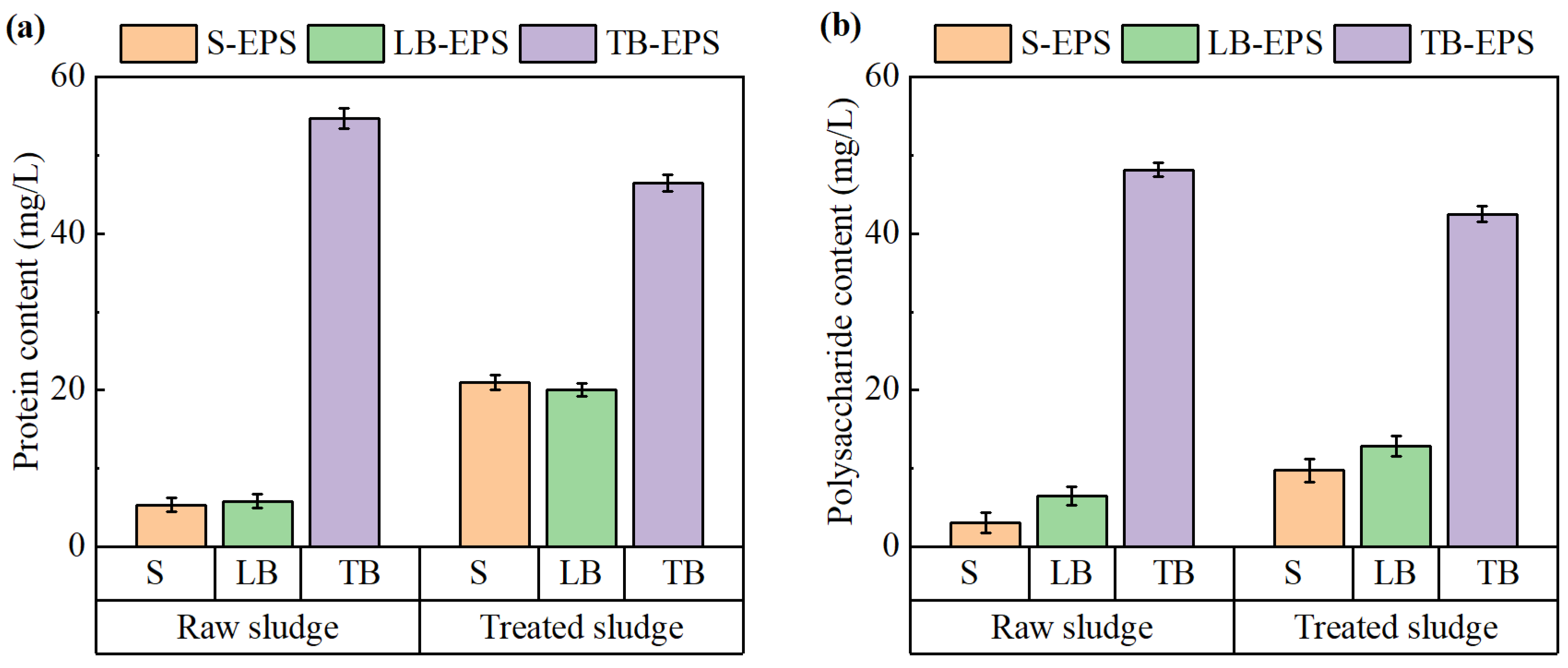
As mentioned, EPS has a great impact on sludge dewatering due to its strong capacity to retain bound water. The content and distribution of organic components within EPS significantly influence dewatering performance. Among these components, proteins and polysaccharides are the most abundant and functionally relevant. To evaluate the impact of the electrochemical treatment on sludge dewaterability, changes in the protein and polysaccharide contents across different EPS layers were analyzed. The results are shown in Figure 8.
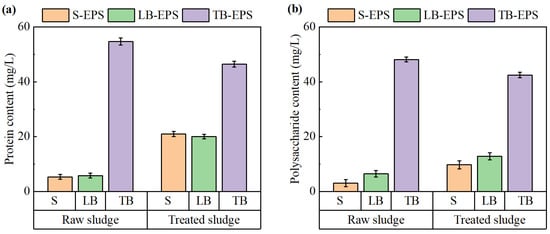
Figure 8.
Changes in (a) protein and (b) polysaccharide contents in EPS before and after electrochemical treatment.
As shown in Figure 8a, the protein content in tightly bound EPS (TB-EPS) was significantly higher than that in soluble EPS (S-EPS) and loosely bound EPS (LB-EPS) of the sludge. Following electrochemical conditioning, the protein content in TB-EPS decreased, whereas the protein content in both S-EPS and LB-EPS increased. A similar trend was observed for polysaccharides, as illustrated in Figure 8b. After the treatment, the polysaccharide contents in S-EPS and LB-EPS also increased, though to a lesser extent compared to proteins.
During the electrochemical treatment process, the ·OH generated in the sludge system promoted the oxidative degradation of TB-EPS, partially converting it into LB-EPS and S-EPS [34]. The conversion and removal of hydrophilic organic matter in TB-EPS reduced the water-holding capacity of the sludge and further improved its dewatering performance. These findings are consistent with the study of Zhang et al. [35].
3.3.3. Change in LDH Concentration
The rupture of sludge cells causes the release of intracellular bound water, which is essential for improving sludge dewaterability. In this study, LDH in the sludge supernatant was measured to evaluate the effect of the electrochemical treatment on cell lysis.
Compared to the raw sludge, the LDH concentration in the supernatant of the sludge after electrochemical conditioning increased from 97.5 μg/L to 135.0 μg/L, representing approximately a 1.4-fold increase. It indicates that the electrochemical pretreatment effectively promoted cell lysis. This effect can be attributed to the generation of ·O2− and ·OH within the system. These highly reactive species can oxidatively attack cell membranes and intracellular components, leading to the release of LDH into the liquid phase.
In conclusion, it can be predicted that the improvement of the overall sludge dewatering performance results from two factors: one is the increased release of bound water from the EPS of sludge, and the other is the increased release of bound water from the ruptured sludge cells.
4. Conclusions
In this study, the effectiveness of the Fe/Ti-based electrochemical treatment combined with the flocculation process for sludge dewatering was evaluated. The sludge moisture content was reduced to 53.2% at an electrode spacing of 2 cm, electrolysis time of 50 min, CaCl2 (1 M) dosage of 3 mL, stirring speed of 500 rpm, and PDMDAAC dosage of 0.32 g/L, significantly lower than that of the untreated sludge (82.4%). The improved dewatering performance was mainly attributed to the removal of bound water from EPS and within the sludge cells. It is speculated that ·OH and other reactive species play a key role in the process. These findings demonstrate the potential of combining Fe/Ti-based electrochemical conditioning with PDMDAAC flocculation as a promising approach for improving sludge dewatering efficiency at the laboratory scale. In future studies, strategies to reduce energy consumption associated with electrolysis and mixing can be explored, and the system’s performance under pilot-scale conditions can be further assessed.
Author Contributions
Conceptualization, X.Z. and B.Y.; methodology, X.Z., X.W., and Q.L.; validation, Y.S., Q.L., and X.W.; formal analysis, B.Y. and Y.S.; investigation, Y.S.; resources, X.Z.; data curation, Y.S.; writing—original draft preparation, B.Y. and Q.L.; writing—review and editing, X.Z.; supervision, X.Z.; funding acquisition, X.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Shenzhen Science and Technology Program (GJHZ20220913143007014).
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gupta, A.; Ramachandran, S.; Mayilswamy, N.; Nighojkar, A.; Kandasubramanian, B. Dye-laden sludge-derived biochar for wastewater remediation: A review on pyrolytic engineering, adsorptive interactions, and environmental prospects. Sustain. Chem. Environ. 2025, 11, 100271. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Ling, Z.C.; Zhao, M.M.; Sha, L.; Li, C.; Lu, X.B. Investigation of the properties and mechanism of activated sludge in acid-magnetic powder conditioning and vertical pressurized electro-dewatering (AMPED) process. Sep. Purif. Technol. 2024, 328, 124973. [Google Scholar] [CrossRef]
- Hyrycz, M.; Ochowiak, M.; Krupinska, A.; Wlodarczak, S.; Matuszak, M. A review of flocculants as an efficient method for increasing the efficiency of municipal sludge dewatering: Mechanisms, performances, influencing factors and perspectives. Sci. Total Environ. 2022, 820, 153328. [Google Scholar] [CrossRef]
- Zhou, P.; Li, D.J.; Zhang, C.; Ping, Q.; Wang, L.; Li, Y.M. Comparison of different sewage sludge pretreatment technologies for improving sludge solubilization and anaerobic digestion efficiency: A comprehensive review. Sci. Total Environ. 2024, 921, 171175. [Google Scholar] [CrossRef]
- Zhang, X.D.; Ye, P.; Wu, Y.J. Enhanced technology for sewage sludge advanced dewatering from an engineering practice perspective: A review. J. Environ. Manag. 2022, 321, 115938. [Google Scholar] [CrossRef]
- Rumky, J.; Deb, A.; Shim, M.J.; Laakso, E.; Repo, E. A review on the recent advances in electrochemical treatment technologies for sludge dewatering and alternative uses. J. Hazard. Mater. Adv. 2023, 11, 100341. [Google Scholar] [CrossRef]
- Heng, G.C.; Isa, M.H. Electrochemical Disintegration of Activated Sludge Using Ti/RuO2 Anode. Appl. Mech. Mater. 2014, 567, 44–49. [Google Scholar] [CrossRef]
- He, Z.Q.; Han, W.; Zhou, X.; Jin, W.B.; Liu, W.T.; Gao, S.H.; Zhao, Z.C.; Chen, Y.D.; Jiang, G.M. Effect of on-site sludge reduction and wastewater treatment based on electrochemical-A/O combined process. Water 2021, 13, 941. [Google Scholar] [CrossRef]
- Sun, Y.J.; Liang, Y.K.; Sun, W.Q.; Zhou, J.; Shah, K.J. Oxidation-flocculation conditioning to improve the performance and mechanism of municipal sludge dewatering. Sep. Purif. Technol. 2024, 347, 127656. [Google Scholar] [CrossRef]
- Jin, L.Y.; Zhang, P.Y.; Zhang, G.M.; Li, J. Study of sludge moisture distribution and dewatering characteristic after cationic polyacrylamide (C-PAM) conditioning. Desalination Water Treat. 2016, 57, 29377–29383. [Google Scholar] [CrossRef]
- Dao, V.H.; Cameron, N.R.; Saito, K. Synthesis, properties and performance of organic polymers employed in flocculation applications. Polym. Chem. 2016, 7, 11–25. [Google Scholar] [CrossRef]
- Leonhartsberger, S.; Carmona, P.; Seidl, B.; Mann, K.J.; Kozich, M.; Sulaeva, I.; Stanetty, C.; Mihovilovic, M.D. Polysaccharide-based green flocculants: A systematic and comparative study of their coagulation-flocculation efficiency. Carbohydr. Polym. 2025, 358, 123527. [Google Scholar] [CrossRef]
- Xie, H.K.; Zhao, C.F.; Yu, W.D.; Shao, S.; Zhang, L.P.; Zhang, Y.L.; Guo, F.; Li, H.G.; Xie, L.N. Study on the radiation synthesis of polydimethyldiallylammonium chloride and its application in the deep dewatering of Sludge deposited. Chem. Res. Appl. 2022, 34, 904–908. [Google Scholar]
- Hu, X.; Shen, Y.H.; Zhang, H.J.; Xia, J.; Kong, F.G.; Zhang, W.H. Insight into the effect of calcium carbonate filler on the dewatering performance of simulated pulp & paper mill sludge. J. Environ. Chem. Eng. 2022, 10, 108863. [Google Scholar] [CrossRef]
- Hoane, A.G.; Zheng, Q.L.; Maldonado, N.D.; Espinosa-Marzal, R.M.; Gewirth, A.A. Impact of multivalent cations on interfacial layering in water-in-salt electrolytes. ACS Appl. Energy Mater. 2024, 7, 5179–5192. [Google Scholar] [CrossRef]
- GB/T 11901-1989; Water Quality-Determination of Suspended Substance-Gravimetric Method. Standards Press of China: Beijing, China, 1989.
- Ministry of Ecology and Environment the People’s Republic of China’s Water and Wastewater Monitoring and Analysis Methods Committee. Water and Wastewater Monitoring and Analysis Method, 4th ed.; China Environmental Science Press Pub.: Beijing, China, 2002. [Google Scholar]
- Li, X.Y.; Yang, S.F. Influence of loosely bound extracellular polymeric substances (EPS) on the flocculation, sedimentation and dewaterability of activated sludge. Water Res. 2007, 41, 1022–1030. [Google Scholar] [CrossRef]
- Ben Hamed, H.; Mainardis, M.; Moretti, A.; Toye, D.; Léonard, A. Extracellular polymeric substances (EPS) in sewage sludge management: A call for methodological standardization. J. Environ. Manag. 2025, 376, 124407. [Google Scholar] [CrossRef]
- Yuan, S.; Wang, J.; Wang, G.; Liu, J.; Zhang, S.; Li, Y.; Wang, X.; Wang, X.; Zhu, S.; Chen, H. Development and protective mechanism of a freeze-drying protectants against freeze-drying for Lactiplantibacillus plantarum W1. Food Bioprod. Process. 2025, 151, 258–267. [Google Scholar] [CrossRef]
- Cao, B.D.; Zhang, T.; Zhang, W.J.; Wang, D.S. Enhanced technology based for sewage sludge deep dewatering: A critical review. Water Res. 2021, 189, 116650. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.R.; Xu, Y.; Wang, J.L.; Qiu, S.X.; Rao, B.Q.; Xu, P. A pore-scale physical model for electric dewatering of municipal sludge based on fractal geometry. J. Environ. Eng. 2023, 149, 04022099. [Google Scholar] [CrossRef]
- Silva, F.L.; Lanza, M.R.V.; Saez, C.; Rodrigo, M.A. Electrochemical dewatering for the removal of hazardous species from sludge. J. Environ. Manag. 2019, 233, 768–773. [Google Scholar] [CrossRef]
- Jiang, L.P.; Hu, Z.; Wang, Y.A.; Ru, D.Y.; Li, J.W.; Fan, J.L. Effect of trace elements on the development of co-cultured nitrite-dependent anaerobic methane oxidation and methanogenic bacteria consortium. Bioresour. Technol. 2018, 268, 190–196. [Google Scholar] [CrossRef]
- Zhu, Y.Q.; Wu, S.Q.; Liu, Z.W.; Zheng, X.Y.; Zhao, M.; Liu, S.H.; Wang, L.J.; Fan, C.Z. Optimization of flocculation-precipitation dewatering treatment for river sludge: Flocculant and conditioner dosage, organic matter content. J. Environ. Chem. Eng. 2025, 13, 118720. [Google Scholar] [CrossRef]
- Yang, Y.H.; Yang, X.F.; Yang, Q.Y.; Zhang, H.N.; Xu, W.X.; Zhu, L.F.; Ma, P.J.; Li, Y.Y. Exploring the feasibility and potential mechanism of synergistic enhancement of sludge dewaterability by ultrasonic cracking, chitosan re-flocculation and sludge-based biochar adsorption of water-holding substances. J. Environ. Chem. Eng. 2022, 10, 108303. [Google Scholar] [CrossRef]
- Tao, S.Y.; Liang, S.; Chen, X.Y.; Zhu, Y.W.; Yu, W.B.; Hou, H.J.; Hu, J.P.; Xiao, K.K.; Yuan, S.S.; Yang, J.K. Enhanced sludge dewatering by PDMDAAC coupled with Fenton-like reaction initiated by Fe-rich sludge biochar with in-situ generation of H2O2: Fe/C structure as an electron shuttle. Resour. Conserv. Recycl. 2023, 198, 107184. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, B.Y.; Yue, Q.Y.; Wei, J.C.; Li, Q. The characterization and flocculation efficiency of composite flocculant iron salts-polydimethyldiallylammonium chloride. Chem. Eng. J. 2008, 142, 175–181. [Google Scholar] [CrossRef]
- Xu, T.Y.; Zhu, R.L.; Shang, H.; Xia, Y.B.; Liu, X.; Zhang, L.Z. Photochemical behavior of ferrihydrite-oxalate system: Interfacial reaction mechanism and charge transfer process. Water Res. 2019, 159, 10–19. [Google Scholar] [CrossRef]
- Ding, X.; Wang, S.Y.; Shen, W.Q.; Mu, Y.; Wang, L.; Chen, H.; Zhang, L.Z. Fe@Fe2O3 promoted electrochemical mineralization of atrazine via a triazinon ring opening mechanism. Water Res. 2017, 112, 9–18. [Google Scholar] [CrossRef]
- Liu, J.L.; Yang, R.; Chai, Y.Q.; Yuan, R. Versatile luminol/dissolved oxygen/Fe@Fe2O3 nanowire ternary electrochemiluminescence system combined with highly efficient strand displacement amplification for ultrasensitive microRNA detection. Anal. Chem. 2021, 93, 13334–13341. [Google Scholar] [CrossRef]
- Zhao, H.L.; Ke, J.; Zhu, S.K.; Li, M.T.; Chen, J.Q.; Yang, Q.L. Natural hematite and oxalic acid co-enhance electrochemical system for degradation of sulfamethoxazole: Role of oxalic acid and ROS generation. Electrochim. Acta 2025, 530, 146399. [Google Scholar] [CrossRef]
- den Hartog, S.; Samanipour, M.; Ching, H.Y.V.; Van Doorslaer, S.; Breugelmans, T.; Hubin, A.; Ustarroz, J. Reactive oxygen species formation at Pt nanoparticles revisited by electron paramagnetic resonance and electrochemical analysis. Electrochem. Commun. 2021, 122, 106878. [Google Scholar] [CrossRef]
- Li, X.Q.; Yu, Z.; Ge, X.L.; Zhang, W.Z.; Fang, Y.K.; Liu, W.Z.; Wang, A.J. Volatile fatty acids bio-production using extracellular polymeric substances disengaged from sludge for carbon source recycling. Bioresour. Technol. 2023, 386, 129565. [Google Scholar] [CrossRef]
- Zhang, W.J.; Chen, J.; Tang, M.Y.; Wu, H.J.; Liu, M.; Ai, J.; Wang, D.S. Citric acid chelated Fe(II) catalyzed peroxidation for simultaneously improving sludge dewaterability and antibiotic resistance genes (ARGs) removal. Sep. Purif. Technol. 2022, 280, 119925. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).