1. Introduction
Access to safe and clean drinking water is a fundamental requirement for the survival and well-being of all living organisms. Despite its importance, water sources are increasingly being contaminated with harmful substances, including heavy metal ions, synthetic dyes, pharmaceutical residues, and pathogenic microorganisms. These pollutants pose significant risks to human health and ecosystems, contributing to a wide range of diseases and environmental degradation. Removing these pollutants is critical for safeguarding both human health and environmental sustainability. Despite existing regulations intended to ensure the proper disposal of industrial effluents, over 80% of the world’s wastewater is still discharged without adequate treatment [
1]. This highlights the urgent need for effective and sustainable water treatment technologies.
Toluidine Blue (TB) is a synthetic dye widely used in biological staining, medical diagnostics, and various industrial applications. However, its release into water bodies poses significant environmental and health risks due to its toxic, non-biodegradable, and potentially carcinogenic nature [
2]. The dye can disrupt aquatic ecosystems by blocking sunlight penetration and reducing photosynthetic activity, while also contaminating drinking water sources [
3]. Consequently, the removal of toluidine blue from wastewater is essential to mitigate its adverse effects. Efforts to remove toluidine blue often involve adsorption, advanced oxidation, or biodegradation methods. Adsorption, in particular, is a cost-effective and efficient approach that utilizes various materials to capture and remove dyes from water. A variety of adsorbents, such as activated carbon [
4], polyurethane foam/zinc oxide nanocomposite [
5], nanoparticles [
3], manganese/graphene coated titaniferous sand [
6], agricultural waste-derived materials [
7], and natural zeolites [
8], among others, have been successfully employed for the elimination of toluidine blue from aqueous solutions. However, the high cost and non-renewable nature of some adsorbents have driven researchers to explore sustainable and eco-friendly alternatives [
9,
10].
In recent years, there has been a growing interest in utilizing low-cost natural materials as adsorbents for dye removal. Agricultural by-products, in particular, have gained attention due to their abundance, biodegradability, and potential for waste valorization [
11]. The black cumin seed press cake, by-product generated in large quantities during oil extraction, has emerged as a promising candidate. This material offers potential as a cost-effective natural biosorbent, addressing both waste management and water purification challenges [
12,
13,
14]. The black cumin seed by-product, obtained after oil extraction, is rich in protein, fiber, and bioactive compounds [
15]. It has been repurposed for various applications, including animal feed, organic fertilizers, and functional food ingredients [
16,
17]. Additionally, its antioxidant and antimicrobial properties make it valuable in nutraceuticals and natural health products [
12,
18]. Recent studies have highlighted the potential of black cumin seed by-products as adsorbents for environmental and industrial applications. Due to its porous structure, high content of organic compounds, and surface functional groups such as hydroxyl (OH), carbonyl (C=O), carboxyl (O–C=O), and amines (NH
2), it can effectively adsorb pollutants such as heavy metals, dyes, and organic contaminants from wastewater [
12,
16,
19]. This makes it a low-cost, eco-friendly alternative to synthetic adsorbents, offering a sustainable solution for water purification and pollution control.
Given the limited studies on low-cost and environmentally friendly adsorbents for toluidine blue removal, the present work investigates the efficiency of SPCN as a biosorbent. To the best of our knowledge, black cumin seed press cake has not yet been explored for the adsorption of toluidine blue, leaving a gap in the current understanding of its potential. Therefore, this study aims to evaluate its adsorption performance and highlight its promise as a sustainable and cost-effective material for wastewater treatment.
2. Materials and Methods
Black cumin seed press cake was provided by Nigella OOD (Dimitrovgrad, Bulgaria). Subsequently, oil residues were removed by Soxhlet extraction with petroleum ether (Sigma-Aldrich Chemie GmbH, Taufkirchen, Germany). The extraction was performed for 1.5 h at a solid-to-liquid ratio of 1:2, and the solvent was evaporated under reflux at approximately 70 °C.
The porous structure of studied material was investigated by low-temperature (−196 °C) nitrogen adsorption using a Quantachrome NOVA 1200 apparatus.
Infrared spectra of the sample before and after TB adsorption were recorded using Thermo Nicolet iS50 FTIR Spectrometer. The analyzed samples (1 to 2 mg) were mixed with spectroscopic grade KBr (0.1 to 0.2 g) in an agate mortar. The mixture was pressed in a hydraulic press for 5 min at 5000–10,000 kg per cm2. The obtained spectra were subjected to the built-in baseline correction in the OMNIC 9.16.1.188 package.
To examine the morphology of the samples, scanning electron microscopy (SEM) was employed using the JEOL JSM 6390 microscope in secondary electron image modus (SEI) at suitable magnifications. Prior to analysis, the dried samples were sputter-coated with a thin gold layer to improve conductivity and enhance image resolution. Elemental composition was determined using an Oxford INCA energy-dispersive X-ray (EDX) spectroscopy system.
The pH drift method was employed to determine the point of zero charge (pHpzc) of SPCN. A 0.1 M KNO3 solution was adjusted to an initial pH of 2–12 using 0.1 M HCl or 0.1 M NaOH, and 0.200 g of the biosorbent was then added to 50 mL aliquots. The suspensions were shaken at 25 °C for 24 h, followed by filtration. Final pH values were measured with a calibrated pH meter, and the pHpzc was taken as the point where ΔpH = 0.
Adsorption experiments were conducted in 50 mL conical flasks, each containing a measured amount of adsorbent and 15 mL of toluidine blue solution. The mixtures were agitated on a rotary shaker for predetermined time intervals and centrifuged to separate the solid and liquid phases upon reaching equilibrium. The supernatant was analyzed using a Thermo Evolution 160 UV–Vis spectrophotometer (Thermo Fisher Scientific Inc., Waltham, MA, USA) at the maximum absorption wavelength (λmax = 632 nm). TB concentrations were determined from a calibration curve linear over the range 2–10 mg L−1. Samples with higher concentrations were appropriately diluted to fall within this range, ensuring accurate quantification.
All chemicals used in the study were of analytical grade. Batch adsorption experiments were conducted to investigate the influence of various parameters, including contact time, pH, initial dye concentration, and temperature, on the adsorption process. The equilibrium adsorption capacity (
Qe, expressed in mg g
−1) was determined using the following equation:
where
C0 and
Ce represent the initial and equilibrium concentrations of TB (mg L
−1), respectively.
V stands for the solution volume (L), and
m corresponds to the mass of the sorbent (g). It is essential to note that each experiment was conducted in duplicate to ensure accuracy and reliability.
3. Results and Discussion
3.1. Texture Parameters
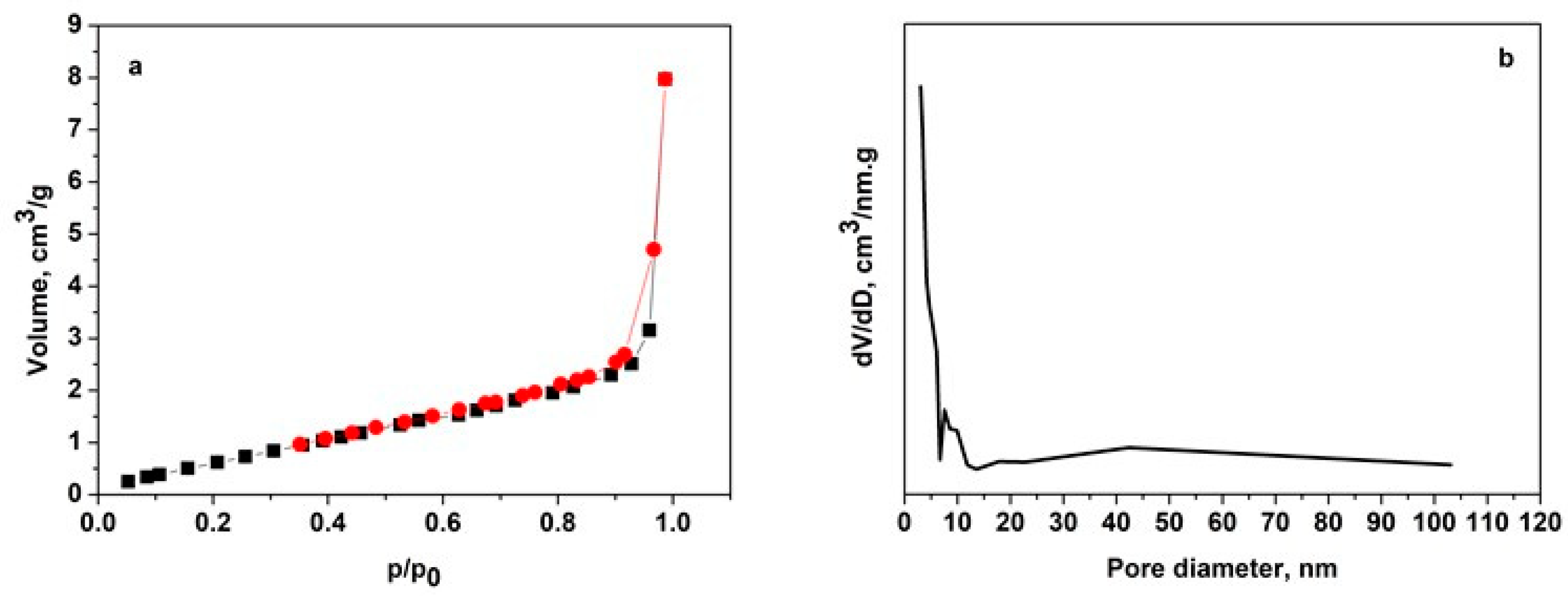
The nitrogen adsorption–desorption isotherm and pore size distribution of the studied biomaterial are presented in
Figure 1.
The nitrogen adsorption–desorption isotherm displays a Type IV isotherm, as classified by IUPAC, accompanied by a distinct hysteresis loop, which confirms the presence of mesoporous structures. The hysteresis loop is characteristic of the H3 type, suggesting the existence of aggregated or slit-like mesopores, with potential contributions from micropores. A steep increase in volume at high relative pressure (p/p0) signifies capillary condensation within the mesopores, further supporting the material’s mesoporous nature. The pore size distribution curve reveals a dominant peak at smaller pore diameters, highlighting the prevalence of micropores and small mesopores. Additionally, the rapid decline in dV/dD values at larger pore diameters indicates a minimal contribution from macropores, confirming that the material is predominantly mesoporous with some microporous features.
Analysis of the nitrogen adsorption–desorption isotherm reveals that the material has a specific surface area of 3 m2/g, a total pore volume of 0.01 cm3/g, and an average pore diameter of 15.6 nm.
3.2. FTIR Study
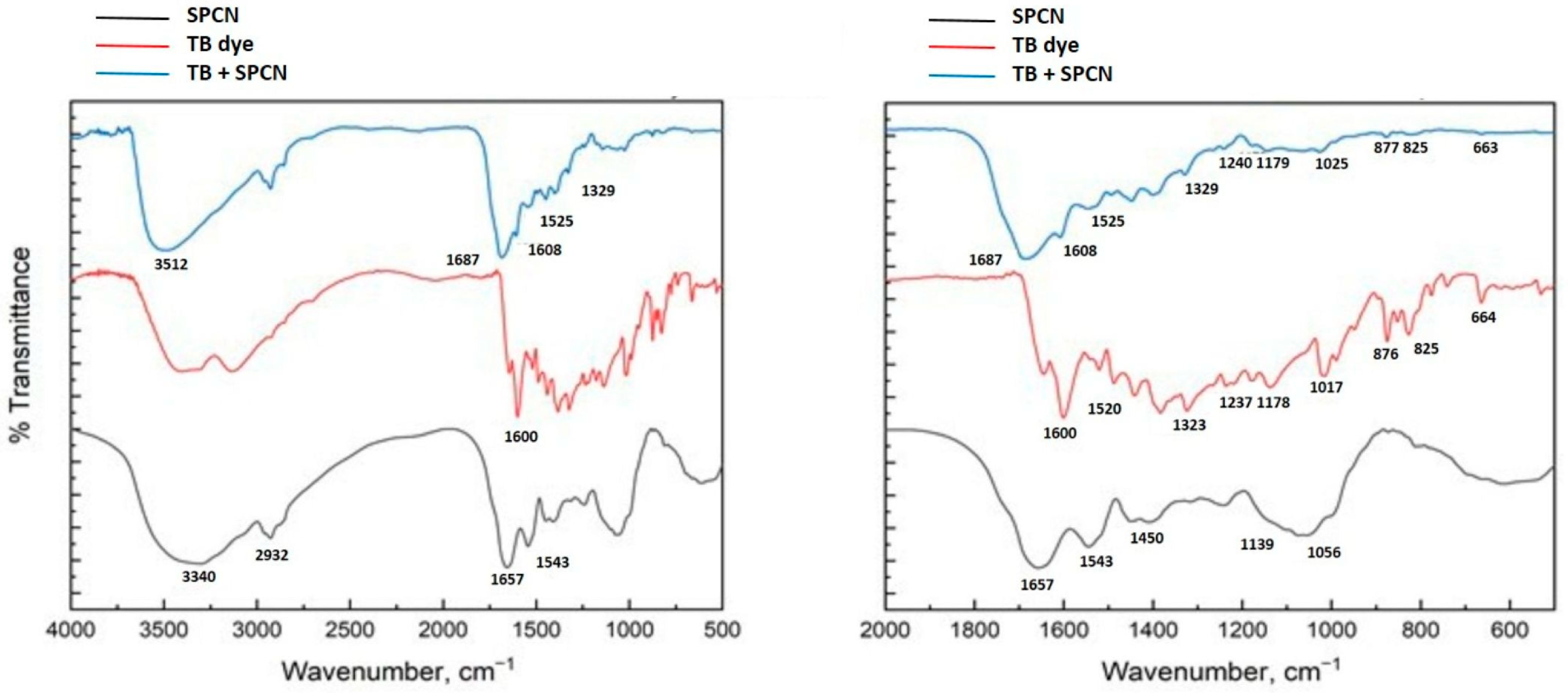
In
Figure 2, the FTIR spectra of pure toluidine blue, black cumin seed press cake (SPCN), and SPCN after TB adsorption are presented, including a full-range spectrum (500–4000 cm
−1) and an expanded view (500–2000 cm
−1) to clearly display all relevant peaks.
The FTIR spectrum of toluidine blue exhibits its characteristic vibrational frequencies, consistent with the literature reports. These include prominent peaks attributable to aromatic C=C stretching, amine bending vibrations, and vibrations associated with sulfonic acid and amino functional groups, which serve as reference bands for identifying changes in interaction after dye adsorption [
20].
The FTIR spectrum of SPCN before dye adsorption shows the following characteristic peaks: A broad absorption band around 3340 cm
−1, corresponding to O–H stretching vibrations, typical of hydroxyl groups from alcohols and phenols. The broadness of the band suggests strong hydrogen bonding, likely due to the polysaccharide or polyphenolic components of SPCN. A small band at 2932 cm
−1, assigned to C–H stretching vibrations of aliphatic –CH
3 and –CH(CH
3)– groups. These are indicative of methyl and isopropyl fragments, consistent with the molecular structure of thymoquinone, the major bioactive compound in cumin seed [
21]. A strong and sharp peak at 1657 cm
−1, associated with C=O stretching vibrations, possibly arising from conjugated carbonyl groups or the quinoid structure of thymoquinone [
22]. A distinct peak at 1543 cm
−1, corresponding to N–H bending or amide II-type vibrations, indicating the presence of amide or amino groups, possibly from residual proteins or structural modifications in SPCN. A peak around 1450 cm
−1, attributed to C–H bending (scissoring). Minor bands at 1139 cm−
1 and 1056 cm−
1, indicating C–O stretching and =C–H bending modes, respectively, which suggest oxygenated aromatic functionalities [
23].
After TB adsorption, the FTIR spectrum of SPCN exhibits significant shifts and new bands, indicating interactions between SPCN and toluidine blue: The O–H stretching band shifts from 3340 cm
−1 to 3512 cm
−1, suggesting disruption or weakening of hydrogen bonding. This may be due to the incorporation of the dye, which alters the hydrogen bonding network through steric hindrance or electronic redistribution [
24]. The C=O stretching band shifts from 1657 cm
−1 to 1687 cm
−1, implying a change in electron density around the carbonyl group. This shift is consistent with complex formation between the carbonyl oxygen and a cationic group—likely the dimethylaminium moiety of toluidine blue—leading to weakened conjugation and thus a higher stretching frequency [
25]. A red shift in the NH
2 bending vibration, from 1543 cm
−1 to 1525 cm
−1, may be attributed to hydrogen bonding between the dye’s amine groups and the hydroxyl/carbonyl functionalities of SPCN. This interaction stabilizes the amine group and lowers its vibrational energy [
26,
27,
28].
In addition, after TB adsorption, the FTIR spectrum of SPCN displays new bands at 1608, 1329, 1240, 1179, 1025, 877, 825, and 663 cm−1, which are characteristic of toluidine blue. The appearance of these bands confirms the successful adsorption of the dye onto SPCN. They correspond to various vibrational modes, including aromatic ring stretching, C–N stretching, and out-of-plane C–H bending in substituted aromatic rings.
These spectral shifts suggest that hydroxyl groups participate in hydrogen bonding with the amine functionalities of TB, while carbonyl groups interact with the cationic dimethylaminium moiety of the dye through electrostatic attraction. In addition, the observed changes in the N–H band indicate possible stabilization of the dye’s amine groups via interactions with oxygenated sites on SPCN.
3.3. SEM Analysis
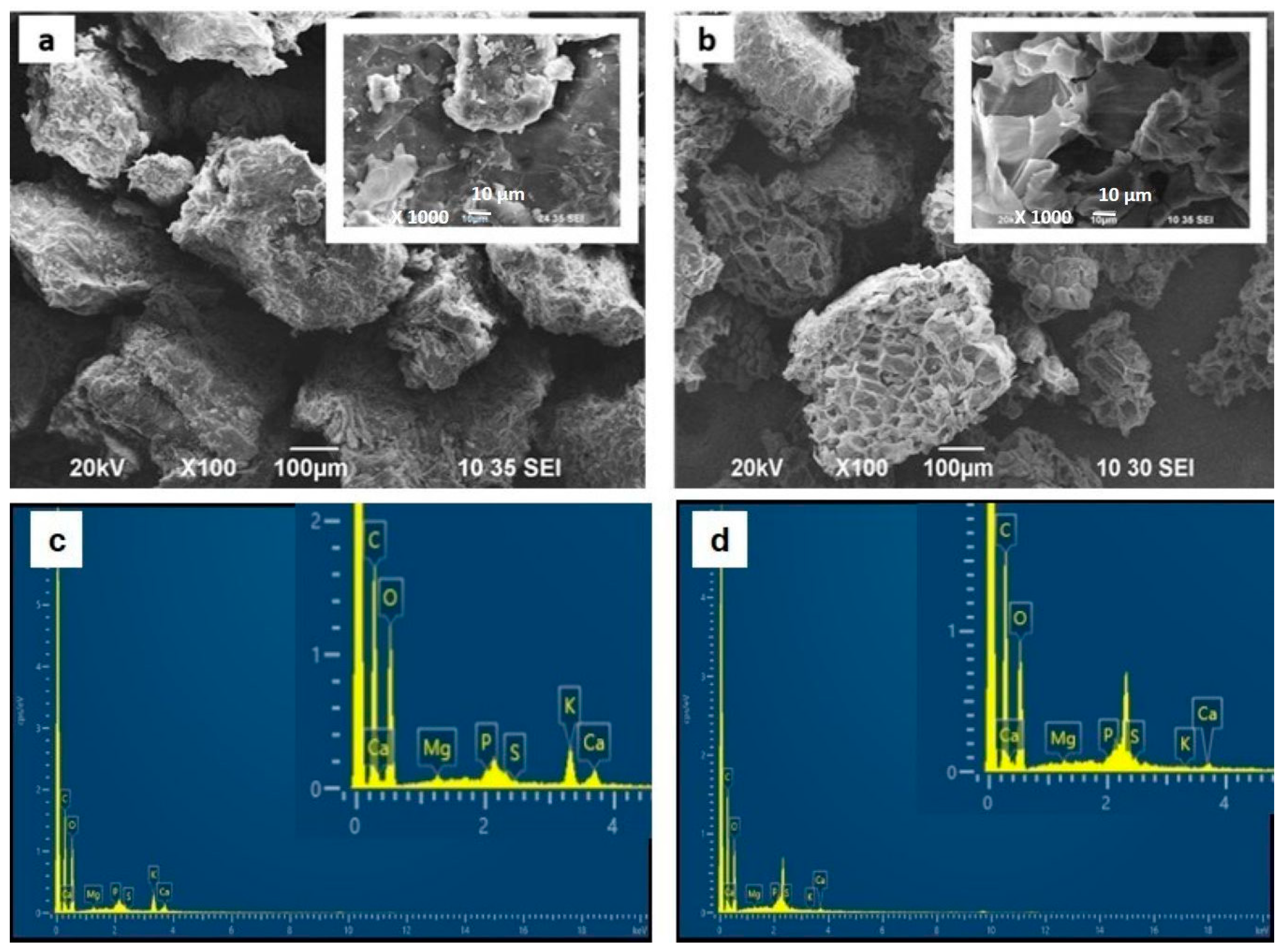
In
Figure 3, the SEM images show the surface morphology of SPCN before and after TB adsorption. The material exhibits a rough and porous structure with irregular morphology (
Figure 3a). After TB adsorption, noticeable changes in surface appearance are observed, including increased coverage and deposition of dye molecules. The inset image highlights that the pores appear more occupied; this visual effect is due to dye molecules adsorbing onto the pore walls and covering the rough surfaces, rather than physically blocking the large pores. Such surface coverage accounts for the observed morphological changes in the SEM images, even though the molecular dimensions of TB are much smaller than the pore size (~50 µm).
The corresponding EDX spectra further confirm this observation (
Figure 3c,d). Raw SPCN shows only a very low sulfur content (0.15 wt.%), whereas SPCN after TB adsorption exhibits a pronounced sulfur peak (3.56 wt.%). Since sulfur is a characteristic element of the toluidine blue molecule, this increase provides direct evidence of dye uptake on the biosorbent surface. Additionally, the relative increase in carbon and decrease in oxygen content suggest effective coverage of the SPCN surface by the organic dye molecules. These combined SEM-EDX results provide both morphological and elemental confirmation of successful adsorption.
3.4. Adsorption Studies
3.4.1. Effect of Contact Time and Adsorption Kinetics
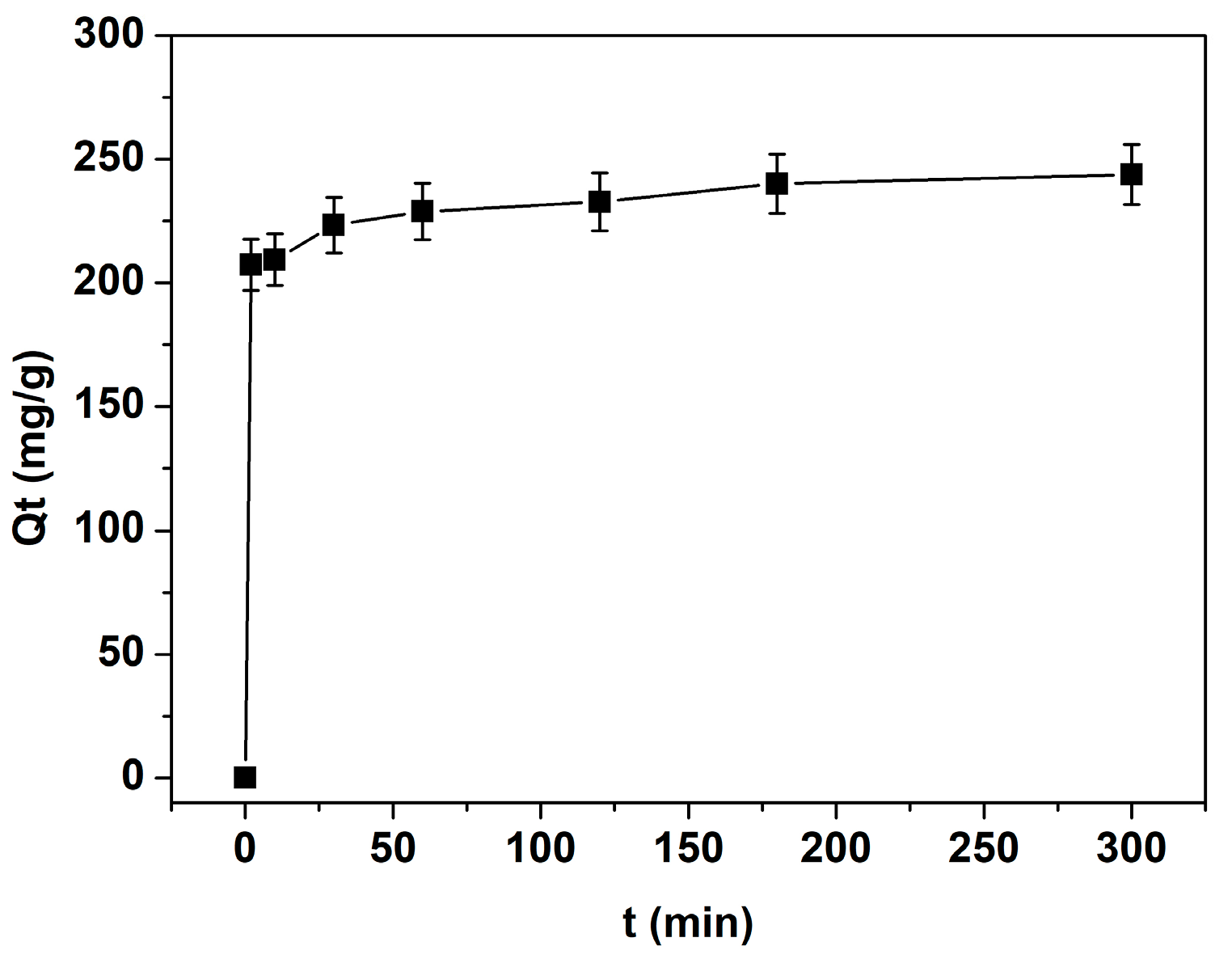
The effect of contact time on TB adsorption was investigated at pH 7 with an initial dye concentration of 1000 mg L−
1 and 50 mg of biosorbent. As shown in
Figure 4, adsorption increased rapidly and reached equilibrium within approximately 30 min, demonstrating a fast adsorption rate. A contact time of 2 h was used in all experiments to ensure complete adsorption under varying conditions of pH, temperature, and initial dye concentration.
To better understand the adsorption mechanism, three kinetic models were applied to the experimental data: the pseudo-first-order, pseudo-second-order, and intra-particle diffusion models. These models are represented by the following equations:
Pseudo-first-order model:
Pseudo-second-order model:
Intraparticle diffusion model:
In these equations,
Qe and
Qt represent the amounts of TB adsorbed at equilibrium and at time
t, respectively. The terms
k1,
k2, and
kid denote the rate constants for each model, while
C is a constant related to the boundary layer thickness. The calculated rate constants and correlation coefficients (
r2) are summarized in
Table 1.
Although the pseudo-first-order model exhibits a considerable correlation coefficient, it is substantially less accurate than the pseudo-second-order model. The calculated equilibrium adsorption capacity from the pseudo-first-order model is significantly lower than the experimental value, further confirming its inadequacy in describing the process. In contrast, the pseudo-second-order model shows a correlation coefficient very close to 1, and its calculated equilibrium adsorption capacity aligns well with the experimental value, indicating an excellent fit. This model assumes that the rate-limiting step involves chemical adsorption, characterized by the sharing or exchange of electrons between toluidine blue and the biosorbent [
29]. The initial adsorption rate,
h = k2Qe2, derived from the pseudo-second-order model, was calculated as 2.21 × 10
4 mg·g
−1·min
−1, confirming the very rapid uptake of TB and supporting the short equilibrium time observed experimentally.
The intra-particle diffusion model also plays a role, as indicated by its relatively high kid value (2.393 mg g−1 min−1/2) and r2 value (0.9353). However, the lower r2 compared to the pseudo-second-order model suggests that intra-particle diffusion is not the sole rate-limiting step. The higher value of C further implies that boundary layer resistance significantly influences the adsorption process, indicating that other mechanisms, such as boundary layer diffusion, may also contribute.
3.4.2. Effect of pH
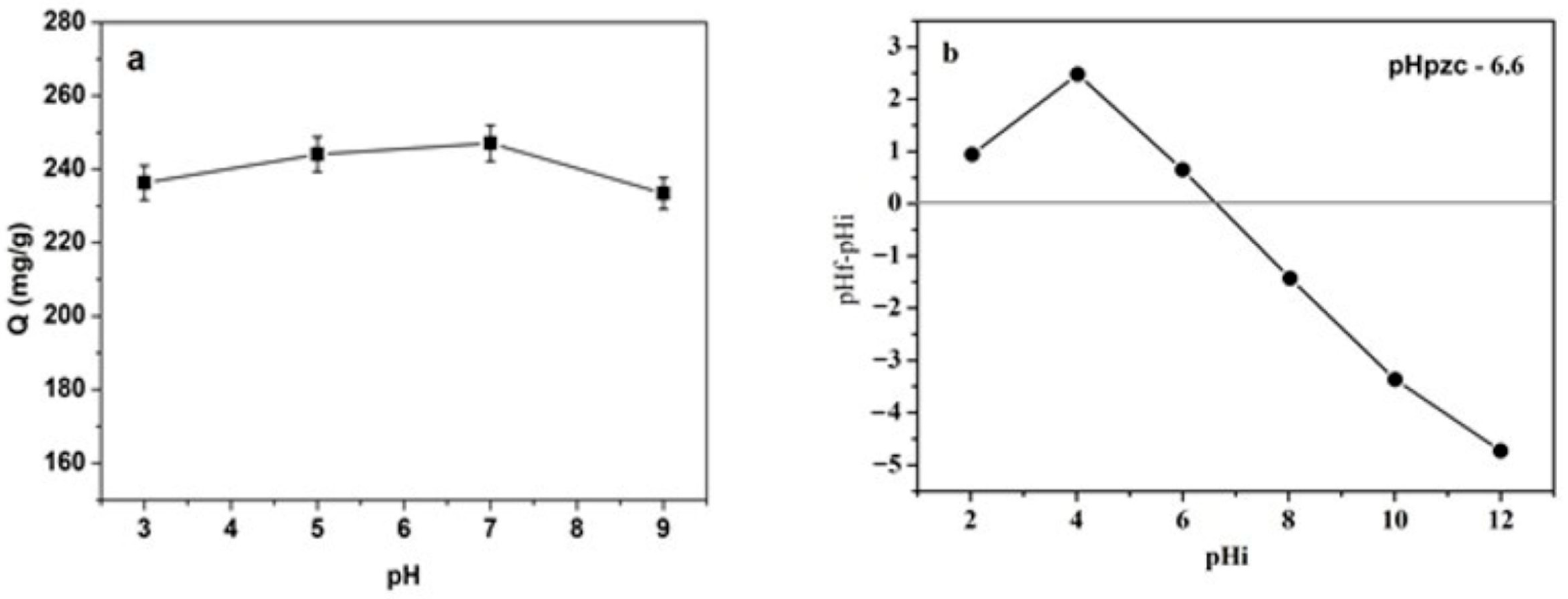
The effect of initial pH on the adsorption capacity of toluidine blue (1000 mg L
−1) onto black cumin seed by-product was investigated using 50 mg of biosorbent, as illustrated in
Figure 5a.
The adsorption capacity remains relatively stable across the investigated pH range, with a slight increase observed at neutral pH (~6–7) and minor decreases at both lower (pH 3–4) and higher (pH 8–9) values.
The point of zero charge (pH
pzc) of SPCN was determined to be 6.6 (see
Figure 5b), which aligns with the observed adsorption trend. At pH values below the pH
pzc, the SPCN surface is predominantly protonated, leading to electrostatic repulsion with cationic TB molecules and a slight reduction in adsorption. Near the pH
pzc (pH ~ 6–7), the surface charge balance favors stronger interactions, resulting in the highest adsorption capacity. Under alkaline conditions (pH > pH
pzc), the surface becomes negatively charged, which in principle favors electrostatic attraction with TB; however, the slight decrease at pH 9 may be due to dye aggregation, changes in speciation, or competitive effects from hydroxyl ions (OH
−). Overall, maximum adsorption occurs around pH 6–7, indicating optimal conditions for TB uptake.
The relatively small variation in adsorption across the pH range suggests that electrostatic interactions, while relevant, are not the dominant mechanism. Instead, hydrogen bonding, hydrophobic interactions, and π–π stacking likely contribute substantially to adsorption, maintaining stability even when electrostatic effects are reduced or altered. This combination of mechanisms enhances the robustness and versatility of SPCN as a biosorbent, a feature particularly advantageous for practical wastewater treatment, where pH conditions can vary considerably.
3.4.3. Adsorption Isotherm
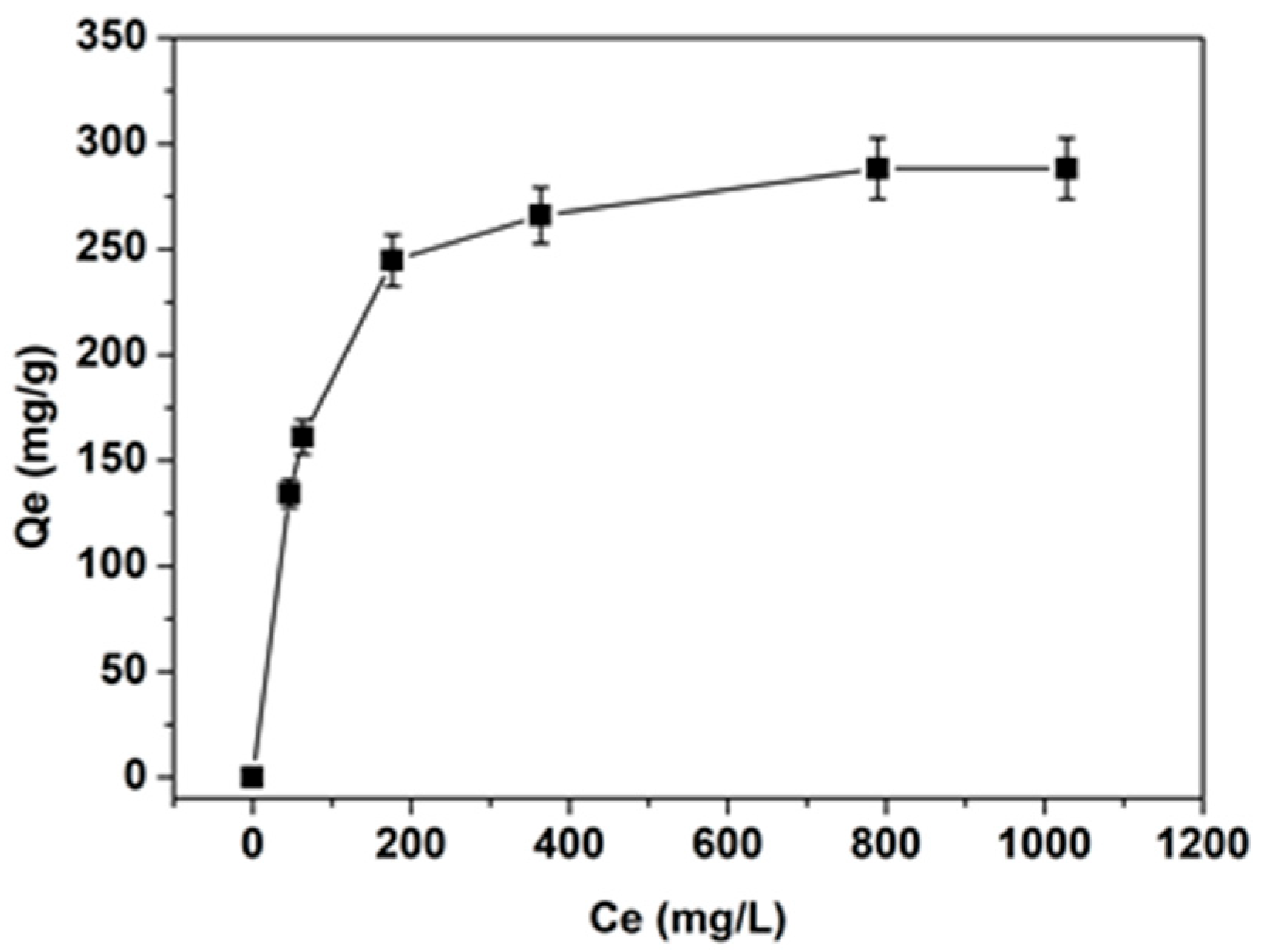
The experimental isotherm data for toluidine blue (initial concentrations of 500–2000 mg L
−1) obtained using 50 mg of biosorbent, together with the calculated model isotherm constants and correlation coefficients, are presented in
Figure 6 and
Table 1. The data in
Figure 6 indicate a Type I (Langmuir-type) isotherm, characteristic of monolayer adsorption on a surface with a finite number of identical sites. The steep slope at lower Ce values reflects the strong affinity between toluidine blue and the black cumin seed–derived adsorbent, likely due to the abundance of active sites available at the initial stages of adsorption. Although SPCN exhibits a very low BET surface area (3 m
2 g
−1) and a total pore volume of only 0.01 cm
3 g
−1, it demonstrates a remarkably high maximum adsorption capacity for toluidine blue. This suggests that, for lignocellulosic biosorbents, adsorption efficiency is governed primarily by surface chemistry rather than porosity. FTIR analysis confirmed the presence of hydroxyl, carbonyl, amine, and aromatic functional groups, which provide multiple binding sites for electrostatic attraction, hydrogen bonding, and π–π interactions with cationic dyes. Furthermore, the material contains transport macropores that facilitate rapid dye diffusion, contributing to the fast adsorption kinetics observed in this study.
Adsorption isotherms illustrate the relationship between the quantity of adsorbate on the adsorbent surface and its equilibrium concentration in the solution. These models provide insights into the interaction mechanisms between adsorbates and adsorbents and enable the prediction of adsorption capacity. In this work, the equilibrium data for TB dye adsorption were evaluated using three widely used isotherm models: Langmuir, Freundlich, and Dubinin–Radushkevich. Each of these models, as described in [
30], is based on distinct theoretical principles and is expressed by the following equations:
Dubinin–Radushkevich model:
where Ce is the dye concentration in the equilibrium solution (mg L−1); Qe is the amount of dye adsorbed (mg) per unit mass of adsorbent (g); Q0 and Qm are the maximum adsorption capacity (mg g−1); KL and kF are the Langmuir and Freundlich constants, β is the adsorption energy constant (mol2 J−2), and ε is the Polanyi potential, E is the mean adsorption energy.
The Langmuir model provides the best fit for the adsorption process, as indicated by the high r2 value (0.9996). This suggests that adsorption occurs primarily as a monolayer on a homogeneous surface. The Freundlich model also offers a reasonable fit, but its lower r2 value (0.8690) and high n value (4.16) imply some degree of surface heterogeneity. The Dubinin–Radushkevich (D-R) model further supports the conclusion that the adsorption process is predominantly physical, as the calculated energy value (below 8 kJ mol−1) typically corresponds to weak van der Waals interactions.
The experimental maximum adsorption capacity of SPCN was found to be 288.14 mg g
−1, closely matching the theoretical value predicted by the Langmuir model (304.88 mg g
−1), confirming the biosorbent’s strong potential for cationic dye removal. As summarized in
Table 2, SPCN shows a significantly higher adsorption capacity than most natural and low-cost biosorbents reported in the literature. Although some engineered materials, such as graphene oxide/bentonite composites (458.7 mg g
−1) and protein-immobilized nanofiber membranes (435.4 mg g
−1), exhibit higher performance, these involve costly precursors or sophisticated fabrication techniques, which may hinder their large-scale application. By contrast, SPCN is an agricultural by-product requiring minimal processing, making it a promising low-cost and sustainable adsorbent with adsorption performance comparable to advanced materials but without the associated complexity and cost.
3.4.4. Effect of Temperature and Thermodynamic Studies
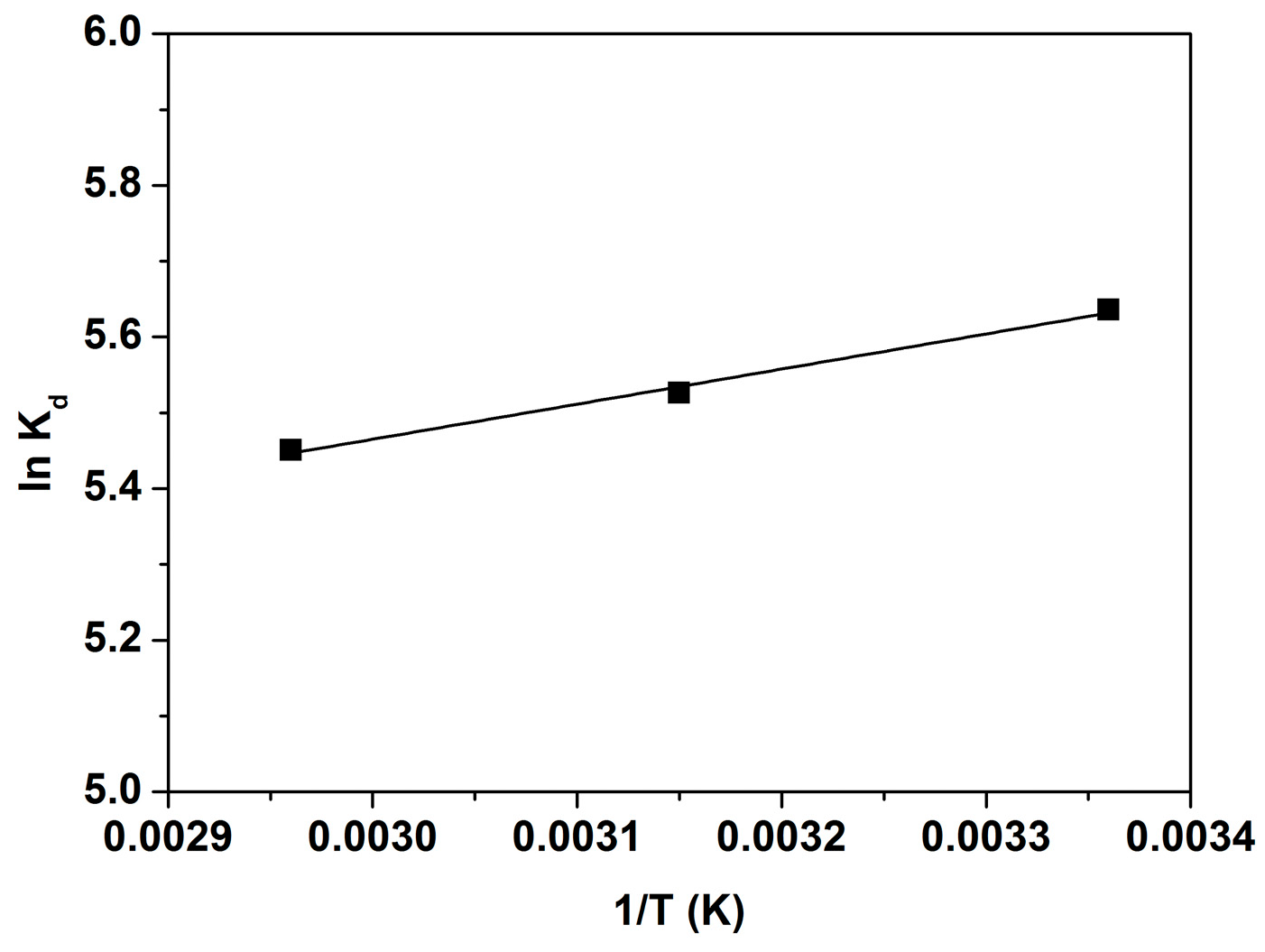
Temperature plays a crucial role in the adsorption process by influencing the kinetic energy of dye molecules. To assess its effect on TB removal, experiments were conducted at 293 K, 303 K, and 333 K. The results showed that TB adsorption decreased with rising temperature, confirming an exothermic process. The distribution coefficient (
Kd) and thermodynamic parameters are described by the following equations:
In these formulas,
Kd represents the dimensionless equilibrium constant,
R is the gas constant (J mol
−1K
−1), and
T is the temperature (K). By plotting
lnKd against 1/
T (
Figure 7), the enthalpy change (Δ
H0) can be determined from the slope, while the entropy change (Δ
S0) is derived from the intercept.
Thermodynamic parameters (ΔG0, ΔH0, and ΔS0) provide valuable insight into the nature and mechanism of TB adsorption on SPCN. The negative ΔG0 values at all temperatures (−13.86, −14.54, and −15.21 kJ mol−1 at 293 K, 313 K, and 333 K, respectively) indicate that the process is spontaneous and thermodynamically favorable. The negative ΔH0 value (−3.85 kJ mol−1) confirms the exothermic nature of adsorption, meaning energy is released when toluidine blue binds to SPCN. The relatively low magnitude of ΔH0 suggests that physical adsorption—such as van der Waals forces or hydrogen bonding—plays a significant role in the adsorption process. These findings are consistent with the adsorption energy (E) value obtained. The positive ΔS0 value (33.64 J mol−1 K−1) indicates increased randomness at the solid-liquid interface. This suggests that toluidine blue molecules are more freely distributed in the adsorbed state, likely due to the displacement of water or solvent molecules from the adsorbent surface. The high affinity of SPCN for toluidine blue further supports this observation.
3.5. Desorption Studies
The desorption of pre-adsorbed toluidine blue from black cumin seed by-product was investigated to evaluate the potential for biosorbent regeneration. For adsorption–desorption (reusability) experiments, a TB concentration of 1000 mg L−1 was used to assess SPCN performance under relatively high dye loading conditions. Batch desorption experiments were carried out using four different desorbing solutions: a 50:50 mixture of ethanol and 1 M HCl, a 50:50 mixture of ethanol and 1 M NaCl, and 1 M HCl and 1 M NaCl individually. Pre-adsorbed SPCN (50 mg) was added to 15 mL of each desorbing solution and stirred at room temperature for 24 h. The solutions were then filtered, and the concentration of desorbed dye was measured to assess desorption efficiency.
The results, summarized in
Table 3, indicate that the highest desorption efficiency (87%) was achieved using the ethanol and 1M HCl mixture. The high desorption percentage suggests that TB adsorption is predominantly governed by physical interactions, such as van der Waals forces or hydrogen bonding, rather than chemical bonding, which aligns with previous findings. Additionally, the data reveal that ethanol has a negligible role in the desorption process, as mixtures containing it did not significantly outperform HCl or NaCl alone. These findings highlight the potential for efficient biosorbent regeneration, making black cumin seeds particularly suitable for wastewater treatment applications.
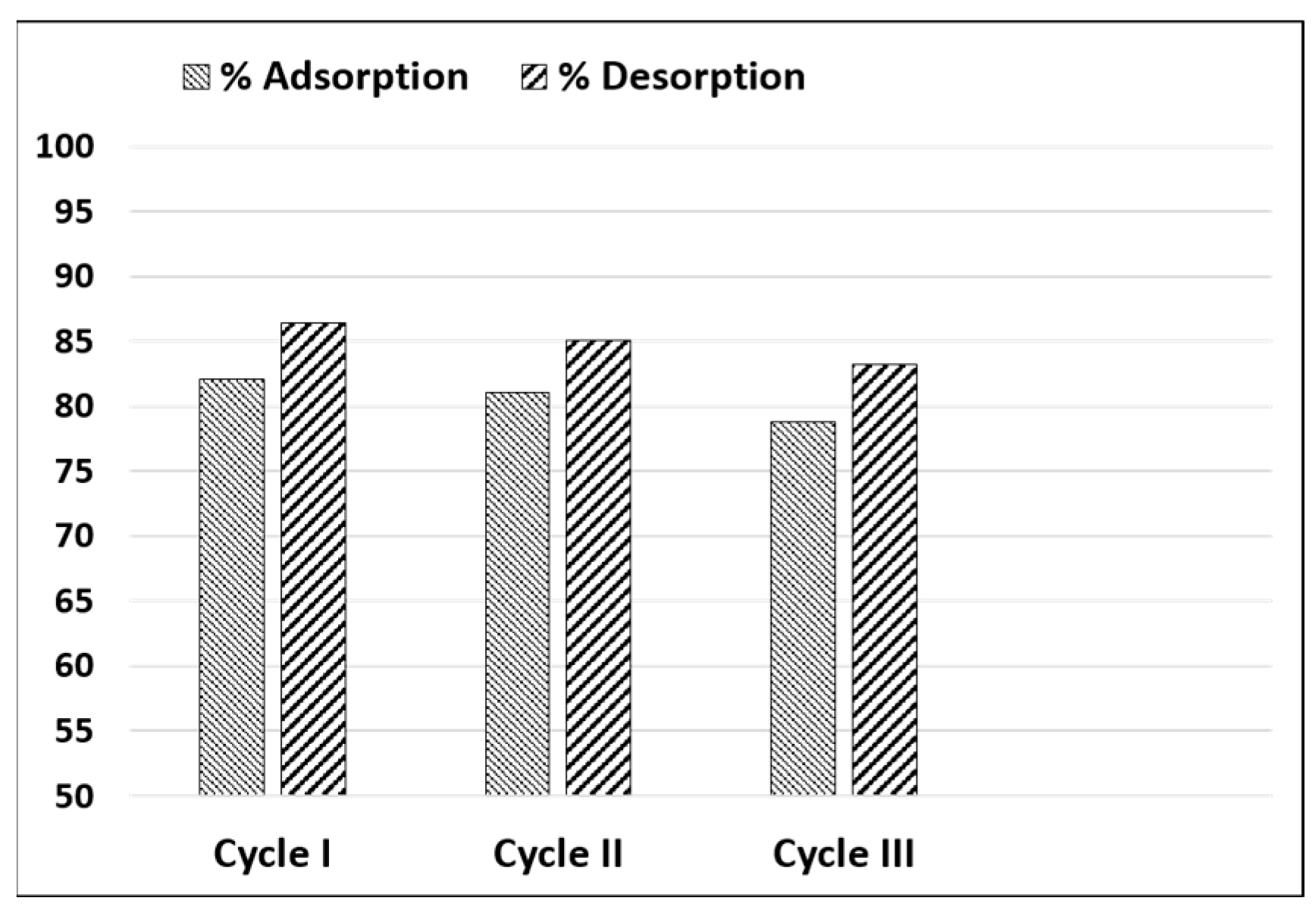
In this context, the ability of a biosorbent to maintain its adsorption capacity over multiple cycles is a key factor for practical applications.
Figure 8 illustrates the performance of SPCN during three consecutive adsorption–desorption cycles, using ethanol/1 M HCl (50:50) as the regenerating eluent. The results indicate only a slight decrease in adsorption efficiency (from 82.1% to 78.6%) and desorption efficiency (from 86.6% to 83.2%) over the cycles. Such minimal loss in performance confirms that SPCN can be effectively regenerated and reused for at least three cycles without significant deterioration. This reusability highlights the reversible adsorption mechanism of SPCN, which is a major advantage for sustainable dye removal. Overall, these findings suggest that SPCN is a promising biosorbent with potential for practical application in wastewater purification.
4. Conclusions
This study evaluated the efficiency of Nigella sativa seed press cake (SPCN) as a biosorbent for removing toluidine blue (TB), a cationic dye, from aqueous solutions. The effects of pH, contact time, initial dye concentration, and temperature on the adsorption process were systematically investigated. The adsorption efficiency remained consistently high across a wide pH range, indicating the material’s practical versatility.
Kinetic and isotherm modeling showed that the adsorption process followed the pseudo-second-order kinetic model and was best described by the Langmuir isotherm, suggesting monolayer coverage on a homogenous surface. The maximum adsorption capacity of SPCN was found to be 305 mg·g−1, surpassing many comparable low-cost adsorbents. Thermodynamic parameters (negative ΔG0 and ΔH0) confirmed that the adsorption is spontaneous and exothermic. The relatively low magnitude of ΔH0, together with high desorption efficiency, indicates that the process is predominantly driven by reversible physical interactions.
FTIR analysis demonstrated the cooperative role of hydroxyl, carbonyl, amine, and aromatic functional groups in adsorption through hydrogen bonding, electrostatic interactions, and π–π stacking. Importantly, despite its low BET surface area (3 m2·g−1) and minimal pore volume (0.01 cm3·g−1), SPCN achieved high adsorption capacity, highlighting that adsorption efficiency in lignocellulosic biosorbents is primarily governed by surface chemistry rather than porosity.
Overall, SPCN is a renewable, cost-effective, and reusable biosorbent with strong potential for large-scale application in the removal of cationic dyes from wastewater, offering performance comparable to advanced engineered materials but without their associated cost or complexity.
Author Contributions
Conceptualization, G.G. and P.V.; methodology, C.T., A.P., M.M., I.Y. and T.V.; software, T.V.; data curation I.Y.; writing—original draft preparation, C.T., A.P. and P.V.; writing—review and editing, G.G., P.V. and C.T.; visualization, C.T. and I.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by MU-Pleven, Project №7/2023.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author(s).
Acknowledgments
The authors express their gratitude for the support of Project №7/2023 “Recovery of waste from oil manufacture”, Research equipment of the Distributed Research Infrastructure INFRAMAT, part of the Bulgarian National Roadmap for Research Infrastructures, supported by the Bulgarian Ministry of Education and Science, was used in this investigation.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sadiku, M.; Selimi, T.; Berisha, A.; Maloku, A.; Mehmeti, V.; Thaçi, V.; Hasani, N. Removal of methyl violet from aqueous solution by adsorption onto halloysite nanoclay: Experiment and theory. Toxics 2022, 10, 445. [Google Scholar] [CrossRef]
- Neag, E.; Malschi, D.; Măicăneanu, A. Isotherm and kinetic modelling of Toluidine Blue (TB) removal from aqueous solution using Lemna minor. Int. J. Phytoremediat. 2018, 20, 1049–1054. [Google Scholar] [CrossRef]
- Brandao, W.Q.; Maciel, B.G.; de Araújo Lima, E.M.; Mojica-Sanchez, L.C.; da Silva, R.J.; de Melo, C.P. Carboxymethylcellulose magnetic composite for adsorptive removal of cationic toluidine blue dye. Mater. Chem. Phys. 2023, 303, 127782. [Google Scholar] [CrossRef]
- Sadiq, M.; Khan, M.; Aman, R.; Sadiq, S.; Ahmad, M.S.; Ali, M.; Ali, R. Thermodynamic and kinetic studies of adsorptive removal of toluidine blue by activated carbon from olive pit. Desalination Water Treat. 2017, 78, 292–299. [Google Scholar] [CrossRef]
- Moawed, E.A.; Eissa, M.S.; Al-Tantawy, S.A. Application of polyurethane foam/zinc oxide nanocomposite for antibacterial activity, detection, and removal of basic dyes from wastewater. Int. J. Environ. Sci. Technol. 2023, 20, 7767–7774. [Google Scholar] [CrossRef]
- Abbaz, M.; Azougarh, Y.; Benafqir, M.; Anfar, Z.; El Alem, N. Removal of toluidine blue by manganese/graphene coated titaniferous sand and optimization of decoloration process via Box-Behnken design. Mediterr. J. Chem. 2018, 7, 1–7. [Google Scholar] [CrossRef]
- Olajire, A.A.; Giwa, A.A.; Bello, I.A. Competitive adsorption of dye species from aqueous solution onto melon husk in single and ternary dye systems. Int. J. Environ. Sci. Technol. 2015, 12, 939–950. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, X.; Wang, X.; Carlson, K.; Li, Z. Removal of toluidine blue and safranin O from single and binary solutions using zeolite. Crystals 2021, 11, 1181. [Google Scholar] [CrossRef]
- Asemave, K.; Thaddeus, L.; Tarhemba, P. Lignocellulosic-Based Sorbents: A Review. Sustain. Chem. 2021, 2, 271–285. [Google Scholar] [CrossRef]
- Vassileva, P.; Uzunov, I.; Voykova, D.; Kovacheva, A.; Avramova, I.; Tyuliev, G.; Kukeva, R.; Mehandjiev, D. Study of silver adsorption on cellulose-based biosorbents. Cellul. Chem. Technol. 2024, 58, 663–673. [Google Scholar] [CrossRef]
- Ali, K.; Zeidan, H.; Amar, R.B. Evaluation of the use of agricultural waste materials as low-cost and eco-friendly sorbents to remove dyes from water: A review. Desalination Water Treat. 2023, 302, 231–252. [Google Scholar] [CrossRef]
- Rathi, G.; Siddiqui, S.I.; Pham, Q. Nigella sativa seeds based antibacterial composites: A sustainable technology for water cleansing—A review. Sustain. Chem. Pharm. 2020, 18, 100332. [Google Scholar]
- Thabede, P.M.; Shooto, N.D. Application of black cumin (Nigella sativa L.) seeds for the removal of metal ions and methylene blue from aqueous solutions. Cogent Eng. 2022, 9, 2013419. [Google Scholar] [CrossRef]
- Shooto, N.D.; Thabede, P.M.; Naidoo, E.B. Simultaneous adsorptive study of toxic metal ions in quaternary system from aqueous solution using low-cost black cumin seeds (Nigella sativa) adsorbents. S. Afr. J. Chem. Eng. 2019, 30, 15–27. [Google Scholar]
- Mohammed, S.J.; Amin, H.H.; Aziz, S.B.; Sha, A.M.; Hassan, S.; Abdul Aziz, J.M.; Rahman, H.S. Structural characterization, antimicrobial activity, and in vitro cytotoxicity effect of black seed oil. Evid.-Based Complement. Altern. Med. 2019, 2019, 6515671. [Google Scholar] [CrossRef]
- Addala, A.; Belattar, N.; Elektorowicz, M. Nigella sativa L. seeds biomass as a potential sorbent of lead from aqueous solutions and wastewaters. Orient. J. Chem. 2018, 34, 638–647. [Google Scholar] [CrossRef]
- Gharby, S.; Harhar, H.; Guillaume, D.; Haddad, A.; Charrouf, Z. The chemical composition and oxidative stability of cold-pressed black cumin seed oil. J. Food Compos. Anal. 2015, 40, 197–202. [Google Scholar]
- Gemeay, A.H.; Aboelfetoh, E.F.; El-Sharkawy, R.G. Immobilization of green synthesized silver nanoparticles onto amino-functionalized silica and their application for indigo carmine dye removal. Water Air Soil Pollut. 2018, 229, 16. [Google Scholar] [CrossRef]
- Benamraoui, F.; Boudiaf, H.; Bouhank, A.; Bencheikh, L.; Bourzami, R.; Gil, A.; Boulahbal, A.-I.; Boutahala, M. Methylene blue removal using black cumin seeds waste: Experimental study and molecular dynamic simulation. Desalination Water Treat. 2023, 300, 167–177. [Google Scholar] [CrossRef]
- Nasher, M.A.; Youssif, M.I.; El-Ghamaz, N.A.; Zeyada, H.M. Linear and nonlinear optical properties of irradiated Toluidine Blue thin films. Opt.-Int. J. Light Electron. Opt. 2019, 178, 532–543. [Google Scholar] [CrossRef]
- Neunert, G.; Kamińska, W.; Nowak-Karnowska, J. Evaluating the Thymoquinone Content and Antioxidant Properties of Black Cumin (Nigella sativa L.) Seed Oil During Storage at Different Thermal Treatments. Appl. Sci. 2025, 15, 377. [Google Scholar] [CrossRef]
- Whaley, A.K.; Burtseva, E.C.; Kuldyrkaeva, E.C.; Novosad, A.S.; Babak, N.L.; Zohova, E.C.; Whaley, A.O.; Goncharov, M.Y.; Terninko, I.A.; Yakovlev, G.P. A method for the isolation of thymoquinone from black caraway seed oil (Nigella sativa L.). Drug Dev. Regist. 2023, 12, 29–40. (In Russian). [Google Scholar] [CrossRef]
- Shafodino, F.S.; Lusilao, J.M.; Mwapagha, L.M. Phytochemical characterization and antimicrobial activity of Nigella sativa seeds. PLoS ONE 2022, 17, e0272457. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.E.; Gomez, M.A.; Elouatik, S.; Demopoulos, G.P. Confocal Raman Imaging of N719 Complex Adsorbed onto Anatase TiO2 Film in High Wavenumber Region: Examining the Involvement of Ti-OH Surface Groups. ECS Trans. 2010, 28, 1–7. [Google Scholar] [CrossRef]
- Ryu, J.H.; Lee, H.; Kim, Y.J.; Kang, Y.S.; Kim, H.S. Facilitated olefin transport by reversible olefin coordination to silver ions in a dry cellulose acetate membrane. Chem.—Eur. J. 2001, 7, 1525–1529. [Google Scholar] [CrossRef]
- Hilario, J.; Kubelka, J.; Keiderling, T.A. Optical spectroscopic investigations of model β-sheet hairpins in aqueous solution. J. Am. Chem. Soc. 2003, 125, 7562–7574. [Google Scholar] [CrossRef]
- Kasat, R.B.; Chin, C.Y.; Thomson, K.T.; Franses, E.I.; Wang, N.H.L. Interpretation of chromatographic retentions of simple solutes with an amylose-based sorbent using infrared spectroscopy and DFT modeling. Adsorption 2006, 12, 405–416. [Google Scholar] [CrossRef]
- Kudo, S.; Ogawa, H.; Yamakita, E.; Watanabe, S.; Suzuki, T.; Nakashima, S. Adsorption of water to collagen as studied using infrared (IR) microspectroscopy combined with relative humidity control system and quartz crystal microbalance. Appl. Spectrosc. 2017, 71, 1621–1632. [Google Scholar] [CrossRef]
- Sahnoun, S.; Boutahala, M. Adsorption removal of tartrazine by chitosan/polyaniline composite: Kinetics and equilibrium studies. Int. J. Biol. Macromol. 2018, 114, 1345–1353. [Google Scholar] [CrossRef]
- Vassileva, P.; Tumbalev, V.; Kichukova, D.; Voykova, D.; Kovacheva, D.; Spassova, I. Study on the dye removal from aqueous solutions by graphene-based adsorbents. Materials 2023, 16, 5754. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, N.B.; Mohamed, E.H.; Ali, F.F.; Al-Hadithy, S. An efficient method for removing toluidine blue and malachite green from industrial wastewater using limestone as an adsorbent surface. Anal. Methods Environ. Chem. J. 2024, 7, 113–126. [Google Scholar] [CrossRef]
- Fosso-Kankeu, E.; Potgieter, J.; Waanders, F.B. Removal of malachite green and toluidine blue dyes from aqueous solution using a clay-biochar composite of bentonite and sweet sorghum bagasse. Int. J. Appl. Eng. Res. 2019, 14, 1324–1333. [Google Scholar]
- Guo, L.; Li, G.; Liu, J.; Ma, S.; Zhang, J. Kinetic and equilibrium studies on adsorptive removal of toluidine blue by water-insoluble starch sulfate. J. Chem. Eng. Data 2011, 56, 1875–1881. [Google Scholar] [CrossRef]
- Rauf, M.A.; Qadri, S.M.; Ashraf, S.; Al-Mansoori, K.M. Adsorption studies of toluidine blue from aqueous solutions onto gypsum. Chem. Eng. J. 2009, 150, 90–95. [Google Scholar] [CrossRef]
- Tural, B.; Ertaş, E.; Enez, B.; Tural, S. Removal of lead (II) and toluidine blue from wastewater with new magnetic Bacillus niacini nano-biosorbent. Int. J. Environ. Sci. Technol. 2024, 21, 7431–7444. [Google Scholar] [CrossRef]
- Huong, D.T.M.; Chai, W.S.; Show, P.L.; Lin, Y.L.; Chiu, C.Y.; Tsai, S.L.; Chang, Y.K. Removal of cationic dye waste by nanofiber membrane immobilized with waste proteins. Int. J. Biol. Macromol. 2020, 164, 3873–3884. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Chen, Y.; Zhang, W.; Li, B. Fabrication of graphene oxide/bentonite composites with excellent adsorption performances for toluidine blue removal from aqueous solution. Adv. Powder Technol. 2019, 30, 493–501. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).