Efficient Adsorptive Removal of Phosphonate Antiscalant HEDP by Mg-Al LDH
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Preparation of Mg-Al LDH
2.3. Mg-Al LDH Characterizations
2.4. HEDP Adsorption Experiment
2.4.1. Influence of Adsorbent Dosage
2.4.2. Influence of Initial HEDP ConcentrationI
2.4.3. Influence of Solution pH
2.4.4. Influence of Coexisting Ions
2.5. Adsorption Kinetic Model
2.5.1. Kinetics Experiment
2.5.2. Adsorption Isotherm Experiment
2.5.3. Adsorption Thermodynamics Experiment
3. Results and Discussion
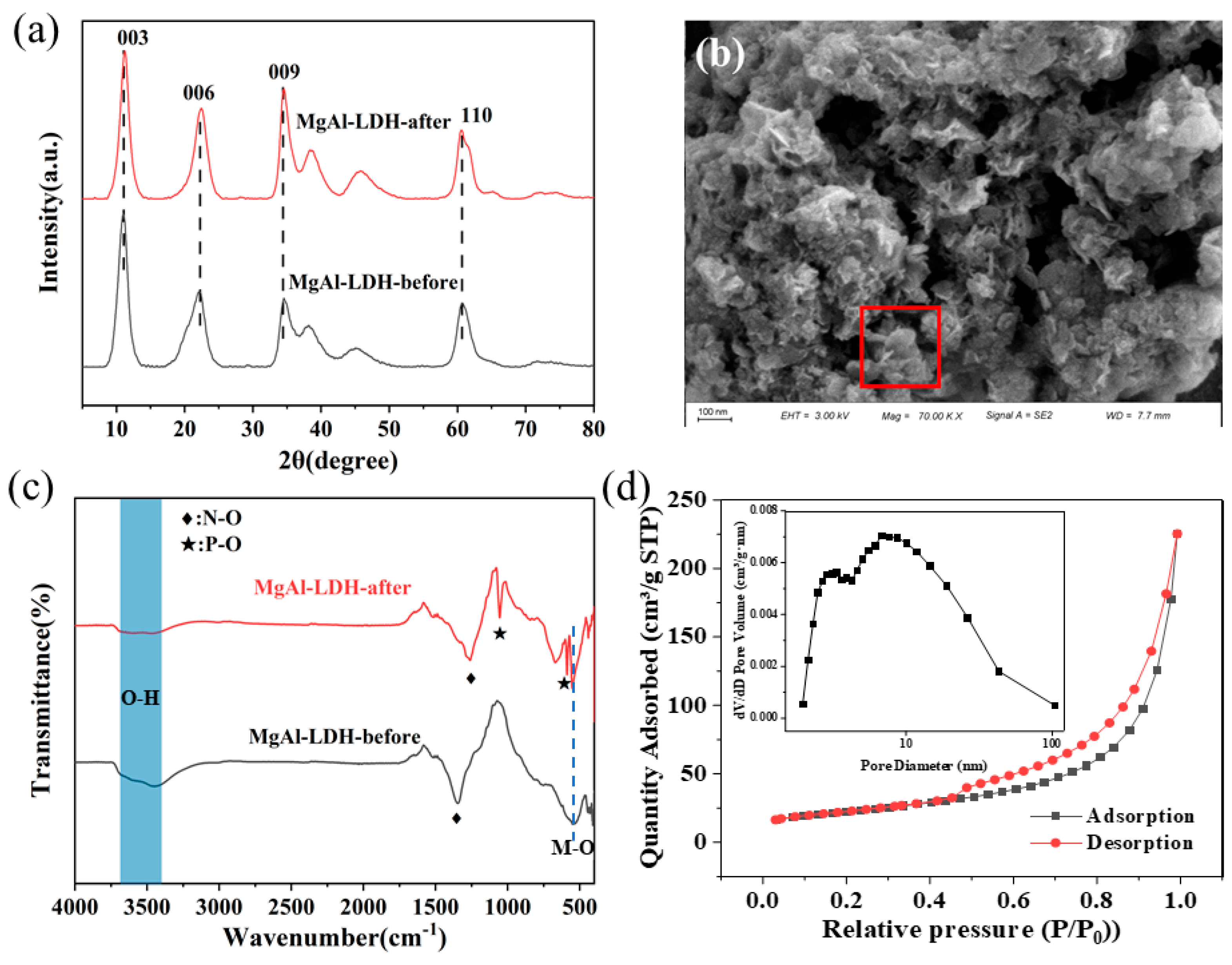
3.1. Characterization of Mg-Al LDH
3.2. HEDP Adsorption
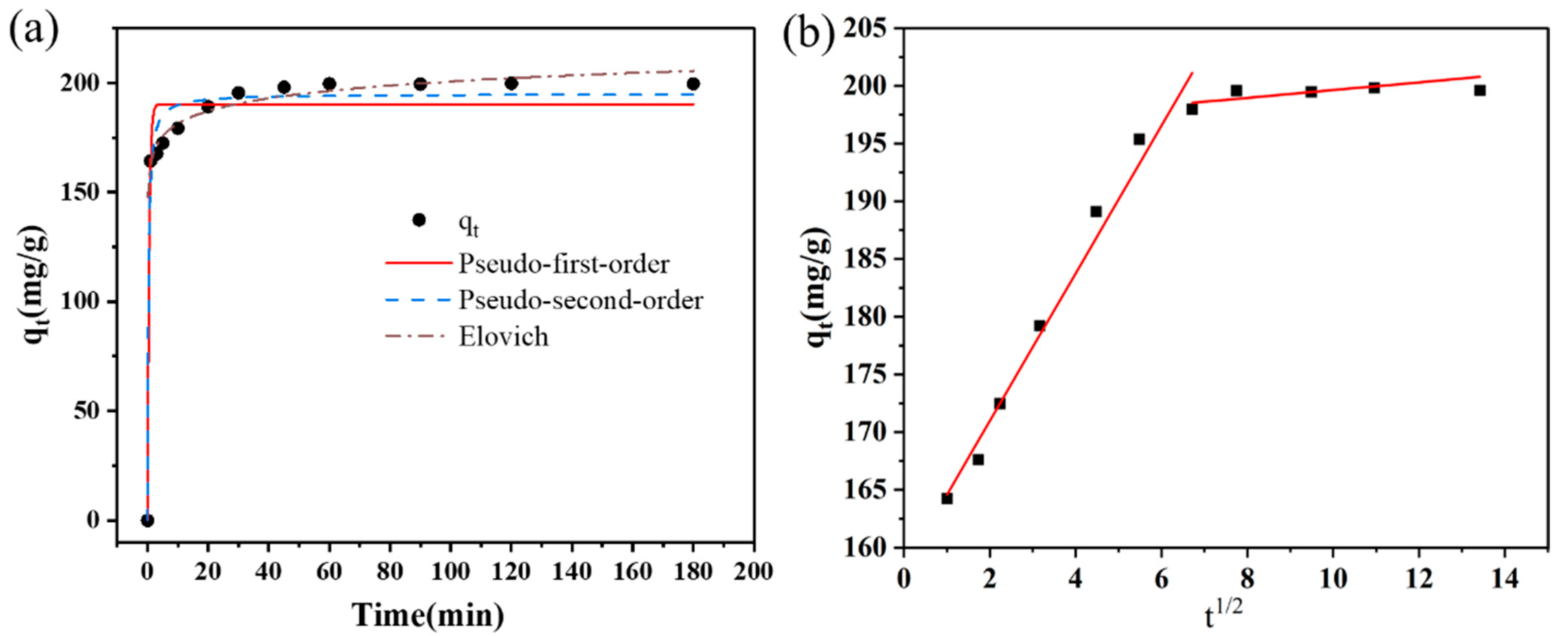
3.2.1. Adsorption Kinetics
3.2.2. Adsorption Isotherm
3.2.3. Adsorption Thermodynamics
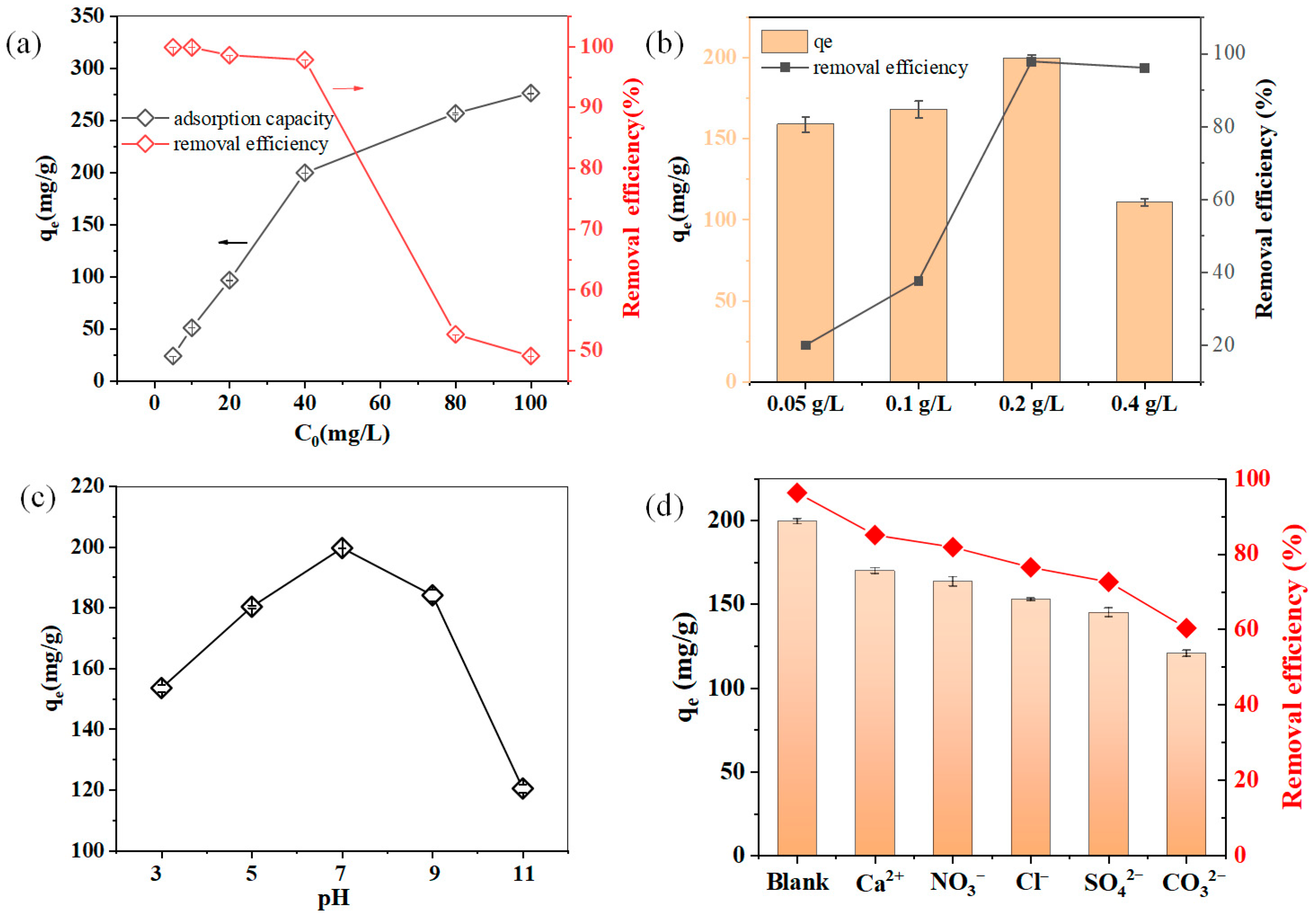
3.3. Effect of Operating Parameters on HEDP Removal by Mg-Al LDH
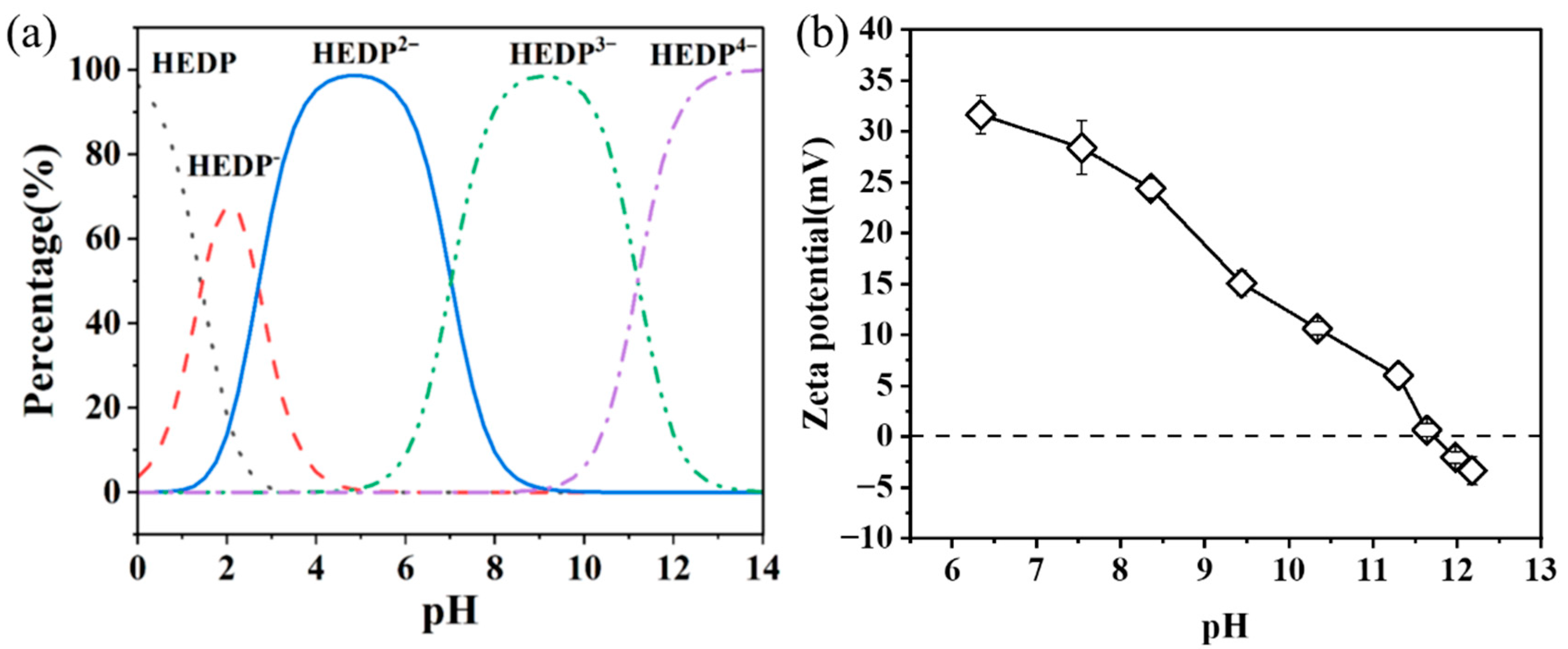
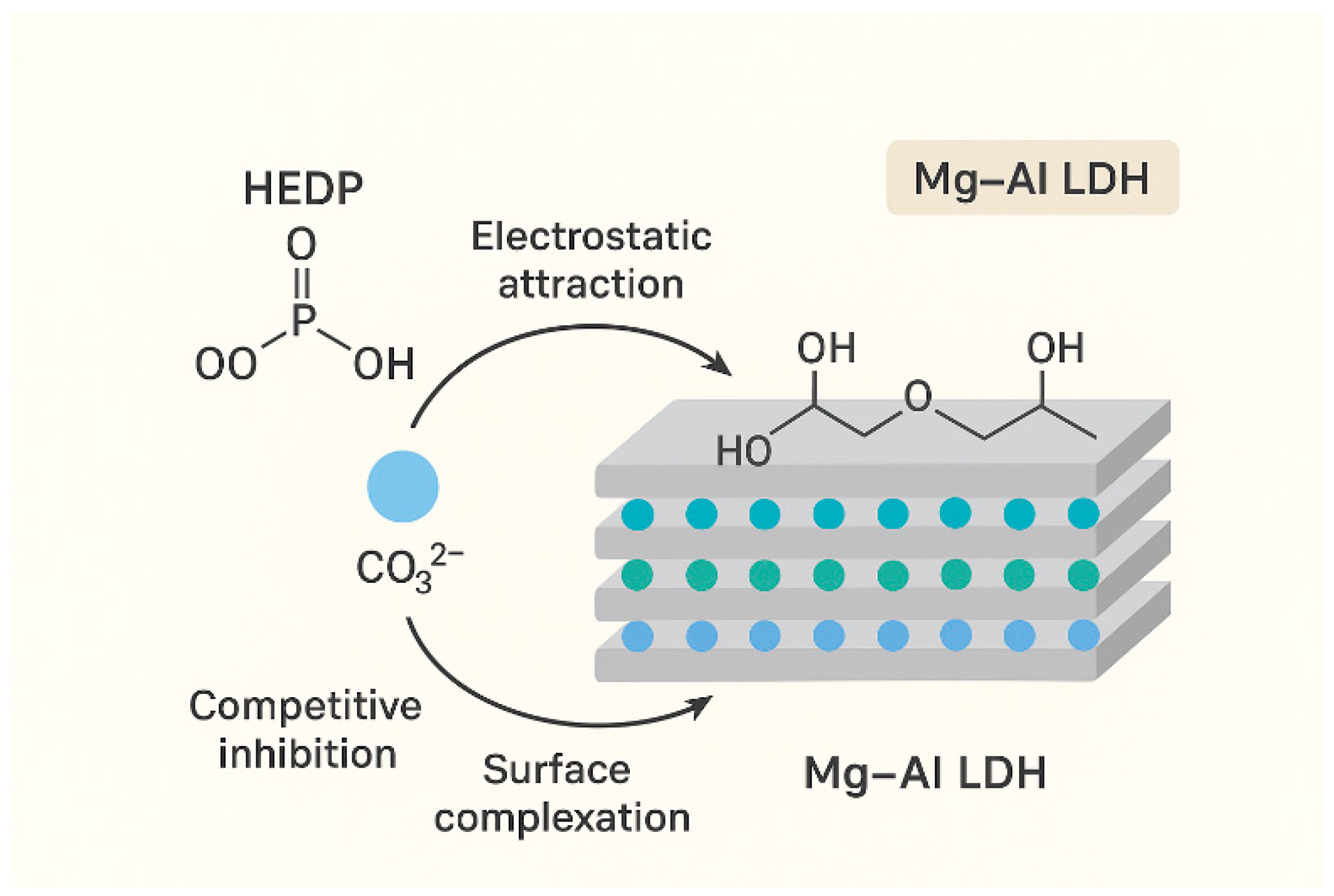
3.4. Adsorption Mechanism
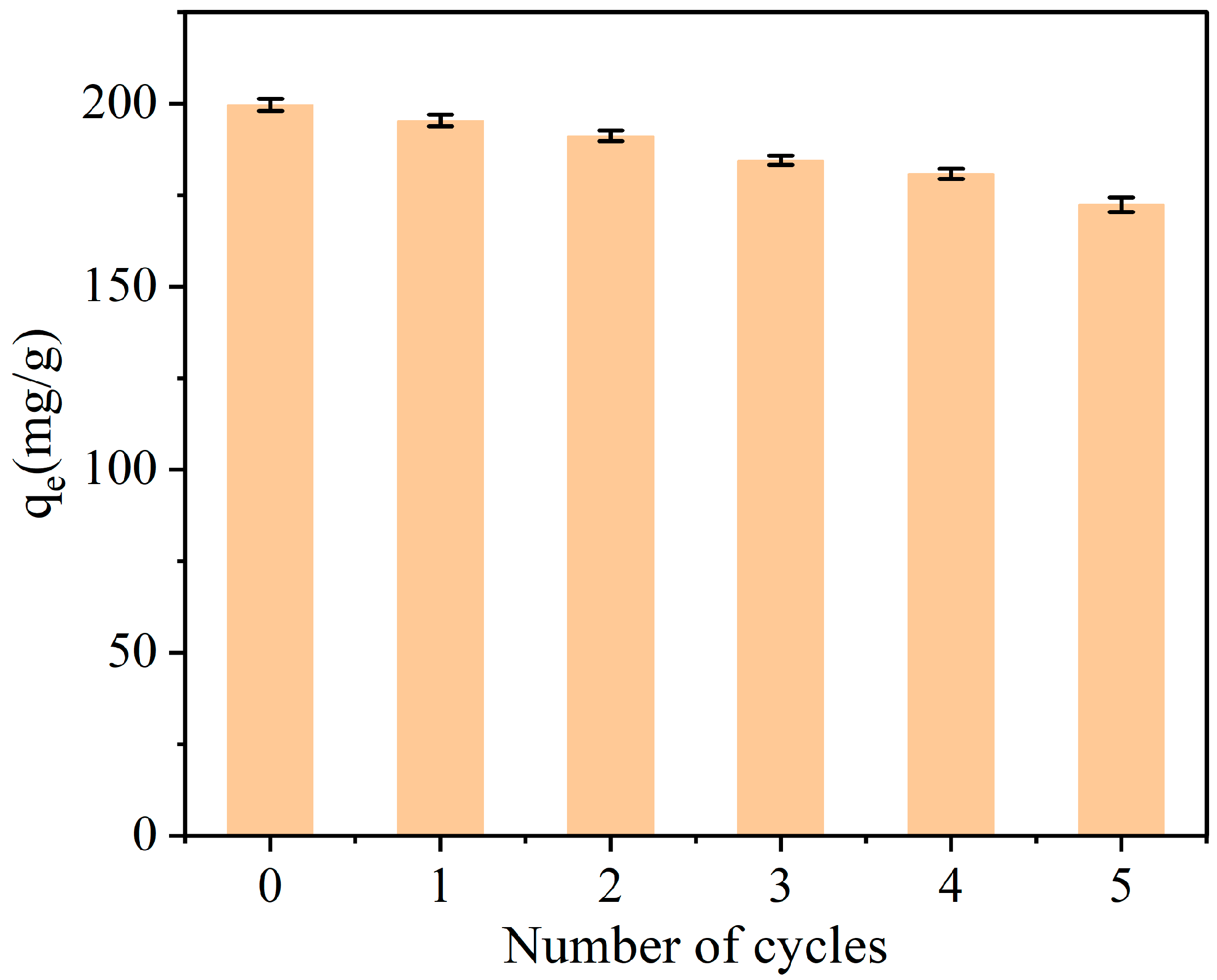
3.5. Comparison with Other Adsorbents and Reusability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sattayatewa, C.; Arnaldos, M.; Pagilla, K. Measurement of organic nitrogen and phosphorus fractions at very low concentrations in wastewater effluents. Water Environ. Res. 2011, 83, 675–683. [Google Scholar] [CrossRef]
- Rott, E.; Minke, R.; Steinmetz, H. Removal of phosphorus from phosphonate-loaded industrial wastewaters via precipitation/flocculation. J. Water Process Eng. 2017, 17, 188–196. [Google Scholar] [CrossRef]
- Nowack, B.; Stone, A.T. Competitive adsorption of phosphate and phosphonates onto goethite. Water Res. 2006, 40, 2201–2209. [Google Scholar] [CrossRef]
- Rott, E.; Steinmetz, H.; Metzger, J.W. Organophosphonates: A review on environmental relevance, biodegradability and removal in wastewater treatment plants. Sci. Total Environ. 2018, 615, 1176–1191. [Google Scholar] [CrossRef]
- Di Capua, F.; de Sario, S.; Ferraro, A.; Petrella, A.; Race, M.; Pirozzi, F.; Fratino, U.; Spasiano, D. Phosphorous removal and recovery from urban wastewater: Current practices and new directions. Sci. Total Environ. 2022, 823, 153750. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Huang, K.; Wang, Z.; An, G.; Zhang, M.; Liu, W.; Fu, S.; Guo, H.; Zhang, B.; Lian, C. Functional Group Engineering of Single-Walled Carbon Nanotubes for Anchoring Copper Nanoparticles Toward Selective CO2 Electroreduction to C2 Products. Small 2025, 21, 2502733. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.-J.; Yu, L.-Q.; Zhang, Q.; Zhao, Y.-H.; Xiong, J.-R.; Zhu, X.-Y.; Fan, N.-S.; Huang, B.-C.; Jin, R.-C. Conversion of municipal wastewater-derived waste to an adsorbent for phosphorus recovery from secondary effluent. Sci. Total Environ. 2020, 705, 135959. [Google Scholar] [CrossRef]
- Yuan, M.-y.; Qiu, S.-k.; Li, M.-m.; Li, Y.; Wang, J.-X.; Luo, Y.; Zhang, K.-q.; Wang, F. Adsorption properties and mechanism research of phosphorus with different molecular structures from aqueous solutions by La-modified biochar. Environ. Sci. Pollut. Res. 2023, 30, 14902–14915. [Google Scholar] [CrossRef]
- Li, C.; Yang, Q.; Nie, H.; Liu, D.; Liu, Y. Adsorption removal of organic phosphonate HEDP by magnetic composite doped with different rare earth elements. Chem. Eng. J. Adv. 2022, 9, 100221. [Google Scholar] [CrossRef]
- Wan, J.; Li, R.; Feng, X.; Yang, J.; Ye, Y.; Jian, S.; Zhang, X. Insights into simultaneous adsorption of orthophosphate (PO43−) and 1-hydroxyethane 1, 1-diphosphonic acid (HEDP) by kaolin/lanthanum carbonate composites: Experimental analysis and DFT calculations. Chem. Eng. J. 2023, 476, 146664. [Google Scholar] [CrossRef]
- Guo, C.; Xu, Y.; Ni, C.; Pan, X.; Tijing, L.D.; Shon, H.K.; Deng, N.; Huang, X. Tailoring pore size to enhance dissolution of layered double oxides for efficient nitrogen and phosphorus recovery via crystallization of struvite from wastewater. J. Colloid Interface Sci. 2025, 692, 137546. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Tao, C.; Yu, F.; Zhao, Z.; Wang, Z.; Deng, N.; Huang, X. Ball-milled layer double hydroxide as persulfate activator for efficient degradation of organic: Alkaline sites-triggered non-radical mechanism. J. Hazard. Mater. 2024, 461, 132219. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhao, Y.; Li, Y.; Yuan, Q.; Wu, Y.; Wang, K.; Sun, K.; Wu, J.; Jiang, J.; Zhang, B. Joule Heating-Driven sp2-C Domains Modulation in Biomass Carbon for High-Performance Bifunctional Oxygen Electrocatalysis. Nano-Micro Lett. 2025, 17, 221. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, M.; Ren, S.; Yu, J.; Li, T. Ultra-High Adsorption Capacity of Calcium–Iron Layered Double Hydroxides for HEDP Removal through Phase Transition Processes. Environ. Sci. Technol. 2024, 58, 19514–19522. [Google Scholar] [CrossRef]
- Huang, X.; Su, M.; Zhou, J.; Shu, W.; Huang, Z.; Gao, N.; Qian, G. Novel activation of persulfate by its intercalation into Mg/Al-layered double hydroxide: Enhancement of non-radical oxidation. Chem. Eng. J. 2017, 328, 66–73. [Google Scholar] [CrossRef]
- Ni, Q.; Zhang, S.; Wang, K.; Guo, H.; Zhang, J.; Wu, M.; Wang, L. Carbon quantum dot-mediated binary metal–organic framework nanosheets for efficient oxygen evolution at ampere-level current densities in proton exchange membrane electrolyzers. J. Mater. Chem. A 2024, 12, 31253–31261. [Google Scholar] [CrossRef]
- Pan, Q.; Zheng, F.; Deng, D.; Chen, B.; Wang, Y. Interlayer spacing regulation of NiCo-LDH nanosheets with ultrahigh specific capacity for battery-type supercapacitors. ACS Appl. Mater. Interfaces 2021, 13, 56692–56703. [Google Scholar] [CrossRef]
- Fu, S.; Tang, B.; Wang, Z.; An, G.; Zhang, M.; Wang, K.; Liu, W.; Guo, H.; Zhang, B.; Wang, L. Hydrophobic carbon quantum dots with Lewis-Basic nitrogen sites for electrocatalyst CO2 reduction to CH4. Chem. Eng. J. 2024, 500, 157207. [Google Scholar] [CrossRef]
- Zeng, B.; Wang, Q.; Mo, L.; Jin, F.; Zhu, J.; Tang, M. Synthesis of Mg-Al LDH and its calcined form with natural materials for efficient Cr (VI) removal. J. Environ. Chem. Eng. 2022, 10, 108605. [Google Scholar] [CrossRef]
- Yang, Z.; Li, S.; Xia, X.; Liu, Y. Hexagonal MgAl-LDH simultaneously facilitated active facet exposure and holes storage over ZnIn2S4/MgAl-LDH heterojunction for boosting photocatalytic activities and anti-photocorrosion. Sep. Purif. Technol. 2022, 300, 121819. [Google Scholar] [CrossRef]
- An, G.; Wang, K.; Yang, M.; Zhang, J.; Zhong, H.; Wang, L.; Guo, H. Rational Design of Metal-Free Nitrogen-Doped Carbon for Controllable Reduction of CO2 to Syngas. Molecules 2025, 30, 953. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, O.P.; De Moraes, S.G.; Duran, N.; Cornejo, L.; Alves, O.L. Evaluation of boron removal from water by hydrotalcite-like compounds. Chemosphere 2006, 62, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, S.; Sasaki, K.; Hirajima, T. Effect of freeze drying on characteristics of Mg–Al layered double hydroxides and bimetallic oxide synthesis and implications for fluoride sorption. Appl. Clay Sci. 2016, 132, 460–467. [Google Scholar] [CrossRef]
- Wang, F.; Wen, Z.; Zheng, Z.; Fang, W.; Chen, L.; Chen, F.; Zhang, N.; Liu, X.; Ma, R.; Chen, G. Memory effect of MgAl layered double hydroxides promotes LiNO3 dissolution for stable lithium metal anode. Adv. Energy Mater. 2023, 13, 2203830. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Khalil, A.K.; Dweiri, F.; Almanassra, I.W.; Chatla, A.; Atieh, M.A. Mg-Al layered double hydroxide doped activated carbon composites for phosphate removal from synthetic water: Adsorption and thermodynamics studies. Sustainability 2022, 14, 6991. [Google Scholar] [CrossRef]
- Ait Ichou, A.; Benhiti, R.; Abali, M.; Dabagh, A.; Carja, G.; Soudani, A.; Chiban, M.; Zerbet, M.; Sinan, F. Characterization and sorption study of Zn2 [FeAl]-CO3 layered double hydroxide for Cu (II) and Pb (II) removal. J. Solid State Chem. 2023, 320, 123869. [Google Scholar] [CrossRef]
- Nguena, K.L.T.; Fotsop, C.G.; Bopda, A.; Tchuifon, D.R.T.; Djioko, F.H.K.; Ngueabouo, A.M.S.; Madu, C.A.; Ezema, F.I.; Oguzie, E.E. Unraveling the sorption mechanism of industrial dyes onto Zr-based MOFs: Computational and experimental modelling for highly efficient removal. Mater. Adv. 2025, 6, 579–597. [Google Scholar] [CrossRef]
- Zhang, Q.; Ji, F.; Zhao, T.; Shen, Q.; Fang, D.; Kuang, L.; Jiang, L.; Ding, S. Systematic screening of layered double hydroxides for phosphate removal and mechanism insight. Appl. Clay Sci. 2019, 174, 159–169. [Google Scholar] [CrossRef]
- Goh, K.-H.; Lim, T.-T. Influences of co-existing species on the sorption of toxic oxyanions from aqueous solution by nanocrystalline Mg/Al layered double hydroxide. J. Hazard. Mater. 2010, 180, 401–408. [Google Scholar] [CrossRef]
- Mavromatis, V.; Pearce, C.R.; Shirokova, L.S.; Bundeleva, I.A.; Pokrovsky, O.S.; Benezeth, P.; Oelkers, E.H. Magnesium isotope fractionation during hydrous magnesium carbonate precipitation with and without cyanobacteria. Geochim. Cosmochim. Acta 2012, 76, 161–174. [Google Scholar] [CrossRef]
- Jia, Z.; Zeng, W.; Xu, H.; Li, S.; Peng, Y. Adsorption removal and reuse of phosphate from wastewater using a novel adsorbent of lanthanum-modified platanus biochar. Process Saf. Environ. Prot. 2020, 140, 221–232. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, Q.; Liu, J.; Chang, N.; Wan, L.; Chen, J. Phosphate adsorption on lanthanum hydroxide-doped activated carbon fiber. Chem. Eng. J. 2012, 185, 160–167. [Google Scholar] [CrossRef]
- Deluchat, V.; Bollinger, J.-C.; Serpaud, B.; Caullet, C. Divalent cations speciation with three phosphonate ligands in the pH-range of natural waters. Talanta 1997, 44, 897–907. [Google Scholar] [CrossRef]
- Lacour, S.; Deluchat, V.; Bollinger, J.-C.; Serpaud, B. Complexation of trivalent cations (Al (III), Cr (III), Fe (III)) with two phosphonic acids in the pH range of fresh waters. Talanta 1998, 46, 999–1009. [Google Scholar] [CrossRef]
- Ni, C.; Chen, N.; He, J.; Pan, M.; Wang, X.; Pan, B. Complexation-based selectivity of organic phosphonates adsorption from high-salinity water by neodymium-doped nanocomposite. Water Res. 2023, 246, 120705. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yang, Q.; Liu, D.; Nie, H.; Liu, Y. Removal of organic phosphonate HEDP by Eu-MOF/GO composite membrane. J. Environ. Chem. Eng. 2021, 9, 106895. [Google Scholar] [CrossRef]
- Li, C.; Yang, Q.; Lu, S.; Liu, Y. Adsorption and mechanism study for phosphonate antiscalant HEDP removal from reverse osmosis concentrates by magnetic La/Zn/Fe3O4@ PAC composite. Colloids Surf. A Physicochem. Eng. Asp. 2021, 613, 126056. [Google Scholar] [CrossRef]
- Shao, Q.; Yi, Y.; Xie, Y.; Guo, J.; Yang, H.; Chen, Y.; Liu, Z. Zirconium-loaded zeolite composites for selective sorption of 1-hydroxyethylidene-1,1-diphosphonic acid (HEDP) from water: Performance and mechanism. Process Saf. Environ. Prot. 2024, 185, 153–163. [Google Scholar] [CrossRef]
- Shao, Q.; Yi, Y.; Xie, Y.; Yang, H.; Guo, J.; Liu, Z. Selective sorption of organic phosphonate HEDP by steel slag: Efficiency and mechanism. Process Saf. Environ. Prot. 2024, 186, 645–655. [Google Scholar] [CrossRef]

| Adsorbent | Specific Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Diameter (nm) |
|---|---|---|---|
| Mg-Al LDH | 78.38 | 0.35 | 13.11 |
| Mg-Al LDH | Pseudo-First-Order Kinetics | Pseudo-Second-Order Kinetics | Elovich | ||||||
|---|---|---|---|---|---|---|---|---|---|
| qe (exp)/ (mg·g−1) | qe (cal)/ (mg·g−1) | k1/ (min−1) | R2 | qe (cal)/ (mg·g−1) | k2/ g·(mg·min)−1 | R2 | α | β | R2 |
| 199.58 | 190.32 | 1.933 | 0.959 | 194.92 | 0.0194 | 0.98 | 2.18 | 0.12 | 0.997 |
| Models | Parameters | 298 K | 308 K | 318 K |
|---|---|---|---|---|
| Langumir | qm (mg·g−1) | 279.00 | 174.14 | 130.15 |
| KL (L·mg−1) | 0.995 | 1.096 | 3.131 | |
| R2 | 0.948 | 0.903 | 0.967 | |
| Freundlich | 1/n | 0.252 | 0.168 | 0.0724 |
| KF (mg g−1) | 109.854 | 91.572 | 98.177 | |
| R2 | 0.824 | 0.923 | 0.696 | |
| Redlich-Peterson | n | 1.216 | 0.887 | 0.959 |
| KRp | 198.396 | 886.177 | 799.656 | |
| R2 | 0.947 | 0.908 | 0.914 |
| T (K) | ∆G° (kJ mol−1) | ∆S° (J (mol·K)−1) | ∆H° (kJ mol−1) |
|---|---|---|---|
| 298 | −11.98 | −345.11 | −114.82 |
| 308 | −8.53 | ||
| 318 | −5.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, C.; Zhang, L.; Zhang, Q.; Ni, C.; Deng, N.; Huang, X. Efficient Adsorptive Removal of Phosphonate Antiscalant HEDP by Mg-Al LDH. Separations 2025, 12, 259. https://doi.org/10.3390/separations12100259
Guo C, Zhang L, Zhang Q, Ni C, Deng N, Huang X. Efficient Adsorptive Removal of Phosphonate Antiscalant HEDP by Mg-Al LDH. Separations. 2025; 12(10):259. https://doi.org/10.3390/separations12100259
Chicago/Turabian StyleGuo, Changjin, Lejiaqi Zhang, Qi Zhang, Congcong Ni, Ning Deng, and Xin Huang. 2025. "Efficient Adsorptive Removal of Phosphonate Antiscalant HEDP by Mg-Al LDH" Separations 12, no. 10: 259. https://doi.org/10.3390/separations12100259
APA StyleGuo, C., Zhang, L., Zhang, Q., Ni, C., Deng, N., & Huang, X. (2025). Efficient Adsorptive Removal of Phosphonate Antiscalant HEDP by Mg-Al LDH. Separations, 12(10), 259. https://doi.org/10.3390/separations12100259





