Efficient Photocatalytic Degradation of Triclosan and Methylene Blue by Synthesized Ag-Loaded ZnO under UV Light
Abstract
1. Introduction
2. Material and Methods
2.1. Chemicals
2.2. Synthesis of Photocatalysts
- Synthesis of ZnO nanoparticles
- b.
- Synthesis of silver-loaded ZnO nanomaterial
2.3. Characterization of Materials
2.4. Photocatalytic Degradation Experiment
3. Results and Discussion
3.1. Characterization
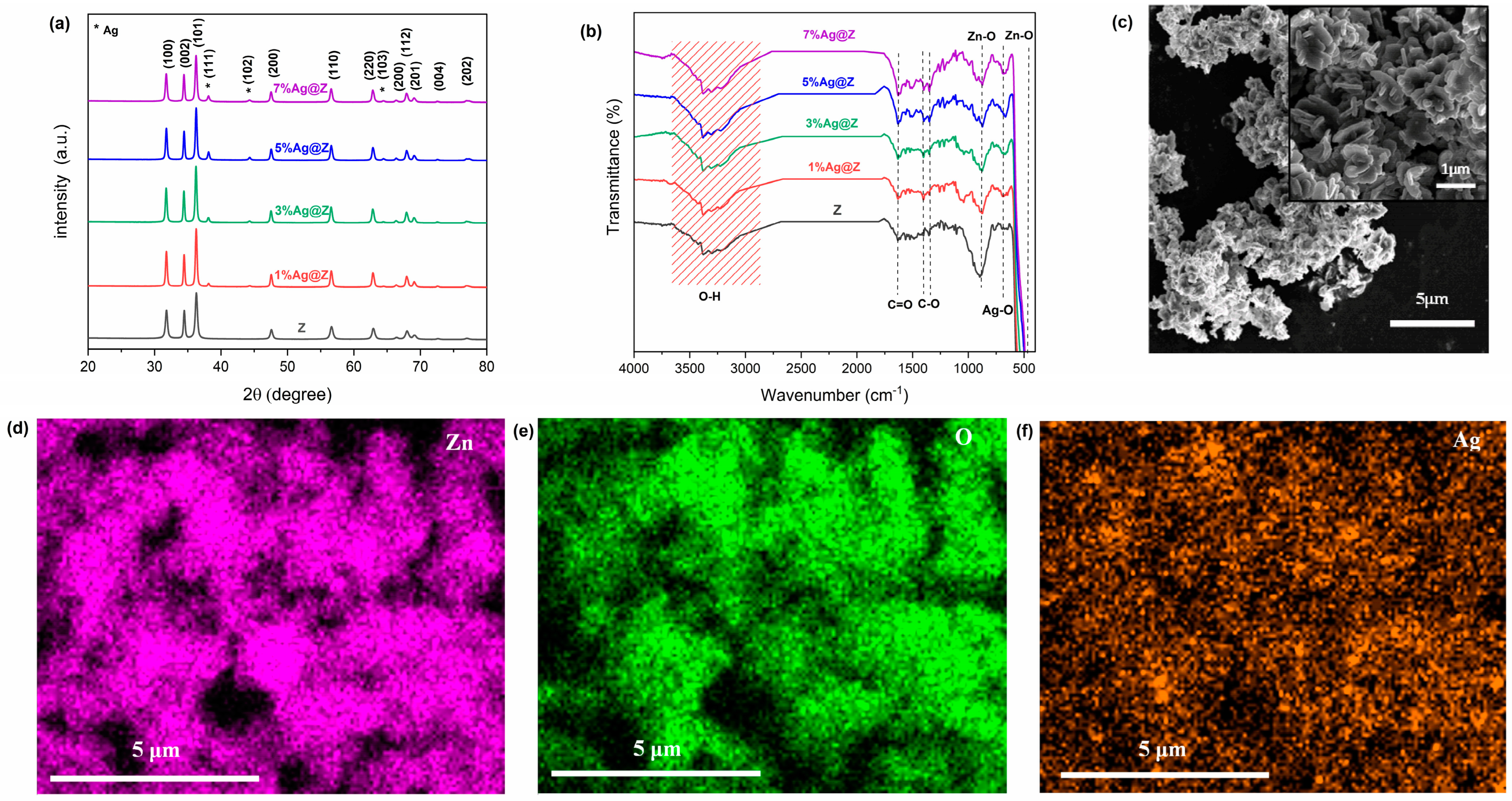
3.1.1. Structural Proprieties
3.1.2. FTIR Analysis
3.1.3. Morphological Proprieties
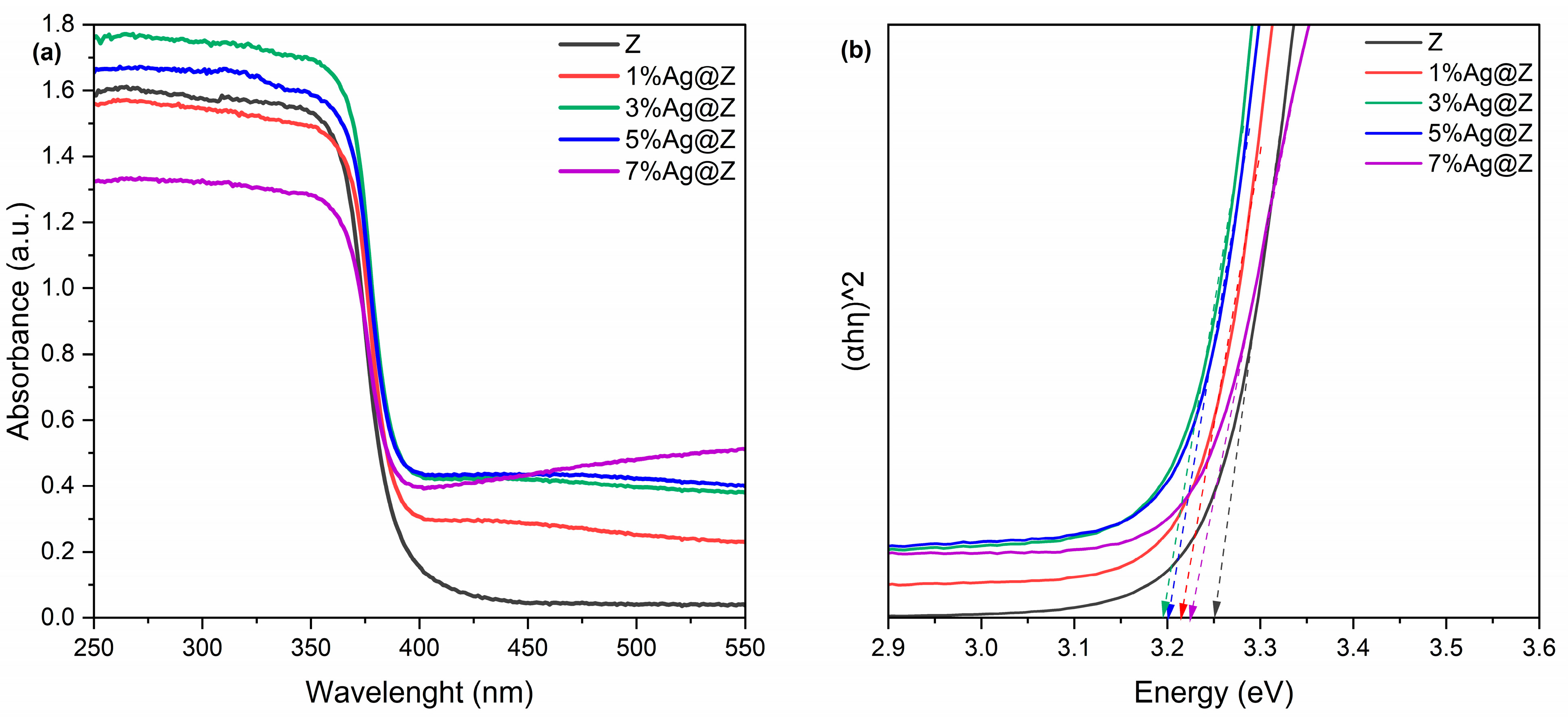
3.1.4. Optical Proprieties
3.1.5. XPS Analysis
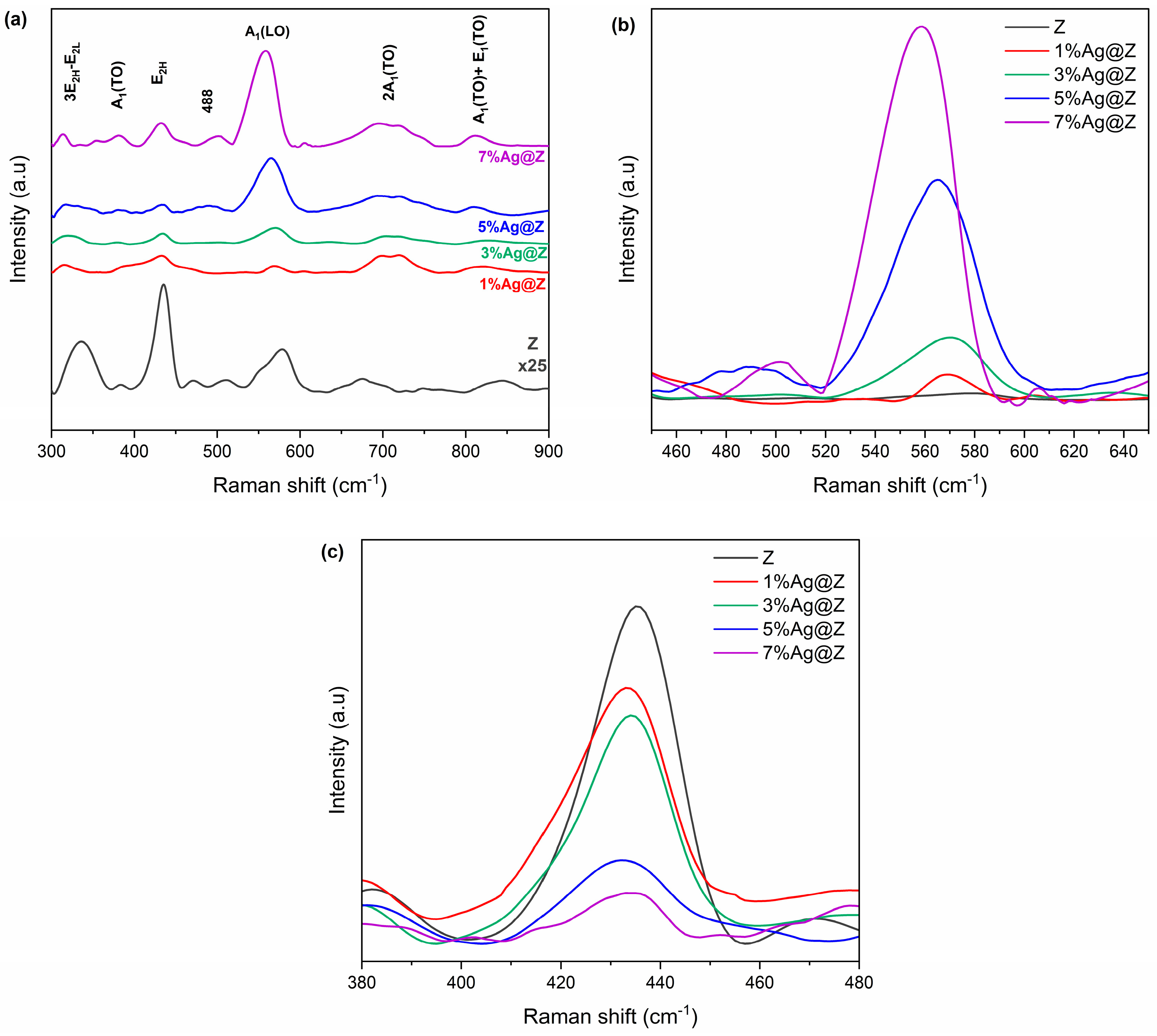
3.1.6. Raman Analysis
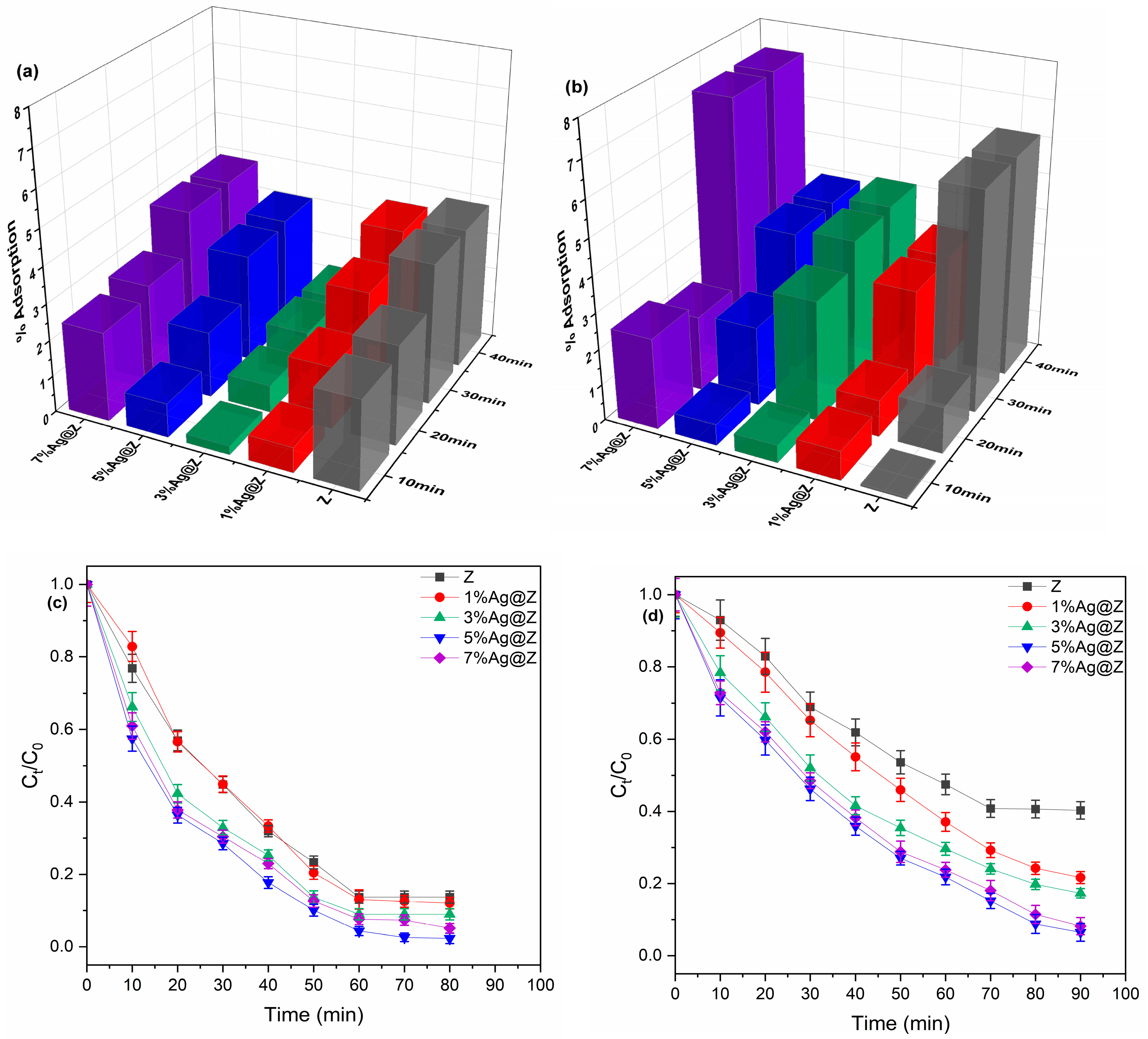
3.2. Photocatalytic Degradation Performance
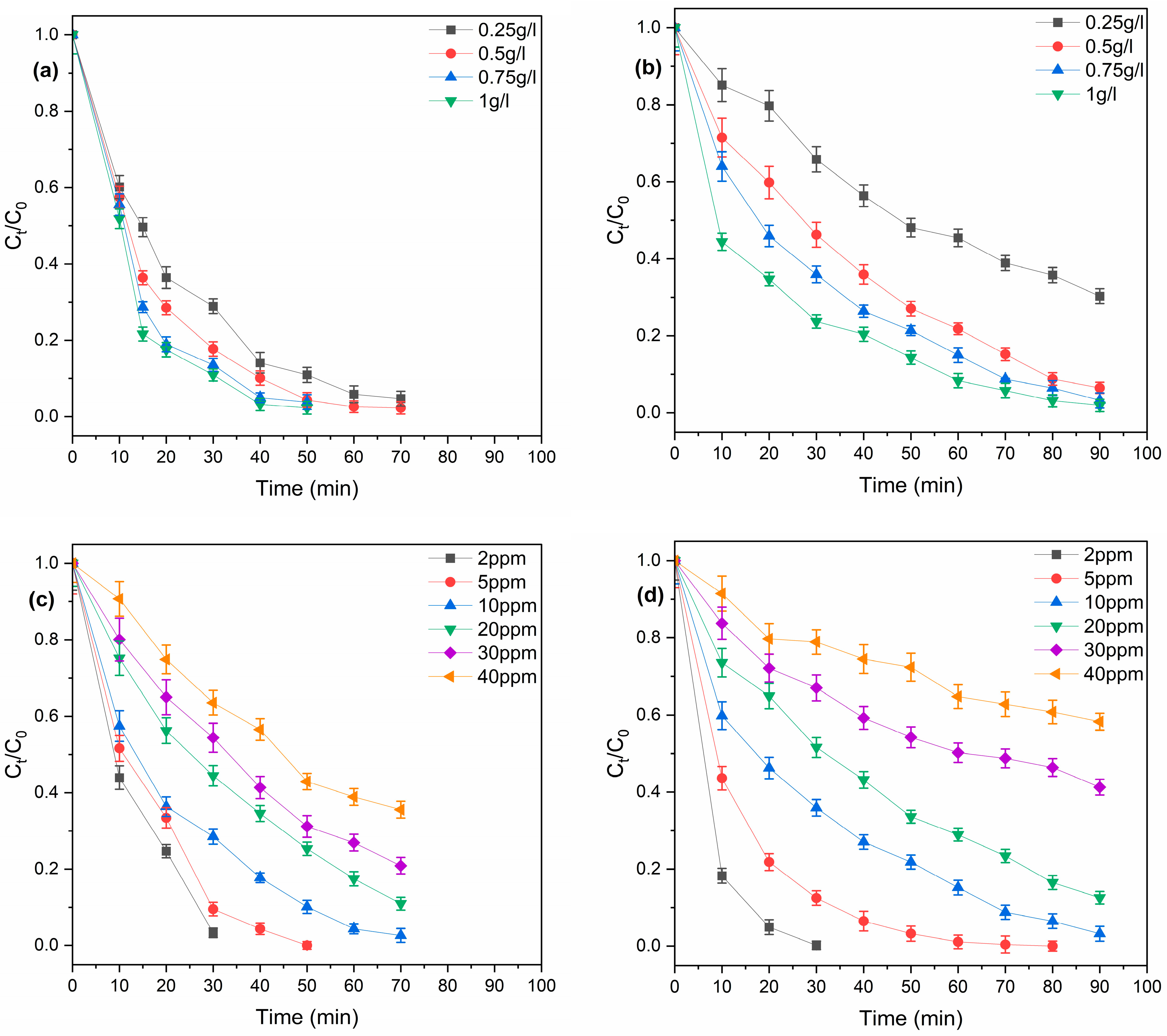
3.3. Catalyst Dosage Effect
3.4. Concentration Effect
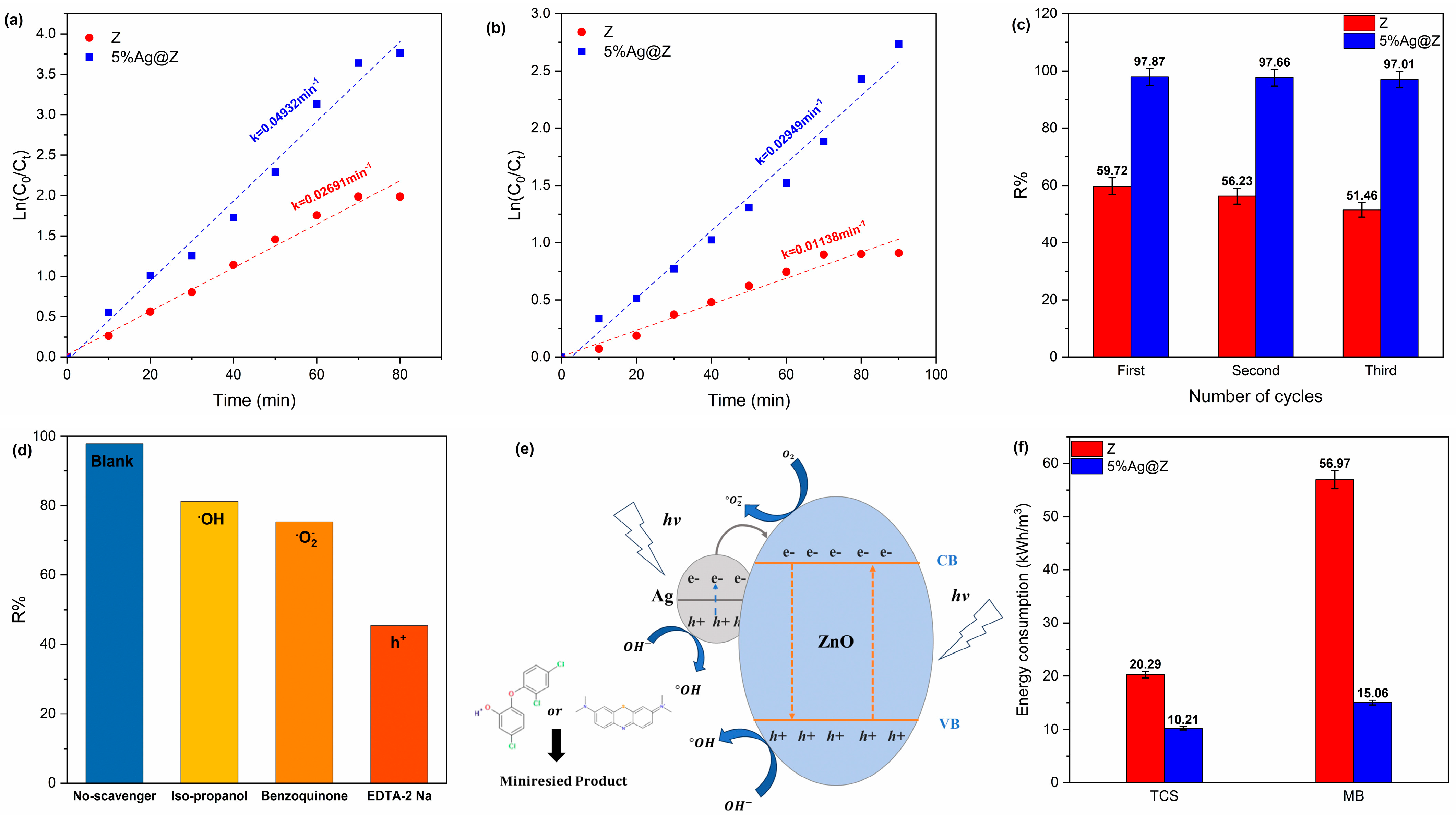
3.5. Kinetics
3.6. Photocatalytic Reusability
3.7. Role of Reactive Species and Photocatalytic Mechanism
3.8. Energy Consumption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, S.; Xia, X.; Song, Q.; Zhao, Y.; Yang, J. Heterogeneous Junction Ni-MOF @ BiOBr Composites: Photocatalytic Degradation of Methylene Blue and Ciprofloxacin. Solid. State Sci. 2023, 138, 107135. [Google Scholar] [CrossRef]
- Lee, K.M.; Lai, C.W.; Ngai, K.S.; Juan, J.C. Recent Developments of Zinc Oxide Based Photocatalyst in Water Treatment Technology: A Review. Water Res. 2016, 88, 428–448. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Zhang, T.; Zhou, Y.; Liu, Z.F.; Zhang, Y.; Bian, Y.; Feng, X.S. Triclosan and Related Compounds in the Environment: Recent Updates on Sources, Fates, Distribution, Analytical Extraction, Analysis, and Removal Techniques. Sci. Total Environ. 2023, 870, 161885. [Google Scholar] [CrossRef]
- Milanović, M.; Đurić, L.; Milošević, N.; Milić, N. Challenges in managing and risk assessment of emerging Comprehensive Insight into Triclosan—From Widespread Occurrence to Health Outcomes. Environ. Sci. Pollut. Res. 2023, 30, 25119–25140. [Google Scholar] [CrossRef] [PubMed]
- Isai, K.A.; Shrivastava, V.S. Photocatalytic Degradation of Methylene Blue Using ZnO and 2%Fe–ZnO Semiconductor Nanomaterials Synthesized by Sol–Gel Method: A Comparative Study. SN Appl. Sci. 2019, 1, 1247. [Google Scholar] [CrossRef]
- Olusakin, P.; Oladiran, T.; Oyinkansola, E.; Joel, O. Results in Engineering Methylene Blue Dye: Toxicity and Potential Elimination Technology from Wastewater. Results Eng. 2022, 16, 100678. [Google Scholar] [CrossRef]
- Khairnar, S.D.; Shrivastava, V.S. Facile Synthesis of Nickel Oxide Nanoparticles for the Degradation of Methylene Blue and Rhodamine B Dye: A Comparative Study. J. Taibah Univ. Sci. 2019, 13, 1108–1118. [Google Scholar] [CrossRef]
- Pavel, M.; Anastasescu, C.; State, R.-N.; Vasile, A.; Papa, F.; Balint, I. Photocatalytic Degradation of Organic and Inorganic Pollutants to Harmless End Products: Assessment of Practical Application Potential for Water and Air Cleaning. Catalysts 2023, 13, 380. [Google Scholar] [CrossRef]
- Kosera, V.S.; Cruz, T.M.; Chaves, E.S.; Tiburtius, E.R.L. Triclosan Degradation by Heterogeneous Photocatalysis Using ZnO Immobilized in Biopolymer as Catalyst. J. Photochem. Photobiol. A Chem. 2017, 344, 184–191. [Google Scholar] [CrossRef]
- da Silva Alves, D.C.; de Farias, B.S.; Breslin, C.; de Almeida Pinto, L.A.; Cadaval, T.R.S.A. Carbon Nanotube-Based Materials for Environmental Remediation Processes. In Advanced Materials for Sustainable Environmental Remediation; Elsevier: Amsterdam, The Netherlands, 2022; pp. 475–513. [Google Scholar] [CrossRef]
- Vaya, D.; Surolia, P.K. Environmental Technology & Innovation Semiconductor Based Photocatalytic Degradation of Pesticides: An Overview. Environ. Technol. Innov. 2020, 20, 101128. [Google Scholar] [CrossRef]
- Wong, K.T.; Kim, S.C.; Yun, K.; Choong, C.E.; Nah, I.W.; Jeon, B.H.; Yoon, Y.; Jang, M. Understanding the Potential Band Position and e–/H+ Separation Lifetime for Z-Scheme and Type-II Heterojunction Mechanisms for Effective Micropollutant Mineralization: Comparative Experimental and DFT Studies. Appl. Catal. B 2020, 273, 119034. [Google Scholar] [CrossRef]
- Khalid, A.; Ahmad, P.; Memon, R.; Gado, L.F.; Khandaker, M.U.; Almukhlifi, H.A.; Modafer, Y.; Bashir, N.; Abida, O.; Alshammari, F.A.; et al. Structural, Optical, and Renewable Energy-Assisted Photocatalytic Dye Degradation Studies of ZnO, CuZnO, and CoZnO Nanostructures for Wastewater Treatment. Separations 2023, 10, 184. [Google Scholar] [CrossRef]
- Zhang, Z.; Zada, A.; Cui, N.; Liu, N.; Liu, M.; Yang, Y.; Jiang, D.; Jiang, J.; Liu, S. Synthesis of Ag Loaded ZnO/BiOCl with High Photocatalytic Performance for the Removal of Antibiotic Pollutants. Crystals 2021, 11, 981. [Google Scholar] [CrossRef]
- Dimitropoulos, M.; Aggelopoulos, C.A.; Sygellou, L.; Tsantis, S.T.; Koutsoukos, P.G.; Yannopoulos, S.N. Unveiling the Photocorrosion Mechanism of Zinc Oxide Photocatalyst: Interplay between Surface Corrosion and Regeneration. J. Environ. Chem. Eng. 2024, 12, 112102. [Google Scholar] [CrossRef]
- Trandafilović, L.V.; Jovanović, D.J.; Zhang, X.; Ptasińska, S.; Dramićanin, M.D. Enhanced Photocatalytic Degradation of Methylene Blue and Methyl Orange by ZnO:Eu Nanoparticles. Appl. Catal. B 2017, 203, 740–752. [Google Scholar] [CrossRef]
- Ersöz, E.; Altintas Yildirim, O. Green Synthesis and Characterization of Ag-Doped ZnO Nanofibers for Photodegradation of MB, RhB and MO Dye Molecules. J. Korean Ceram. Soc. 2022, 59, 655–670. [Google Scholar] [CrossRef]
- Assimeddine, M.; Farid, Z.; Abdennouri, M.; Barka, N.; Lemdek, E.M.; Sadiq, M. Improvement of Photocatalytic Degradation of Methyl Orange by Impregnation of Natural Clay with Nickel: Optimization Using the Box-Behnken Design (BBD). Environ. Sci. Pollut. Res. 2023, 30, 62494–62507. [Google Scholar] [CrossRef] [PubMed]
- Ramesan, M.T.; Greeshma, K.P.; Parvathi, K.; Anilkumar, T. Structural, Electrical, Thermal, and Gas Sensing Properties of New Conductive Blend Nanocomposites Based on Polypyrrole/Phenothiazine/Silver-Doped Zinc Oxide. J. Vinyl Addit. Technol. 2020, 26, 187–195. [Google Scholar] [CrossRef]
- Alnehia, A.; Al-Sharabi, A.; Al-Hammadi, A.H.; Al-Odayni, A.B.; Alramadhan, S.A.; Alodeni, R.M. Phyto-Mediated Synthesis of Silver-Doped Zinc Oxide Nanoparticles from Plectranthus Barbatus Leaf Extract: Optical, Morphological, and Antibacterial Properties. Biomass Convers. Biorefin 2023, 14, 17041–17053. [Google Scholar] [CrossRef]
- Stan, M.; Popa, A.; Toloman, D.; Silipas, T.D.; Vodnar, D.C. Antibacterial and Antioxidant Activities of ZnO Nanoparticles Synthesized Using Extracts of Allium Sativum, Rosmarinus Officinalis and Ocimum Basilicum. Acta Metall. Sin. (Engl. Lett.) 2016, 29, 228–236. [Google Scholar] [CrossRef]
- Zeferino, R.S.; Barboza-flores, M.; Pal, U. Photoluminescence and Raman Scattering in Ag-Doped ZnO Nanoparticles. J. Appl. Phys. 2011, 109, 014308. [Google Scholar] [CrossRef]
- Chauhan, A.; Verma, R.; Kumari, S.; Sharma, A.; Shandilya, P. Photocatalytic Dye Degradation and Antimicrobial Activities of Pure and Ag-Doped ZnO Using Cannabis Sativa Leaf Extract. Sci. Rep. 2020, 10, 781. [Google Scholar] [CrossRef] [PubMed]
- Varudkar, H.A.; Umadevi, G.; Nagaraju, P.; Dargad, J.S.; Mote, V.D. Fabrication of Al-Doped ZnO Nanoparticles and Their Application as a Semiconductor-Based Gas Sensor for the Detection of Ammonia. J. Mater. Sci. Mater. Electron. 2020, 31, 12579–12585. [Google Scholar] [CrossRef]
- Aggelopoulos, C.A.; Dimitropoulos, M.; Govatsi, A.; Sygellou, L.; Tsakiroglou, C.D.; Yannopoulos, S.N. Influence of the Surface-to-Bulk Defects Ratio of ZnO and TiO2on Their UV-Mediated Photocatalytic Activity. Appl. Catal. B 2017, 205, 292–301. [Google Scholar] [CrossRef]
- Merlin, R. Raman Studies of Fibonacci, Thue-Morse, and Random Superlattices. In Light Scattering in Solids V: Superlattices and Other Microstructures; Cardona, M., Güntherodt, G., Eds.; Springer: Berlin/Heidelberg, Germany, 1989; Volume 66/1, pp. 214–232. ISBN 978-3-540-50400-9. [Google Scholar]
- Ma, X.; Li, H.; Liu, T.; Du, S.; Qiang, Q.; Wang, Y.; Yin, S.; Sato, T. Comparison of Photocatalytic Reaction-Induced Selective Corrosion with Photocorrosion: Impact on Morphology and Stability of Ag-ZnO. Appl. Catal. B 2017, 201, 348–358. [Google Scholar] [CrossRef]
- Balarak, D.; Mengelizadeh, N.; Rajiv, P.; Chandrika, K. Photocatalytic Degradation of Amoxicillin from Aqueous Solutions by Titanium Dioxide Nanoparticles Loaded on Graphene Oxide. Environ. Sci. Pollut. Res. 2021, 28, 49743–49754. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Guo, T.; Zhang, Y.; Yin, L.; Dai, Y. Chemosphere Progress on Simultaneous Photocatalytic Degradation of Pollutants and Production of Clean Energy: A Review. Chemosphere 2023, 339, 139486. [Google Scholar] [CrossRef]
- Al-Musawi, T.J.; Yilmaz, M.; Ramírez-Coronel, A.A.; Al-Awsi, G.R.L.; Alwaily, E.R.; Asghari, A.; Balarak, D. Degradation of Amoxicillin under a UV or Visible Light Photocatalytic Treatment Process Using Fe2O3/Bentonite/TiO2: Performance, Kinetic, Degradation Pathway, Energy Consumption, and Toxicology Studies. Optik 2023, 272, 170230. [Google Scholar] [CrossRef]
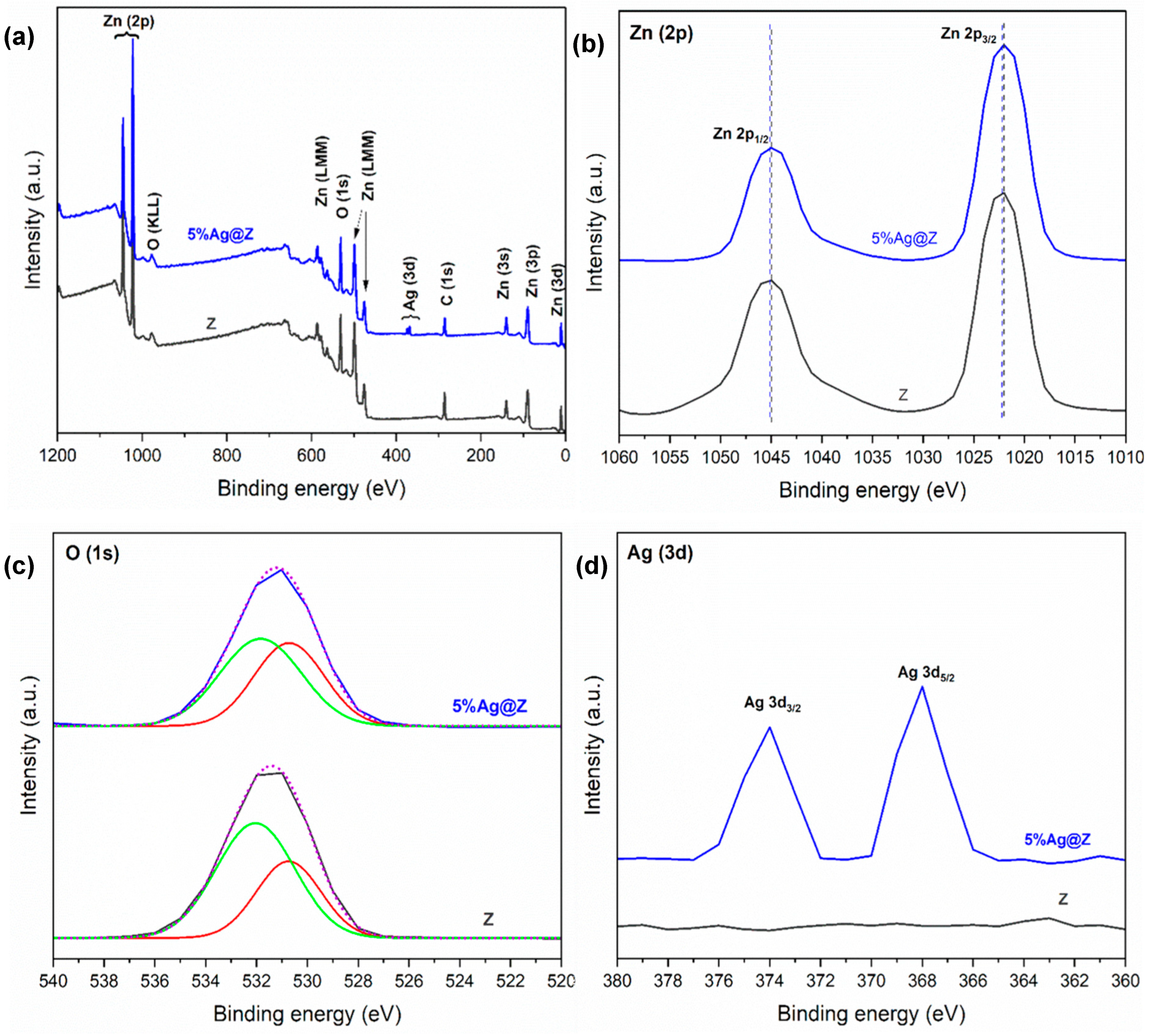
| Atomic Percentage (At %) | ||||
|---|---|---|---|---|
| Zn (2p) | O (1s) | Ag (3d) | Element Ratio Zn:O:Ag | |
| Z | 80.05 | 19.95 | - | 4:1:0 |
| 5% Ag@Z | 81.65 | 15.28 | 3.07 | 26.6:5:1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chems, M.; González-Fernández, L.A.; Sanchez Polo, M.; Anouar, A.; Castillo Ramos, V. Efficient Photocatalytic Degradation of Triclosan and Methylene Blue by Synthesized Ag-Loaded ZnO under UV Light. Separations 2024, 11, 221. https://doi.org/10.3390/separations11080221
Chems M, González-Fernández LA, Sanchez Polo M, Anouar A, Castillo Ramos V. Efficient Photocatalytic Degradation of Triclosan and Methylene Blue by Synthesized Ag-Loaded ZnO under UV Light. Separations. 2024; 11(8):221. https://doi.org/10.3390/separations11080221
Chicago/Turabian StyleChems, Myriam, Lázaro Adrián González-Fernández, Manuel Sanchez Polo, Abdellah Anouar, and Ventura Castillo Ramos. 2024. "Efficient Photocatalytic Degradation of Triclosan and Methylene Blue by Synthesized Ag-Loaded ZnO under UV Light" Separations 11, no. 8: 221. https://doi.org/10.3390/separations11080221
APA StyleChems, M., González-Fernández, L. A., Sanchez Polo, M., Anouar, A., & Castillo Ramos, V. (2024). Efficient Photocatalytic Degradation of Triclosan and Methylene Blue by Synthesized Ag-Loaded ZnO under UV Light. Separations, 11(8), 221. https://doi.org/10.3390/separations11080221










