Abstract
Phyllostachys glauca McClure leaves (PML), as economical natural product material, contain abundant phenols, particularly flavonoids, with significant biological activities that contribute to their widespread applications in food, pharmaceutical, and cosmetic industries. To study the significant phenols in PML, an ultrasound-assisted deep eutectic solvent extraction method with high efficiency and low toxicity was established for extracting the phenols from PML and the bamboo leaves of 17 other bamboo species. Using the Ultra Performance Liquid Chromatography (UPLC) method, the content of phenols in the leaves of the 18 bamboo species was determined. PML were found to contain neochlorogenic acid, chlorogenic acid, cryptochlorogenic acid, isoorientin, and orientin at contents of 0.793 ± 00.17 mg/g, 0.491 ± 0.0081 mg/g, 0.485 ± 0.0043 mg/g, 0.465 ± 0.0021 mg/g, and 0.044 ± 0.0005 mg/g, respectively, with a total content of 2.278 mg/g. These contents were significantly higher than those found in the leaves of 17 other bamboo species. Additionally, the significant phenols, neochlorogenic acid, chlorogenic acid, and cryptochlorogenic acid were found simultaneously in the leaves of 15 bamboo species, especially in PML. Therefore, PML can be viewed as the natural product material with considerable application values, owing to its abundant phenols, and can exhibit its importance in utilizing neochlorogenic acid, chlorogenic acid, and cryptochlorogenic acid as resources.
1. Introduction
Bamboo belongs to the Gramineae plant, and has excellent environmental adaptability and extremely fast rate of growth [1,2]. Therefore, it can be viewed as a kind of economical natural product material. Otherwise, Bamboo leaves are rich in phenols [3], which exhibit numerous biological activities, including anti-inflammatory [4], antibacterial [5], anticancer [6], and antiviral [7] activities. In recent studies, flavonoids have been identified as the most predominant phenol in bamboo leaves with excellent antioxidant activity and anti-inflammatory activity [8,9], especially being isoorientin [10,11,12,13,14,15]. Similarly, Phyllostachys glauca McClure leaves (PML), reported to be a natural antioxidant [16], are abundant in flavonoids and some phenolic acids [17], making them a subject of extensive investigation in the field of natural products.
However, the major phenols from PML still need to be further explored using a reasonable extraction method. At present, most of the extraction methods of phenols from bamboo leaves are the traditional organic solvent extraction method and common ultrasonic extraction method with methanol or ethanol as solvent. The former has the disadvantages of low extraction efficiency, high toxicity, and laborious operation. The latter only uses ordinary solvent to extract, which still has the disadvantages of high toxicity and low extraction efficiency [18,19,20,21]. Fortunately, deep eutectic solvent (DES), proposed by Abott in 1999 [22], can be used as a novel solvent with green economy and high extraction efficiency to replace common solvents, such as methanol and ethanol [23,24]. Therefore, in this study, the ultrasonic-assisted deep eutectic solvent method was used to extract phenols from bamboo leaves, with the advantages of high extraction efficiency and low toxicity. Otherwise, an established Ultra Performance Liquid Chromatography (UPLC) method was used to determine the phenols content.
This study is aimed at revealing the major phenols from PML with overwhelming contents compared with other bamboo species leaves, and in exploring the connotative findings using contents data. Otherwise, establishing an ultrasound-assisted deep eutectic solvent extraction method with high extraction efficiency is also significant for the efficient utilization of nature products.
2. Materials and Methods
2.1. Materials
2.1.1. Solvents
Methanol (HPLC grade, J.T. Baker, Shanghai, China), Acetonitrile (LC-MS grade, J.T. Baker, Shanghai, China), Choline chloride (AR grade, Shanghai Aladdin Biochemical Science and Tech-nology Co., Ltd., Shanghai, China), Formic acid (LC-MS grade, Fisher Scientific, Waltham, MA, USA), Ethylene glycol (AR grade, J.T. Baker, Shanghai, China), Anhydrous ethanol (AR grade, Beijing Tongguang Fine Chemical, Beijing, China), Deionized water, Neochlorogenic acid standard (HPLC grade, so-dium columbite bio, Chengdu, Sichuan, China), Chlorogenic acid standard (HPLC grade, sodium columbite bio, Chengdu, Sichuan, China), Cryptochlorogenic acid standard (HPLC grade, sodium columbite bio, Chengdu, Sichuan, China), Isoorientin standard (HPLC grade, Source Leaf Bio, Shanghai, China.), and Orientin standard (HPLC grade, Source Leaf Bio, Shanghai, China).
2.1.2. Instruments
A Yunbang YB-500 multifunctional pulverizer (Yongkang Speed Front Industry & Trade Co., Ltd., Jinhua, Zhejiang, China), JXFSTPRP-24 Automatic sample rapid grinder (Shanghai Jingxin Business Development Co., Shanghai, China), MP5002 electronic balance (Shanghai Hengping Scientific Instrument Co., Ltd., Shanghai, China), ISO9001 precision electronic balance (Sartorius, Gottingen, Niedersachsen, Germany), HH.SY11-Ni Electrothermal constant-temperature water bath pot (Beijing Changfeng Instrumentation Co., Ltd., Beijing, China), Intelligent digital display constant-temperature water bath (Tianjin Sateri Experi-mental and Analytical Instrument Manufacturing Factory, Tianjin, China), KQ-800E Ultrasonic Cleaner (Kunshan Ultrasonic Instrument Co., Ltd., Suzhou, Jiangsu, China), TB1677-MD 4C NT Vacuum pump (Vacuubrand, Wertheim, Baden, Germany), 1290 Infinitely series ultra-high–performance liquid chromatography (UPLC) (Agilent Technologies, Santaclara, CA, USA), and a TGL-16C Benchtop Centrifuge (Shanghai Anting Scientific Instruments, Shanghai, China).
2.1.3. Bamboo Leaves
Eighteen mature, disease-free, pest-free, intact bamboo leaves were harvested from the National Botanical Garden (Beijing, China) in October 2023 and authenticated by researcher Wang Jinge of the National Botanical Garden, including Phyllostachys glauca f. yunzhu, Phyllostachys glauca McClure, Phyllostachys glauca var. variabilis, Phyllostachys propinqua, Phyllostachys aureosulcata “Spectabilis”, Phyllostachys bissetii McClure, Phyllostachys aureosulcata McClure, Phyllostachys iridescens C. Y. Yao, Pleioblastus kongosanensis f. aureostriatus, Pleioblastus gozadakensis Nakai, Pseudosasaja-ponica (Sieb. et Zucc.) Makino, Pleioblastus argenteostriatus, Pleioblastus fortunei v.Houtte Nakai, Shibataea chinensis Nakai, Shibataea lanceifolia C. H. Hu, Hibanobambus tranguillans f. shiroshima H. Okamura, Indocalamus decorus Q. H. Dai, and Indocalamus tessellatus (Munro) P. C. Keng.
All of the bamboo leaves were stored at the International Centre for Bamboo and Rat tan (ICBR), Beijing, China.
2.2. Experimental Preparation
2.2.1. Preparation of DES
The preparation of DES referred to the literature [25]. Choline chloride (AR grade) and ethylene glycol (AR grade) were accurately weighed in a 1:2 molar ratio and placed in a 1000 mL flat-bottomed flask, which was heated in an electrically heated thermostatic water bath at 80 °C with stirring for 1 h until the solution was translucent, homogeneous, and clarified.
2.2.2. Extraction of Phenols from Bamboo Leaves
Bamboo leaves were cleaned with deionized water and shade-dried at room temperature. Then, dried bamboo leaves were placed in a multifunctional pulverizer to smash into the bamboo leaf residues. The obtained bamboo leaf residues were put into the automatic sample rapid-grinding machine in batches of 1 min, with a pre-set frequency of 60 Hz to grind into bamboo powder. The obtained bamboo powders were stored in the refrigerator at −80 °C for use.
One gram of bamboo powder was extracted using the ultrasonic extraction method with the DES (Choline chloride and ethylene glycol in molar ratio 1:2) each time at a temperature of 50 °C. Subsequently, the crude extract was subjected to vacuum suction filtration using a Buchholz funnel for 5 min.
2.3. Establishment of the UPLC Method
2.3.1. Preparation of Sample Solution
The extract obtained after vacuum suction filtration was filtrated through a 0.22 µm filter membrane for analysis using UPLC, with an extraction time of 30 min, water content in DES of 40%, and solid-to-liquid ratio of 1:15.
2.3.2. Chromatographic Conditions
An Agilent Proroshell 120 EC-C18 chromatographic column (150 mm × 2.1 mm, 2.7 μm) was used. Formic acid solution (0.1%) was used as mobile phase A, and acetonitrile was used as mobile phase B. The elution conditions were 0–40 min and 10–15% mobile phase B. The column temperature was set at 35 °C. The injection volume was 1 μL, and the flow rate was 0.08 mL/min. The wavelength of the diode array detector (DAD) for the determination of neochlorogenic acid, chlorogenic acid, and cryptochlorogenic acid was 325 nm, and the wavelength for the determination of isoorientin and orientin was 345 nm.
2.3.3. Preparation of the Mixed Standard Stock Solution
Standard solutions of neochlorogenic acid, chlorogenic acid, cryptochlorogenic acid, isoorientin, and orientin were individually prepared in 10 mL volumetric flasks using methanol as the solvent to yield concentrations of 0.25 mg/mL. Sequentially, 1 mL of each solution was transferred and combined to create a mixed standard stock solution.
Chromatogram comparison and methodological verification: DAD chromatograms of the sample solution and mixed standard stock solution were compared at 325 nm and 345 nm. Retention time and ultraviolet (UV) spectral data were used for the qualitative identification of the 5 phenols in PML. The linear relation and sensitivity were determined by diluting each standard solution by half 7 times. Furthermore, the precision, sample stability, measurement reproducibility, and accuracy were investigated.
2.3.4. Quantitative Determination Using UPLC
The content of phenols in sample solutions was determined using UPLC, and each measurement was conducted parallelly in triplicate.
2.4. Optimization of the Process Conditions of Ultrasound-Assisted Deep Eutectic Solvent Extraction
2.4.1. Single-Factor Test
The effects of extraction time, water content in DES, and solid-to-liquid ratio on the total phenol content were studied using a single-factor test.
2.4.2. Orthogonal Test
L9 (33) was used to conduct an orthogonal test, building upon the results from the single-factor test. Thus, the most optimal process conditions, as well as the degree of dominance and significance of each factor, were determined. The orthogonal test factor level is shown in Table 1.

Table 1.
Orthogonal factors level table.
Next, a comparison was made among the common ultrasonic extraction method using methanol or ethanol as solvents, the traditional organic solvent extraction method, and the ultrasound-assisted deep eutectic solvent extraction method in terms of total phenol content.
A traditional organic solvent extraction method referred to the literature [26]. Fifty grams of bamboo powder was placed in a 1000 mL round-bottom flask, adding 500 mL of anhydrous ethanol for three hot reflux extractions. Each extraction was 6 h. The extraction temperature was set at 60 °C. Then, the filtrates were combined to reduced pressure filtration. Subsequently, we transferred 1 mL of the extract passed through a 0.22 μm filter membrane, and injected it for detection.
2.5. Determination of the Content in the Leaves of Different Bamboo Species
The content of phenols in bamboo leaves was analyzed using the UPLC method established in this study.
3. Results and Discussion
3.1. UPLC Characterization
Neochlorogenic acid, chlorogenic acid, cryptochlorogenic acid, isoorientin, and orientin were identified in the PML. The five phenols have positive biological activities [27,28,29,30,31], involving antioxidant, anti-inflammatory, antibacterial, anti-cancer, and antiviral activities. The structures of these compounds are illustrated in Figure 1.
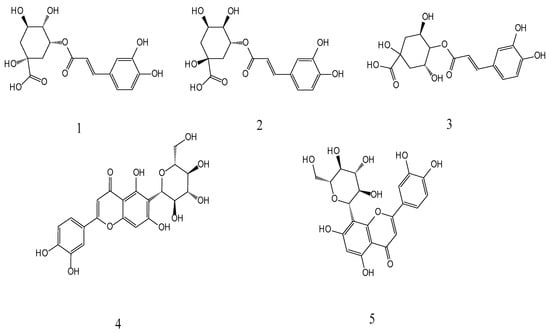
Figure 1.
Structures of the five phenols: 1-neochlorogenic (5-Caffeoylquin), 2-chlorogenic (3-Caffeoylquinic acid), 3-cryptochlorogenic (4-Caffeoylquinic acid), 4-issorientin (Luteo-lin-6-C-glucoside), and 5-orientin (Luteolin-8-C-glucoside).
DAD chromatograms of the sample solution and mixed standards are shown in Figure 2.
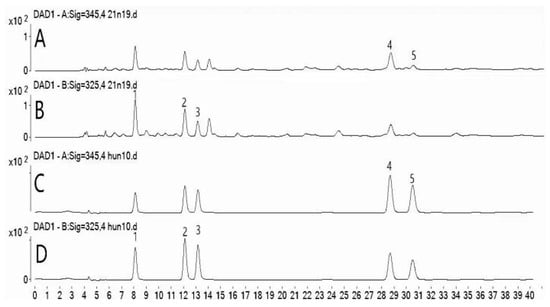
Figure 2.
1-neochlorogenic acid, 2-chlorogenic acid, 3-cryptochlorogenic acid, 4-isoorientin, and 5-orientin. (A) DAD chromatogram of sample solution at 345 nm. (B) DAD chromatogram of sample solution at 325 nm. (C) DAD chromatogram of mixed standards at 345 nm. (D) DAD chromatogram of mixed standards at 325 nm.
Compounds 1, 2, 3, 4, and 5 in PML were qualitatively identified based on their retention times in the DAD chromatogram and UV spectral data. The relative standard deviations (RSDs) of the retention times of compounds 1, 2, 3, 4, and 5 were 0.09%, 0.06%, 0.11%, 0.15%, and 0.10%, respectively.
3.2. Methodological Verification
3.2.1. Linear Relation and Sensitivity
Standard linear equations were established using concentration as the abscissa and peak area as the ordinate. A strong correlation between the peak area and concentration, as well as the high sensitivity of the instrument, can be seen in Table 2.

Table 2.
Linear relation and sensitivity.
3.2.2. Instrument Precision, Sample Stability, Measurement Reproducibility, and Accuracy
The RSDs of the peak areas for compounds 1, 2, 3, 4, and 5 were 0.46%, 0.29%, 0.19%, 0.34%, and 0.07%, respectively, as determined by five consecutive injections of the standard mixing stock solution. These findings demonstrate the excellent precision of the instrument.
The sample solution was injected five times at 3 h intervals in one day to assess the intra-day stability based on the RSDs of the peak areas. Similarly, the inter-day stability was assessed by injecting the sample solution at the same time over five consecutive days. The RSDs of the peak areas of compounds 1, 2, 3, 4, and 5 for assessing intra-day stability were 0.36%, 0.18%, 0.64%, 1.08%, and 1.75%, respectively. Similarly, the RSDs of the peak areas of compounds 1, 2, 3, 4, and 5 for assessing inter-day stability were 0.72%, 0.88%, 1.44%, 0.47%, and 1.15%, respectively. These findings demonstrate the excellent stability of the sample solution.
Six sample solutions prepared in different batches were injected to assess their reproducibility by determining the RSDs of the peak areas of compounds 1, 2, 3, 4, and 5. The RSDs of the peak areas of compounds 1, 2, 3, 4, and 5 were 1.37%, 1.07%, 1.19%, 0.89%, and 1.14%, respectively, which demonstrated the excellent reproducibility of the extraction method.
Nine samples of bamboo powders (1 g each) of known content were precisely weighed and divided into three groups and labeled as A, B, and C. Each group was supplemented with a known standard solution at low, medium, and high concentrations, respectively, and the recovery was calculated. The results are shown in Table 3.

Table 3.
The results of recovery.
The recovery outcomes illustrate the high accuracy of the determined content. The contents of compounds 1, 2, 3, 4, and 5 from PML under the process conditions of 30 min of extraction time, 40% of water content in DES, and 1:15 of solid-to-liquid ratio were 0.618 ± 0.0038 mg/g, 0.383 ± 0.0017 mg/g, 0.368 ± 0.0012 mg/g, 0.385 ± 0.0026 mg/g, and 0.038 ± 0.0020 mg/g, respectively, and the total content was 1.792 mg/g.
3.3. Optimization of Process Conditions
3.3.1. The Single-Factor Test
The effects of extraction time, water content in DES, and solid-to-liquid ratio on the content of total phenols are shown in Figure 3, Figure 4 and Figure 5.
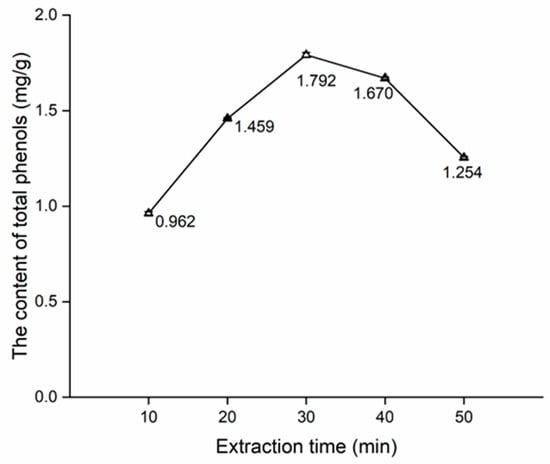
Figure 3.
Change in the content of total phenols with extraction time.
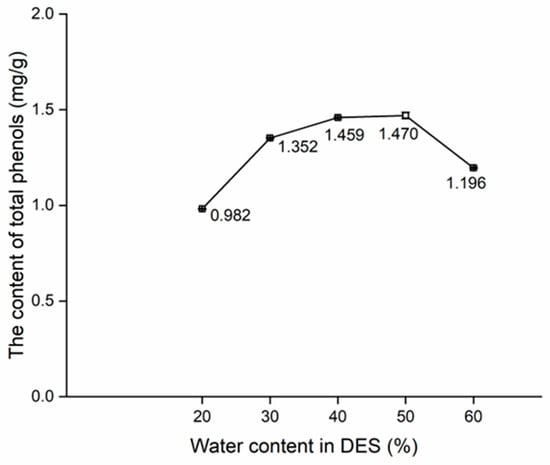
Figure 4.
Change in the content of total phenols with water content in DES.
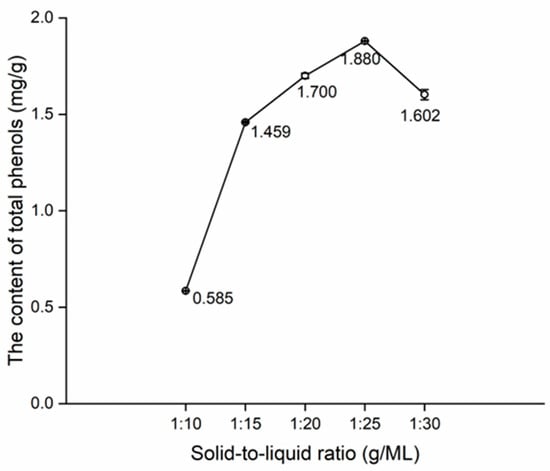
Figure 5.
Change in the content of total phenols with the solid-to-liquid ratio.
Figure 3 shows a notable increase in the content of total phenols from 10 min to 30 min, with the total content peaking at 30 min. Next, the content of total phenols exhibits a steady decline from 30 min to 40 min. Finally, there is a noticeable decrease from 40 min to 50 min. This trend likely stems from the challenge of achieving complete extraction at 10 min and 20 min. On the other hand, phenols undergo decomposition at excessively prolonged extraction times such as 40 min and 50 min. Consequently, 30 min of the extraction time was associated with the highest content (Figure 3), and 20, 30, and 40 min of extraction times were used for the orthogonal test.
Apart from the range of 20% to 30%, the upward trend in the content of total phenols (Figure 4) was less steep than 30% to 50% (Figure 3), with the content of total phenols peaking at 50%. On the other hand, a notable downward trend of 50% to 60% was noted. This trend could be reasonably attributed to the reduction in the viscosity of DES after the addition of water, which enhanced the solubility of phenols in DES (Figure 4). Consequently, there was a consistent increase in the content of total phenols from 30% to 50%. Nevertheless, the saturation of the dissolution ability of DES after the addition of water led to a stagnation in the increasing trend in total phenol content, causing it to drop from 50% to 60% [32,33]. In summary, the orthogonal test using 30%, 40%, and 50% of water content in DES were conducted.
In Figure 5, a surge in the content of total phenols can be observed from 1:10 to 1:15, which is attributable to the challenge of achieving complete infiltration of bamboo powders using DES, resulting in a low total phenol content at 1:10. Subsequently, a steady increase in the content of total phenols can be noted from 1:15 to 1:25, culminating in an inflection point at 1:25. The increase in solid-to-liquid ratio may decrease the viscosity of DES, thereby enhancing the dissolution capacity of DES until saturation [34]. Consequently, 1:20, 1:25, and 1:30 of solid-to-liquid ratios were used for the orthogonal tests.
3.3.2. The Single-Factor Test
Results from the orthogonal test are shown in Table 4.

Table 4.
Results from the orthogonal test.
SSPS statistical software version 26 (IBM) was used to perform the main effect analysis, and the results are shown in Table 5.

Table 5.
Results from the main effect analysis.
The order of dominance in the effects on the content of total phenols caused by extraction time, water content in DES, and solid-to-liquid ratio was extraction time > solid-to-liquid ratio > water content in DES, as determined using range analysis. The significance of each factor was examined using a variance analysis. Extraction time has a significant effect on the content of total phenols, whereas the water content in DES and the solid-to-liquid ratio did not have a significant effect. On the other hand, the optimal process conditions were identified based on the K1, K2, and K3 values, which correspond to 30 min of extraction time, 50% of water content in DES, and 1:25 of solid-to-liquid ratio. The final contents of compounds 1, 2, 3, 4, and 5 from PML were determined to be 0.793 ± 0.0017 mg/g, 0.491 ± 0.0081 mg/g, 0.485 ± 0.0043 mg/g, 0.465 ± 0.0021 mg/g, and 0.044 ± 0.0005 mg/g, respectively, resulting in a total content of 2.278 mg/g. Additionally, the results from the common ultrasonic extraction method using methanol or ethanol as a solvent and the traditional organic solvent extraction method are shown in Table 6.

Table 6.
The results from common ultrasonic extraction method and traditional organic solvent extraction method.
The common ultrasonic extraction method using methanol as a solvent resulted in the content of total phenols that was 19.49% lower than the method used in this study. The content of total phenols obtained after common ultrasonic extraction using ethanol as a solvent was 13.26% lower than the method used in this study. Otherwise, the content of total phenols obtained in this study was 1.10% higher than the traditional organic solvent extraction method undergoing hot reflux for a long time. These findings demonstrate the advantage of ultrasound-assisted deep eutectic solvent extraction on extraction efficiency compared with the common ultrasonic extraction method and the traditional organic solvent extraction method. Therefore, this method could be effectively used to extract phenols from bamboo leaves owing to its high extraction efficiency and low toxicity.
3.4. Analysis of the Contents of 5 Phenols from the Leaves of 18 Bamboo Species
Detailed information on the contents of five phenols from the leaves of 18 bamboo species are shown in Table 7.

Table 7.
Information on the contents of five phenols from the leaves of 18 bamboo species.
PML were found to contain the highest contents of five phenols, which were 0.793 ± 00.17 mg/g, 0.491 ± 0.0081 mg/g, 0.485 ± 0.0043 mg/g, 0.465 ± 0.0021 mg/g, and 0.044 ± 0.0005 mg/g for compounds 1, 2, 3, 4, and 5, respectively, and the total content was 2.278 mg/g. The content of the five phenols in the leaves of all bamboo species was predominantly presented in PML regardless of whether it pertained to the individual or total content. Therefore, PML have significant application values owing to the presence of abundant and significant phenols. Otherwise, the average contents of compounds 1, 2, 3, 4, and 5 in the leaves of the 18 bamboo species were calculated to be 0.189 mg/g, 0.134 mg/g, 0.145 mg/g, 0.168 mg/g, and 0.003 mg/g, respectively, and compounds 1, 2, and 3, which are commonly identified in honeysuckle [35], were detected simultaneously in the leaves of the 15 bamboo species. Hence, compounds 1, 2, and 3 can also be identified as the significant phenols in the tested bamboo leaves in addition to flavonoids. In summary, PML, as a kind of economical natural product material, can be considered as a significant source of the five phenols with significant application values, and can also be regarded as a novel approach for using compounds 1, 2, and 3 to expand its application potential in food [36], pharmaceutical [37], and cosmetic [38] industries. However, other bamboo species having higher phenol content in leaves are still waiting to be found. Otherwise, the specific application of PML needs to be furthermore explored under the conclusion of this study.
4. Conclusions
Ultrasound-assisted deep eutectic solvent extraction showed low toxicity and high extraction efficiency for extracting phenols from bamboo leaves compared with common ultrasonic extraction using methanol or ethanol as a solvent and traditional organic solvent extraction methods. The contents of neochlorogenic acid, chlorogenic acid, cryptochlorogenic acid, isoorientin, and orientin from PML measured using UPLC were determined to be 0.793 ± 00.17 mg/g, 0.491 ± 0.0081 mg/g, 0.485 ± 0.0043 mg/g, 0.465 ± 0.0021 mg/g, and 0.044 ± 0.0005 mg/g, respectively, and the total content was 2.278 mg/g, which was found to be the highest among all the bamboo leaves examined. Furthermore, compounds 1, 2, and 3 were simultaneously detected in the 15 leaves of bamboo species, especially in PML. Therefore, compounds 1, 2, and 3 can be considered as the primary phenols in the tested bamboo leaves. Overall, this study highlights the significant values of the use of PML in food, pharmaceutical, and cosmetic industries, and offers a novel approach to the resource utilization of compounds 1, 2 and 3, which is attributable to the abundant and significant phenols content and economy of PML.
Author Contributions
Funding acquisition, Supervision, Project administration, Validation, X.G. and X.Y.; Investigation, Methodology, Formal Analysis, Writing—original draft, J.H.; Software, Visualization, J.W. (Jianjun Wang); Resources, J.W. (Jin Wang) and D.Z.; Writing—review, W.S. and H.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National key research and development program project [2022YFD2200905 to Xue-Feng Guo].
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Iroegbu, A.; Ray, S. Bamboos: From Bioresource to Sustainable Materials and Chemicals. Sustainability 2021, 13, 12200. [Google Scholar] [CrossRef]
- Basak, M.; Dutta, S.; Biswas, S.; Chakraborty, S.; Sarkar, A.; Rahaman, T.; Dey, S.; Biswas, P.; Das, M. Genomic Insights into Growth and Development of Bamboos: What Have We Learnt and What More to Discover? Trees 2021, 35, 1771–1791. [Google Scholar] [CrossRef]
- Andressa Almeida Farias, C.; Rodrigues dos Reis, A.; Rodrigues de Morais, D.; Alves Camponogara, J.; Bettio, L.; Albieri Pudenzi, M.; Augusto Ballus, C.; Teixeira Barcia, M. Phenolic Diversity and Antioxidant Potential of Different Varieties of Bamboo Leaves Using LC-ESI-QTOF-MS/MS and LC-ESI-QqQ-MS/MS. Food Res. Int. 2024, 179, 114025. [Google Scholar] [CrossRef] [PubMed]
- Magdalena, P.A.; Florin, I.; Loredana, S.; Carmen, C.; Florin, F.; Ionut, G.O.; Iren, S.A. Comprehensive and Critical View on the Anti-Inflammatory and Immunomodulatory Role of Natural Phenolic Antioxidants. Eur. J. Med. Chem. 2024, 265, 116075. [Google Scholar]
- Xue, J.; Guo, X.; Xu, G.; Chen, X.; Jiao, L.; Tang, X. Discovery, Identification, and Mode of Action of Phenolics from Marine-Derived Fungus Aspergillus Ustus as Antibacterial Wilt Agents. J. Agric. Food Chem. 2024, 72, 2989–2996. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, L.; El-Ansari, M.; Sharaf, M. Natural Phenolics: A Source of Anticancer Agents. Egypt. Pharm. J. 2019, 18, 1. [Google Scholar] [CrossRef]
- Loaiza-Cano, V.; Monsalve-Escudero, L.M.; Filho, C.d.S.M.B.; Martinez-Gutierrez, M.; de Sousa, D.P. Antiviral Role of Phenolic Compounds against Dengue Virus: A Review. Biomolecules 2020, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Ren, T.; Li, H.; Lu, Q.; Di, X. Antioxidant Activity and Mechanism Exploration for Microwave-Assisted Extraction of Flavonoids from Scutellariae Radix Using Natural Deep Eutectic Solvent. Microchem. J. 2024, 200, 110300. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Sahana, G.R.; Nagella, P.; Joseph, B.V.; Alessa, F.M.; Al-Mssallem, M.Q. Flavonoids as Potential Anti-Inflammatory Molecules: A Review. Molecules 2022, 27, 2901. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, M.; Huo, X.; Xu, Q.; Wu, L.; Wang, L. Separation of Flavonoids and Purification of Chlorogenic Acid from Bamboo Leaves Extraction Residues by Combination of Macroporous Resin and High-Speed Counter-Current Chromatography. Molecules 2023, 28, 4443. [Google Scholar] [CrossRef]
- Li, H.; Tan, X.; Huang, W.; Zhu, X.; Yang, X.; Shen, Y.; Yan, R. Enzymatic Acylation of Flavonoids from Bamboo Leaves: Improved Lipophilicity and Antioxidant Activity for Oil-Based Foods. J. Agric. Food Chem. 2023, 71, 4817–4824. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Cheng, J.; Zhao, J.; Shi, R.; He, L.; Li, Q.; Chen, Y. Efficient Purification of Flavonoids from Bamboo Shoot Residues of Phyllostachys edulis by Macroporous Resin and Their Hypoglycemic Activity. Food Chem. X 2022, 16, 100505. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Pan, F.; Yao, L.; Fang, H.; Cheng, Y.; Zhang, Z.; Chen, Y.; Zhang, A. Isolation, Characterization of Bamboo Leaf Flavonoids by Size Exclusion Chromatography and Their Antioxidant Properties. Chem. Biodivers. 2022, 19, e202200506. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Shi, X.; Liu, J.; Jiang, Y.; Wu, Y.; Xu, Y.; Yang, C. The Effects of Bamboo Leaf Flavonoids on Growth Performance, Immunity, Antioxidant Status, and Intestinal Microflora of Chinese Mitten Crabs (Eriocheir sinensis). Anim. Feed Sci. Technol. 2022, 288, 115297. [Google Scholar] [CrossRef]
- Li, X.; Tao, W.; Xun, H.; Yao, X.; Wang, J.; Sun, J.; Yue, Y.; Tang, F. Simultaneous Determination of Flavonoids from Bamboo Leaf Extracts Using Liquid Chromatography-Tandem Mass Spectrometry. Rev. Bras. Farmacogn. 2021, 31, 347–352. [Google Scholar] [CrossRef]
- Fei, Z.; Xie, D.; Wang, M.; Zhang, Y.; Zhang, H.; Du, Q.; Jin, P. Enhanced Biotransformation of Bioactive Components and Volatile Compounds of Bamboo (Phyllostachys glauca McClure) Leaf Juice Fermented by Probiotic Streptococcus thermophiles. Lebenson. Wiss. Technol. 2023, 173, 114363. [Google Scholar] [CrossRef]
- Guo, X.-F.; Yue, Y.-D.; Tang, F.; Wang, J.; Yao, X.; Sun, J. Simultaneous Determination of Seven Flavonoids in Dan Bamboo Phyllostachys glauca McClure Leaf Extract and in Commercial Products by HPLC-DAD: A New Determination Method of Flavonoids. J. Food Biochem. 2013, 37, 748–757. [Google Scholar] [CrossRef]
- Mattonai, M.; Massai, P.; Ribechini, E. Sustainable Microwave-Assisted Eutectic Solvent Extraction of Polyphenols from Vine Pruning Residues. Microchem. J. 2024, 197, 109816. [Google Scholar] [CrossRef]
- Jiang, L.; Belwal, T.; Huang, H.; Ge, Z.; Limwachiranon, J.; Zhao, Y.; Li, L.; Ren, G.; Luo, Z. Extraction and Characterization of Phenolic Compounds from Bamboo Shoot Shell under Optimized Ultrasonic-Assisted Conditions: A Potential Source of Nutraceutical Compounds. Food Bioproc. Technol. 2019, 12, 1741–1755. [Google Scholar] [CrossRef]
- Buelvas-Puello, L.M.; Franco-Arnedo, G.; Martínez-Correa, H.A.; Ballesteros-Vivas, D.; Sánchez-Camargo, A.D.P.; Miranda-Lasprilla, D.; Narváez-Cuenca, C.-E.; Parada-Alfonso, F. Supercritical Fluid Extraction of Phenolic Compounds from Mango (Mangifera indica L.) Seed Kernels and Their Application as an Antioxidant in an Edible Oil. Molecules 2021, 26, 7516. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.-T.; Wang, C.; Guo, M.F.; Aprà, E.; Ma, R.; Ragauskas, A.J.; Zhang, X. Lignin with Controlled Structural Properties by N-Heterocycle-Based Deep Eutectic Solvent Extraction. Proc. Natl. Acad. Sci. USA 2023, 120, e2307323120. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Munro, H.L.; Rasheed, R.K.; Tambyrajah, V. Preparation of Novel, Moisture-Stable, Lew-Is-Acidic Ionic Liquids Containing Quaternary Ammonium Salts with Functional Side Chains. Chem. Commun. 2001, 19, 2010–2011. [Google Scholar] [CrossRef] [PubMed]
- Morrison, H.G.; Sun, C.C.; Neervannan, S. Characterization of Thermal Behavior of Deep Eutectic Solvents and Their Potential as Drug Solubilization Vehicles. Int. J. Pharm. 2009, 378, 136–139. [Google Scholar] [CrossRef] [PubMed]
- Omar, K.A.; Sadeghi, R. Database of Deep Eutectic Solvents and Their Physical Properties: A Review. J. Mol. Liq. 2023, 384, 121899. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Tang, F. Optimization of Extraction Process of Flavone C-glycosides from Bamboo Leaves by Deep Eutectic Solvent-Ball Mill Method. Chem. Ind. For. Prod. 2023, 43, 60–66. [Google Scholar] [CrossRef]
- An, R.; Yuan, T.; Guo, X. Quantification and Antioxidant Activity of Flavonoids in Bamboo Leaves Extract of Bambusa. Chem. Ind. For. Prod. 2023, 43, 97–103. [Google Scholar] [CrossRef]
- Kim, M.; Choi, S.-Y.; Lee, P.; Hur, J. Neochlorogenic Acid Inhibits Lipopolysaccharide-Induced Activation and pro-Inflammatory Responses in BV2 Microglial Cells. Neurochem. Res. 2015, 40, 1792–1798. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Wang, L.-L.; Xue, N.-N.; Li, C.; Guo, H.-H.; Ren, T.-K.; Zhan, Y.; Li, W.-B.; Zhang, J.; Chen, X.-G.; et al. Chlorogenic Acid Effectively Treats Cancers through Induction of Cancer Cell Differentiation. Theranostics 2019, 9, 6745–6763. [Google Scholar] [CrossRef] [PubMed]
- Ganzon, J.G.; Chen, L.-G.; Wang, C.-C. 4-O-Caffeoylquinic Acid as an Antioxidant Marker for Mulberry Leaves Rich in Phenolic Compounds. J. Food Drug Anal. 2018, 26, 985–993. [Google Scholar] [CrossRef]
- Anilkumar, K.; Reddy, G.V.; Azad, R.; Yarla, N.S.; Dharmapuri, G.; Srivastava, A.; Kamal, M.A.; Pallu, R. Evaluation of Anti-Inflammatory Properties of Isoorientin Isolated from Tubers of Pueraria tuberosa. Oxidative Med. Cell. Longev. 2017, 2017, 5498054. [Google Scholar] [CrossRef]
- Dhakal, H.; Lee, S.; Choi, J.K.; Kwon, T.K.; Khang, D.; Kim, S.-H. Inhibitory Effects of Orientin in Mast Cell-Mediated Allergic Inflammation. Pharmacol. Rep. 2020, 72, 1002–1010. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, M.; Wang, Q.; Du, H.; Zhang, L. Deep Eutectic Solvent-Based Microwave-Assisted Method for Extraction of Hydrophilic and Hydrophobic Components from Radix Salviae miltiorrhizae. Molecules 2016, 21, 1383. [Google Scholar] [CrossRef] [PubMed]
- García, G.; Aparicio, S.; Ullah, R.; Atilhan, M. Deep Eutectic Solvents: Physicochemical Properties and Gas Separation Applications. Energy Fuels 2015, 29, 2616–2644. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Ou, H.; Gregersen, H.; Zuo, J. Deep Eutectic Solvent-Based Ultrasound-Assisted Extraction of Polyphenols from Cosmos sulphureus. J. Appl. Res. Med. Aromat. Plants 2023, 32, 100444. [Google Scholar] [CrossRef]
- Xie, W.; Yu, Y.; Hou, M.; Zhang, Y.; Yu, H.; Zhang, H.; Zhang, G.; Jing, H.; Chen, A. Simultaneous Separation and Determination of Five Chlorogenic Acid Isomers in Honeysuckle by Capillary Electrophoresis Using Self-synthesized Ionic Liquid [N-Methylimidazole-β-cyclodextrin] [Bromide] as Separation Selector. J. Sep. Sci. 2022, 45, 3197–3207. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef] [PubMed]
- Scott, K.A.; Cox, P.B.; Njardarson, J.T. Phenols in Pharmaceuticals: Analysis of a Recurring Motif. J. Med. Chem. 2022, 65, 7044–7072. [Google Scholar] [CrossRef]
- de Lima Cherubim, D.J.; Buzanello Martins, C.V.; Oliveira Fariña, L.; da Silva de Lucca, R.A. Polyphenols as Natural Antioxidants in Cosmetics Applications. J. Cosmet. Dermatol. 2020, 19, 33–37. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).