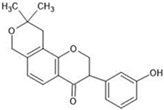
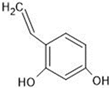
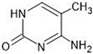
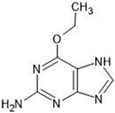
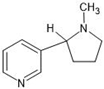
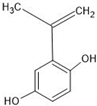
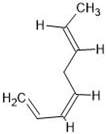
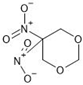
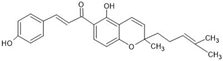
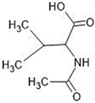
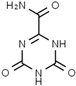
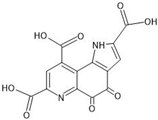
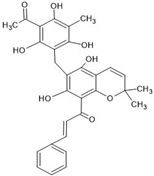
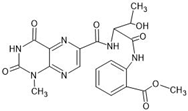
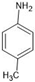
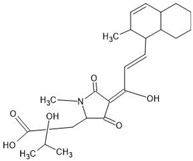
Abstract
Plants serve as reservoirs of bioactive compounds endowed by nature, rendering them promising subjects for investigating chemical diversity. Despite their potential, much remains untapped, whether in standardized extracts or isolated pure compounds. This unexplored terrain has paved the way for significant discoveries in pharmaceuticals. Notably, research has delved into the medicinal properties of Mallotus philippensis, a prominent plant in South Asia. Employing meticulous extraction techniques such as maceration, the fruit of this plant underwent initial antimicrobial screening, revealing encouraging results. Subsequent fractionation of the plant’s extracts via liquid–liquid extractions, utilizing dichloromethane and absolute ethanol, facilitated further analysis. Evaluating these fractions for antibacterial activity demonstrated efficacy against various pathogenic microorganisms, particularly Pseudomonas aeruginosa and Escherichia coli, notably by the ethanolic and dichloromethane extracts. Furthermore, a comprehensive phytochemical analysis unveiled the presence of alkaloids, flavonoids, saponins, glycosides, phenols, and tannins. An assessment of the extracts’ antioxidant potential via the 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging assay showcased significant activity, with a radical scavenging rate of 97%. This underscores the significance of utilizing fruit remnants, which are often rich in valuable chemical constituents yet commonly discarded, thereby adding value to both the species and the environment. Further investigation focused on the composition of Mallotus philippensis fruit, encompassing volatile and non-volatile metabolites through HPLC-MS analysis. Additionally, this study introduced the application of ionic liquid-loaded polysulfone microcapsules to enrich target constituents from crude extracts. An exploration of the key separation conditions, results, and recycling performance of these microcapsules provided insights for future research endeavors. Overall, this comprehensive study of Mallotus philippensis fruit extracts establishes a foundation for the ongoing exploration and development of this medicinal plant.
1. Introduction
Medicinal plants are reservoirs rich in bioactive compounds that are highly regarded for their therapeutic potential in natural environments. The extraction and characterization of these valuable constituents from medicinal flora are integral to advancing innovative healthcare products. These compounds are renowned for their substantial therapeutic efficacy and ability to address various medical conditions [1]. Throughout human history, traditional therapeutics have tapped into a vast wealth of natural constituents, spanning terrestrial flora, animal byproducts, marine organisms, and microbial fermentation derivatives. This enduring reliance on natural remedies, with their proven therapeutic efficacy, has spurred the meticulous extraction of bioactive compounds from traditional medicinal botanicals. As a result, natural products play a crucial role, serving as primary reservoirs in the early stages of pharmaceutical exploration within our modern medical paradigms [2]. Mallotus philippensis belongs to the Euphorbiaceae family, a taxonomic group characterized by its diverse genus hosting a plethora of plant species indigenous to tropical and sub-tropical locales, spanning arboreal and shrub varieties across the globe [3]. Often referred to as Kamala, Kampillaka, or Shendri, Mallotus philippinensis is a perennial shrub or small tree typically endemic to the outer Himalayas, flourishing at elevations up to 1500 m. A distinctive feature of this plant is its fruit, which bears glandular hairs meticulously harvested and processed into reddish-brown powders. These powders are obtained through the manual agitation and rubbing of the fruits, with the resultant residue collected on the fabric. Traditionally, Kamala has been utilized as a natural dye for coloring silk. Furthermore, the powders derived from this botanical specimen are believed to harbor a spectrum of medicinal properties. Within the domain of Ayurvedic medicine, Kamala finds application in alleviating a myriad of symptoms, including cough, constipation, wounds, and ulcers. Moreover, it is administered topically to address various dermatological afflictions such as sores, dermatoses, and parasitic infestations. In the Indian subcontinent, the powders derived from the leaves and bark are commonly employed as a poultice for treating skin disorders, with approximately 20 recognized species exhibiting medicinal uses [4]. Table S1 of the Supplementary Information (S.I.) comprehensively describes Mallotus philippinensis. Kamala, characterized by its crimson-hued powders composed of glandular hairs from the fruit capsule, is commonly utilized for its anthelmintic and cathartic properties, and various other pharmacological applications [5]. The plant holds many steroids, diterpenoids, triterpenoids, flavonoids, phenols, proteins, saponins, alkaloids, and carbohydrates [6]. Medicinal plants are an exceptional resource for acquiring antimicrobial medications [7]. Hence, conducting further study on these plants is imperative to understand better their properties, safety, and effectiveness [8]. According to Ayurvedic principles, leaves exhibit bitterness, offer cooling properties, and serve as appetizers. Diverse botanical components, such as glands and hairs found in capsules or fruits, are harnessed for their warming, purgative, anthelmintic, vulnerary, cleansing, ripening, carminative, and alexiteric attributes. These constituents have effectively addressed bronchitis, abdominal disorders, and splenomegaly. When consumed with milk or yogurt, they can notably aid in the expulsion of tapeworms [9]. Alternatively called Kampillakah, Kamala is commonly used as an orally administered medicinal substance. This botanical specimen has a longstanding application history due to its anthelmintic and purgative properties [10,11]. In the northern regions of Thailand, the fruits and bark have assumed multifaceted roles in traditional medicine and as a reservoir of natural dye. Researchers have extracted numerous bioactive compounds from these fruits, unveiling a spectrum of pharmacological effects, including but not limited to antiallergic, anti-inflammatory, antifungal, and antibiotic properties [12]. Moreover, the powders and specific constituents extracted from Kamala are utilized as supplementary agents in external therapeutic interventions designed to facilitate the healing of ulcers and wounds. These components specifically address dermatological ailments triggered by parasites, encompassing conditions like scabies, ringworms, and herpes. In India, formulations derived from Kamala leaves and bark are frequently employed as poultices for managing skin disorders [13,14]. This research entails a comprehensive phytochemical investigation targeting alkaloids, flavonoids, saponins, glycosides, phenols, and tannins in the fruit extract of the medicinal plant Mallotus philippinensis. Furthermore, it reports findings on the fruit extract’s potential antibacterial properties. Additionally, the DPPH method, a commonly employed technique for such assessments, was utilized to gauge the antioxidant capacity of ethanolic extracts derived from the fruit of M. philippinensis [15,16]. After a comprehensive assessment of the notable selectivity and separation efficacy exhibited by ionic liquids (ILs), they were utilized to augment the concentration of targeted components from the crude extract, facilitating their subsequent utilization in associated domains.
2. Materials and Methods
2.1. Chemicals and Reagents
Ethanol (99%), methanol (99%), and dichloromethane (99%) were provided by Alad-din Company (Shanghai, China) and used for extraction. Tryptone, soy peptone, sodium chloride, and agar were supplied by Aladdin Company (Shanghai, China), and ultra-pure water was used for bacterial culture media preparations. Gentamicin (100 mg/mL) (MA0322), ampicillin (100 mg/mL) (MA0317), and ofloxacin (50 mg/mL) (82419-36-1) were bought from Meilun Biotechnology Co., Ltd. (Dalian China) and used as antibiotics. E. coli (J0053DX), S. aureus (230508S01), and P. aeruginosa (230213S01) bacteria were acquired from Guangdong Huankai Microbial Sci & Tech., Co., Ltd. (Guangzhou, China). Hydrochloric acid magnesium ribbon, sodium hydroxide, chloroform, sulfuric acid, acid anhydrides, pyridine, sodium nitroprusside, dinitro benzoic acid, ferric chloride, sodium nitrite, phthalic anhydride, and lead acetate were also bought from Meilun Biotechnology Co., Ltd. (Dalian China); 1,1-Diphenyl-2-picrylhydrazyl free radical (DPPH-D273092, 97%) was obtained from Aladdin Company (Shanghai, China). Experimental ultrapure water was made by the UPH-I-10T series ultrapure water producer, which was provided by ULUPURE Technology Co., Ltd. (Chengdu, China). Ionic liquids were all directly provided by Aladdin Chemicals Inc. (Shanghai, China). The LC-MS 8040 Series instrument was supplied by Shimadzu (Kyoto, Japan).
2.2. Plant Collection and Identification
The research utilized the raw material derived from M. philippensis, sourced from the fruit of the plant under study. Fresh specimens of M. philippensis were procured from elevated terrain in Palo Dheri, Rustam, District Mardan, Khyber Pakhtunkhwa, Pakistan. Plant samples were collected during the flowering period spanning March and April 2023.
The medicinal plant M. philippensis sample resource, a spurge family member, was authenticated by Professor Yanfang Li from the Department of Pharmaceutical and Biological Engineering at Sichuan University. Authentication was conducted through meticulous comparison with existing literature surveys.
2.3. Plant Materials
Following the separation of fruits from the plant, the raw material underwent a meticulous washing process before being finely ground into small fragments. Subsequently, the fragmented fruits were carefully subjected to shade drying for 20–30 days. This process ensures protection from external contaminants and dust by minimizing exposure to light, thereby maintaining the purity of the raw material. The dried raw material was then finely pulverized into powder form (60 mesh) utilizing a stainless-steel mini laboratory mill grinder. Finally, the resultant fine powders were stored in small polyethylene laboratory bags at ambient temperature.
2.4. Extraction (Maceration)
By the widely employed maceration technique [17], 20 gm of desiccated and pulverized fruit materials were enclosed within a sealed reagent bottle constructed from Pyrex glass. Subsequently, 200 mL of absolute ethanol was introduced utilizing a graduated cylinder. The reagent bottle was covered with aluminum foil and kept for up to 2–3 weeks at room temperature, and frequent shaking was performed daily to release plant-soluble phytoconstituents. The extract acquired via wetting was filtered through a standard What-man filter paper to collect concentrated ethanolic extract and evaporated solvents at 40 °C using a laboratory rotary evaporator. Furthermore, 20 g of air-dried powders of fruit M. philippensis were kept in a conical flask, and 200 dichloromethane was added. The conical flask was covered with the help of aluminum foil and transparent cotton tape, and the conical flask was kept for 2 weeks at room temperature with continuous shaking (300 rpm). The plant fruit powders release inorganic soluble phytochemicals in a conical flask. These extracts were obtained by filtration using Whatman filter paper and subsequent solvent removal using a rotary evaporator under vacuum.
2.5. Investigation on Bioactivities for the Samples
2.5.1. Antibacterial Assay
The McFarland standard was used to prepare for the suspension of microorganisms [17]. In analyzing antibacterial sensitivity testing, 3 g tryptone, 1 g soy peptone, 1 g sodium chloride, 3 g agar, and 200 mL UP water were used in bacterial media preparation. The solution was mixed thoroughly and boiled to dissolved agar powders to obtain a gelatinous solution. Then, the bacterial media was autoclaved at 121 °C temperature for 15 min. The press was allowed to cool at room temperature and then poured into the sterile Petri dishes, and the Petri dishes were left for 1 h to solidify. The bacteria were spread in each Petri dish with the help of cotton swabs that covered the whole media without leaving any gaps. Four sterile filter paper discs were placed in each Petri plate, separated from each other by a 3 cm distance. Then, 0.01 mm (10 µL) fraction was loaded in the first discs; antibiotics ofloxacin, gentamicin, and ampicillin were loaded in the second, third, and fourth discs. After that, all the Petri plates were stored in an incubator at 37 °C for 24 h.
2.5.2. DPPH Radical Scavenging Assay
This study used ethanolic extracts of M. philippinesis for the antioxidant analysis. It was created to dilute DPPH in methanol. A UV-1800PC spectrophotometer (MAPADA Instruments Co. Ltd., Shanghai, China) was employed to assess the mixture’s absorbance at 517 nm after 24 h of dark incubation at room temperature. The DPPH was diluted in methanol to create the blank [18].
2.6. Phytochemical Analysis for Related Samples
2.6.1. Preliminary Analysis with Various Tests
A total of 2 mL of ethanolic and dichloromethane fruit extracts was added to separate test tubes, and phytochemical tests were conducted to detect bioactive compounds. Moderately adjusted from the reported methods, all the experiments and related details in preliminary analysis for bioactive constituents can be found in Table S2 of SI.
2.6.2. Analysis of Alkaloids and Flavonoids Using Thin-Layer Chromatography (TLC)
Here, the thin chromatographic analysis was first employed to examine the ethanolic extracts of M. philippinesis by using 50 × 100 mm silica gel (Sil-G) plates from Ocean Chemical Co., Ltd. (Qingdao, China), with layer thickness from 0.20 to 0.25 mm. As part of the technique, 5 mg of the extracts was weighed out, dissolved in 10 mL of methanol, and then homogenized before applying aliquots to the plates, with a 1 cm gap between each application. The method used Sil-G plates developed with the upper phase of ethyl acetate-water (7:3) for alkaloid identification. Furthermore, a solution of water/n-butanol/acetic acid (4:4:2) was applied as a developing reagent for flavonoid identification. Then, the KH-3000 plus T.L.C. scanner (Kezhe Inc., Shanghai, China) was used.
2.6.3. Liquid Chromatography–Mass Spectroscopy (LC-MS) Assessments
The LC-MS 8040 Series instrument (Shimadzu Kyoto, Japan) was employed to annotate the unknown metabolites in the crude ethanolic and dichloromethane extracts of M. philippensis fruit. The dry material was first dissolved in 10 mg/mL of methanol and di-chloromethane and then filtered through common Whatman filter paper and microporous membrane in sample preparation for analysis. The LC-MS system consisted of a stationary phase, an Agilent Eclipse plus C18 column (2.1 × 150 mm, 3.5 µm). The mobile phase involved a gradient of acetonitrile and 0.1% volume/volume formic acid dissolved in water. The instrument was configured with n = 3 levels of fragmentation and used the Turbo Detection Data Scanning (TDDS) feature to analyze the fragmentation pattern of eluted chemicals. Literature data were analyzed for comparison purposes. The quantification of identified unknown compounds was carried out utilizing linear calibration curves. The examination was conducted three times, and the outcomes were presented as the mean plus standard deviation (S.D.).
3. Results and Discussion
3.1. Antibacterial Activities
Table 1 and Table 2 show the results of the antibacterial activity of two extracts. The mean ± standard deviation indicates the inhibited zone for each fraction and active drug measured. Extracted plant antibiotics are antimicrobial compounds from diverse plants known for their robust antibacterial properties. These natural antibiotics are safe and demonstrate efficacy in combating bacterial infections. They generally entail minimal side effects, rendering them a preferred alternative to synthetic antibiotics [19]. Active phytochemicals, potent compounds abundantly present in plants, exert a significant influence in eliciting biological activities, including their notable antimicrobial efficacy against diverse pathogens. Their pivotal contribution to the continual quest for novel antibiotic drug discovery and development is substantial [16,20,21]. The current study highlights the effectiveness of ethanolic and dichloromethane fruit extracts of M. philippensis as potent antibacterial agents. The ethanolic and dichloromethane extracts of M. philippensis fruit showed significant growth inhibition of the test bacteria. The dichloromethane extract derived from M. philippensis fruit exhibited the highest efficacy at a 10 µL dosage (see Table 1 and Figure 1A and Figure 2B), showing 3.2 ± 0.2 mm, 2.7 ± 0.2 mm, and 2.6 ± 0.2 mm for E. coli., P. aeruginosa, and S. aureus., respectively. The M. philippensis fruit absolute ethanolic extracted fraction also showed obvious inhibition at 10 µL, and the results were 3.2 ± 0.2 mm against P. aeruginosa, with a 2.8 ± 0.2 mm and 2.3 ± 0.2 mm inhibited zone for S. aureus and E. coli, respectively (see Table 2 and Figure 1B and Figure 2A). Absolute ethanolic and dichloromethane extracted fractions showed the highest inhibition zone with P. aeruginosa and E. coli, at 3.2 ± 0.2 mm (shown in Table 2 and Figure 1B and Figure 2A) and 3.2 ± 0.2 mm (see Table 1 and Figure 1A and Figure 2B), as compared with those of the standard antibiotics ofloxacin (2.7 ± 0.2 mm) and gentamicin (3.0 ± 0.2 mm) with significant inhibition activity. In comparison, ampicillin exhibited an inhibition zone diameter of 3.3 ± 0.2 mm (shown in Table 2 and Figure 1B and Figure 2A).

Table 1.
Results of antibacterial activity of dichloromethane extract.

Table 2.
Results of antibacterial activity of absolute ethanolic extract.
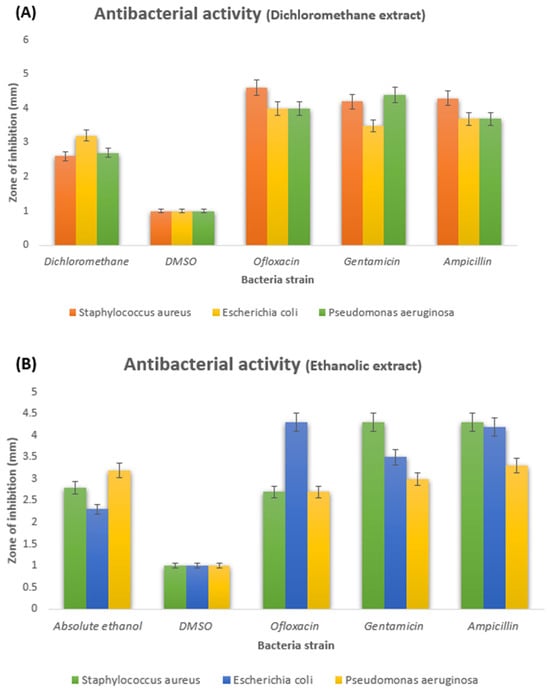
Figure 1.
(A) Inhibited zone diameters of dichloromethane fruit extract and (B) absolute ethanolic fruit extract of M. philippensis against bacterial strain.
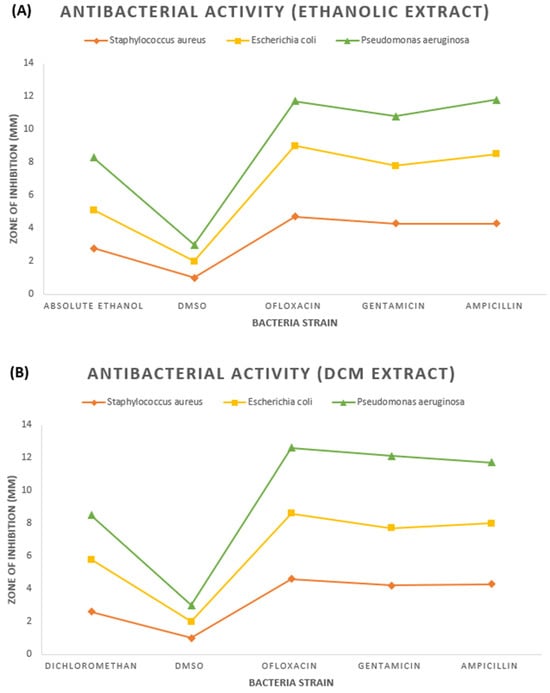
Figure 2.
(A) Phase diagram of inhibited zone shown by ethanolic fruit extract and (B) dichloromethane fruit extract of M. philippensis against bacterial strain.
Plants possess a rich repository of medicinal compounds, including many bioactive substances, which have captured substantial interest from researchers and herbal practitioners alike. Terpenoids, steroids, saponins, tannins, and flavonoids stand out as pivotal constituents in the pharmacological arsenal of the plant realm [22]. A comprehensive investigation has scrutinized the antimicrobial potency of extracts derived from Mollotus philippensis fruits. These extracts are enriched with a spectrum of bioactive compounds intrinsic to the plant, comprising flavonoids, tannins, and phenolic compounds. Each of these constituents exhibits remarkable antibacterial efficacy against prevalent pathogens such as Escherichia coli, Staphylococcus aureus, and Pseudomonas aeruginosa [23].
3.2. Antioxidant Activity by DPPH Free Radical Scavenging Assay
The advocated method [24] assessed the plant extracts’ antioxidant capacity against DPPH. A methanolic solution of DPPH (250 mg) at a concentration of 4 M was prepared. Afterward, 1 mL portions were extracted from each sample within the methanolic extract. Samples were extracted at 2, 3, 4, and 5 mg/mL concentrations, respectively (see Table 3). Four replicates were generated for each sample at every concentration, with 3 mL of the methanolic DPPH dilution subsequently introduced into each aliquot. The findings regarding milligrams of quercetin per milligram of dry weight equivalence are presented. The calibration curve was generated using the subsequent quercetin concentrations: 0.002, 0.003, 0.004, and 0.005 mg/mL (see Table 3). The following formula was employed to calculate the percentage of radical scavenging assay (% R.S.A.) as Equation (1).

Table 3.
Antioxidant activity by DPPH of fruit methanolic extract of M. philippensis.
The antioxidant activity of the methanolic extract of M. philippensis fruit was investigated using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging assay. Prior studies in the literature have yet to be conducted on the antioxidant activities. As presented in Table 3, the methanolic extract showed significant activity of IC50: 19.39 µg/mL and 10.6 µg/mL at 50 and 20 µg/mL concentrations.
3.3. Preliminary Phytochemicals Analysis
Concurrently modulating numerous cellular processes within plants, they substantively influence a plant’s distinctive hue, aroma, and taste. Moreover, an expanding corpus of scholarly investigation underscores the indispensable medicinal advantages offered by phytochemicals, often accompanied by minimal adverse effects. The delineation and assessment of phytochemicals have become pivotal prerequisites in developing plant-derived pharmaceuticals. These bioactive compounds are initially extracted from plant matter, subsequently identified, and quantified through established methodologies adhering to standardized protocols for phytochemical analysis [25]. Examining the comprehensive outcomes from phytochemical analysis for the two extracts of fruit M. philippinensis (Table 4 and Figure 3), the ethanolic extract revealed significant levels of alkaloids, flavonoids, steroids, saponin, and phenols. At the same time, the D.C.M extract specifically exhibited elevated glycoside content.

Table 4.
Results of phytochemical screening of M. philippinensis fruit.
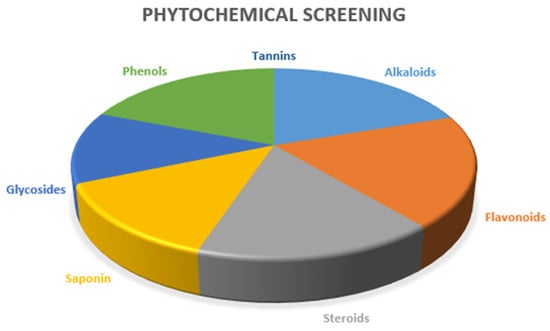
Figure 3.
Pie diagram of phytochemical screening of two extracts of M. philippinensis fruit.
In alkaloid analysis, the extracts from M. philippensis fruits in ethanol and dichloromethane showed distinct color changes with Mayer’s, Wagner’s, and Hager’s reagents, confirming alkaloid presence (as depicted in Table 4 and Figure 3). As diverse nitrogen-containing compounds, alkaloids are crucial for a plant’s defense and make up approximately 60% of plant-based drugs, with notable pharmacological effects [26]. They are commonly found in various plant families, including Amaryllidaceae, Apocynaceae, Papaveraceae, Asteraceae, Solanaceae, Rutaceae, Fabaceae, and Rubiaceae [27]. Plant alkaloids constitute a robust treatment modality for chronic afflictions such as cancer, diabetes, and neurological disorders. Originally evolved as plant defenses, these compounds demonstrate remarkable efficacy in combating infections, exemplifying many therapeutic advantages that transcend conventional medicinal applications [28,29]. Plant alkaloids also curb inflammation by blocking vital proinflammatory protein complexes in relevant signaling pathways [30,31]. Alkaloids show potential in treating neurodevelopmental disorders by inhibiting M.A.O., acetylcholinesterase, and butyrylcholinesterase. They also act as NMDA receptor antagonists and muscarinic and adenosine receptor agonists [32].
Other phytochemicals recognized for their biological and pharmacological activities include a group of compounds known as flavonoids (shown in Table 4 and Figure 3). The copious and biologically active compounds have incited comprehensive investigation, elucidating various characteristics, including anticancer, anti-inflammatory, antioxidant, antimutagenic, antithrombotic, antiviral, antibacterial, and vasodilator effects [33,34]. As plant-made pigments, flavonoids can shield against U.V. exposure [35,36]. A red ring formed after adding concentrated sulfuric acid to the M. philippensis fruit extracts con-firmed the presence of steroids (Table 4 and Figure 3). Medicinal plants and herbs offer potential for new therapies and inspire the creation of synthetic drugs [37]. They rely on analyzing two crucial groups of isoprenoid compounds: steroids (including phytosterols) and triterpenoids. Despite being present in low concentrations, these compounds are essential for biological activity and pharmacological properties. They often work alone or in synergy with other bioactive compounds, such as polyphenols, to amplify their effects [38,39]. Phytosterols reduce blood lipid and cholesterol levels, including harmful LDL-C [40]. They show clinical promise in preventing cardiovascular diseases, fatty liver, inflammation, rheumatoid arthritis, and obesity-related illnesses while improving insulin resistance and lipid metabolism. Likewise, triterpenoids offer diverse bioactive properties due to their varied structures [41,42,43]. Such constituents provide numerous benefits, such as anti-inflammatory, antimicrobial, antiviral, hepato-protective, antidiabetic, and anticarcinogenic effects. Their wide array of bioactivities makes them indispensable in pharmaceutical and industrial applications. They also gained attention as potential weapons against multidrug-resistant microbes and fungi [44,45,46,47,48].
Besides that, saponins are important bioactive secondary metabolites with bubbling behavior [49,50]. Existing in more than 500 plant species, saponins are amphiphilic glycosides. They comprise hydrophilic glycones (sugar units) linked to hydrophobic aglycones (steroids or terpenoids) [51,52,53,54,55]. Saponins form micelles and reduce surface tension when dissolved in a solvent. Thus, they are considered naturally occurring surface-active compounds that easily blend into ecological systems [56,57].
On the other hand, they are known to exhibit hemolytic potential [58,59]. They also demonstrate antimicrobial effects against specific bacteria and viruses affecting mammals. These properties, determined by the aglycone structure and sugar unit count, make saponins a key ingredient in various preparations [60,61,62].
Cardiac glycosides were detected as green, and concentrated sulfuric acid was added at the end using the Keller–Kiliani test. Medicinal plants contain diverse natural glycosides, which serve as valuable reservoirs for therapeutic agents characterized by reduced toxicity and fewer side effects. C-glycosides and their derivatives constitute a unique class of carbohydrate patterns prevalent in many natural compounds as potential bioactive pharmaceuticals and specialized chemicals [63,64,65,66,67,68,69,70,71]. Extracting and refining glycosides from medicinal plants is crucial for pharmacological research and developing new drugs [72,73,74]. Finally, central tests, including litmus, ferric chloride, Liebermann’s, and phthalein dye tests, were conducted to identify phenols in fruit extracts of M. philippensis. These tests confirmed the presence of phenols in the sample. Phenol and its derivatives are of interest due to their prevalence in surface water as primary components of humic substances. Plants produce various phenolic compounds, which are also valuable for the pharmaceutical or dye industry [75,76,77]. Tannins were detected in the sample post ferric chloride, gelatinous solution, and lead acetate tests. They comprise diverse chemical compositions [78]. As a high-molecular-weight phenolic in plants, tannins vary from 500 to over 20,000 Da. There are over 8000 variations of tannins, which can be found both free and bound within plant cells [79].
3.4. Thin-Layer Chromatography (TLC) for Alkaloids and Flavonoids Analysis
Following the studies, thin-layer chromatography (T.L.C.) was used in the phytochemical research of ethanolic extracts of M. philippinensis based on preliminary tests; alkaloids and flavonoids were found in the fruit extract. When compared to the applicable standards, the samples’ intensity and color indicated their presence (see Figure 4). The outcomes of the alkaloids and flavonoid determination assays validated the T.L.C. results [80]. The following formula calculated the Rf value of flavonoids and alkaloids as Equation (2).
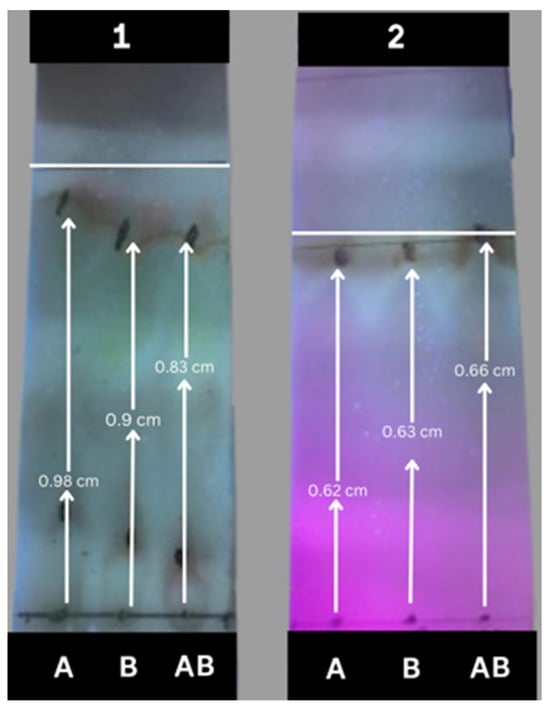
Figure 4.
Thin-layer chromatography (TLC) for absolute ethanolic extract (A: 50 wt.%, B: 70 wt.%, AB: 100 wt.%) of M. philippinesis developed with (1) the upper phase of ethyl acetate/water (7:3) and (2) acetone/ethanol/water (4:2:4).
After completing the direct observation mentioned above, the spots of alkaloids and flavonoids were assigned and located on the thin-layer plate using Mayer’s reagent and alkaline reagent in Table S2. After labeling these relevant spots, thin-layer scanning was performed with a TLC scanner and the contents of the two kinds of components were determined by the percentage of their integrated area of the corresponding spots to the total area. The related results are shown in Figure 5, indicating that the content of alkaloids was higher than that of flavonoids in the ethanolic extract.
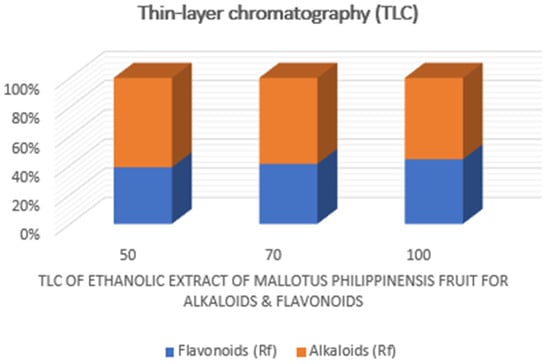
Figure 5.
Chemical analysis on ethanolic extracts derived from the fruit of M. philippinensis based on TLC, scanning.
3.5. High Performance Liquid Chromatography–Mass Spectrometry (HPLC-MS) Profiling
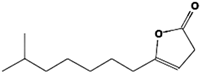
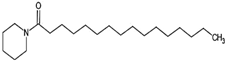
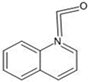
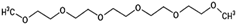
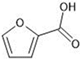
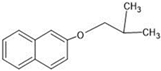
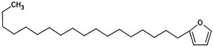
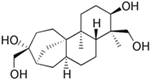
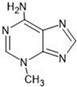
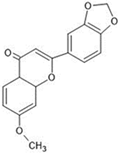
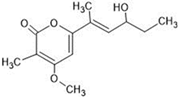
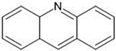
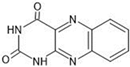
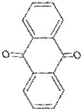
The annotation of secondary compounds in M. philippensis fruit absolute ethanol and dichloromethane crude extracts was conducted using the HPLC-MS technique. In total, compounds agreed from the ethanolic extract and D.C.M. extracts. First, it should be noted that due to limited data in our LC-MS molecular library or the influence of testing conditions, some compounds belonging to the previously discovered structural types were not detected, which is also a predictable situation. Numerous studies in the literature focused on qualitative analysis using LC-MS/MS for its powerful performance [81,82]. Therefore, a precise L.C.–MS/MS method was developed to detect 26 compounds in the ethanol extract (Table 5 and Figure 6) and 15 compounds in the D.C.M. extract (Table 6 and Figure 6) derived from M. philippinensis. In this study, the ionization mode was employed to analyze the compounds. Upon examining the comprehensive results of the LC-MS/MS analysis, it is evident that phenolic and non-phenolic compounds are abundant in the ethanolic and D.C.M. extracts of M. philippinensis fruit (Table 5 and Table 6, and Figure 6). Notably, considerable variations were noted in the flavonoid levels of M. philippinensis fruit [83]. Furthermore, several compounds, such as 2-furoic acid, 2-octadecyl furan, 3-methyladenine, 4-methoxy acetanilide, 4-vinyl resorcinol, all oxazine, acridine, anthraquinone, cortistatin l, diminazens, and nicotine, were detected in the ethanol extract; meanwhile, isoquinoline, spool, ox-on amide, rottlerin, toluidine, and zopfiellamide A were detected in the D.C.M. extract. Furthermore, the ethanolic extract was much richer than the D.C.M. extract [84]. In the literature survey, a few studies regarding the phenolic and flavonoid constituents of M. philippinensis were determined using HPLC and G.C.–M.S. techniques. In a previous study [85], the researchers isolated and further identified different phenolic compounds such as chalcones, phloroglucinol, rottlerin, 4′-hydroxyrottlerin, isorottlerin, 4′-hydroxyisorottlerin, iso-allorottlerin, and mallotophilippen F. These results enrich the findings for valuable compounds from this plant together with our research.

Table 5.
Results of HPLC-MS of tentative annotation of ethanolic extracts from the fruit of M. philippinensis.
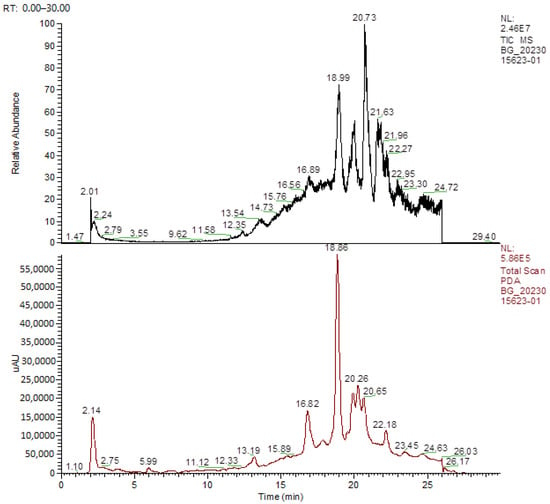
Figure 6.
HPLC-MS profiles of ethanol (upper) and D.C.M (lower) extracts from M. philipiensis fruit.

Table 6.
Results of HPLC-MS of tentative identification of D.C.M extracts from the fruit of M. philipiensis.
3.6. Enrichment of Target Components with Ionic Liquid-Loaded Microcapsules
As the current hot point of green solvents, ionic liquids can be freely designed for different separation tasks and easily tailored by flexible cationic–anion combinations. A series of ILs have been successfully used to extract and separate hundreds of target compounds from natural products [86]. However, their application to extract M. philippinesis has yet to be reported. Based on the above studies, many N-containing compounds have been found in its extract, so the alkaloids were selected as the target constituents for the following enrichment from ethanolic extract by using ionic liquid, which can be separated from those coexisting non-alkaloid compounds and show higher activities for higher purity. In this section, we prepared polysulfone microcapsules according to our reported method [87], which was used as the carrier of potential ILs. Then, the candidate ILs were loaded in them by an ultrasonic wave (100 W) for 3 h, and the mixture was then shaken in the shaker (500 rpm) overnight. Before use, the residual ILs on the surface of the microcapsules were washed with ethanol and further dried. After that, the ionic liquid-loaded microcapsules were added to the aqueous solution of M. philippinesis ethanolic extract, which was stirred (500 rpm) for thorough adsorption. During this process, different ILs ([Amim][Br], [Bmim][Br], [Bmim][PF6], [Bmim][CH3SO3], [Bmim][HSO4]), the solid/liquid ratio (dosage of ionic liquid-loaded microcapsules, mg/mL), the initial concentration (mg/mL) of crude extract, and time (h) were investigated for their effects on the adsorption efficiency (%) of alkaloids. The potential impact of pH and temperature were not explored because we aimed to make the separation operation more convenient and friendly (less acid/base and energy consumption). After the saturation of adsorption was achieved, the number of unabsorbed alkaloids in the supernatant was calculated via its residual concentration. For the quantitative analysis, 3-methyladenine in Table 5 was selected as the standard compound for developing the working curve of total alkaloids, which was detected at 272 nm in water using U.V. spectroscopy [88]. The whole results can be found in Figure 7.
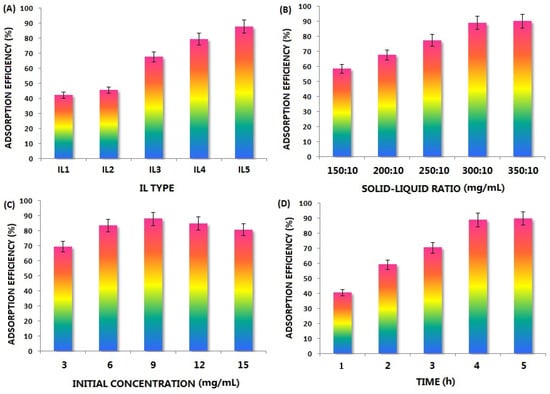
Figure 7.
Effect of (A) IL type (IL1: [Amim][Br], IL2: [Bmim][Br], IL3: [Bmim][PF6], IL4: [Bmim][CH3SO3], IL5: [Bmim][HSO4], (B) solid-liquid ratio: 300:10 mg/mL, (C) initial concentration: 9 mg/mL, 4 h); (B) solid-liquid ratio ([Bmim][HSO4]), initial concentration: 9 mg/mL, (D) Time (h).
As shown in Figure 7A, various loaded ILs exhibited a different performance, and the order of adsorption efficiency was [Bmim][HSO4] > [Bmim][CH3SO3] > [Bmim][PF6] > [Bmim][Br] > [Amim][Br]. When the investigated cations of ILs were changed, the difference was not obvious; simultaneously, the effect of anions was more significant. It can be found that the stronger the anion acidity, the higher the adsorption efficiency. As alkaline compounds, the target constituents will interact with acidic absorbents more easily. Secondly, the solid liquid is another key factor; if the sorbent dosage is insufficient, its enrichment on alkaloids will be inadequate. On the other hand, if the adsorption saturation has been reached, an excessive solid-liquid ratio only results in an unnecessary excess of adsorbent. According to the trend in Figure 7B, it can be found that the adsorption efficiency of alkaloids rises significantly with an increasing dosage of the [Bmim][HSO4]-loaded microcapsule, which should be ascribed to the greater number of adsorption chances available for these target molecules. Furthermore, when the solid/liquid ratio is higher than 35:1, the adsorption efficiency is not improved, which means that the adsorption equilibrium has been reached. Moreover, Figure 7C reflects the effect of the crude extract’s initial concentration (mg/mL) on the adsorption efficiency. When the solid/liquid ratio is constant, overly concentrated extraction solutions are dense and unsuitable for mass transfer. Still, they may also exceed the processing capacity of IL-loaded microcapsules. In contrast, an overly diluted sample solution will reduce the separation efficiency of each enrichment process, so the concentration level in the middle is appropriate. As a result, should be the optimal concentration (mg/mL) of the ethanolic extract aqueous solution. Finally, enough adsorption duration should be ensured, which is important to achieve transfer phenomena in micropores and microchannels. Compared to the adsorption time on commonly used sorbent particles, it will be longer for microcapsules. It can be observed from Figure 7D that the adsorption efficiency becomes higher first and then reaches a stable level with the increase of time after 240 min, indicating that the adsorption equilibrium is achieved at that time. In this situation, surface adsorption is faster, intracapsular diffusion is slower, and the latter determines the whole separation speed. A scientific post-treatment is necessary to recover the enriched alkaloids and reuse the IL and microcapsules after adsorption. For the adsorbed alkaloids, the corresponding microcapsules containing them were first collected with filtration and then placed in an empty glass chromatography column with a plunger. Subsequently, the mixture of methanol/acetone/ethyl acetate (1:1:4, v/v) was added to the system to elute them thoroughly during desorption. After continuous and sufficient contact between the fluent and microcapsules, the alkaloids in the ionic liquid will diffuse from the microcapsules to the outer environment. When the color of the effluent becomes very light, it indicates that the desorption process is over. All the desorbed liquid was collected and combined, and related alkaloids were obtained after solvent removal under vacuum. Under the same conditions in Table 1 and Table 2, the inhibition zone diameter of the enriched alkaloids reached 3.9 ± 0.2 mm, 3.7 ± 0.2 mm, and 3.4 ± 0.2 mm for S. aureus, E. coli, and P. aeruginosa, respectively. These data were more ideal than those of the crude extract and were close to or even exceeded those of the control drugs. Plant age plays an important role in containing phenolic compounds. In mature plants, discernible distinctions exist between young and mature leaves. Young leaves exhibit higher concentrations of ferulic acid and its precursor caffeic acid, purportedly rendering them more resilient to bacterial infections than mature leaves. However, variations in phenolic acid levels induced by bacterial infections are marginal and lack statistical significance in mature plants [89].
4. Conclusions
According to the above research, it has been confirmed that extracts of the plant Mallotus philippinensis have great antimicrobial potential and can be used for microbial infections (bacterial strain). They also show the potential to be used as an antibiotic and traditional medicine. The dichloromethane and absolute ethanolic extracts of the plant M. philippinensis fruit showed obvious antibacterial activities. To comprehensively discover the potential chemical substances as much as possible, a series of test methods and analytical techniques were all employed, including classical reagents, thin-layer chromatography, and liquid chromatography–mass spectrometry. The phytochemical activities of M. philippinensis fruit extracts proved that the plant has good potential for alkaloids, flavonoids, phenols, tannins, steroids, saponins, and glycosides. Therefore, the evaluation of M. philippinensis, a valuable medicinal plant, holds significant importance, as it has the potential to aid in the exploration and development of novel antibiotic drugs for the market. These results showed that M. philippinensis has a high potential for antioxidant and antimicrobial activities; further evaluation is crucial. We also examined the composition of the fruit of M. philipiensis, specifically the volatile and non-volatile secondary metabolites, by LC-MS.
In summary, different methods have their features and limitations. LC-MS has high sensitivity. However, the number of compounds in its commercial database is limited, and many compounds cannot be recognized. Traditional methods have low sensitivity. However, they are easy to operate, and the featured phenomenon of specificity has been widely applied in daily work and has undergone many tests. Combining the two ways can complement each other’s strengths, maximize the advantages, and comprehensively reflect the chemical composition.
Finally, the enrichment of target components with ionic liquid-loaded microcapsules was successfully achieved using the crude extract. This study is the first to report a comprehensive chemical profile of this plant species, as prior studies have only reported a limited amount of chemicals, particularly for non-volatile metabolites. Besides that, it also included the preparation technique of related valuable components with the combination of green solvents, which is expected to provide the reader with more meaningful reference.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/separations11060165/s1, Table S1: Botanical description of M. philippensis; Table S2: The test methods and key details for potential constituents.
Author Contributions
A.A.: methodology, experiments, investigation, writing—original draft. H.C.: validation, writing—review. H.X.: quantitation. S.W.: data analysis. S.Y.: conceptualization, supervision. All authors have read and agreed to the published version of the manuscript.
Funding
The preparation of this paper was supported by the Chengdu Key Research & Development Supporting Project (2022-YF05-00910-SN), and the Sichuan University-Luzhou Science and Technology Innovation Platform Construction Project (2022CDLZ-20).
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
The authors, Ali, A., Chen, H., Xu, H., Wang, S. and Yao, S. would like to present special thanks to Yanping Huang from the Center of Engineering Experimental Teaching, School of Chemical Engineering, Sichuan University for the help of LC-MS analysis.
Conflicts of Interest
The authors affirm that they have no conflicts of interest pertaining to the publication of this paper.
References
- Huie, C.W. A review of modern sample-preparation techniques for the extraction and analysis of medicinal plants. Anal. Bioanal. Chem. 2002, 373, 23–30. [Google Scholar] [CrossRef]
- Shibata, M.; Khan, I.A.; Iinuma, M.; Shirai, T. Natural products for medicine. J. Biomed. Biotechnol. 2012, 2012, 147120. [Google Scholar] [CrossRef]
- Widen, C.J.; Puri, H.S. Natural occurrence and chemical’ variability of phloroglucinols in Kamala. Planta Med. 1980, 40, 284–287. [Google Scholar] [CrossRef]
- Singh, R.; Singhal, K.C. Antifilarial activity of Mallotus philippinensis Lam. on Setaria cervie (Nematoda: Filarioidea) in-vitro. J. Physiol. Pharmacol. 1997, 40, 397–403. [Google Scholar]
- Gangwar, M.; Gautam, M.K.; Ghildiyal, S.; Nath, G.; Goel, R.K. Mallotus philippinensis Muell. Arg fruit glandular hairs extract promotes wound healing on different wound model in rats. BMC Complement. Altern. Med. 2015, 15, 123. [Google Scholar] [CrossRef]
- Bilal, M.; Parveen, A.; Fiaz, A.; Mazhar, M. In vitro phytochemical analysis, antimicrobial and antioxidant activity of Mallotus philippinensis. Inter. J. Nat. Med. Health Sci. 2022, 2, 11–16. [Google Scholar] [CrossRef]
- Rios, J.L.; Recio, M.C. Medicinal plants and antimicrobial activity. J. Ethnopharmaco. 2005, 100, 80–84. [Google Scholar] [CrossRef]
- Nascimento, G.G.F.; Lacatelli, J.; Freitas, P.C.; Silva, G.L. Antibacterial activity of plant extracts and phytochemicals on antibiotic-resistant bacteria. Braz. J. Microbiol. 2000, 3, 886–891. [Google Scholar] [CrossRef]
- Zaidi, S.F.H.; Yamada, K.; Kadowaki, M.; Usmanghani, K.; Sugiyama, T. Bactericidal activity of medicinal plants, employed for the treatment of gastrointestinal ailments, against Helicobacter pylori. J. Ethnopharmacol. 2009, 121, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Hishikar, K. Purgative and anthelmintic effects of Mallotus phllippinensis in rats against tape worm. Indian J. Physiol. Pharmacol. 1984, 28, 63–66. [Google Scholar]
- Kumar, A.; Patil, M.; Kumar, P.; Bhatti, R.C.; Kaur, R.; Sharma, N.K.; Singh, A.N. Mallotus philippensis (Lam.) Müll. Arg.: A review on its pharmacology and phytochemistry. J. Herbmed Pharmacol. 2020, 10, 31–50. [Google Scholar] [CrossRef]
- Cheenpracha, S.; Pyne, S.G.; Patrick, B.O.; Andersen, R.J.; Maneerat, W.; Laphookhieo, S. Mallopenins A–E antibacterial phenolic derivatives from the fruits of Mallotus philippensis. J. Nat. Prod. 2019, 82, 2174–2180. [Google Scholar] [CrossRef]
- Rana, S.; Prakash, V.; Sagar, A. Antibacterial activity of Malotus philippensis. J. Med. Plants Stud. 2016, 4, 104–106. [Google Scholar]
- Daikonya, A.; Katsuki, S.; Wu, J.B.; Kitanaka, S. Anti-allergic agents from natural sources: Anti-allergic activity of new phloroglucinol derivatives from Mallotus philippensis (Euphorbiaceae). Chem. Pharm. Bull. 2002, 50, 1566–1569. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Schaich, K.M.; Tian, X.; Xie, J. Hurdles and pitfalls in measuring antioxidant efficacy: A critical evaluation. J. Funct. Foods 2015, 14, 111–125. [Google Scholar] [CrossRef]
- Hassan, A.; Ullah, H. Antibacterial and antifungal activities of the medicinal plant Veronica biloba. J. Chem. 2019, 2019, 5264943. [Google Scholar] [CrossRef]
- Cuong, D.X.; Xuan, H.N.; Dong, D.H.; Thủy, L.T.M.; Van Thanh, N.; Ha, H.T.; Tuyen, D.T.T.; Chinh, D.X. Tannins: Extraction from plants. In Tannins—Structural Properties, Biological Properties, and Current Knowledge; IntechOpen: London, UK, 2020; Volume 1, 148p. [Google Scholar] [CrossRef]
- Hussain, A.; Khan, M.N.; Iqbal, Z.; Sajid, M.S. An account of the botanical anthelmintics used in traditional veterinary practices in Sahiwal district of Punjab, Pakistan. J. Ethnopharmacol. 2008, 119, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.; Ahmad, I. Antibacterial, antifungal, and insecticidal potentials of Oxalis corniculata and its isolated compounds. Int. J. Anal. Chem. 2015. [Google Scholar] [CrossRef]
- Sharifi-Rad, J. Herbal antibiotics: Moving back into the mainstream as an alternative for “superbugs. Cell. Mol. Biol. 2016, 62, 1–2. [Google Scholar] [PubMed]
- Alghazeer, R.; El-Saltani, H.; Saleh, N.; Al-Najjar, A.; Hebail, F. Antioxidant and antimicrobial properties of five medicinal Libyan plants extracts. Nat. Sci. 2012, 4, 324–335. [Google Scholar] [CrossRef]
- Edziri, H.; Mastouri, M.; Cheraif, I.; Aouni, M. Chemical composition and antibacterial, antifungal and antioxidant activities of the flower oil of Retama raetam (Forssk.) webb from Tunisia. Nat. Prod. Res. 2010, 24, 789–796. [Google Scholar] [CrossRef]
- Wang, M.; Shi, Q.; Shi, H.; Li, J.; Yang, Y.; Li, Q.; Shao, S.; Gao, S. The promotion effect of phenolic acid compound on the photo-removal of estrogen from water under simulated sunlight irradiation. Chem. Eng. J. 2020, 387, 123999. [Google Scholar] [CrossRef]
- Egbuna, C.; Ifemeje, J.; Maduako, M.; Tijjani, H.; Udedi, S.; Nwaka, A.; Ifemeje, M. Phytochemical Test Methods: Qualitative, Quantitative, and Proximate Analysis; Apple Academic Press: Palm Bay, FL, USA, 2019. [Google Scholar]
- Venkategowda, A.; Gowda, L. Isolation, and characterization of antimicrobial alkaloids from Plumeria alba flowers against food borne pathogens. Am. J. Life Sci. 2014, 2, 1–6. [Google Scholar]
- Yang, L.; Stöckigt, J. Trends for diverse production strategies of plant medicinal alkaloids. Nat. Prod. Rep. 2010, 27, 1469–1479. [Google Scholar] [CrossRef]
- Adamski, Z.; Blythe, L.L.; Milella, L.; Bufo, S.A. Biological activities of alkaloids: From toxicology to pharmacology. Toxins 2020, 12, 210. [Google Scholar] [CrossRef]
- Ng, Y.P.; Or, T.C.T.; Ip, N.Y. Plant alkaloids as drug leads for Alzheimer’s disease. Neurochem. Int. 2015, 89, 260–270. [Google Scholar] [CrossRef]
- Yatoo, M.I.; Gopalakrishnan, A.; Saxena, A.; Parray, O.R.; Tufani, N.A.; Chakraborty, S.; Tiwari, R.; Dhama, K.; Iqbal, H.M.N. Anti-inflammatory drugs, and herbs with special emphasis on herbal medicines for countering inflammatory diseases and disorders—A review. Recent. Pat. Infla. 2018, 12, 39–58. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, L.; Di, S.N.; Xu, Q.; Ren, Q.C.; Chen, S.Z.; Huang, N.; Jia, D.; Shen, X.F. Steroidal alkaloid solanine A from Solanum nigrum Linn. exhibits antiinflammatory activity in lipopolysaccharide/interferon γ-activated murine macrophages and animal models of inflammation. Biomed. Pharmacother. 2018, 105, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Hussain, G.; Rasul, A.; Anwar, H.; Aziz, N.; Razzaq, A.; Wei, W.; Ali, M.; Li, J.; Li, X. Role of plant derived alkaloids and their mechanism in neurodegenerative. Int. J. Biol. Sci. 2018, 14, 341–357. [Google Scholar] [CrossRef] [PubMed]
- Tarahovsky, Y.S.; Kim, Y.A.; Yagolnik, E.A.; Muzafarov, E.N. Flavonoid-membrane interactions: Involvement of flavonoid-metal complexes in raft signaling. BBA-Biochim. Biophys. Acta 2014, 1838, 1235–1246. [Google Scholar] [CrossRef]
- Hussain, G.; Zhang, L.; Rasul, A.; Anwar, H.; Sohail, M.U.; Razzaq, A.; Aziz, N.; Shabbir, A.; Ali, M.; Sun, T. Role of plant-derived flavonoids and their mechanism in attenuation of Alzheimer’s and Parkinson’s diseases: An update of recent data. Molecules 2018, 23, 814. [Google Scholar] [CrossRef]
- Mierziak, J.; Kostyn, K.; Kulma, A. Flavonoids as important molecules of plant interactions with the environment. Molecules 2014, 19, 16240–16265. [Google Scholar] [CrossRef]
- Jäger, A.; Saaby, L. Flavonoids and the CNS. Molecules 2011, 16, 1471–1485. [Google Scholar] [CrossRef]
- Fitzgerald, M.; Heinrich, M.; Booker, A. Medicinal plant analysis: A historical and regional discussion of emergent complex techniques. Front. Pharmacol. 2020, 10, 1480. [Google Scholar] [CrossRef]
- Wrońska, N.; Szlaur, M.; Zawadzka, K.; Lisowska, K. The synergistic effect of triterpenoids and flavonoids—New approaches for treating bacterial infections. Molecules 2022, 27, 847. [Google Scholar] [CrossRef]
- Tonga, J.L.; Kamdem, M.H.K.; Pagna, J.I.M.; Fonkui, T.Y.; Tata, C.M.; Fotsing, M.C.D.; Nkengfack, E.A.; Mmutlane, E.M.; Ndinteh, D.T. Antibacterial activity of flavonoids and triterpenoids isolated from the stem bark and sap of Staudtia kamerunensis Warb. Arab. J. Chem. 2022, 15, 104150. [Google Scholar] [CrossRef]
- Fang, S.; Belwal, T.; Li, L.; Limwachiranon, J.; Liu, X.; Luo, Z. Phytosterols and their derivatives: Potential health promoting uses against lipid metabolism and associated diseases, mechanism, and safety issues. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1243–1267. [Google Scholar] [CrossRef]
- Ansari, P.; Akther, S.; Hannan, J.M.A.; Seidel, V.; Nujat, N.J.; Abdel-Wahab, Y.H. Pharmacologically active phytomolecules isolated from traditional antidiabetic plants and their therapeutic role for the management of diabetes mellitus. Molecules 2022, 27, 4278. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xin, Y.; Mo, Y.; Marozik, P.; He, T.; Guo, H. The bioavailability and biological activities of phytosterols as modulators of cholesterol metabolism. Molecules 2022, 27, 523. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, A.; Hamid, K.; Kam, A.; Wong, K.H. The pentacyclic triterpenoids in herbal medicines and their pharmacological activities in diabetes and diabetic complications. Curr. Med. Chem. 2013, 20, 908–931. [Google Scholar]
- Paduch, R.; Kandefer-Szerszeń, M. Antitumor and antiviral activity of pentacyclic triterpenes. Mini-Rev. Org. Chem. 2014, 11, 262–268. [Google Scholar] [CrossRef]
- Xiao, S.; Tian, Z.; Wang, Y.; Si, L.; Zhang, L.; Zhou, D. Recent progress in the antiviral activity and mechanism study of pentacyclic triterpenoids and their derivatives. Med. Res. Rev. 2018, 38, 951–976. [Google Scholar] [CrossRef]
- Ghosh, S. Triterpenoids: Structural diversity, biosynthetic pathways and bioactivity. Stud. Nat. Prod. Chem. 2020, 67, 411–461. [Google Scholar]
- Tolufashe, G.F.; Lawal, M.M.; Govender, K.K.; Shode, F.O.; Singh, T. Exploring the bioactivity of pentacyclic triterpenoids as potential antimycobacterial nutraceutics: Insights through comparative biomolecular modeling. J. Mol. Graph. Model. 2021, 105, 1007. [Google Scholar] [CrossRef] [PubMed]
- Harley, B.K.; Neglo, D.; Tawiah, P.; Pipim, M.A.; Mireku-Gyimah, N.A.; Tettey, C.O.; Amengor, C.D.; Fleisher, T.C.; Waikhom, S.D. Bioactive triterpenoids from Solanum torvum fruits with antifungal, resistance modulatory and antibiofilm formation activities against fluconazole-resistant Candida albicans strains. PLoS ONE 2021, 16, e0260956. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.C.; Xue, C.H.; Zhang, T.T.; Wang, Y.M. Saponins from sea cucumber and their biological activities. J. Agric. Food Chem. 2018, 66, 7222–7237. [Google Scholar] [CrossRef] [PubMed]
- Svigelj, R.; Dossi, N.; Grazioli, C.; Toniolo, R. Deep Eutectic Solvents (DESS) and their application in biosensor development. Sensors 2021, 21, 4263. [Google Scholar] [CrossRef] [PubMed]
- Negi, J.S.; Negi, P.S.; Pant, G.J.; Rawat, M.S.M.; Negi, G.J. Naturally occurring saponins: Chemistry and biology. J. Poisonous Med. Plant Res. 2013, 1, 1–6. [Google Scholar]
- Sabri, N.; Moulai-Mostefa, N. Formulation and characterization of oil-in-water emulsions stabilized by saponins extracted from Hedera helix Algeriensis using response surface method. Biointerface Res. Appl. Chem. 2020, 10, 6282–6292. [Google Scholar]
- Rai, S.; Acharya-Siwakoti, E.; Kafle, A.; Devkota, H.P.; Bhattarai, A. Plant-derived saponins: A review of their surfactant properties and applications. Sci 2021, 3, 44. [Google Scholar] [CrossRef]
- Bottcher, S.; Drusch, S.; Oleszek, W.; Hamed, A. Saponins—Self-assembly and behavior at aqueous interfaces. Adv. Colloid Interface Sci. 2017, 243, 105–113. [Google Scholar] [CrossRef]
- Grzywaczyk, A.; Smułek, W.; Smułek, G.; Ślachciński, M.; Kaczorek, E. Application of natural surfactants for improving the leaching of zinc and copper from different soils. Environ. Technol. Innov. 2021, 24, 101926. [Google Scholar] [CrossRef]
- Bottcher, S.; Drusch, S. Interfacial properties of saponin extracts and their impact on foam characteristics. Food Biophys. 2016, 11, 91–100. [Google Scholar] [CrossRef]
- Wisetkomolmat, J.; Suppakittpaisarn, P.; Sommano, S.R. Detergent plants of Northern Thailand: Potential sources of natural saponins. Resources 2019, 8, 10. [Google Scholar] [CrossRef]
- Zaynab, M.; Sharif, Y.; Abbas, S.; Afzal, M.; Qasim, M.; Khalofah, A.; Ansari, M.J.; Khan, K.A.; Tao, L.; Li, S. Saponin toxicity as key player in plant defense against pathogens. Toxicon 2021, 193, 21–27. [Google Scholar] [CrossRef]
- Morozov, V.A.; Weiss, R.A. Two types of HTLV-1 particles are released from MT-2 cells. Virology 1999, 255, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Tyagi, A.; Bhansali, P.; Pareek, S.; Singh, V.; Ilyas, A.; Mishra, R.; Poddar, N.K. Saponins: Extraction bio-medicinal properties and way forward to anti-viral representatives. Food Chem. Toxicol. 2021, 150, 1120. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Kajimoto, T.; Kinjo, J.; Nakayama, K.; Nohara, T. Chemical transglycosylation of functional bioactive glyco-linkages. Tetrahedron Lett. 1998, 39, 3513–3516. [Google Scholar] [CrossRef]
- Shida, W.; Tahara, Y.; Morikawa, S.; Monde, K.; Koga, R.; Ohsugi, T.; Otsuka, M.; Ikemoto, A.; Tateishi, H.; Ikeda, T.; et al. The unique activity of saponin: Induction of cytotoxicity in HTLV-1 infected cells. Bioorgan. Med. Chem. 2023, 91, 117408. [Google Scholar] [CrossRef]
- Zhang, L.; Wei, W. Anti-inflammatory and immunoregulatory effects of paeoniflorin and total glucosides of paeony. Pharmacol. Ther. 2020, 207, 107452. [Google Scholar] [CrossRef]
- Liao, H.; Ma, J.; Yao, H.; Liu, X. Recent progress of C-glycosylation methods in the total synthesis of natural products and pharmaceuticals. Org. Biomol. Chem. 2018, 16, 1791–1806. [Google Scholar] [CrossRef]
- Taylor, R.J.; McAllister, G.D.; Franck, R.W. The Ramberg–Bäcklund reaction for the synthesis of C-glycosides C-linked-disaccharides and related compounds. Carbohyd. Res. 2006, 341, 1298–1311. [Google Scholar] [CrossRef]
- Xu, L.; Fan, N.; Hu, X. Recent development in the synthesis of C-glycosides involving glycosyl radicals. Org. Biomol. Chem. 2020, 18, 5095–5109. [Google Scholar] [CrossRef]
- Henschke, J.P.; Lin, C.; Wu, P.; Tsao, W.; Liao, J.; Chiang, P. β-Selective C-arylation of diisobutylaluminum hydride modified 1,6-anhydroglucose: Synthesis of canagliflozin without recourse to conventional protecting groups. J. Org. Chem. 2015, 80, 5189–5195. [Google Scholar] [CrossRef]
- Guo, C.; Hu, M.; DeOrazio, R.J.; Usyatinsky, A.; Fitzpatrick, K.; Zhang, Z.; Maeng, J.-H.; Kitchen, D.B.; Tom, S.; Luche, M.; et al. The design and synthesis of novel SGLT2 inhibitors: C-glycosides with benzyltriazolopyridinone and phenylhydantoin as the aglycone moieties. Bioorgan. Med. Chem. 2014, 22, 3414–3422. [Google Scholar] [CrossRef]
- Hahn, F.; Guth, F.M. The ambruticins and jerangolids—Chemistry, biology and chemoenzymatic synthesis of potent antifungal drug candidates. Nat. Prod. Rep. 2020, 37, 1300–1315. [Google Scholar] [CrossRef]
- Bowen, J.I.; Wang, L.; Crump, M.P.; Willis, C.L. Ambruticins: Tetrahydropyran ring formation and total synthesis. Org. Biomol. Chem. 2021, 19, 6210–6215. [Google Scholar] [CrossRef]
- Kobayashi, T.; Regens, C.S.; Denmark, S.E. Total synthesis of papulacandin d, In Strategies and Tactics. Org. Synth. 2012, 08, 79–126. [Google Scholar]
- Shimura, J.; Ando, Y.; Ohmori, K.; Suzuki, K. Total synthesis and structure assignment of saptomycin. Org. Lett. 2022, 24, 1439–1443. [Google Scholar] [CrossRef] [PubMed]
- Yuan, K.; Chen, S.; Chen, X.; Yao, S.; Wang, X.; Song, H.; Zhu, M. High effective extraction of selected anthraquinones from Polygonum multiflorum using ionic liquids with ultrasonic assistance. J. Mol. Liq. 2020, 314, 1439–1443. [Google Scholar] [CrossRef]
- Qi, Y.; Fox, C.B. A Two-step orthogonal chromatographic process for purifying the molecular adjuvant QS-21 with high purity and yield. J. Chromatogr. A 2021, 1635, 461705. [Google Scholar] [CrossRef] [PubMed]
- Oyedemi, B.O.; Shinde, V.; Shinde, K.; Kakalou, D.; Stapleton, P.D.; Gibbons, S.J. Novel R-plasmid conjugal transfer inhibitory and antibacterial activities of phenolic compounds from Mallotus philippensis (Lam.) Mull. Arg. Glob. Antimicrob. Resist. 2016, 5, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Leong, W.F.; Elias, R.J.; Tikekar, R.V. UV-C irradiated gallic acid exhibits enhanced antimicrobial activity via generation of reactive oxidative species and quinone. Food Chem. 2019, 287, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yao, J.; Wang, S.; Pan, X.; Xiao, R.; Huang, Q.; Wang, Z.; Qu, R. Phototransformation of estrogens mediated by Mn (III), not by reactive oxygen species, in the presence of humic acids. Chemosphere 2018, 201, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Martín, J.; Beltrán-Heredia, J.; Gibello-Pérez, P. Adsorbent biopolymers from tannin extracts for water treatment. Chem. Eng. J. 2011, 168, 1241–1247. [Google Scholar] [CrossRef]
- Das, A.K.; Islam, M.N.; Faruk, M.O.; Ashaduzzaman, M.; Dungani, R. Review on tannins: Extraction processes, applications, and possibilities. S. Afri. J. Bot. 2020, 135, 58–70. [Google Scholar] [CrossRef]
- De Sousa, B.C.M.; Gomes, D.D.A.; Da Silva Viana, A.F.; Da Silva, B.A.; Barata, L.E.S.; Sartoratto, A.; Lustosa, D.C.; Vieira, T.A. Phytochemical analysis, and antioxidant activity of ethanolic extracts from different parts of Dipteryx punctata (S. F. Blake) Amshoff. Appl. Sci. 2023, 13, 9600. [Google Scholar] [CrossRef]
- Cavaliere, C.; Foglia, P.; Pastorini, E.; Samperi, R.; Lagana, À. Identification and mass spectrometric characterization of glycosylated flavonoids in Triticum durum plants by high-performance liquid chromatography coupled with tandem mass spectrometry. Rapid Commun. Mass. Spectrom. 2015, 19, 3143–3158. [Google Scholar] [CrossRef]
- Ertas, A.; Boga, M.; Yılmaz, M.A.; Yesil, Y.; Hasimi, N.; Kaya, M.S.; Temel, H.; Kolak, U. Chemical compositions by using LC–MS/MS and GC–MS and biological activities of Sedum sediforme (Jacq.). Pau. J. Agric. Food Chem. 2014, 62, 4601–4609. [Google Scholar] [CrossRef]
- Ertaş, A.; Yılmaz, M.A.; Fırat, M. Chemical profile by LC–MS/MS, GC/MS and antioxidant activities of the essential oils and crude extracts of two Euphorbia species. Nat. Prod. Res. 2014, 29, 529–534. [Google Scholar] [CrossRef]
- Miyuki, F.; Yoshimi, I.; Toŝhiyuki, T.; Tetsuro, I.; Kenichi, N.; Iliya, I.; Masayoshi, O.; Munekazu, I.; Yoshiaki, S.; Yoshikazu, T. Novel, complex flavonoids from Mallotus philippensis (kamala tree). Helv. Chim. Acta 2005, 88, 1048–1058. [Google Scholar]
- Agar, O.T.; Dikmen, M.; Ozturk, N.; Yilmaz, M.A.; Temel, H.; Turkmenoglu, F.P. Comparative studies on phenolic composition, antioxidant, wound healing and cytotoxic activities of selected Achillea L. species growing in Turkey. Molecules 2015, 20, 17976–18000. [Google Scholar] [CrossRef]
- Ventura, S.P.M.; e Silva, F.A.; Quental, M.V.; Mondal, D.; Freire, M.G.; Coutinho, J.A.P. Ionic-liquid-mediated extraction and separation processes for bioactive compounds: Past, present, and future trends. Chem. Rev. 2017, 117, 6984–7052. [Google Scholar] [CrossRef]
- He, Q.; Guo, Z.; Cao, Y.; Yang, M.; Yao, S. Selective separation of main flavonoids by combinational use of ionic liquid-loaded microcapsules from crude extract of Tartary buckwheat. Food Chem. 2021, 362, 130255. [Google Scholar] [CrossRef]
- Alam, M.J.; Ahmad, S. Quantum chemical and spectroscopic investigations of 3-methyladenine. Spectrochim. Acta. A 2014, 128, 653–664. [Google Scholar] [CrossRef]
- Dadáková, K.; Heinrichová, T.; Lochman, J.; Kašparovský, T. Production of defense phenolics in tomato leaves of different age. Mol./Mol. Online/Mol. Annu. 2020, 25, 4952. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).