Hyphenation of Thermodesorption into GC × GC-TOFMS for Odorous Molecule Detection in Car Materials: Column Sets and Adaptation of Second Column Dimensions to TD Pressure Constraints
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
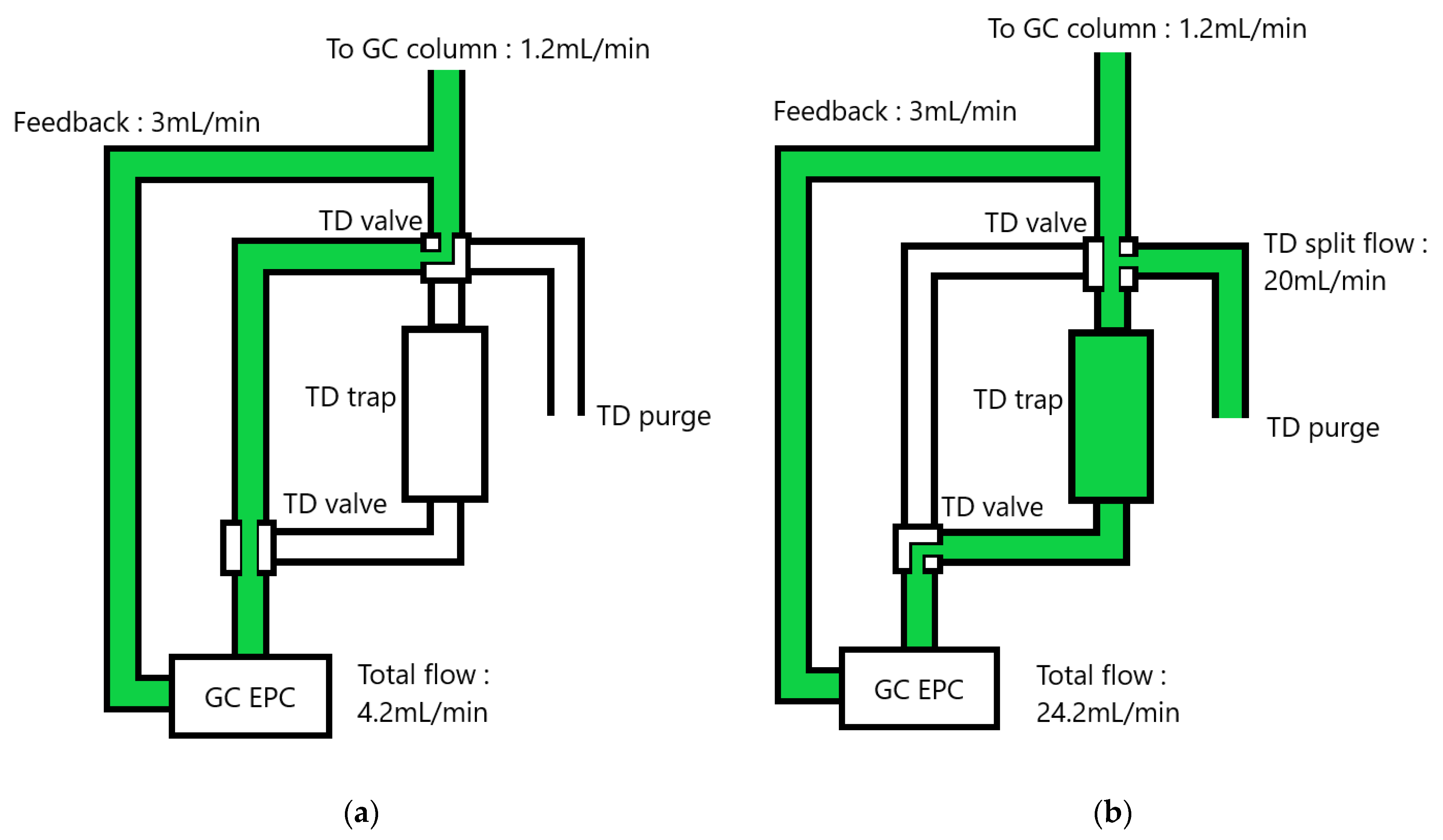
2.2. TD-GC Hyphenation
2.3. Global GC × GC–TOFMS Settings
3. Results
3.1. Part A, Choice of Set of Column
3.1.1. Preliminary Experiments
3.1.2. Column Phases Selection
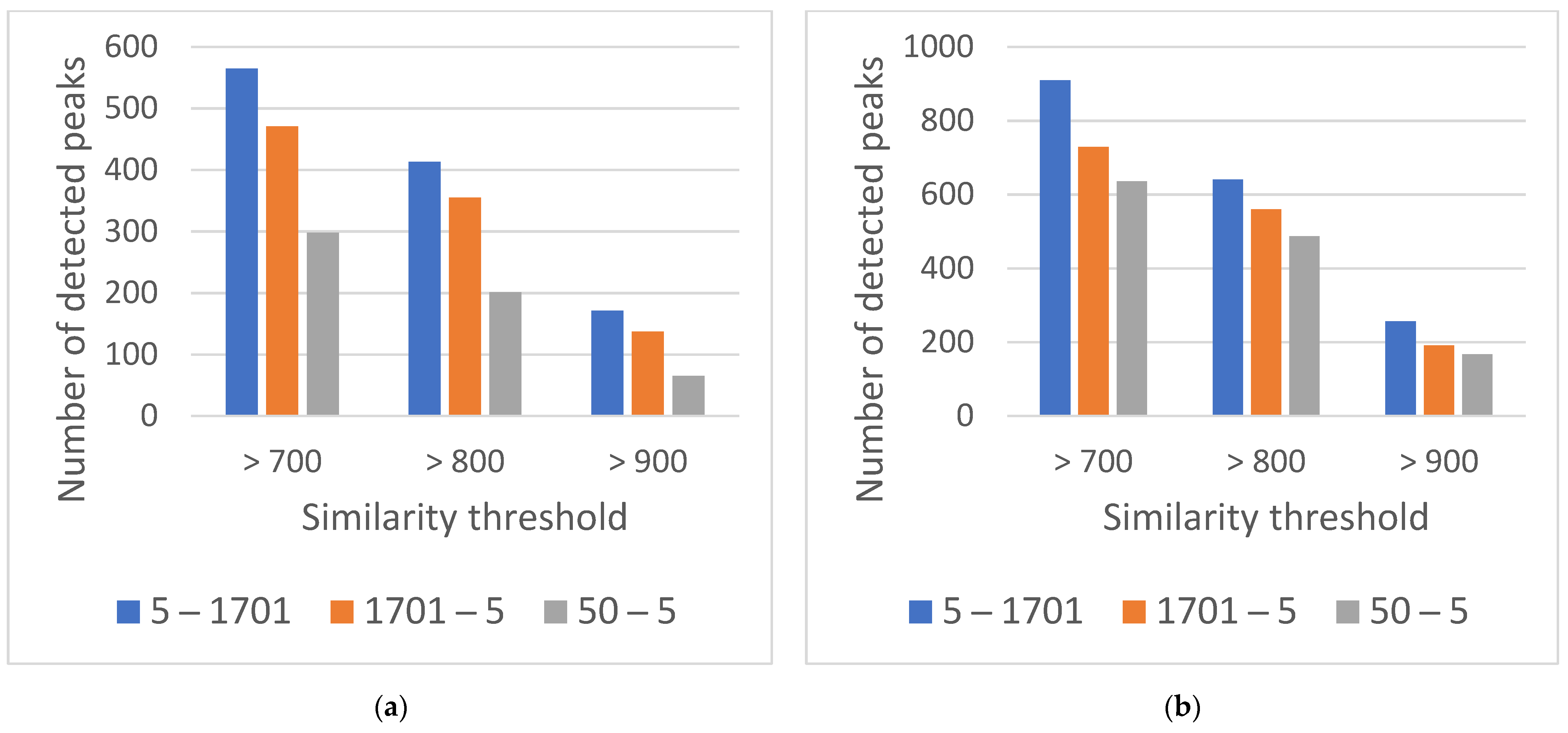
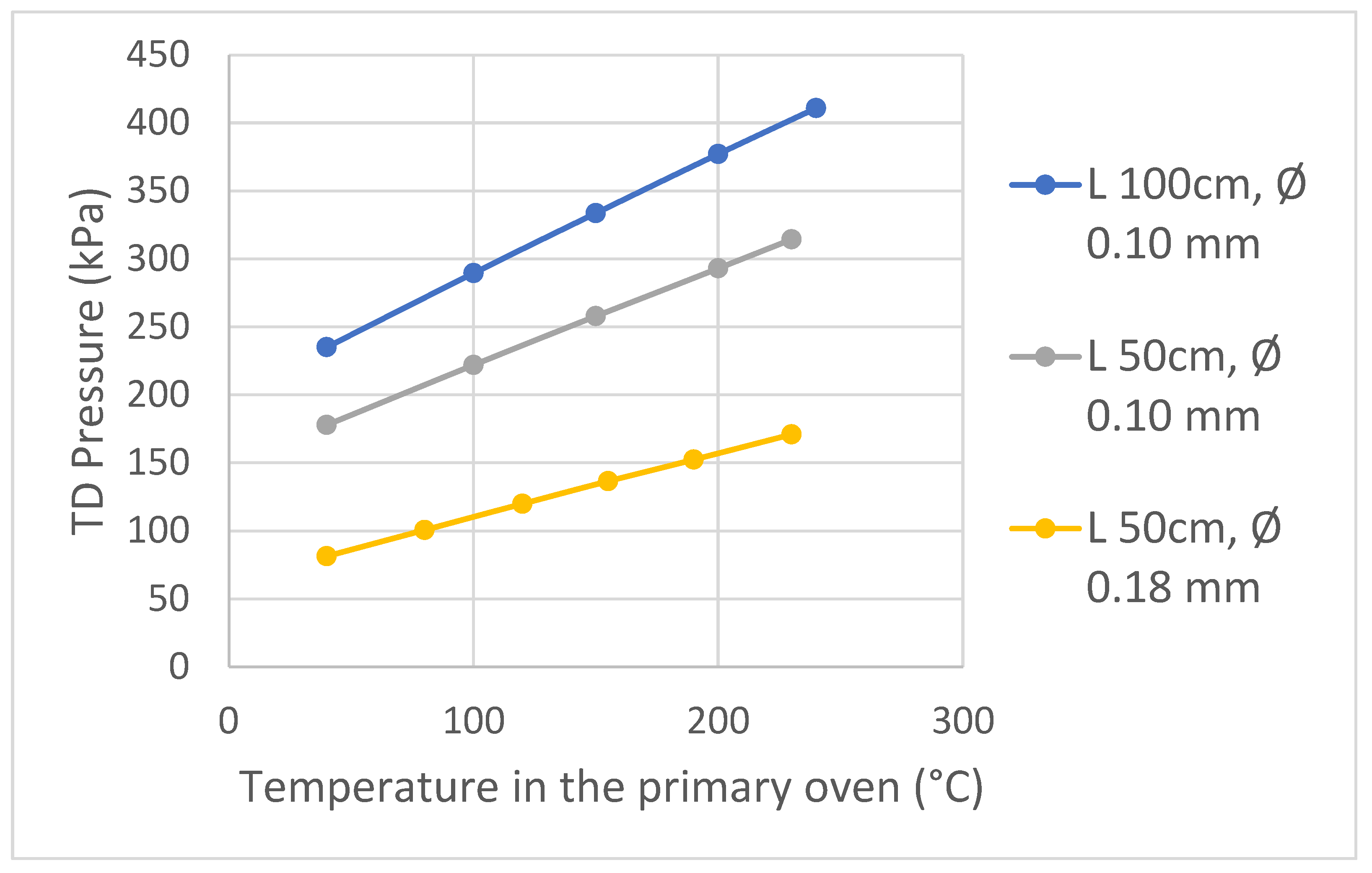
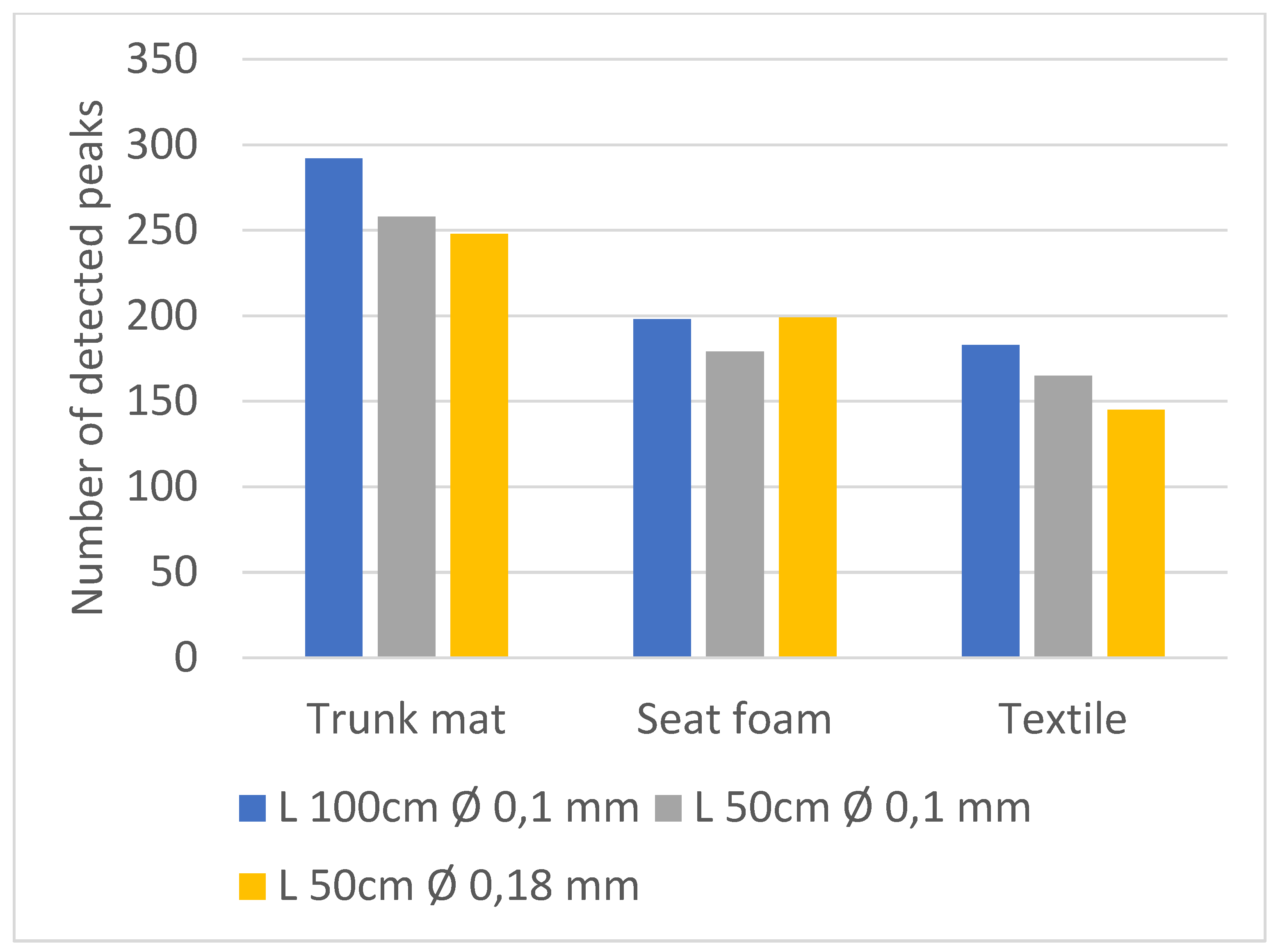
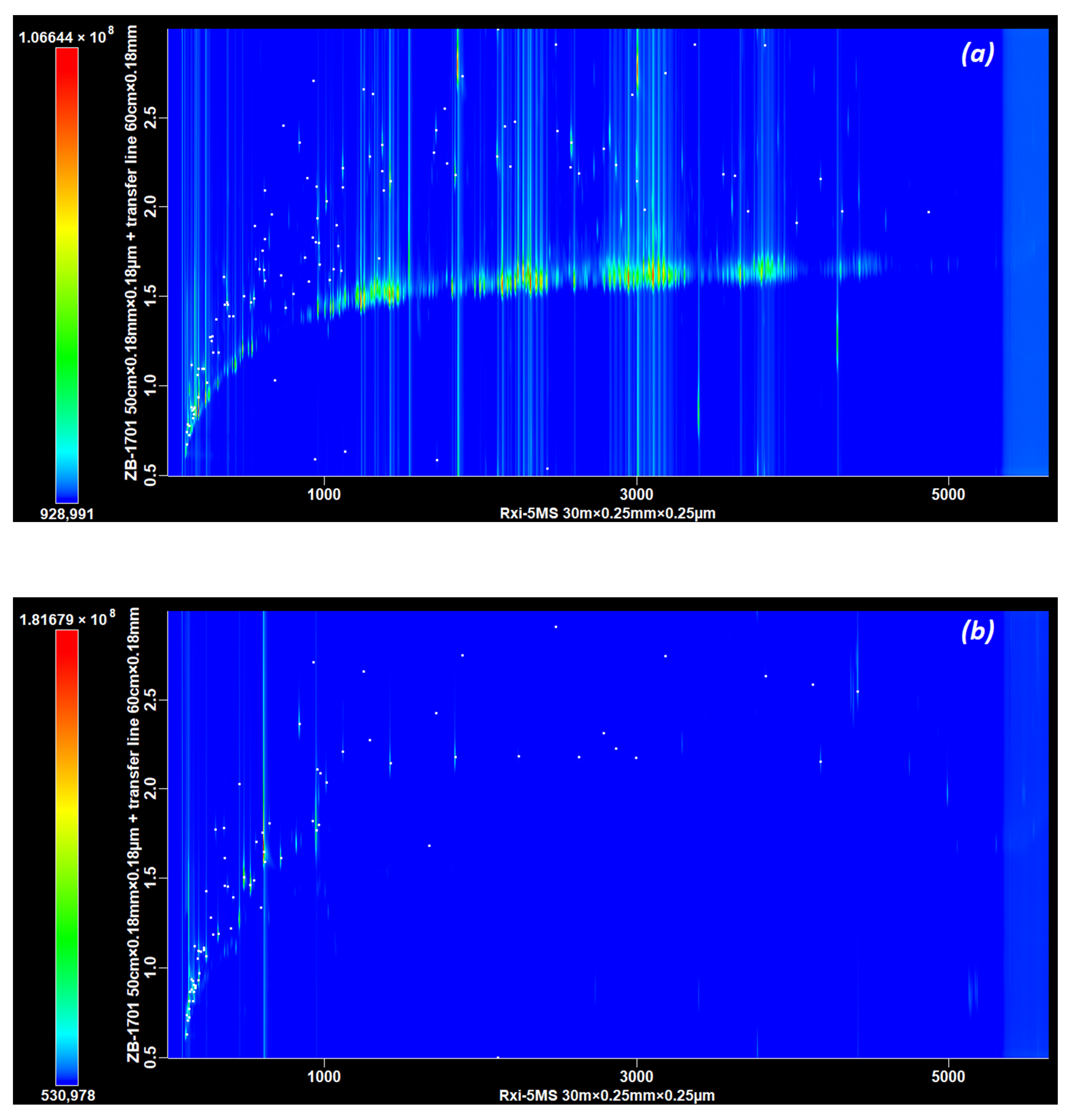
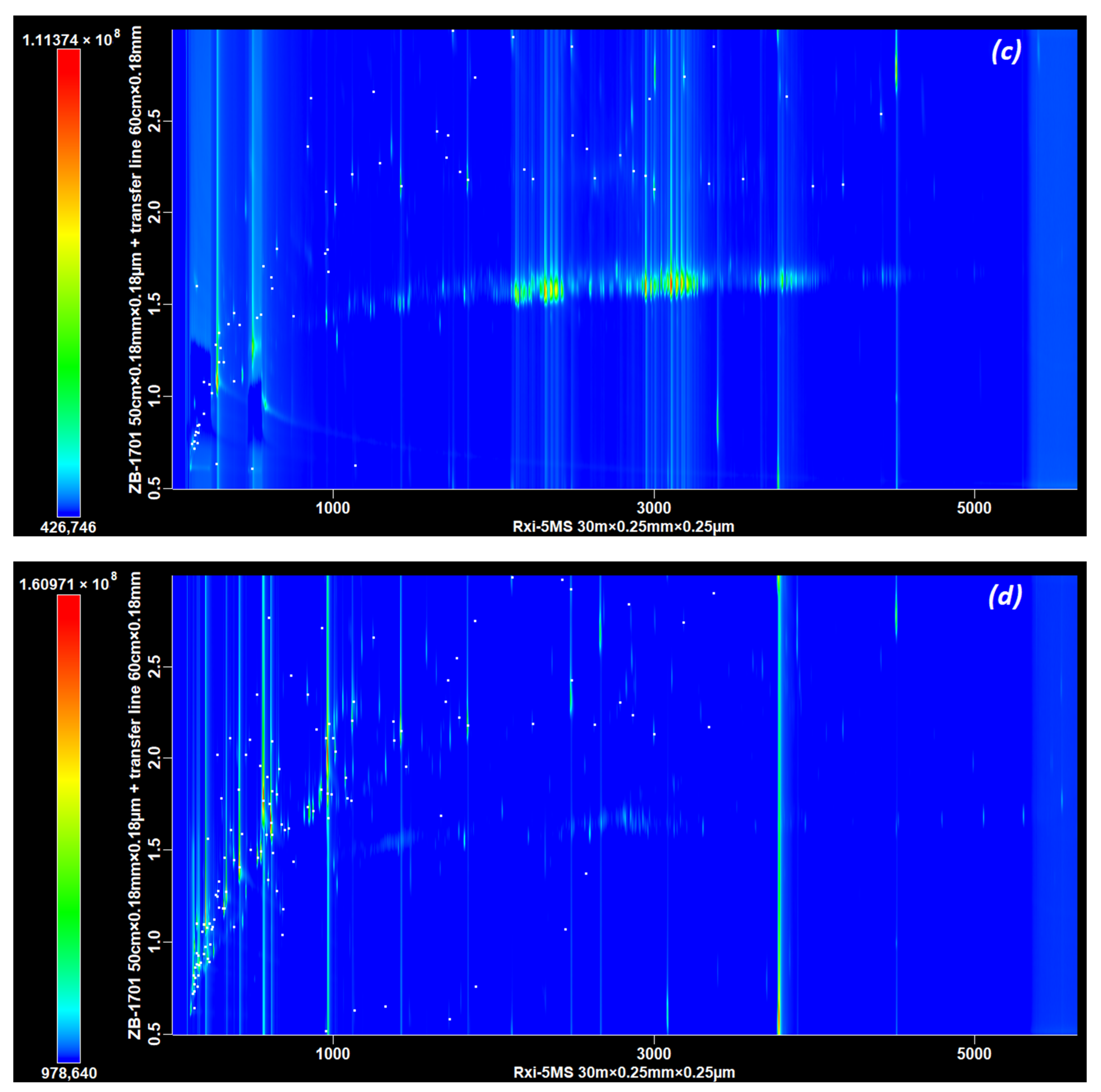
3.2. Part B, Selecting the Dimensions of the 2D Column
The Influence of the Length and Diameter of the Second Dimension Column
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Brancher, M.; Griffiths, K.D.; Franco, D.; De Melo Lisboa, H. A review of odour impact criteria in selected countries around the world. Chemosphere 2017, 168, 1531–1570. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.-S.; Li, T.-T.; Luo, M.; Liu, J.-F.; Liu, Z.-R.; Bai, Y.-H. Air pollution in the microenvironment of parked new cars. J. Affect. Disord. 2008, 43, 315–319. [Google Scholar] [CrossRef]
- Faber, J.; Brodzik, K.; Gołda-Kopek, A.; Łomankiewicz, D. Benzene, toluene and xylenes levels in new and used vehicles of the same model. J. Environ. Sci. 2013, 25, 2324–2330. [Google Scholar] [CrossRef] [PubMed]
- Brodzik, K.; Faber, J.; Łomankiewicz, D.; Gołda-Kopek, A. In-vehicle VOCs composition of unconditioned, newly produced cars. J. Environ. Sci. 2014, 26, 1052–1061. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Matsunaga, I. A case study on identification of airborne organic compounds and time courses of their concentrations in the cabin of a new car for private use. Environ. Int. 2006, 32, 58–79. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Matsunaga, I.; Tomioka, K.; Kumagai, S. Interior Air Pollution in Automotive Cabins by Volatile Organic Compounds Diffusing from Interior Materials: I. Survey of 101 Types of Japanese Domestically Produced Cars for Private Use. Indoor Built Environ. 2006, 15, 425–444. [Google Scholar] [CrossRef]
- Yoshida, T.; Matsunaga, I.; Tomioka, K.; Kumagai, S. Interior Air Pollution in Automotive Cabins by Volatile Organic Compounds Diffusing from Interior Materials: II. Influence of Manufacturer, Specifications and Usage Status on Air Pollution, and Estimation of Air Pollution Levels in Initial Phases of Delivery as a New Car. Indoor Built Environ. 2006, 15, 445–462. [Google Scholar] [CrossRef]
- Janicka, A.B.; Reksa, M.; Sobianowska-Turek, A. The impact of car vehicle class on volatile organic compounds (VOC’s) concentration in microatmosphere of car cabin. J. KONES 2010, 17, 207–212. [Google Scholar]
- Chen, X.; Feng, L.; Luo, H.; Cheng, H. Analyses on influencing factors of airborne VOCS pollution in taxi cabins. Environ. Sci. Pollut. Res. 2014, 21, 12868–12882. [Google Scholar] [CrossRef]
- Xu, B.; Chen, X.; Xiong, J. Air quality inside motor vehicles’ cabins: A review. Indoor Built Environ. 2016, 27, 452–465. [Google Scholar] [CrossRef]
- Buchecker, F.; Loos, H.M.; Buettner, A. Investigations on the impact of hardening on the odour of an aqueous cavity preservation for automotive applications using sensory and instrumental analysis. Talanta Open 2022, 5, 100095. [Google Scholar] [CrossRef]
- VDA 276-3 (05/2022), VDA Webshop. Available online: https://webshop.vda.de/VDA/vda-276-3-052022 (accessed on 6 December 2022).
- ISO 12219-1:2021; Interior Air of Road Vehicles—Part 1: Whole Vehicle Test Chamber—Specification and Method for the Determination of Volatile Organic Compounds in Cabin Interiors. ISO: Geneva, Switzerland. Available online: https://www.iso.org/standard/72410.html (accessed on 15 May 2022).
- Buchecker, F.; Baum, A.; Loos, H.M.; Buettner, A. Follow your nose—Traveling the world of odorants in new cars. Indoor Air 2022, 32, e13014. [Google Scholar] [CrossRef]
- Boyle, J.A.; Djordjevic, J.; Olsson, M.J.; Lundström, J.N.; Jones-Gotman, M. The Human Brain Distinguishes between Single Odorants and Binary Mixtures. Cereb. Cortex 2008, 19, 66–71. [Google Scholar] [CrossRef]
- Laing, D.G.; Eddy, A.; Best, D.J. Perceptual characteristics of binary, trinary, and quaternary odor mixtures consisting of unpleasant constituents. Physiol. Behav. 1994, 56, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Jaubert, J.-N.; Gordon, G.; Dore, J.-C. Une organisation du champ des odeurs. I: Recherche de critères objectifs. Parfums Cosmét. Arômes 1987, 77, 53–56. [Google Scholar]
- Jaubert, J.-N.; Gordon, G.; Dore, J.-C. Une organisation du champ des odeurs. II: Modèle descriptif de l’organisation de l’espace odorant. Parfums Cosmét. Arômes 1987, 71–82. [Google Scholar]
- Verriele, M.; Plaisance, H.; Vandenbilcke, V.; Locoge, N.; Jaubert, J.N.; Meunier, G. Odor evaluation and discrimination of car cabin and its components: Application of the “field of odors” approach in a sensory descriptive analysis. J. Sens. Stud. 2012, 27, 102–110. [Google Scholar] [CrossRef]
- Agapakis, C.M.; Tolaas, S. Smelling in multiple dimensions. Curr. Opin. Chem. Biol. 2012, 16, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Kaeppler, K.; Mueller, F. Odor Classification: A Review of Factors Influencing Perception-Based Odor Arrangements. Chem. Senses 2013, 38, 189–209. [Google Scholar] [CrossRef]
- Brattoli, M.; Cisternino, E.; Dambruoso, P.R.; De Gennaro, G.; Giungato, P.; Mazzone, A.; Palmisani, J.; Tutino, M. Gas Chromatography Analysis with Olfactometric Detection (GC-O) as a Useful Methodology for Chemical Characterization of Odorous Compounds. Sensors 2013, 13, 16759–16800. [Google Scholar] [CrossRef]
- Delahunty, C.M.; Eyres, G.; Dufour, J.-P. Gas chromatography-olfactometry. J. Sep. Sci. 2006, 29, 2107–2125. [Google Scholar] [CrossRef] [PubMed]
- Giungato, P.; Di Gilio, A.; Palmisani, J.; Marzocca, A.; Mazzone, A.; Brattoli, M.; Giua, R.; de Gennaro, G. Synergistic approaches for odor active compounds monitoring and identification: State of the art, integration, limits and potentialities of analytical and sensorial techniques. TrAC Trends Anal. Chem. 2018, 107, 116–129. [Google Scholar] [CrossRef]
- Mondello, L.; Tranchida, P.Q.; Dugo, P.; Dugo, G. Comprehensive two-dimensional gas chromatography-mass spectrometry: A review. Mass Spectrom. Rev. 2008, 27, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.A. Qualitative and quantitative approaches in comprehensive two-dimensional gas chromatography. J. Chromatogr. A 2012, 1261, 58–68. [Google Scholar] [CrossRef]
- Lee, A.L.; Bartle, K.D.; Lewis, A.C. A Model of Peak Amplitude Enhancement in Orthogonal Two-Dimensional Gas Chromatography. Anal. Chem. 2001, 73, 1330–1335. [Google Scholar] [CrossRef]
- Czerny, M.; Brueckner, R.; Kirchhoff, E.; Schmitt, R.; Buettner, A. The Influence of Molecular Structure on Odor Qualities and Odor Detection Thresholds of Volatile Alkylated Phenols. Chem. Senses 2011, 36, 539–553. [Google Scholar] [CrossRef]
- Chastrette, M. Data Management in Olfaction Studies. SAR QSAR Environ. Res. 1998, 8, 157–181. [Google Scholar] [CrossRef] [PubMed]
- Blomberg, J.; Schoenmakers, P.J.; Beens, J.; Tijssen, R. Compehensive two-dimensional gas chromatography (GC × GC) and its applicability to the characterization of complex (petrochemical) mixtures. J. High Resolut. Chromatogr. 1997, 20, 539–544. [Google Scholar] [CrossRef]
- Ochiai, N.; Sasamoto, K. Selectable one-dimensional or two-dimensional gas chromatography–olfactometry/mass spectrometry with preparative fraction collection for analysis of ultra-trace amounts of odor compounds. J. Chromatogr. A 2010, 1218, 3180–3185. [Google Scholar] [CrossRef]
- ISO 12219-3:2012; Interior Air of Road Vehicles—Part 3: Screening Method for the Determination of the Emissions of Volatile Organic Compounds from Vehicle Interior Parts and Materials—Micro-Scale Chamber Method. ISO: Geneva, Switzerland. Available online: http://www.iso.org/cms/render/live/en/sites/isoorg/contents/data/standard/05/48/54866.html (accessed on 30 August 2019).
- Jores, C.D.S.; Klein, R.; Legendre, A.; Dugay, J.; Thiébaut, D.; Vial, J. Preparation of Thermodesorption Tube Standards: Comparison of Usual Methods Using Accuracy Profile Evaluation. Separations 2022, 9, 226. [Google Scholar] [CrossRef]
- Wilson, R.B.; Siegler, W.C.; Hoggard, J.C.; Fitz, B.D.; Nadeau, J.S.; Synovec, R.E. Achieving high peak capacity production for gas chromatography and comprehensive two-dimensional gas chromatography by minimizing off-column peak broadening. J. Chromatogr. A 2011, 1218, 3130–3139. [Google Scholar] [CrossRef]
- Shellie, R.; Marriott, P.; Morrison, P.; Mondello, L. Effects of pressure drop on absolute retention matching in comprehensive two-dimensional gas chromatography. J. Sep. Sci. 2004, 27, 503–512. [Google Scholar] [CrossRef]
- Mommers, J.; van der Wal, S. Column Selection and Optimization for Comprehensive Two-Dimensional Gas Chromatography: A Review. Crit. Rev. Anal. Chem. 2021, 51, 183–202. [Google Scholar] [CrossRef]
| Column Set | Short Name | 1D phase | 2D Dimensions (cm × mm × µm) | 2D Phase | Configuration | Modulation Period (s) |
|---|---|---|---|---|---|---|
| Rxi-5MS–ZB-WAX | 5–WAX | 5% diphenyl | 195 × 0.1 × 0.1 | 100% polyethylene-glycol | Normal | 12 |
| Rxi-5MS–ZB-50 | 5–50 | 5% diphenyl | 175 × 0.1 × 0.1 | 50% diphenyl | Normal | 10 |
| DB-17MS–DB-5 | 50–5 | 50% diphenyl | 115 × 0.1 × 0.1 | 5% diphenyl | Reverse | 6 |
| Rxi-5MS–DB-210 | 5–210 | 5% diphenyl | 118 × 0.1 × 0.1 | 50% trifluoropropyl | Normal | 5 |
| ZB-1701–DB-5 | 1701–5 | 14%-cyanopropyl-phenyl | 84 × 0.1 × 0.1 | 5% diphenyl | Reverse | 2 |
| Rxi-5MS–ZB-1701 | 5–1701 | 5% diphenyl | 122 × 0.1 × 0.1 | 14%-cyanopropyl-phenyl | Normal | 6 |
| (a) PP | ||||
|---|---|---|---|---|
| Name | CAS Number | Area | 1D Retention Time (s) | 2D Retention Time (s) |
| Tetrahydrofuran | 109-99-9 | 5.31 × 108 | 169.989 | 0.884 |
| Diphenyl ether | 101-84-8 | 3.89 × 108 | 2577.336 | 2.362 |
| Benzoic acid, methyl ester | 93-58-3 | 3.04 × 108 | 1369.914 | 2.351 |
| Nonanal | 124-19-6 | 2.06 × 108 | 1419.912 | 2.145 |
| Ethylbenzene | 100-41-4 | 1.96 × 108 | 527.466 | 1.468 |
| 1-Butanol | 71-36-3 | 1.94 × 108 | 194.9874 | 1.099 |
| acetone | 67-64-1 | 1.92 × 108 | 124.992 | 0.79 |
| Benzene | 71-43-2 | 1.83 × 108 | 194.9874 | 0.939 |
| Naphthalene | 91-20-3 | 1.71 × 108 | 1712.388 | 2.43 |
| 3-Penten-2-one, 4-methyl- | 141-79-7 | 1.56 × 108 | 377.4756 | 1.471 |
| 1-Hexanol, 2-ethyl- | 104-76-7 | 1.54 × 108 | 1117.428 | 2.218 |
| p-Xylene | 106-42-3 | 1.54 × 108 | 549.9648 | 1.49 |
| 1-Decanol | 112-30-1 | 1.50 × 108 | 2104.866 | 2.283 |
| Decanal | 112-31-2 | 1.42 × 108 | 1834.884 | 2.179 |
| Pyridine, 2,4,6-trimethyl- | 108-75-8 | 1.33 × 108 | 954.936 | 1.942 |
| Acetic acid | 64-19-7 | 9.30 × 107 | 147.4908 | 1.119 |
| 1-Dodecanol | 112-53-8 | 7.23 × 107 | 2864.814 | 2.237 |
| Phenol | 108-95-2 | 7.17 × 107 | 944.94 | 1.804 |
| Benzaldehyde, 3,4-dimethyl- | 5973-71-7 | 7.16 × 107 | 1882.38 | 2.691 |
| Furan, 2,3-dihydro- | 1191-99-7 | 6.91 × 107 | 147.4908 | 0.801 |
| (b) ABS | ||||
| Name | CAS Number | Area | 1D Retention Time (s) | 2D Retention Time (s) |
| Styrene | 100-42-5 | 2.83 × 109 | 612.462 | 1.652 |
| Phenol | 108-95-2 | 2.29 × 109 | 947.442 | 1.768 |
| 2-Propenenitrile | 107-13-1 | 1.89 × 109 | 132.4914 | 0.821 |
| Benzene, chloro- | 108-90-7 | 1.47 × 109 | 482.469 | 1.509 |
| Ethylbenzene | 100-41-4 | 9.96 × 108 | 524.9664 | 1.464 |
| Methyl methacrylate | 80-62-6 | 6.08 × 108 | 242.4846 | 1.069 |
| Benzene, (1-methylethyl)- | 98-82-8 | 4.32 × 108 | 719.952 | 1.614 |
| Toluene | 108-88-3 | 3.92 × 108 | 319.9794 | 1.193 |
| Ethyl Acetate | 141-78-6 | 3.68 × 108 | 162.4896 | 0.871 |
| 1-Butanol | 71-36-3 | 2.84 × 108 | 194.9874 | 1.101 |
| Propanenitrile | 107-12-0 | 2.50 × 108 | 147.4908 | 0.941 |
| Benzaldehyde | 100-52-7 | 2.47 × 108 | 837.444 | 2.366 |
| Benzene | 71-43-2 | 1.79 × 108 | 194.9874 | 0.936 |
| Carbon disulfide | 75-15-0 | 1.71 × 108 | 134.9916 | 0.731 |
| 2-butanone | 78-93-3 | 1.65 × 108 | 154.9902 | 0.888 |
| Pyrrole | 109-97-7 | 1.44 × 108 | 302.4804 | 1.773 |
| Methane, dichloro- | 75-09-2 | 1.10 × 108 | 132.4914 | 0.776 |
| Nonanal | 124-19-6 | 8.88 × 107 | 1419.912 | 2.146 |
| Formic acid | 64-18-6 | 8.02 × 107 | 119.9922 | 0.741 |
| Acetic acid | 64-19-7 | 7.79 × 107 | 167.4894 | 1.125 |
| (c) Dashboard | ||||
| Name | CAS Number | Area | 1D Retention Time (s) | 2D Retention Time (s) |
| Pyridine | 110-86-1 | 2.01 × 108 | 284.982 | 1.351 |
| Furfural | 98-01-1 | 1.39 × 108 | 454.971 | 2.009 |
| Acetic acid | 64-19-7 | 1.21 × 108 | 149.9904 | 1.604 |
| Nonanal | 124-19-6 | 1.16 × 108 | 1419.912 | 2.146 |
| Decanal | 112-31-2 | 8.78 × 107 | 1834.884 | 2.182 |
| Dodecanoic acid | 143-07-7 | 8.49 × 107 | 3184.794 | 2.738 |
| Octanoic acid | 124-07-2 | 7.95 × 107 | 1744,89 | 2.991 |
| Nonanoic acid | 112-05-0 | 7.93 × 107 | 2117.364 | 2.956 |
| Sulphur dioxide | 7446-09-5 | 6.95 × 107 | 494.9682 | 0.61 |
| Decanoic acid | 334-48-5 | 6.89 × 107 | 2484.84 | 2.904 |
| 1-Hexanol, 2-ethyl- | 104-76-7 | 5.61 × 107 | 1117.428 | 2.209 |
| Formic acid | 64-18-6 | 5.13 × 107 | 117.4926 | 0.744 |
| Hexanal | 66-25-1 | 4.76 × 107 | 379.9758 | 1.453 |
| 2-Tridecanone | 593-08-8 | 4.52 × 107 | 2944.812 | 2.201 |
| Furan, 2,5-dimethyl- | 625-86-5 | 4.15 × 107 | 242.4846 | 1.021 |
| 1-Dodecanol | 112-53-8 | 3.72 × 107 | 2867.316 | 2.228 |
| 2(5H)-Furanone | 497-23-4 | 3.48 × 107 | 744.954 | 0.959 |
| Benzaldehyde | 100-52-7 | 3.04 × 107 | 839.946 | 2.36 |
| Carbon disulfide | 75-15-0 | 2.99 × 107 | 134.9916 | 0.72 |
| Heptanal | 111-71-7 | 2.80 × 107 | 647.46 | 1.806 |
| (d) Decor | ||||
| Name | CAS Number | Area | 1D retention Time (s) | 2D Retention Time (s) |
| 1-Methoxy-2-propyl acetate | 108-65-6 | 6.14 × 109 | 562.464 | 1.772 |
| Styrene | 100-42-5 | 3.27 × 109 | 612.462 | 1.651 |
| Acetic acid, butyl ester | 123-86-4 | 2.81 × 109 | 412.4736 | 1.409 |
| 2-Propanol, 1-methoxy- | 107-98-2 | 2.66 × 109 | 212.4864 | 1.08 |
| Isobutyl acetate | 110-19-0 | 2.43 × 109 | 332.4786 | 1.273 |
| Ethyl Acetate | 141-78-6 | 1.80 × 109 | 164.9892 | 0.879 |
| Benzene, 1,2,4-trimethyl- | 95-63-6 | 1.38 × 109 | 964.938 | 1.812 |
| p-Xylene | 106-42-3 | 1.19 × 109 | 549.9648 | 1.494 |
| Triethylamine | 121-44-8 | 1.16 × 109 | 217.4862 | 0.915 |
| Toluene | 108-88-3 | 1.08 × 109 | 319.9794 | 1.186 |
| Acetic acid | 64-19-7 | 1.08 × 109 | 149.9904 | 1.107 |
| 2-Propenenitrile | 107-13-1 | 1.06 × 109 | 129.9918 | 0.826 |
| o-Xylene | 95-47-6 | 8.42 × 108 | 617.46 | 1.583 |
| Ethylbenzene | 100-41-4 | 8.21 × 108 | 527.466 | 1.461 |
| Mesitylene | 108-67-8 | 6.09 × 108 | 874.944 | 1.713 |
| Nonanal | 124-19-6 | 5.89 × 108 | 1419.912 | 2.149 |
| 1-Hexanol, 2-ethyl- | 104-76-7 | 4.74 × 108 | 1117.428 | 2.206 |
| α-Methylstyrene | 98-83-9 | 3.93 × 108 | 924.942 | 1.827 |
| Benzene, 1,2,3-trimethyl- | 526-73-8 | 3.74 × 108 | 1074.93 | 1.892 |
| Ethanol, 2-butoxy- | 111-76-2 | 2.95 × 108 | 659.958 | 1.946 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klein, R.; Dugay, J.; Vial, J.; Thiébaut, D.; Colombet, G.; Barreteau, D.; Gruntz, G. Hyphenation of Thermodesorption into GC × GC-TOFMS for Odorous Molecule Detection in Car Materials: Column Sets and Adaptation of Second Column Dimensions to TD Pressure Constraints. Separations 2024, 11, 162. https://doi.org/10.3390/separations11060162
Klein R, Dugay J, Vial J, Thiébaut D, Colombet G, Barreteau D, Gruntz G. Hyphenation of Thermodesorption into GC × GC-TOFMS for Odorous Molecule Detection in Car Materials: Column Sets and Adaptation of Second Column Dimensions to TD Pressure Constraints. Separations. 2024; 11(6):162. https://doi.org/10.3390/separations11060162
Chicago/Turabian StyleKlein, Romain, José Dugay, Jérôme Vial, Didier Thiébaut, Guy Colombet, Donatien Barreteau, and Guillaume Gruntz. 2024. "Hyphenation of Thermodesorption into GC × GC-TOFMS for Odorous Molecule Detection in Car Materials: Column Sets and Adaptation of Second Column Dimensions to TD Pressure Constraints" Separations 11, no. 6: 162. https://doi.org/10.3390/separations11060162
APA StyleKlein, R., Dugay, J., Vial, J., Thiébaut, D., Colombet, G., Barreteau, D., & Gruntz, G. (2024). Hyphenation of Thermodesorption into GC × GC-TOFMS for Odorous Molecule Detection in Car Materials: Column Sets and Adaptation of Second Column Dimensions to TD Pressure Constraints. Separations, 11(6), 162. https://doi.org/10.3390/separations11060162







