1. Introduction
As the atmospheric CO
2 concentration continues to increase, there is a growing risk of severe and irreversible impacts due to the dramatic progression of climate change [
1]. In response to these concerns, the United Nations Framework Convention on Climate Change facilitated the development of the Paris Agreement, a landmark international accord on climate change [
2]. Under this agreement, participating countries submitted Nationally Determined Contributions (NDCs) outlining their commitments to reduce greenhouse gas emissions. However, analyses suggest that adopting the existing commitments will result in a temperature increase of around 2.4–2.6 °C by the end of the century, considering both conditional and unconditional NDCs [
3]. Therefore, there is a growing recognition of the need for effective carbon dioxide removal (CDR) strategies to complement emission reduction efforts and achieve the desired climate goals. CDR encompasses various approaches that aim to reduce atmospheric CO
2 levels, including methods for direct extraction of CO
2 from the atmosphere and enhancing carbon sinks on land and in the oceans to enhance CO
2 removal [
4].
Primarily, six technical CDR approaches have been identified for the removal and sequestration of carbon dioxide: coastal blue carbon, terrestrial carbon removal and sequestration, bioenergy with carbon capture and storage (BECCS), carbon mineralization, geological sequestration, and direct air capture (DAC) [
5]. Each of the aforementioned technical methods presents its own set of advantages and disadvantages. Yet, factors such as the land area required, water demand, technology learning curve, scalability, and life-cycle considerations cause DAC to garner more attention than coastal blue carbon and BECCS as a method for carbon removal [
6]. Furthermore, since DAC can also be used in the CO
2 utilization industry, which helps climate mitigation efforts, there is a growing interest in developing and scaling up DAC technology [
7].
Despite this growing interest in DAC technology, the industry is still in its infancy, with a limited number of major players such as Carbon Engineering, Climeworks, and Global Thermostat and a plethora of upcoming startups. However, these companies have their own drawbacks, such as high costs and low scale of operations [
8].
One way to drastically reduce the costs is to bring down the energy requirements of the CO
2 capture process. This, as indicated by several research papers, boils down to choosing the most suitable active material that captures CO
2 and the subsequent method of adsorption–desorption [
9]. Several different functional materials are being used in the industry or investigated at research institutes, for example, aqueous alkali hydroxide solutions, solid amine-functionalized adsorbents, solid oxide-based adsorbents, and membrane-based filters [
10]. Similarly, there are various methods proposed for adsorption–desorption, for example, temperature swing adsorption (TSA), temperature vacuum swing adsorption (TVSA), pressure vacuum swing adsorption (PVSA), moisture swing adsorption (MSA), electro-swing process, and electrolysis [
9,
11,
12]
Amine-functionalized adsorbents that work on TVSA are often proposed as active materials for DAC CO
2 capture [
13]. It is known that the characterization, comparison, and evaluation of solid amine-functionalized adsorbents are critical steps in determining their suitability for CO
2 capture applications. To characterize any adsorbent for CO
2 capture, two types of properties are typically measured: intrinsic properties and performance parameters. Intrinsic properties are textural features of the material that are determined after synthesis and depend on the material’s structure. These properties include pore size, surface area, and pore volume, which play a critical role in the adsorption process. Performance parameters, on the other hand, describe the functional behaviour of the adsorbent, such as the CO
2 uptake capacity, selectivity, degradation over lifetime, and regeneration potential. These parameters are used to evaluate the adsorbent’s effectiveness for CO
2 capture applications [
14].
However, assessing adsorbents for CO2 capture can be challenging because there are many different types of adsorbents available, and each has its own unique properties. Furthermore, different research studies may use varying test conditions, making it difficult to compare the adsorbent’s performance parameters accurately. For example, the temperature, pressure, and gas composition used in the experiments can significantly affect the adsorbent’s performance.
Hence, this study aimed to develop a suitable lab setup and a testing method that can help evaluate and compare amine-functionalized adsorbents under TVSA-DAC conditions for a range of performance parameters. Performance parameters such as temperature, relative humidity, CO
2 partial pressure, and adsorption kinetics are tested to assess the efficiency of the adsorbent. The collected data are then used to explore models that explain the multilayer adsorption of both CO
2 and water (H
2O) on these materials, which is crucial for improving TVSA processes [
15]. The results will be compared with thermogravimetric analysis (TGA) for H
2O and CO
2 adsorption on amines to see if this lab unit offers advantages over the conventional methods of measuring adsorption kinetics. This comprehensive approach aids in developing more efficient materials for carbon capture and utilization applications.
The design of the test equipment is based on state-of-the-art knowledge of DAC technology and amine-functionalized adsorbent materials, as well as practical considerations such as ease of use and integrability. For this study, Lewatit VP OC 1065®, a solid amine-functionalized adsorbent, is used. The equipment, methods, and models developed in this work should also apply to other amine-functionalized adsorbents. The final goal of this research is to contribute to the scaling up DAC technology globally by providing a pathway to develop a suitable setup for testing and developing efficient and cost-effective adsorbents.
2. Material and Methods
A novel DAC unit on a laboratory scale at TU Wien was built and used for the experimental investigations of carbon capture potential of amine-functionalized sorbents under direct air capture conditions. The purpose of this facility was to create an environment in which an adsorbent can be loaded and unloaded with CO2 under specific conditions, thus providing information on the optimum operating conditions for different adsorbents.
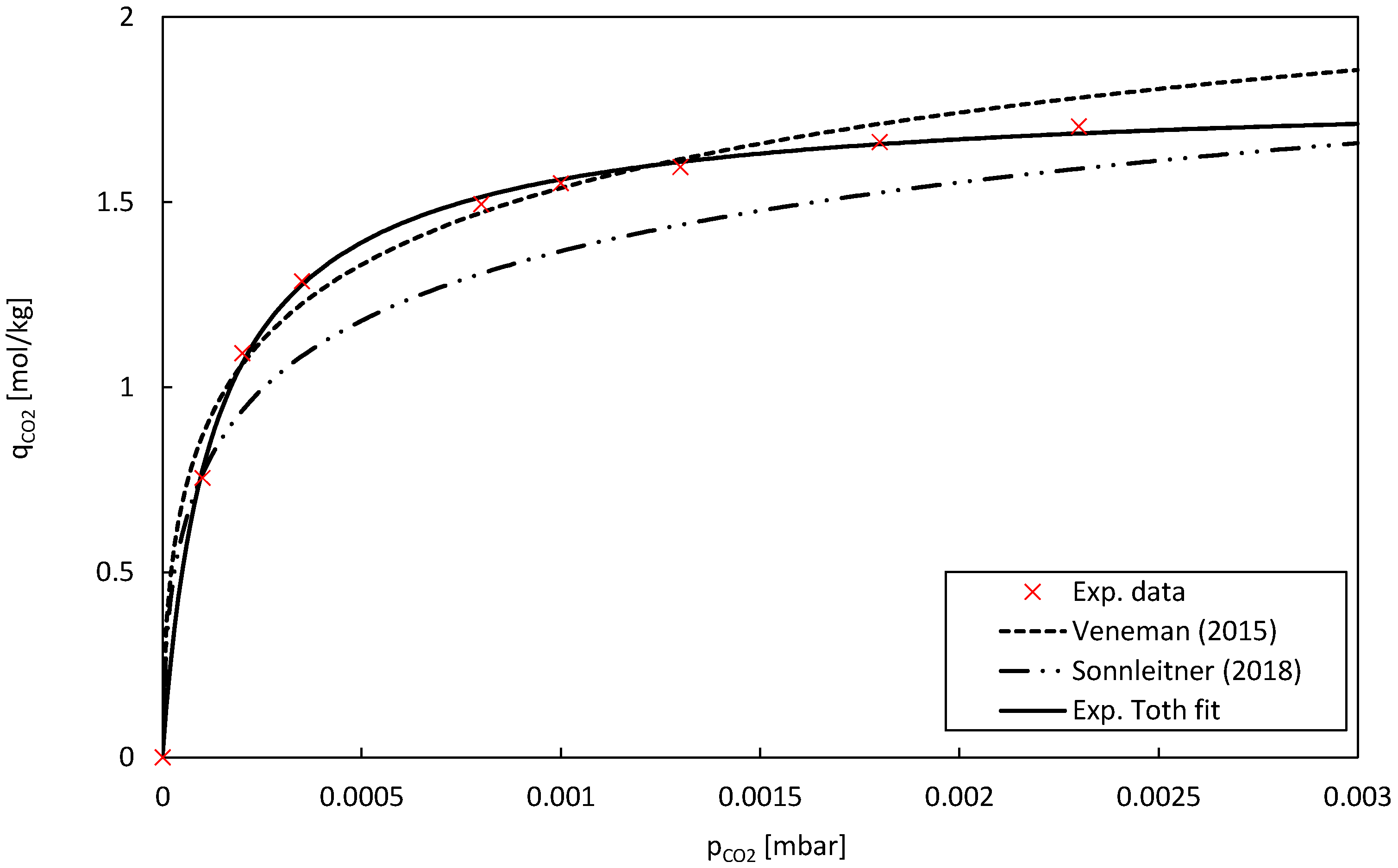
The material used in this study, Lewatit, consists of a polystyrene polymer cross-linked with divinylbenzene and functionalized with primary amine groups. This adsorbent was proposed in a variety of CO
2 capture processes, for example, by Veneman et al. [
16] and Sonnleitner et al. [
17] in a continuous TSA CO
2 capture process, or by Low et al. [
18] in DAC applications, with the latter providing detailed BET analysis of the adsorbent. The key material data obtained from the material datasheet [
19] are presented in
Table 1.
2.1. Laboratory Unit Setup
The experimental setup of the laboratory unit can be divided into two process modes: adsorption and desorption. This unit captures CO2 in batches rather than continuously. For research purposes, this has the advantage that the mass and energy balance can be calculated for both process modes separately.
Figure 1 shows a picture of the DAC unit operating in its desorption mode.
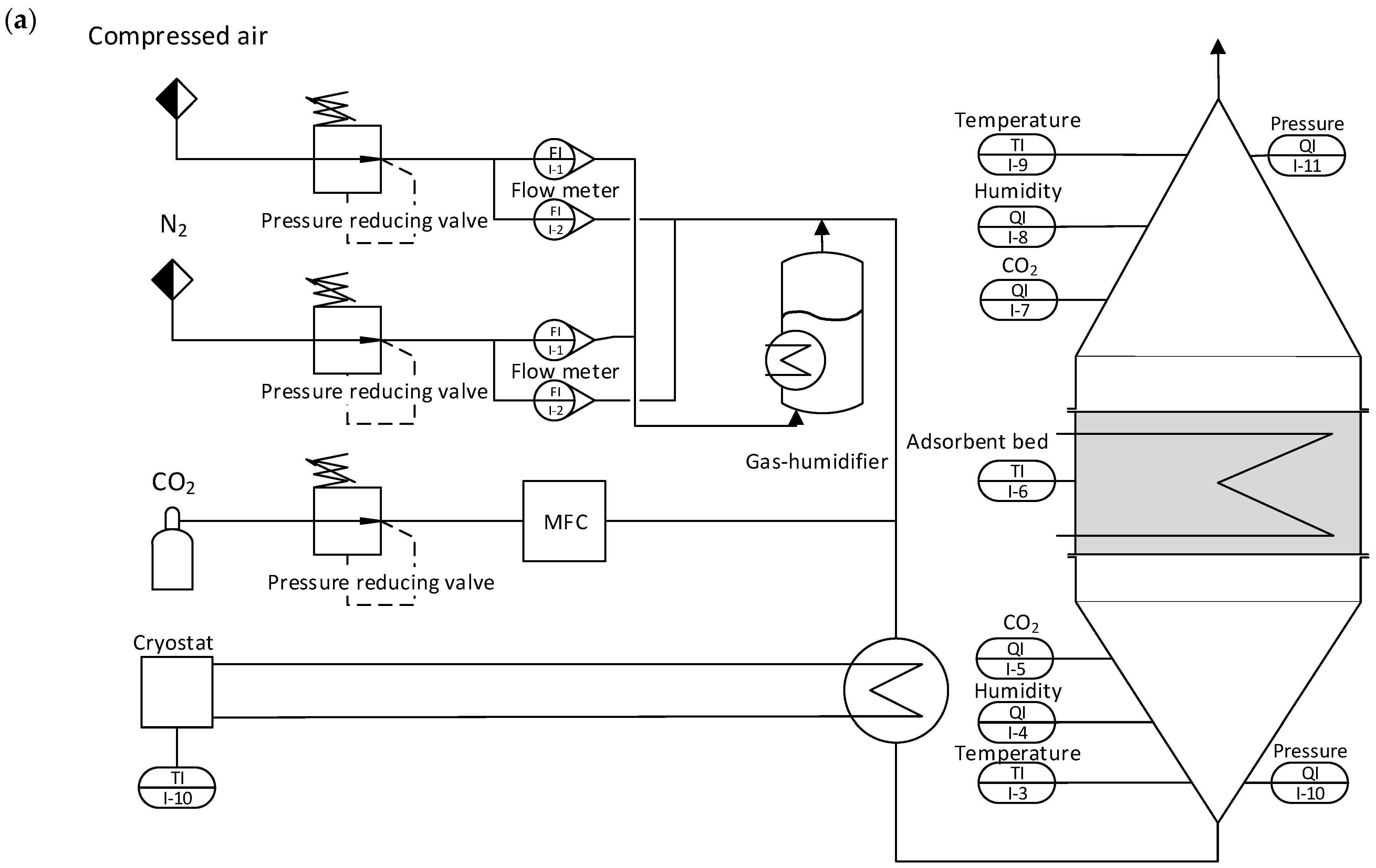
Figure 2a shows a schematic flow diagram of the adsorption setup, and
Figure 2b shows the desorption setup. For the CO
2 loading of the adsorbent, a stream of dry compressed air containing 450 ± 30 ppm of CO
2 is used. To reduce or enhance the CO
2 loading of the gas stream, pure CO
2 or nitrogen can be added via a mass flow controller (MFC). Part of the humidity of the dry gas stream can be sent through a temperature-regulated humidifier to increase the humidity. Before the gas stream reaches the fixed adsorbent bed, it passes through a honeycomb heat exchanger. This enables precise control over the gas stream’s temperature. The fixed bed containing the adsorbent is located between two cones and sealed off, ensuring the adsorbent is only in contact with the gas stream from the heat exchanger.
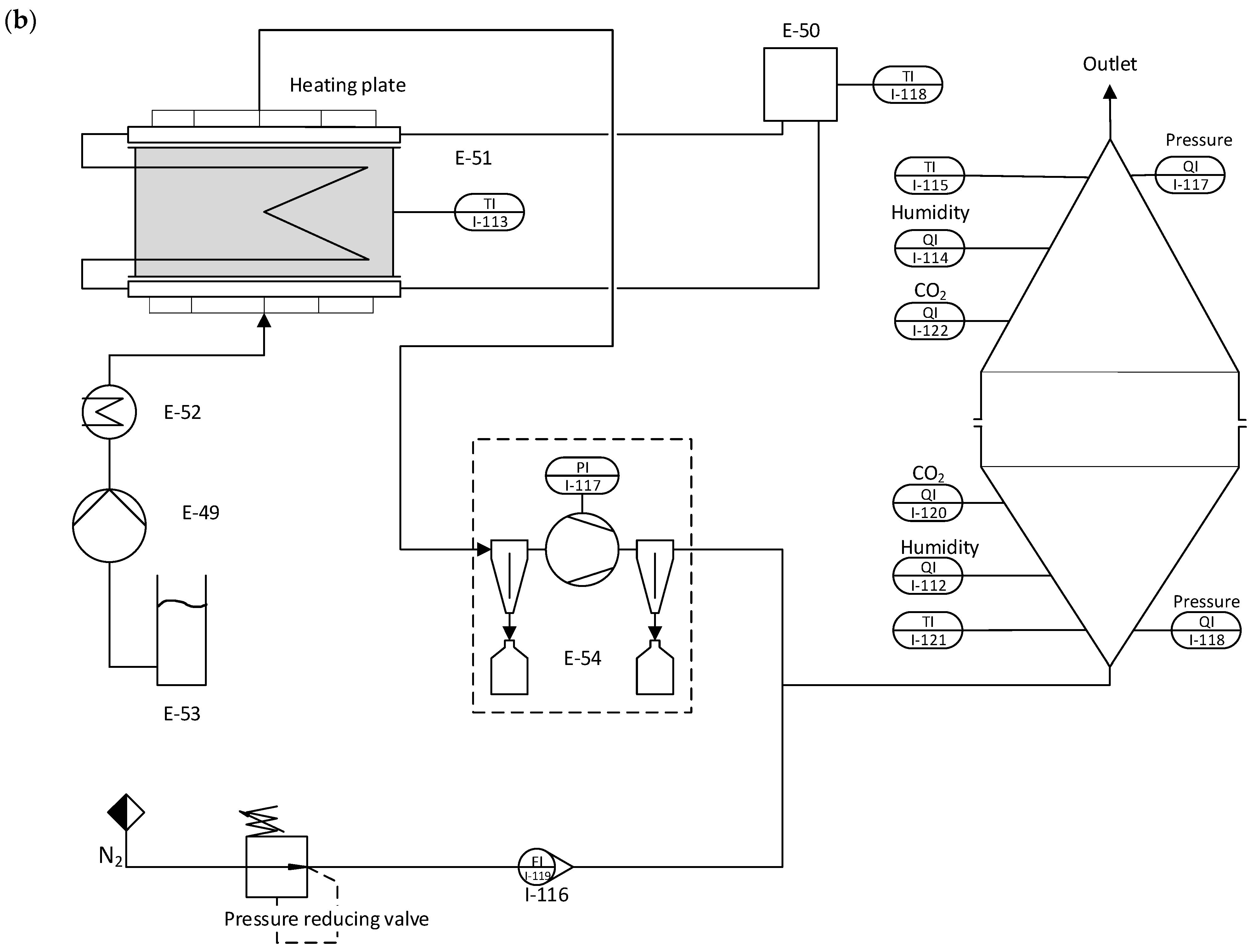
The adsorbent bed is mounted between two heating plates made of aluminum in the regeneration setup, as shown in
Figure 2b. These heating plates feature fine perforations and an intricate gas channelling system. This design efficiently extracts released gas from the fixed bed while ensuring the uniform distribution of purge gas and preventing the formation of channels. Furthermore, the heating plates have additional channels allowing water or thermal oil to be pumped through, allowing the system to regulate the temperature precisely. It is essential to mention that these two channel systems are separated, and no molecular exchange is happening between the water-saturated CO
2 gas stream and the thermal oil. The released gas can be pumped off at the top side on the upper plate, while the purge gas can be introduced at the lower plate. During regeneration, the influence of this purge gas on the desorption process is to be investigated. For this purpose, the apparatus is equipped with a steam generator connected to a membrane pump. The released gas mixture is pumped off via a downstream vacuum pump. A heat exchanger for cooling is installed on the suction side to prevent condensation and overheating in the pump. This heat exchanger collects residual water in the gas stream via condensation. The nitrogen flow is selected so that the downstream CO
2 measurement operates in the intended measuring range (0–10,000 ppm). From the N
2 flow and CO
2 concentration, the absolute amount of CO
2 can finally be determined in the originally extracted gas flow.
Pt100 sensors with a diameter of 3 mm are used to measure the temperature in the fixed bed as well as the temperature of the supply air after the heat exchanger. Due to the low inert mass of the sensors, they react immediately to temperature changes and provide reliable data. The humidity sensors, which are installed in the conical pipes, measure not only the relative humidity but also the temperature. Pressure sensors in the conical pipes monitor the pressure loss across the fixed bed. However, due to the low bed height and low gas velocity, the pressure loss is <1 mbar and can therefore be neglected.
Table 2 shows a list of the sensors used for the investigations. This is sufficient for adsorption measurements before and after the fixed bed. However, since the gas stream from the vacuum pump is almost pure CO
2, it is mixed with a defined quantity of nitrogen. To obtain a homogeneous mixing of the gases and to avoid introducing counterpressure into the vacuum pump, the nitrogen is fed straight through a T-piece. At the same time, the CO
2 flows in laterally through a taper.
2.2. CO2 Adsorption Experiments
All CO2 adsorption measurements were conducted in an aluminum fixed bed column. Approximately 500 g of adsorbent inside the column was tightly packed and fixed in position with wire meshes. The adsorbent was fully desorbed after each adsorption step. All experiments were carried out using TVSA. The experiment consisted of the adsorbent loading and regeneration phase. The adsorbent loading or adsorption phase was initiated by introducing a CO2/N2- or H2O/N2-loaded air stream for the pure component adsorption data or a CO2/H2O-loaded air stream for the co-adsorption data. During each adsorption step, all operating parameters were kept constant and were recorded at 2 s intervals. Equilibrium was reached when the partial pressure of CO2 measured by sensors before and after the adsorbent bed was consistent, indicating that no further adsorption or desorption was occurring. Various heating and cooling systems were used to ensure isothermal conditions during adsorption. The fluidizing humidifier (bubbler) was held at a constant temperature using a PID controller. The separated dry and humid gas streams are reunited before passing through a heat exchanger, which is temperature-controlled via a cryostat.
After the adsorption was completed, the adsorbent bed was placed onto the desorption apparatus, and the initial evacuation step was initiated. This purging step was crucial to removing residual air inside the adsorbent bed and all other connected tubes. This was realized by connecting the adsorbent bed to a vacuum pump and setting an absolute pressure of 50 mbar for one minute. Although some CO2 can be desorbed through physisorption even during an isothermal evacuation, no measurable CO2 was detected in this step. The adsorbent’s regeneration started once the system’s absolute pressure did not rise when the vacuum pump was turned off. This ensured that the system had no leaks and, thus, no false air was passing through the adsorbent bed.
The heating of the adsorbent during regeneration was realized in two different ways. Two aluminum heating plates transfer thermal energy to the fixed bed via heat conduction. Additionally, the fixed bed is heated from the inside via a heat exchanger in the form of bent 6 mm aluminum tubes. As a purging agent, nitrogen or steam can be used. For the latter, the membrane pump transports a defined amount (75 g/h in this study) of water through an evaporator and into the fixed bed. The vacuum pump was set to a specified pressure setting for the desorption duration to ensure a constant gas stream through the adsorbent bed. After fully regenerating, the adsorbent was cooled to approximately 55 °C to avoid sorbent degradation. The desorption conditions were kept the same for the entire duration of the experiment as presented in
Table 3.
2.3. Applied Methods
2.3.1. Adsorption Capacity
The equilibrium loading is determined by balancing according to Equation (1). Adapted for CO
2, the formula is as follows.
where
X is the equilibrium loading of CO
2,
min and
mout are the mass of CO
2 entering and leaving the adsorbent bed during the adsorption period,
mads is the total mass of CO
2 adsorbed, and
madsorbent is the total mass of the adsorbent inside the fixed bed when it is not loaded (fully desorbed). For the entire duration of the experiment, it is assumed that there is a constant mass of CO
2 flowing into the reactor, and thus the total mass of incoming CO
2 min can be calculated. This assumption is justified because no change is made to the reactor inlet stream during this time. To determine the incoming CO
2, the duration of adsorption is multiplied by the concentration measured upstream of the reactor after the experiment, using the ideal gas equation.
where
c is the CO
2 concentration,
is the volumetric flow,
p is the total pressure (1 atm.),
R is the ideal gas constant,
T is the temperature, and
M is the molar mass of CO
2.
The same procedure can be followed for the mass of CO
2 leaving the reactor. Here, the total duration of the adsorption is divided into discrete time intervals with a length of 2 s. For these n time intervals, the concentration measured at the outlet is then used to determine an outgoing mass in this time interval. These partial masses are summed up as shown in Equation (3).
The calculation of the adsorption capacity
follows Equation (4):
2.3.2. Pure Component Adsorption Isotherms
The pure component CO
2 adsorption isotherms were modelled using the temperature-dependent Toth isotherm. For amine functionalized adsorbents, this model has proven accurate for higher CO
2 partial pressures [
16,
20,
21], as well as in low partial pressure regions, as is the case for direct air capture [
9,
22]:
where
ns is the maximum adsorption capacity,
b is the adsorption affinity,
is the partial pressure of CO
2 in the gas phase, and
τ is an exponential factor describing the heterogeneity of the adsorbent called the Toth constant. To obtain the temperature-dependent Toth equation, the aforementioned parameters must also be based on temperature-dependent calculations:
where
ns,0,
b0, and
τ0 represent the values of the Toth parameters at a reference temperature
T0.
χ and
α are dimensionless parameters and ∆
H0 is the isosteric heat of adsorption at zero fractional loading.
For CO
2 capture applications, water adsorption onto solid species is an essential field of study [
23,
24,
25]. The Guggenheim–Anderson–de Boer (GAB) model, an extension of the Brunauer–Emmet–Teller (BET) model, has proven to be the most accurate regarding solid sorbent adsorption. The equation for the GAB model is:
where
is the adsorption capacity at a relative humidity
φ and
nm is the monolayer adsorption capacity of H
2O. The parameters
cg and
Kads are temperature dependent and are calculated as follows:
where Δ
HC and Δ
Hk are the adsorption enthalpies of mono- and multilayer adsorption, and
c0 and
K0 are dimensionless parameters [
26,
27,
28,
29].
2.3.3. Co-Adsorption Isotherms
Previous research has shown that the presence of water enhances the CO
2 uptake of amine-based adsorbents [
16,
30,
31,
32], meaning the CO
2 isotherm becomes steeper, especially in low partial pressure ranges, and the overall maximum uptake increases. There are different approaches in the literature when it comes to describing the co-adsorption of CO
2 and H
2O. In the following paragraphs, three different models are compared to describe this phenomenon.
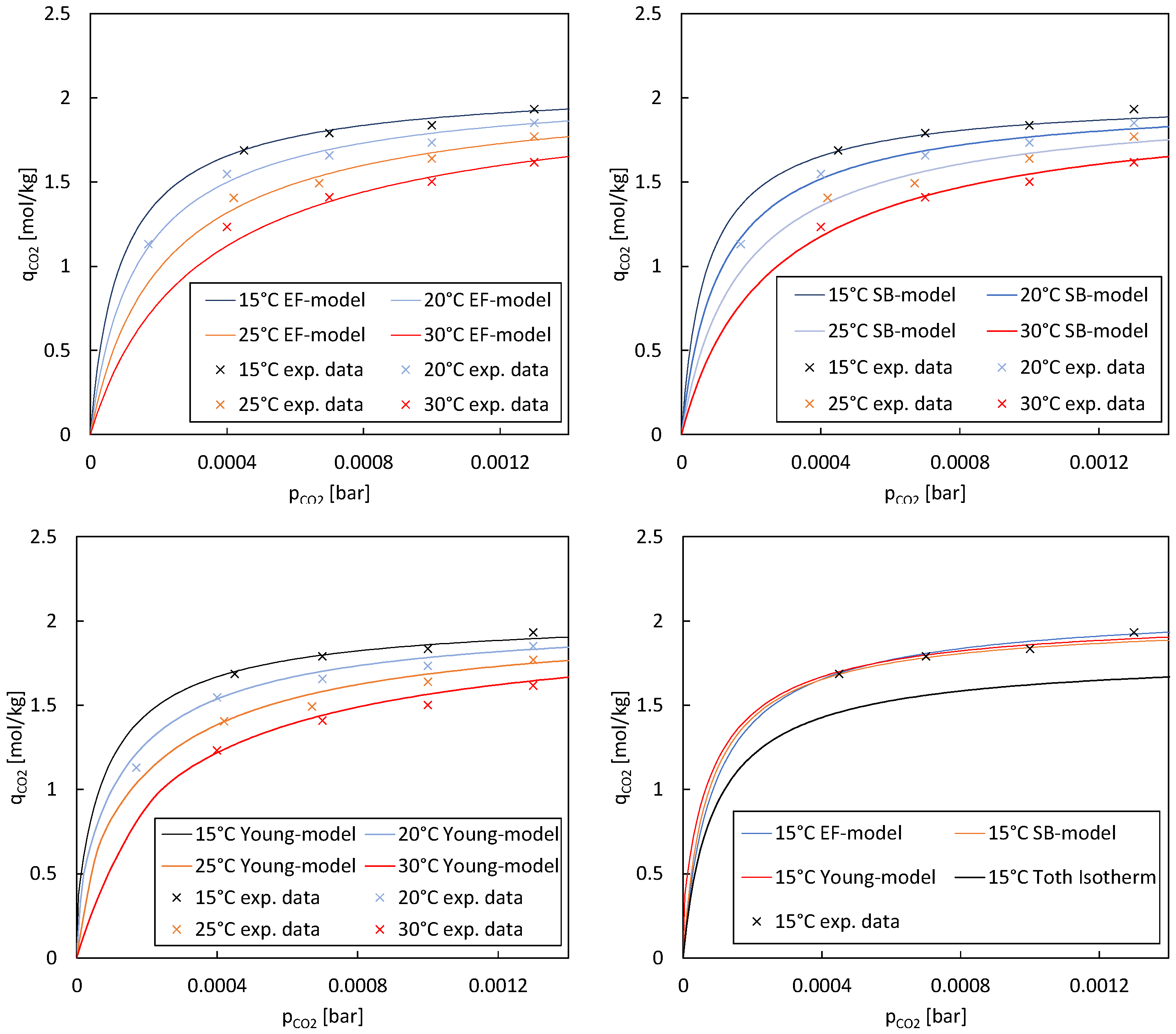
The first approach is empirical, where an enhancement factor (EF) is introduced to describe the adsorption capacity. Wurzbacher et al. [
29] used a similar approach to describe binary CO
2 and H
2O adsorption onto their adsorbent. An enhancement factor
βEF is introduced based on previous adsorption data from Sonnleitner et al. [
17]. This factor includes a constant
k and the relative humidity
φ:
A different approach was followed by Stampi-Bombelli et al. [
28], where a new isotherm model (SB model) based on the Toth isotherm was proposed. The model accounts for the water uptake dependency in the maximum uptake term
ns and the affinity coefficient
b:
This model results in an increased maximum CO2 uptake and isotherm affinity when water is present in the gas and it reduces to the single component Toth model when water is absent.
The third approach is the weighted average dual-site Toth (WADST) co-adsorption model introduced by Young et al. [
27]. This approach is based on the different availability of water molecules at sites on the adsorbent where one site has a water molecule available, and the other does not. The probability that one site has a water molecule available is described via an Arrhenius-style equation described by a critical water loading parameter
A.
The first part of the equation simply describes the Toth model shown in Equation (5) including the fitted parameters from the pure component adsorption isotherms, while the wet site is defined by the same equations and fitted to co-adsorption experiments. While the same model for the adsorption capacity
is used, Equation (9) describes the temperature dependency of
cg and
Kads according to Anderson’s derivation [
33] as follows:
where
E1 refers to the heat of adsorption for the 1st layer,
E2–9 represents the heat of adsorption for the 2nd to 9th layers, and
E10+ corresponds to the heat of adsorption for the 10th layer and beyond, which is comparable to the latent heat of water condensation. The unknown dependencies of temperature (C, D, F, G) on
E1,
E2–9, and
E10+ were empirically fitted to experimental water isotherms for Lewatit.
4. Discussion
In this study, we evaluated three different models, namely, the EF model, SB model, and Young model, to describe the adsorption isotherms of a particular system across various temperatures. We selected these three distinct co-adsorption models from the literature because each offers a fundamentally different approach. By choosing models with diverse theoretical foundations, we aimed to facilitate a comprehensive comparison and evaluation of their respective strengths and limitations. Our analysis focused on the coefficient of determination (R
2) as a measure of how well each model fitted the experimental data (see
Table 7). Our findings revealed notable differences in the performance of the models across different temperatures. The EF model exhibited higher R
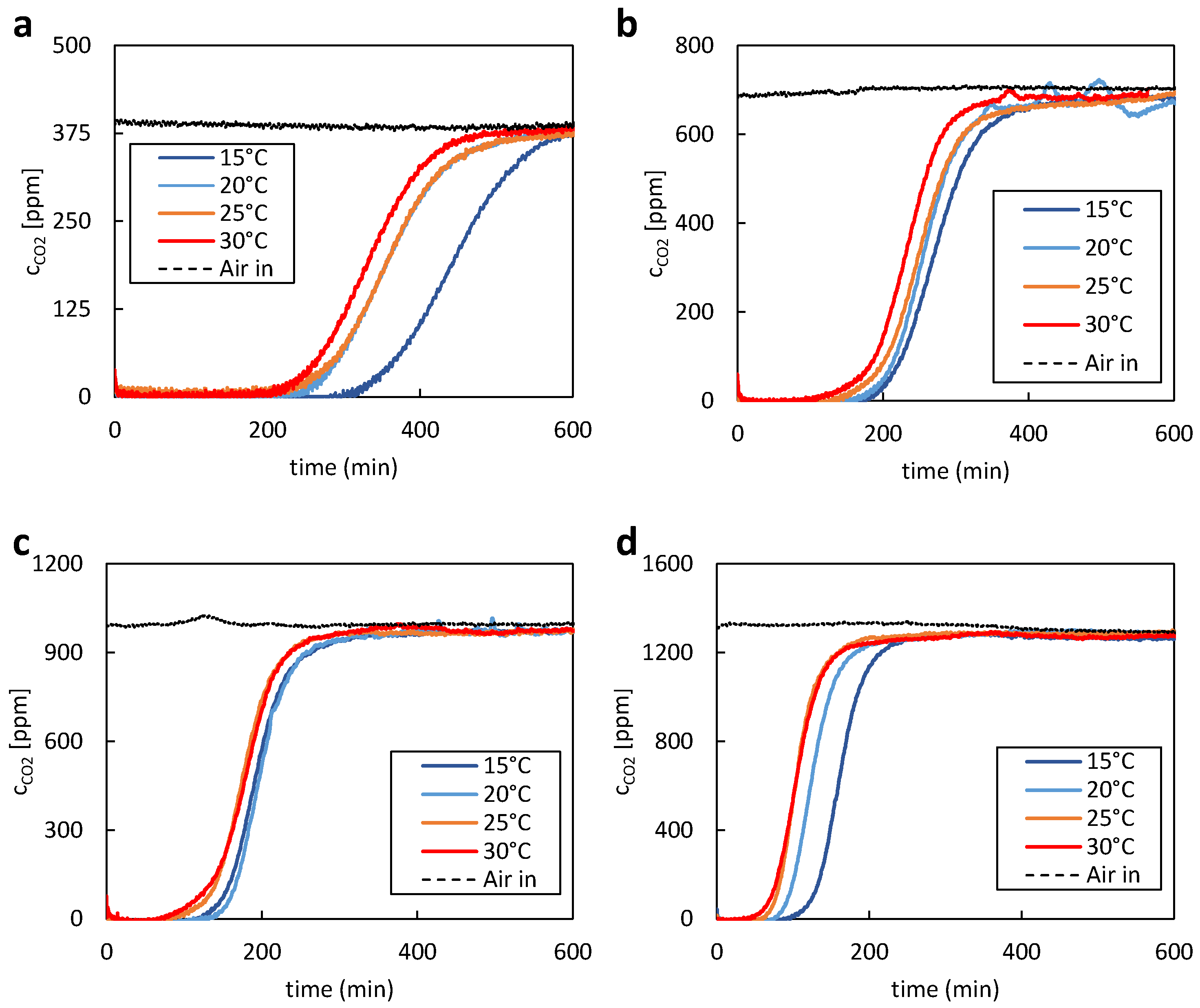
2 values at lower temperatures, such as 15 °C, indicating a better fit to the experimental data in these conditions. Conversely, the SB model and Young model demonstrated more consistent performance across a range of temperatures.
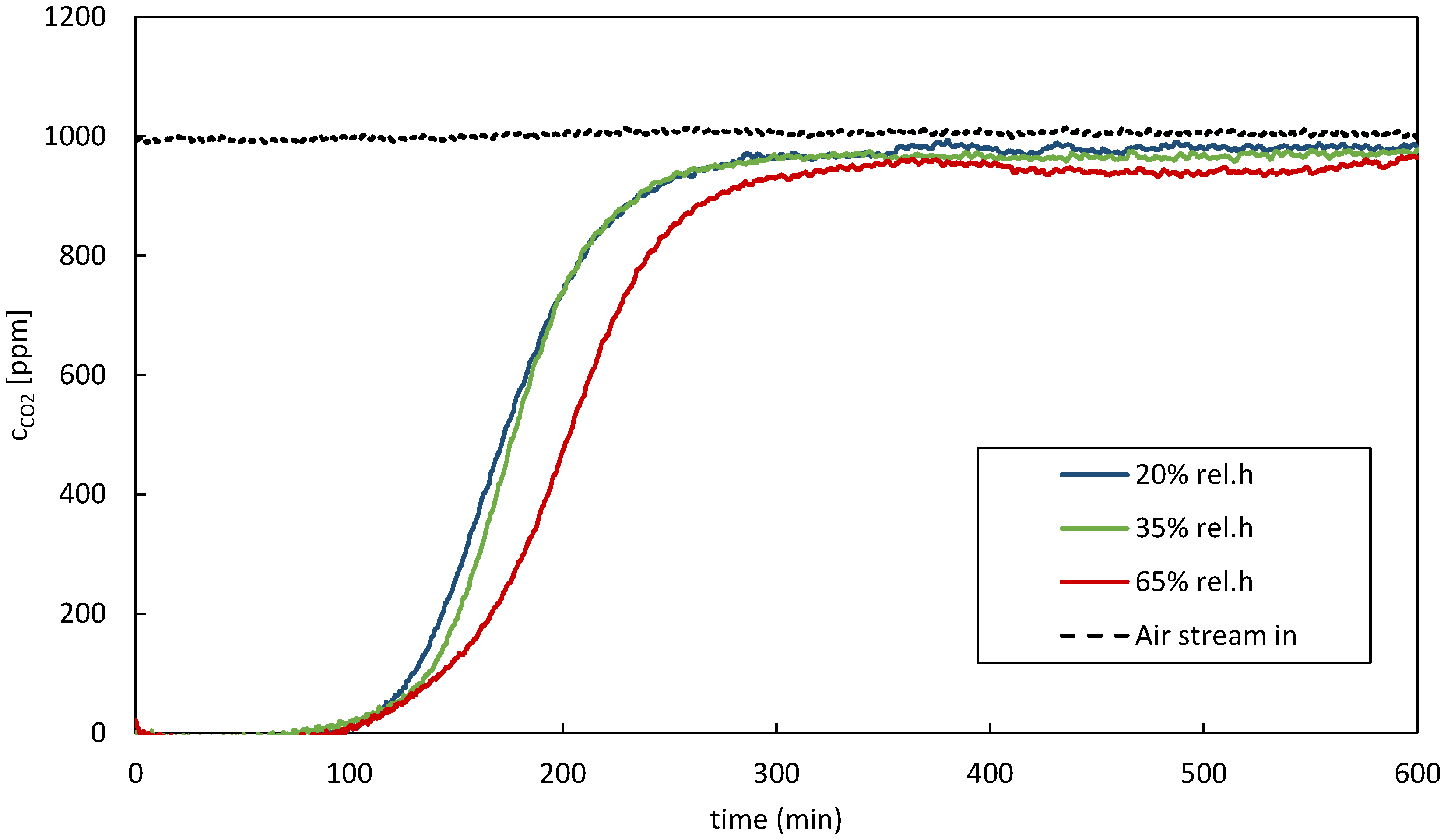
When considering the overall fit across all temperatures, the SB model and Young model emerged as strong contenders, with comparable R2 values suggesting robust performance across varying temperature conditions. These models provided a good representation of the adsorption isotherms, capturing a significant proportion of the variance in the experimental data. These results underscore the importance of evaluating model performance across different temperature regimes. The temperature sensitivity observed in the EF model highlights the necessity of considering temperature effects when selecting an appropriate model for describing adsorption processes. The experimental data exhibited variations and potential sources of inaccuracies. Maintaining constant parameters like temperature, relative humidity, and CO2 partial pressure while investigating the impact of a single parameter proved challenging. It has been found that experimental conditions have a more prolonged effect on adsorption results than just one adsorption and regeneration cycle. To ensure accuracy, multiple measurements were performed under controlled conditions, and the results were validated through comparison with known standards or the literature data. Overall, while experimental measurements may have inherent variability, efforts were made to minimize uncertainties and ensure the reliability of the equilibrium loading and CO2 uptake capacity values reported in the study. However, additional investigations are required to assess the impact of relative humidity on the isotherms.
While the R2 values offer valuable insights into model performance, it is essential to interpret them in the context of other factors. Factors such as the physical basis of the models, simplicity versus complexity, and their ability to generalize to new conditions should also be considered in model selection.
In conclusion, our study provides valuable insights into the suitability of different models for describing adsorption isotherms across varying temperature conditions. The findings contribute to a better understanding of adsorption processes and aid in the selection of appropriate models for predictive purposes in practical applications.
When evaluating the suitability of the laboratory unit compared to TGA, it is important to consider different aspects. The laboratory unit offers several advantages. First, it allows greater flexibility in adjusting parameters such as pressure, temperature, and gas composition. In comparison, TGA usually offers more limited adjustment options. Real-time analysis of dynamic processes cannot be achieved using standard TGA setups, which can be a limitation when studying fast reactions or phenomena. In addition, the laboratory unit can often simulate more realistic conditions specific to the system or process under study. This allows for more accurate modelling of actual adsorption conditions. Another vital advantage of the laboratory plant is that it can accommodate co-adsorption isotherms. In contrast, TGA usually focuses on the adsorption of a single gas. The laboratory system can provide a more realistic representation of complex adsorption processes in which multiple gas components are adsorbed simultaneously by recording co-adsorption isotherms. This is particularly relevant for applications such as gas purification or DAC.
The ability to record co-adsorption isotherms allows more accurate characterization of the interactions between the different gas components and the adsorbent. This allows, for example, a better understanding of synergistic effects or competitive phenomena during adsorption and desorption. This understanding is essential to improve the efficiency of adsorption processes and to determine optimal conditions for the adsorption of specific gas components. Additional conditions and measurements may vary depending on the study’s specific objectives. For example, variations in temperature and pressure can be used to study the effects on adsorption capacity and other adsorption properties. Isotherm measurements at different partial pressures of the adsorbate molecule can be performed to determine adsorption isotherms and better understand adsorption behaviour. Desorption studies allow the investigation of desorption behaviour and provide information on the stability and reusability of the adsorbent.
5. Conclusions
This study aimed to develop a laboratory-scale DAC unit for evaluating and comparing amine-based adsorbents under TSA conditions. The unit was designed to provide information about the optimum operating conditions for different adsorbents and assess their effectiveness and efficiency in capturing CO2 from air.
The experimental campaign conducted with the lab unit allowed for the determination of equilibrium loadings, CO2 uptake capacities, and other performance parameters of the adsorbents. The Toth isotherm model was used to characterize the pure component CO2 adsorption isotherms, while the Guggenheim–Anderson–de Boer model was applied to study water co-adsorption onto the adsorbents. Co-adsorption isotherms were also examined, considering the enhanced CO2 uptake in the presence of water. Three approaches, namely, the EF model, SB model, and Young model, were compared to describe the co-adsorption phenomenon. The results and discussions provided insights into the adsorption performance of the amine-based adsorbents under various test conditions, including temperature, CO2 concentration, and relative humidity. The experiments demonstrated the effect of these parameters on adsorption behaviour and maximum CO2 uptake. In addition, the experimental data validated the SB and Young model to be used in simulation studies. Comparing the results of the lab unit with TGA analysis shows several advantages. The lab unit allows greater flexibility in adjusting performance parameters such as pressure, temperature, and gas composition which allows for more accurate modelling of real-life adsorption conditions. The biggest advantage of this lab setup is that it can accommodate co-adsorption isotherms.
The behaviour of adsorption capacity concerning relative humidity largely depends on the specific properties of the adsorbent material and the gases involved in the process. Overall, relative humidity can impact DAC processes’ performance, energy requirements, and water management aspects. Therefore, it is an important factor to be taken into account when designing and optimizing DAC systems.
Overall, this study contributes to developing more efficient and cost-effective amine-based adsorbents for DAC applications. Providing a comprehensive evaluation and comparison of adsorbents under TSA-DAC conditions paves the way for scaling up the DAC industry globally. Further research can build upon this work by exploring additional adsorbent materials, optimizing operating conditions, and investigating the scalability of the developed lab unit. The continued advancement of DAC technology and the identification of effective adsorbents are crucial steps in mitigating climate change and reducing CO2 levels in the atmosphere.