Phosphate Recovery Mechanism from Low P-Containing Wastewaters via CaP Crystallization Using Apatite as Seed: Seed Adsorption, Surface-Induced Crystallization, or Ion Clusters Aggregation?
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. P Adsorption Experiments
2.3. Titration Experiments
2.4. P recovery Experiments
2.5. Characterization and Analysis
3. Results and Discussion
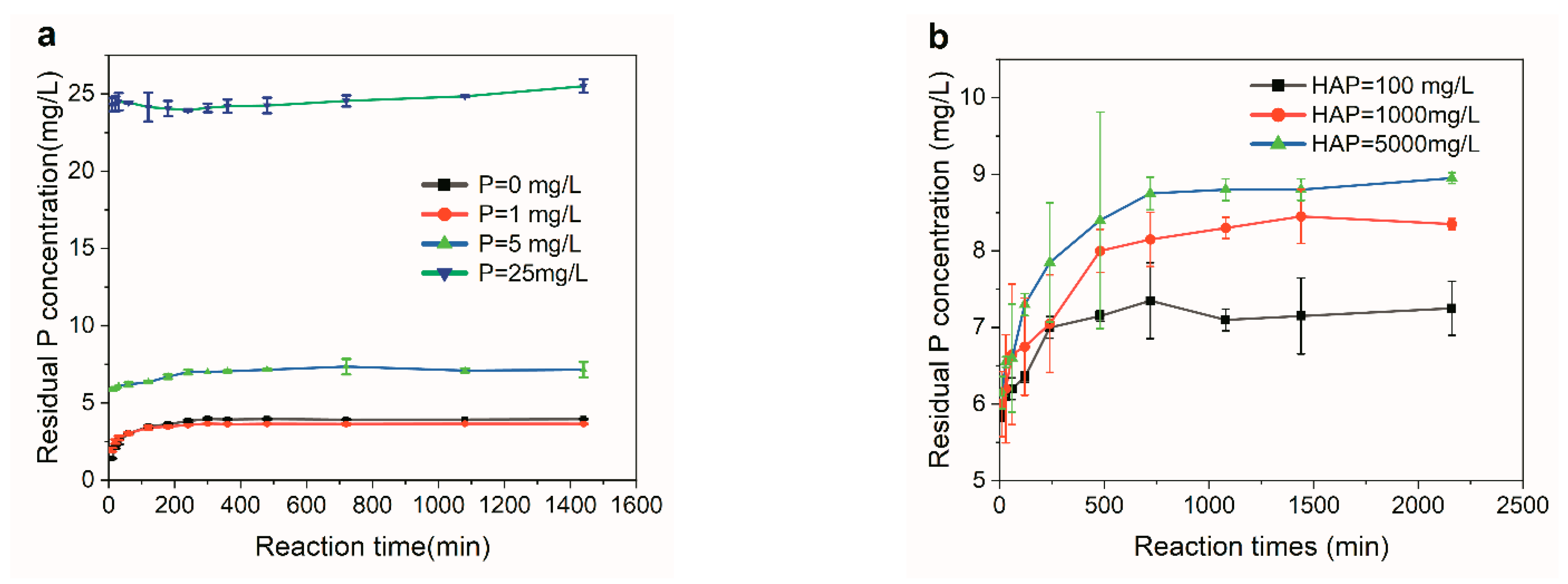
3.1. Kinetics of P Adsorption in LPWs
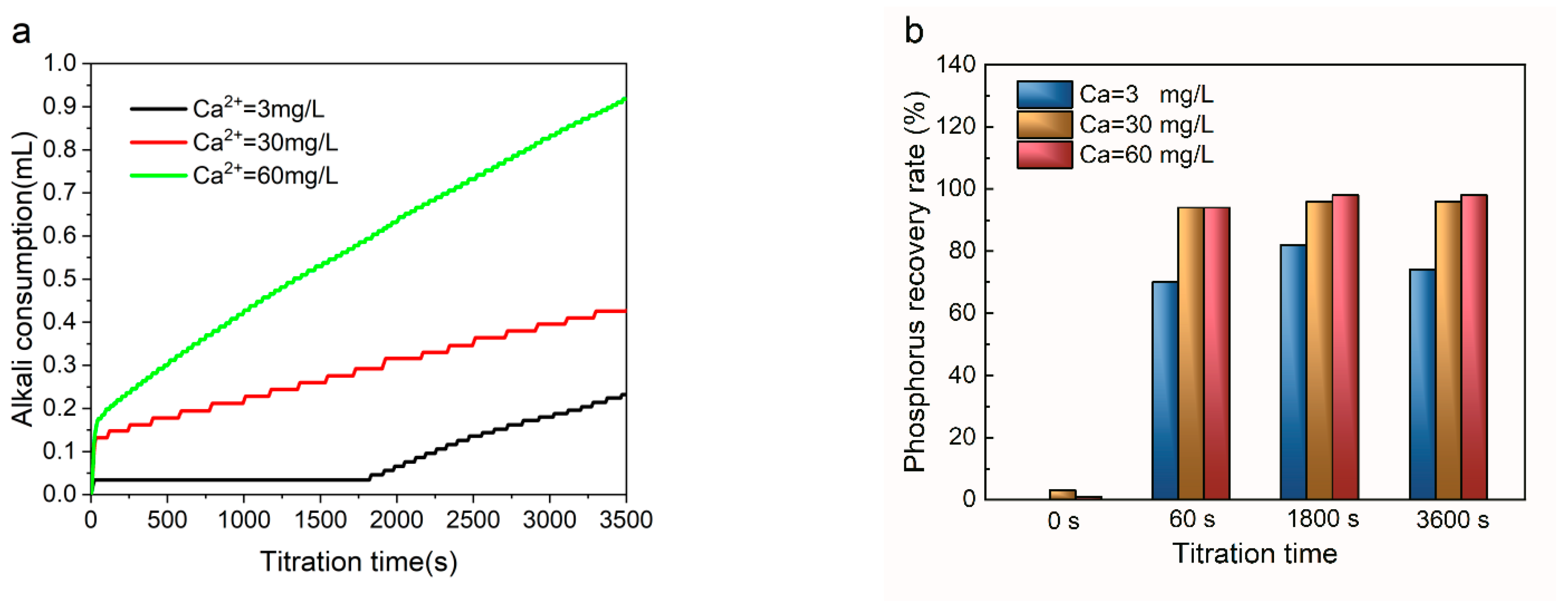
3.2. Role of Ca2+ Introduction
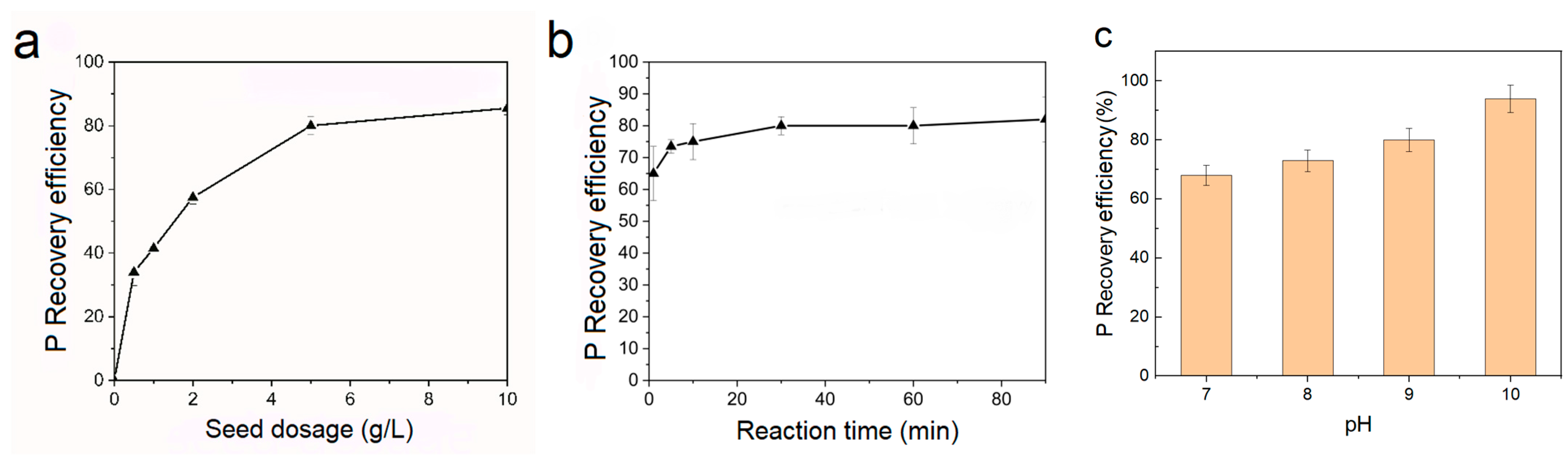
3.3. Effect of Seed Dosage, Crystallization Time, and pH on P Recovery
3.4. Characteristics of Seed Particles and P Recovery Products
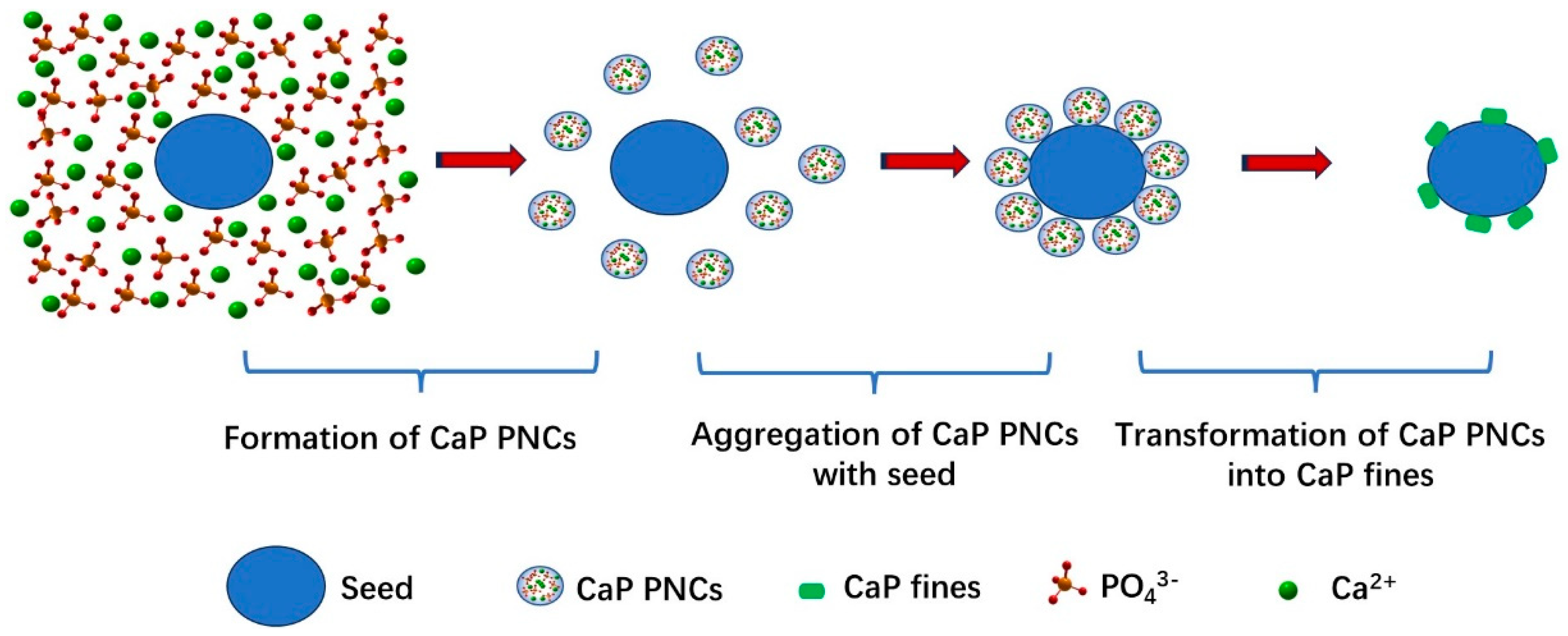
3.5. P Recovery Mechanism from LPWs via CaP Using Apatite
4. Conclusions
- It was HAP dissolution, rather than P adsorption, that occurred when the initial P concentration of LPWs was no higher than 25 mg/L, ruling out the adsorption mechanism for P recovery from LPWs via CaP crystallization using HAP as the seed.
- The dissolution of HAP in LWPs with an initial P concentration no higher than 25 mg/L, an exothermic and entropy reduction driven process, followed the pseudo-second-order kinetic model well. Introducing Ca2+ suppressed the dissolution of HAP effectively, and CaP crystallization occurred.
- It is PNCs’ aggregation, not surface-induced crystallization, that plays the key role in P recovery from LPWs via CaP crystallization using HAP as the seed.
- During PNCs aggregation, P aggregates with Ca2+ quickly, generating CaP PNCs; then, CaP PNCs aggregate with seed particles, followed by CaP PNCs fusion, and they ultimately transform into rod-shaped fines attached to the seed surface.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pap, S.; Zhang, H.Y.; Bogdan, A.; Elsby, D.T.; Gibb, S.W.; Bremner, B.; Taggart, M.A. Pilot-scale phosphate recovery from wastewater to create a fertiliser product: An integrated assessment of adsorbent performance and quality. Water Res. 2022, 228, 119369. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.L.; Yang, J.S.; Weber, M.; Yan, J.; Li, R.H.; Chan, T.S.; Jiang, Y.; Xiao, T.F.; Li, X.Y.; Li, X.D. Phosphate interactions with iron-titanium oxide composites: Implications for phosphorus removal/recovery from wastewater. Water Res. 2023, 234, 119804. [Google Scholar] [CrossRef] [PubMed]
- U.S. Geological Survey. Mineral Commodity Summaries 2021; U.S. Geological Survey: Reston, VA, USA, 2021.
- Capua, F.D.; Sario, S.D.; Ferraro, A.; Petrella, A.; Race, M.; Pirozzi, F.; Fratino, U.; Spasiano, D. Phosphorous removal and recovery from urban wastewater: Current practices and new directions. Sci. Total. Environ. 2022, 823, 153750. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Yin, Z.; Wang, R.D.; Zhu, W.; Zhang, Z.P.; Wang, Y.; Yang, Z.; Liu, F.Q.; Yang, W.B. New Strategy to Remove Phosphate from Low Concentration Solution by MOFs-Modified Resin: High Affinity and Thermal Desorption. Chem. Eng. J. 2023, 465, 142864. [Google Scholar] [CrossRef]
- Singh, A.K.; Kumar, A.; Bilal, M.; Chandra, R. Organometallic pollutants of paper mill wastewater and their toxicity assessment on Stinging catfish and sludge worm. Environ. Technol. Inno. 2021, 24, 101831. [Google Scholar] [CrossRef]
- Xu, M.; Gao, P.; Chen, H.Q.; Huang, X.H.; Xue, Z.X.; Shen, X.X.; Li, C.; Cao, J.S. Spatiotemporal distribution of microorganisms in a full-scale anaerobic baffled reactor–anoxic/oxic treatment plant for printing and dyeing wastewater. J. Water. Process. Eng. 2022, 49, 103090. [Google Scholar] [CrossRef]
- Zou, R.; Tang, K.; Hambly, A.C.; Wünsch, U.J.; Andersen, H.R.; Angelidaki, I.; Zhang, Y.F. When microbial electrochemistry meets UV: The applicability to high-strength real pharmaceutical industry wastewater. J. Hazard. Mater. 2022, 423, 127151. [Google Scholar] [CrossRef] [PubMed]
- Hermassi, M.; Valderrama, C.; Dosta, J.; Cortina, J.L.; Batis, N.H. Evaluation of hydroxyapatite crystallization in a batch reactor for the valorization of alkaline phosphate concentrates from wastewater treatment plants using calcium chloride. Chem. Eng. J. 2015, 267, 142–152. [Google Scholar] [CrossRef]
- Xie, M.; Shon, H.K.; Gray, S.R.; Elimelech, M. Membrane-based processes for wastewater nutrient recovery: Technology, challenges, and future direction. Water Res. 2016, 89, 210–221. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, Y.; Li, D. Adsorptive removal of phosphate from water using mesoporous materials: A review. J. Environ. Manage. 2017, 193, 470–482. [Google Scholar] [CrossRef]
- Rotta, E.H.; Bitencourt, C.S.; Marder, L.; Bernardes, A.M. Phosphorus recovery from low phosphate-containing solution by electrodialysis. J. Membrane. Sci. 2019, 573, 293–300. [Google Scholar] [CrossRef]
- Cheng, P.; Liu, Y.; Yang, L.; Wang, X.; Chi, Y.B.; Yuan, H.L.; Wang, S.B.; Ren, Y.X. Adsorption and recovery of phosphate from aqueous solution by katoite: Performance and mechanism. Colloid. Surface. A 2022, 655, 130285. [Google Scholar] [CrossRef]
- Pronk, W.; Biebow, M.; Boller, M. Electrodialysis for Recovering salts from a urine solution containing micropollutants. Environ. Sci. Technol. 2006, 40, 2414–2420. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Wang, Y.M.; Zhang, X.; Feng, H.Y.; Li, C.R.; Xu, T.W. Phosphate recovery from excess sludge by Conventional Electrodialysis (CED) and electrodialysis with Bipolar Membranes (EDBM). Ind. Eng. Chem. Res. 2013, 52, 15896–15904. [Google Scholar] [CrossRef]
- Zhang, Y.; Desmidt, E.; Looveren, A.V.; Pinoy, L.; Meesschaert, B.; Bruggen, B.V.D. Phosphate separation and recovery from wastewater by novel electrodialysis. Environ. Sci. Technol. 2013, 47, 5888–5895. [Google Scholar] [CrossRef] [PubMed]
- Bellier, N.; Chazarenc, F.; Comeau, Y. Phosphorus removal from wastewater by mineral apatite. Water Res. 2006, 40, 2965–2971. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, Y.Y. Hydroxyapatite crystallization-based phosphorus recovery coupling with the nitrogen removal through partial nitritation/anammox in a single reactor. Water Res. 2020, 187, 116444. [Google Scholar] [CrossRef]
- Xiao, H.Y.; Nie, X.B.; Wan, J.L.; Deng, Q.Q.; Wang, Y.R.; Long, Y.N.; Jiang, C.B. Phosphorous recovery from wastewater with low phosphorous concentration by means of HAP-seeded crystallization of calcium phosphate. China Environ. Sci. 2022, 42, 1681–1687. (In Chinese) [Google Scholar]
- Song, Y.H.; Donnert, D.; Berg, U.; Weidler, P.G.; Nueesch, R. Seed selections for crystallization of calcium phosphate for phosphorus recovery. J. Environ. Sci. 2007, 19, 591–595. [Google Scholar] [CrossRef]
- Karapinar, N.; Hoffmann, E.; Hahn, H.H. Magnetite seeded precipitation of phosphate. Water Res. 2004, 38, 3059–3066. [Google Scholar] [CrossRef]
- Kim, E.H.; Lee, D.W.; Hwang, H.K.; Yim, S. Recovery of phosphates from wastewater using converter slag: Kinetics analysis of a completely mixed phosphorus crystallization process. Chemosphere 2006, 63, 192–201. [Google Scholar]
- Dai, H.L.; Lv, X.W.; Gao, Q.N. Phosphorus recovery from anaerobic supernatant of EBPR process based on HAP crystallization. J. Southeast Univ. 2016, 46, 1020–1026. (In Chinese) [Google Scholar]
- Jang, H.; Kang, S.H. Phosphorus removal using cow bone in hydroxyapatite crystallization. Water Res. 2002, 36, 1324–1330. [Google Scholar]
- Molle, P.; Liénard, A.; Grasmick, A.; Iwema, A.; Kabbabi, A. Apatite as an interesting seed to remove phosphorus from wastewater in constructed wetlands. Water Sci. Technol. 2005, 51, 193–203. [Google Scholar] [CrossRef]
- Boeykens, S.P.; Piol, M.N.; Legal, L.S.; Saralegui, A.B.; Vázquez, C. Eutrophication decrease: Phosphate adsorption processes in presence of nitrates. J. Environ. Manage. 2017, 203, 888–895. [Google Scholar]
- Park, J.H.; Wang, J.J.; Xiao, R.; Zhou, B.Y.; Delaune, R.D.; Seo, D.C. Effect of pyrolysis temperature on phosphate adsorption characteristics and mechanisms of crawfish char. J. Colloid. Interf. Sci. 2018, 525, 143–151. [Google Scholar]
- Søvik, A.K.; Kløve, B. Phosphorus retention processes in shell sand filter systems treating municipal wastewater. Ecol. Eng. 2005, 25, 168–182. [Google Scholar] [CrossRef]
- Lu, S.G.; Bai, S.Q.; Zhu, L.; Shan, H.D. Removal mechanism of phosphate from aqueous solution by fly ash. J. Hazard. Mater. 2009, 161, 95–101. [Google Scholar]
- Leng, L.J.; Zhang, J.Q.; Xu, S.Y.; Xiong, Q.; Xu, X.W.; Li, J.N.; Huang, H.J. Meat & bone meal (MBM) incineration ash for phosphate removal from wastewater and afterward phosphorus recovery. J. Clean. Prod. 2019, 238, 117960. [Google Scholar]
- Yang, M.G.; Shi, J.; Xu, Z.W.; Zhu, S.Y.; Cui, Y.X. Phosphorus removal and recovery from fosfomycin pharmaceutical wastewater by the induced crystallization process. J. Environ. Manage. 2019, 231, 207–212. [Google Scholar] [CrossRef]
- Deng, Q.Q.; Nie, X.B.; Wan, J.L.; Xiao, H.Y.; Xiao, D.F.; Liao, M.F.; Liu, W.Q. Influence of induced-crystallization reactor styles on phosphorous recovery from wastewater with low phosphorous concentration. China Environ. Sci. 2023, 43, 2296–2302. (In Chinese) [Google Scholar]
- Gao, Z.G.; Wu, Y.; Wu, Y.Y.; Gong, J.B.; Bao, Y.; Wang, J.K.; Rohani, S. Self-induced nucleation during the antisolvent crystallization process of candesartan cilexetil. Cryst. Growth. Des. 2018, 18, 7655–7662. [Google Scholar] [CrossRef]
- Gebauer, D.; Kellermeier, M.; Cölfena, H.; Gale, J.D.; Bergström, L. Pre-nucleation clusters as solute precursors in crystallisation. Chem. Soc. Rev. 2014, 43, 2348–2371. [Google Scholar] [CrossRef]
- Kellermeier, M.; Gebauer, D.; Melero-García, E.; Drechsler, M.; Talmon, Y.; Kienle, L.; Cölfen, H.; García-Ruiz, J.M.; Kunz, W. Colloidal stabilization of calcium carbonate prenucleation clusters with Silica. Adv. Funct. Mater. 2012, 22, 4301–4311. [Google Scholar] [CrossRef]
- Ge, X.F.; Wang, L.J.; Zhang, W.J.; Putnis, C.V. Molecular Understanding of Humic Acid-Limited Phosphate Precipitation and Transformation. Environ. Sci. Technol. 2020, 54, 207–215. [Google Scholar] [CrossRef]
- Carino, A.; Ludwig, C.; Cervellino, A.; Müller, E.; Testino, A. Formation and transformation of calcium phosphate phases under biologically relevant conditions: Experiments and modeling. Acta. Biomater. 2018, 74, 478–488. [Google Scholar] [CrossRef]
- Ito, N.; Kamitakahara, M.; Yoshimura, M.; Ioku, K. Importance of nucleation in transformation of octacalcium phosphate to hydroxyapatite. Mat. Sci. Eng. C Mater. 2014, 40, 121–126. [Google Scholar] [CrossRef]
- Song, Y.H.; Hahn, H.H.; Hoffmann, E. The effect of carbonate on the precipitation of calcium phosphate. Environ. Technol. 2002, 23, 207–215. [Google Scholar] [CrossRef]
- Song, Y.H.; Hahn, H.H.; Hoffmann, E. Effects of solution conditions on the precipitation of phosphate for recovery A thermodynamic evaluation. Chemosphere 2002, 48, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.L.; Lv, X.W.; Peng, Y.H.; Zou, H.M.; Shi, J. An efficient approach for phosphorus recovery from wastewater using series-coupled air-agitated crystallization reactors. Chemosphere 2016, 165, 211–220. [Google Scholar] [CrossRef]
- Pap, S.; Gaffney, P.P.J.; Bremner, B.; Sekulic, M.T.; Maletic, S.; Gibb, S.W.; Taggart, M.A. Enhanced phosphate removal and potential recovery from wastewater by thermo-chemically calcinated shell adsorbents. Sci. Total. Environ. 2022, 814, 152794. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.L.; Zhao, C.S.; Wang, Y.P.; Sheng, G.P. Observing the Biologically Induced Phosphate Precipitation by Sludge Extracellular Polymeric Substances in Enhanced Biological Phosphorus Removal. ACS. EST. Eng. 2022, 2, 1514–1522. [Google Scholar] [CrossRef]
- Wang, L.J.; Li, S.; Ruiz-Agudo, E.; Putnis, C.V.; Putnis, A. Posner’s cluster revisited: Direct imaging of nucleation and growth of nanoscale calcium phosphate clusters at the calcite-water interface. Cryst. Eng. Comm. 2012, 14, 6252–6256. [Google Scholar] [CrossRef]
- Garcia, N.A.; Malini, R.I.; Freeman, C.L.; Demichelis, R.; Raiteri, P.; Sommerdijk, N.A.J.M.; Harding, J.H.; Gale, J.D. Simulation of calcium phosphate prenucleation clusters in aqueous solution: Association beyond ion pairing. Cryst. Growth. Des. 2019, 19, 6422–6430. [Google Scholar] [CrossRef] [PubMed]
- Vasenko, L.; Qu, H.Y. Enhancing the recovery of calcium phosphates from wastewater treatment systems through hybrid process of oxidation and crystallization. J. Environ. Chem. Eng. 2019, 7, 102828. [Google Scholar] [CrossRef]
- Cao, X.; Harris, W. Carbonate and magnesium interactive effect on calcium phosphate precipitation. Environ. Sci. Technol. 2008, 42, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Burhenne, L.; Giacomin, C.; Follett, T.; Ritchie, J.; McCahill, J.S.J.; Mérida, W. Characterization of reactive CaCO3 crystallization in a fluidized bed reactor as a central process of direct air capture. J. Environ. Chem. Eng. 2017, 5, 5968–5977. [Google Scholar] [CrossRef]
- Dey, A.; Bomans, P.H.H.; Muller, F.A.; Will., J.; Frederik, P.M.; With, G.D.; Sommerdijk, N.A.J.M. The role of prenucleation clusters in surface-induced calcium phosphate crystallization. Nat. Mater 2010, 9, 1010–1014. [Google Scholar] [CrossRef]
- Yang, X.; Wang, M.Z.; Yang, Y.; Cui, B.L.; Xu, Z.J.; Yang, X.N. Physical origin underlying the prenucleation-cluster-mediated nonclassical nucleation pathways for calcium phosphate. Phys. Chem. Chem. Phys. 2019, 21, 14530–14540. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nie, X.; Li, Y.; Wan, J.; Ouyang, S.; Wang, Z.; Wang, G.; Jiang, H. Phosphate Recovery Mechanism from Low P-Containing Wastewaters via CaP Crystallization Using Apatite as Seed: Seed Adsorption, Surface-Induced Crystallization, or Ion Clusters Aggregation? Separations 2024, 11, 138. https://doi.org/10.3390/separations11050138
Nie X, Li Y, Wan J, Ouyang S, Wang Z, Wang G, Jiang H. Phosphate Recovery Mechanism from Low P-Containing Wastewaters via CaP Crystallization Using Apatite as Seed: Seed Adsorption, Surface-Induced Crystallization, or Ion Clusters Aggregation? Separations. 2024; 11(5):138. https://doi.org/10.3390/separations11050138
Chicago/Turabian StyleNie, Xiaobao, Yinan Li, Junli Wan, Shuai Ouyang, Zhengbo Wang, Guoqi Wang, and Heng Jiang. 2024. "Phosphate Recovery Mechanism from Low P-Containing Wastewaters via CaP Crystallization Using Apatite as Seed: Seed Adsorption, Surface-Induced Crystallization, or Ion Clusters Aggregation?" Separations 11, no. 5: 138. https://doi.org/10.3390/separations11050138
APA StyleNie, X., Li, Y., Wan, J., Ouyang, S., Wang, Z., Wang, G., & Jiang, H. (2024). Phosphate Recovery Mechanism from Low P-Containing Wastewaters via CaP Crystallization Using Apatite as Seed: Seed Adsorption, Surface-Induced Crystallization, or Ion Clusters Aggregation? Separations, 11(5), 138. https://doi.org/10.3390/separations11050138




