Abstract
Bioleaching of Cu from the copper concentrate of Armanis gold-bearing polymetallic ore (Armenia) was investigated. The main objective was revealing high active bacteria and their association, as well as optimizing the bioleaching process with their application to ensure the most efficient recovery of copper from the tested concentrate. To obtain optimal bacterial associations, bottom-up and top-down approaches were used. Bioleaching of copper concentrate was carried out using pure cultures of iron- and sulfur-oxidizing bacteria and their mixed culture, as well as indigenous bacterial consortium. Comparative studies of copper bioleaching by mixed cultures of Acidithiobacillus caldus, Leptospirillum ferriphilum CC, Sulfobacillus thermosulfidooxidans 6, and indigenous consortium Arm of iron-oxidizing bacteria were performed. At the beginning of bioleaching, the amounts of extracted copper by mixed culture and Arm consortium were equal; afterward, between 20–27 days, the Arm indigenous consortium showed significantly higher activity in terms of copper extraction. In parallel, mineralogical and liberation analyses of feed material and bioleaching residues were performed.
1. Introduction
Copper is a metal that is widely used in everyday life [1]. It is required for various applications, including construction, electric power transmission, electronic communications, aerospace, and scientific equipment. Due to the rising global demand for copper and the ongoing depletion of high-grade copper deposits, the mining industry requires methods for the exploitation of low-grade primary copper reservoirs and secondary copper resources (electronic and other copper-containing waste materials and scrap copper) [2,3].
There has been great interest in hydrometallurgical methods for extracting metals from sulfide minerals over the past few decades. For the recovery of metals from ores, concentrates, and recycled or residual materials, bioleaching, a branch of hydrometallurgy, uses the activity of microorganisms in aqueous extractive metallurgy [4]. The advantages of bioleaching over conventional techniques include minimal investment, comfortable operating conditions, energy efficiency, and sustainability [5,6]. Bioleaching is an effective alternative for increasing copper recoveries, particularly from low-grade ores and refractory concentrates that are difficult to process using conventional technologies.
Microorganism-based metal recovery from low-grade ores and mineral concentrates is an important and rapidly developing field of biotechnology [7]. Using iron- and sulfur-oxidizing microorganisms to catalyze the dissolution of valuable metals from sulfide ores or concentrates is known as bioleaching [8,9,10,11].
More than 40 different types of bioleaching microbial species have been identified, according to Baba et al., 2011 [12]. The leaching process is influenced by a wide range of physical and chemical factors, including pH, temperature, redox potential, mineral composition, and particle size distribution. One of the main elements influencing the efficiency of bioleaching is microorganisms. The primary agents in the bioleaching process are acidophilic bacteria and archaea that can metabolize iron and reduce inorganic sulfur compounds (RISC). These microorganisms help the material dissolve by producing the oxidizing agent ferric iron (Fe(III)) and then oxidizing the liberated sulfur compounds from the mineral to sulfuric acid [13]. The utilization of natural communities that are already acclimated to the conditions of the ore appears to be more effective than the application of exogenous cultures of acidophilic mesophiles and moderate and extreme thermophiles [1].
Acidithiobacillus ferrooxidans is a significant microorganism that acts through both direct and indirect mechanisms. The first refers to the oxidation of sulfur and sulfide by bacteria attached to a particle surface. The latter is related to the bacterial oxidation of ferrous ions, which results in the oxidation of sulfur compounds by ferric ions [14,15]. A. ferrooxidans is an extremophilic Gram-negative bacteria able to grow under mesophilic conditions, fixing CO2 and oxidizing iron and/or sulfur compounds. This species is typically found around the world in acidic environments and has undergone substantial research over the years [16,17]. Additionally, its uses in biomining are well-reported [18,19,20]. Leptospirillum ferrooxidans is more prevalent than A. ferrooxidans under various conditions, and it appears to be the dominating bacteria in industrial continuous-flow bio-oxidation tanks [21]. In both natural and technological environments, it is believed that the highly acidophilic bacterium Acidithiobacillus thiooxidans is a key player in the oxidation of sulfur and RISCs [22,23]. At the moment, several other obligate autotrophic acidophilic bacteria capable of oxidizing elemental sulfur have been isolated [24,25,26]. Among them, only Thiobacillus albertensis [25] and Acidithiobacillus caldus [27] are described as distinct species different from A. thiooxidans. Some researchers claim that the primary sulfur-oxidizing bacterium in the reactors used for the bio-oxidation of gold concentrates, which run at 40 °C, is A. caldus [28,29]. According to the authors, A. caldus serves as a member of the community of chemolithotrophic bacteria by bio-oxidizing arsenopyrite to dissolve the sulfur-based inhibitory layer that forms on the mineral surface or by facilitating the mixotrophic and heterotrophic growths of other sulfur- and iron-oxidizing bacteria [30].
Through cooperative bioleaching, sulfur- and iron-oxidizing microorganisms are frequently mixed and inoculated into leaching systems. This is an effective method for increasing the efficiency of sulfide leaching [12,31,32,33,34]. A. ferrooxidans, A. thiooxidans, and L. ferrooxidans are three acidophilic bacterial consortia that have recently demonstrated promising results for copper extraction from low-grade ores and have been recommended for use in large-scale heap bioleaching processes [35,36].
Another successful strategy for increasing leaching effectiveness is the use of native microorganisms [37]. Using indigenous microorganisms, numerous researchers have achieved higher leaching efficiencies [38,39]. Native microorganisms are anticipated to improve leaching efficiency and metal recoveries more than exogenous strains because they are more suited to the site’s mineral composition. This is predicated based on the existence of poisonous trace elements found in the deposit, which must have modified a population with distinct and enhanced resistance mechanisms [40]. Indigenous bacteria in the ore, however, are frequently not eliminated and may therefore contribute to bioleaching. It is frequently seen that indigenous bacteria that have been accustomed to higher concentrations of particular metals in their environment function better as bioleaching catalysts [41]. Native microorganisms collected from the same location are thought to be more effective at recovering metals from ores because indigenous bacteria are more compatible with the mineralogy of the rocks [42].
A “bacterial consortium” is a combination of various bioleaching bacterial species. Bacterial consortia are known to be modified according to a particular type of mineral and its environmental conditions [14]. Each type of ore has distinct properties, such as a particular strain or a group of bacterial strains, that work best with a specific mineral but are useless with other ore types [43]. Because of this, the industry continues to invest in a range of research to uncover novel strains that accomplish optimal bacterial bioleaching, even though numerous bioleaching strains have already been found and patented [14].
Bioleaching processes are already in use in the commercial sector, particularly for copper recovery [44,45]. Despite an increase in copper recovery through bioleaching across the globe, Armenia has no commercial experience with bioleaching operations, and only a few local ores have been examined at the bench scale.
Recovery of suitable native microbes from environmental samples is difficult. The study on batch leaching experiments was extended in this paper to assess the feasibility of using native indigenous consortia to maximize the rates of Cu solubilization from copper concentrate. A comparison of copper concentrate leaching using native indigenous isolates and previously isolated strains was presented. To ensure the most effective copper recovery during the bioleaching of the tested copper concentrate, this work aimed to establish an ideal bacterial association using bottom-up and top-down approaches via pure cultures and native indigenous consortia. Additionally, this research was aimed at determining whether it is feasible to use natural indigenous consortia to extract copper from copper concentrate in Armanis. The Armanis deposit has been mined since 2007, first in small underground tunnels and then almost continuously in open-pit mining until 2013. Concentrate samples were taken in the Armanis concentrator plant in 2022. Leaching tests were conducted to assess copper recovery under abiotic circumstances. To maximize biohydrometallurgical methods for the recovery of copper from Armanis and other copper-rich deposits, this study seeks to be a beneficial resource.
2. Materials and Methods
2.1. Ore Sample
The copper concentrate used in this study was obtained from the Armanis gold-polymetallic mine (Lori region, Armenia) and was ground to obtain a particle size of 45–80 µm. The chemical composition (Cu, Fe, Si, Al, Mg, Ca, Mn, Ni, Co, Ti, and Mo) of the concentrate is presented in Table 1.

Table 1.
Content of base and associated metals in copper concentrate samples and corresponding bioleaching residues. (SD = standard deviation).
2.2. Microorganisms and Culture Conditions
A. caldus (ATCC 51756), Leptospirillum feriphillum CC (OM272948), Sulfobacillus thermosulfidooxidans 6 pure acidophilic, and a newly obtained indigenous consortium “Arm” were used in this study. All strains were cultivated aerobically in a sterile Mackintosh medium (MAC) [46]. FeSO4·7H2O (20 g/L) was used as an energy source for ferrous oxidizers and S0 (10 g/L) for sulfur oxidizers. In addition, 2.0% (w/v) yeast extract was added for the moderate thermophile S. thermosulfidooxidans 6.
Acid mine drainage (AMD) water samples of the Armanis gold–polymetallic mine were used to obtain microbial enrichments (Figure 1).

Figure 1.
AMD generation in Armanis gold–polymetallic mine.
For this purpose, MAC medium containing FeSO4·7H2O and S0 as sources of energy at pH 2.0 were inoculated by AMD water samples and incubated at 37 °C for 5–7 days. The obtained indigenous consortium Arm was used for the bioleaching of copper concentrate.
L. feriphillum CC, S. thermosulfidooxidans 6, and indigenous consortium “Arm” were separately grown in a 1 L MAC medium containing FeSO4·7H2O as a source of energy for 5–7 days. Additionally, in the case of S. thermosulfidooxidans 6, 0.02% yeast extract was added to the medium. Sulfur-oxidizing bacteria A. caldus was grown in a MAC medium containing S0 as a source of energy.
In the logarithmic phase of growth, the cells were collected by centrifugation at 10,000× g for 20 min. The biomass collected was washed with an acidified MAC medium without Fe2+ and resuspended in the same medium. The inoculum of each culture containing 105–108 cells/mL was added to the flasks for bioleaching experiments.
Bacterial cell counts were performed using phase-contrast microscopy (Optika, Ponteranica, Italy) and a Thoma counting chamber, as well as the 10-fold dilution method. The McCready Tables were used to calculate the most probable number (MPN) of bacterial cells [47].
Total DNA from the indigenous consortium Arm was extracted and sequenced by 16S rDNA to analyze the microbial community.
2.3. Morphology and SEM Images
For SEM studies, the indigenous consortium Arm was grown in a MAC medium, at pH 3.0, containing ferrous iron at 40 °C. Then, bacterial culture was filtered onto a 0.2 µm pore-size membrane. Sample preparation for SEM observation was as follows: the surfaces were exposed to 3% (w/v) glutaraldehyde buffered with 0.1 mol/L phosphate buffer for 2 h and dehydrated in gradient ethanol solutions (50, 70, 90, and 100%) for 15 min. The plates were then dried to a critical point and coated with gold. To observe the samples, a Hitachi SU8100 cold field emission scanning electron microscope operating at 15 kV was used.
2.4. Bioleaching Assays
For bioleaching assays, a sample of copper concentrate was crushed and ground in successive steps until particle diameters ranged from 45–80 μm.
The bioleaching of Armanis copper concentrate was carried out in 250 mL Erlenmeyer flasks containing 100 mL of MAC medium, with an initial pH of 1.5–2.0, 1.0, 2.5, and 5% pulp densities (PD), and with an 80 µm particle size. Each culture was inoculated with 10% (v/v) inoculum. The experiments were carried out at 40 °C and at 180 rpm. In addition, the experimental procedure for the abiotic control was also conducted, which was identical to the bioleaching experiments, except that the inoculum was not added. Bioleaching experiments lasted for 31 days. All tests were conducted in triplicate.
The efficiency of bioleaching was determined by measuring soluble copper, total and ferrous iron, redox potential, and pH over time. A digital pH meter (Mettler Toledo, Riga, Latvia) was used to determine the pH value. A Pt electrode was used to measure ORP to an Ag/AgCl reference electrode (mV (vs. Ag/AgCl)). Atomic absorption spectrophotometry AAS was used to determine the amount of copper and total iron in the solution (released from the concentrate during the assays), while complexometric titration with EDTA was used to determine the concentration of ferrous and ferric iron [48]. The pulp density was calculated as the copper concentrate mass ratio to the medium volume.
2.5. Mineralogy and Geochemistry of Concentrates and Residues
Concentrates from the Armanis open-pit mining operation situated in the Lori Province, northern Armenia, were used for bioleaching tests in the present investigation. The determination of modal mineralogical and liberation analyses was carried out on polished thick sections (300 mm thickness) prepared from rock specimens. The geochemical composition was determined on granular sample splits using a portable X-ray fluorescence analyzer (Niton XL3 GOLDD, Thermo Scientific, Waltham, MA, USA), with 10 readings for each sample (settings: main filter, 30 s reading time) and normalization by approved standards.
Scanning electron microscopy (SEM) was used to identify grain properties. SEM-based automated mineralogy is a well-established tool in process mineralogy [49,50]. Mineral distribution analyses, as well as free surface liberation of chalcopyrite particles, were obtained using an Advanced Mineral Identification and Characterization System (AMICS) on a Hitachi TM4000 + SEM equipped with a Bruker XFlash 630H EDX-detector. SEM-based parameters for the image segmentation were 1024 px resolution, 150 ms dwell time per segment, and 15 kV excitation voltage. Data from at least 8 measurement areas per sample were averaged.
3. Results and Discussion
3.1. Taxonomic Composition
The microbial composition of the Arm indigenous consortium showed that the native sample was dominated by genera of Leptospirillum and Acidithiobacillus. The proportion of Leptospirillum (approximately 70%) in the Arm was much higher than Acidithiobacillus (10%) (Figure 2).
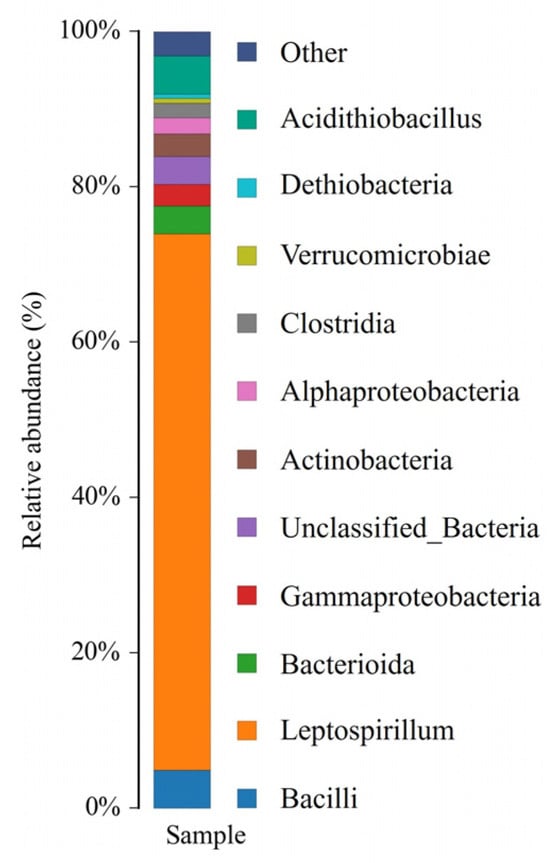
Figure 2.
Microbial composition of the Arm indigenous consortium.
3.2. SEM Micrograph
Rod- and vibro-shaped cells were clearly visible in the SEM micrograph, which is typical for the genera of Leptospirillum and Acidithiobacillus. Thus, as shown in Figure 3, vibrio-shaped cells dominated in the Arm indigenous consortium of iron-oxidizing bacteria.
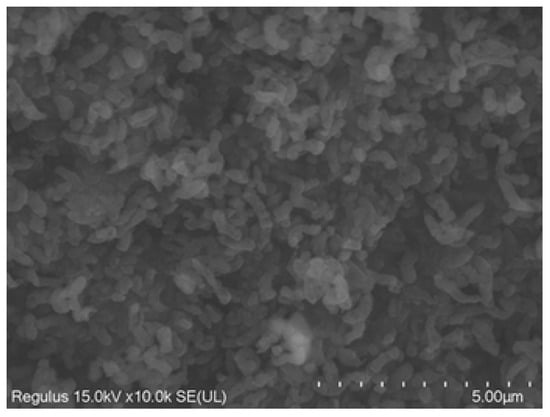
Figure 3.
SEM micrograph of the indigenous consortium of iron-oxidizing bacteria Arm. Cells were grown on ferrous iron at 40 °C for 5 days. Bar represents 5 µm.
3.3. Influence of Pulp Density (PD) on Bioleaching of Copper Concentrate from Armanis Gold-Polymetallic Mine
Bioleaching of copper concentrate was performed by the indigenous consortium of iron-oxidizing bacteria Arm, depending on pulp density (PD). As shown in Figure 3, a significantly higher percentage of extracted copper throughout the dissolution period was observed at PDs of 2.5 and 5.0% for 20 h (Figure 4).
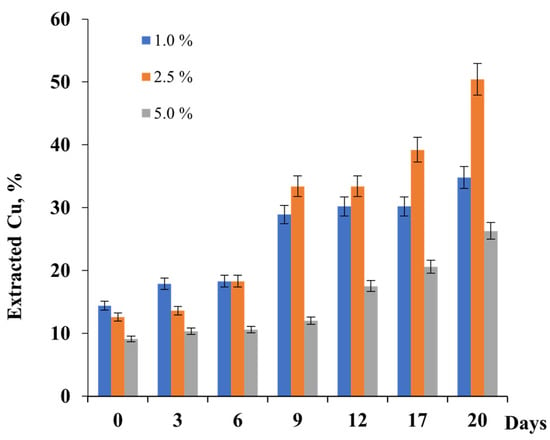
Figure 4.
Bioleaching of copper from copper concentrate by the consortium of iron-oxidizing bacteria Arm-12 at different PDs.
The extents of copper extraction by the Arm indigenous consortium were 34.8, 50.4, and 26.3% at 1, 2.5, and 5.0% of PD for 20 days of bioleaching (Figure 4). As shown, data obtained increasing in PD from 1 to 2.5% led to an enhancement of copper recovery. In the case of 5.0% PD, a decrease in copper extraction from the copper concentrate was observed. Thus, the optimal PD for copper extraction was 2.5%. Olubambi et al., 2007 [51] and Rouchalova et al., 2020 [52] showed in their studies that at a higher value of pulp density, the percentage dissolution of copper decreased. This was also confirmed in the studies of Makita et al., 2004 [53] and Abhilash et al., 2013 [54], when a higher percentage of Cu was achieved with a smaller amount of solid. The conclusions of our studies corresponded to the literature data, where the increasing of pulp density resulted in a decrease in metal leaching.
3.4. Mineralogical Analysis of Feed Material and Residue after Bioleaching
The composition of Armanis copper concentrate (feed material) and bioleaching residues are presented in Table 1.
The concentrates from the Armanis concentrator plant were rich in base metal sulfides and mainly composed of chalcopyrite (CuFeS2), galena (PbS), pyrite (FeS2), and sphalerite (ZnS), as well as gangue minerals such as quartz and orthoclase (Table 2). As shown in Table 2, they contained approximately 36 wt.% chalcopyrite, 12 wt.% galena, 21.6 wt.% pyrite, and 14.4 wt.% sphalerite.

Table 2.
Modal mineralogy of concentrates and corresponding bioleaching samples. Standard deviations are in brackets.
Residues after bioleaching by indigenous consortium Arm also contained significant amounts of jarosite (KFe3(SO4)2(OH)6), which is formed during the bioleaching process. Accessory minerals comprised mainly chlorite and titanite.
Table 3 shows the recovery of sulfide minerals achieved by bioleaching tests that quantify their content in the bioleaching to their initial content in the feed concentrate. The recovery represents the proportion of a mineral dissolved in the individual leaching experiment. The dissolution of chalcopyrite was very limited, generally below 20%, whereas at 5% PD, the leaching rate was the highest (19.1%).

Table 3.
Recovery rates of base metal sulfides in bioleaching residues of Armanis concentrates.
The leaching efficiency for galena was low, due to its leaching resistance caused by rapid amouring by lead sulfate in acid conditions. Therefore, galena generally showed a relative enrichment in the bioleaching residues. The dissolution of pyrite was limited to 22.7% at maximum at PD 2.5%, but the lowest recovery rate of pyrite (11.6%) was observed in the bioleaching residue with PD 1.0%. Sphalerite showed the most advanced dissolution and thus, the best recovery rates, in particular with nearly complete dissolution (>95%) at 2.5% and 5% PD; just a 63.9% recovery of sphalerite was achieved at the bioleaching test with 1.0% PD.
The liberation analysis revealed the intergrowth state of grains of a specific mineral with other minerals quantitatively. Therefore, liberation classes were used to indicate the degree of liberation, from completely overgrown (0% free grain surface = locked) to completely liberated (81–100% free grain surface) mineral grains. The mineral of interest was chalcopyrite, due to the focus on copper extraction in this study, and the results are displayed in Table 4.

Table 4.
Liberation of chalcopyrite grains in concentrates and corresponding bioleaching samples categorized in liberation classes.
The concentrate sample contained chalcopyrite grains with mainly liberated grain surfaces, as displayed in Figure 5. More than 60% of chalcopyrite grains possessed grain surfaces with 60% and more free grain surfaces, and intergrowths with other sulfide or gangue minerals occurred subordinately.
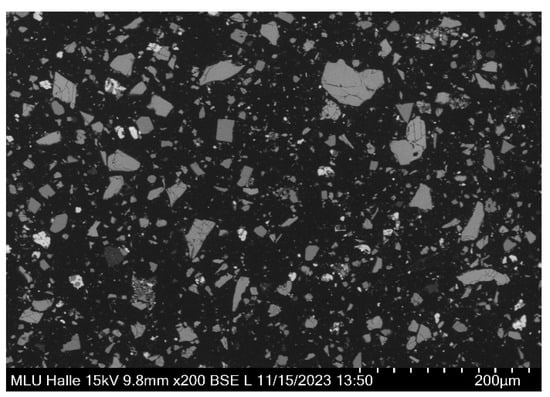
Figure 5.
General view of a polished thick section from a concentrated sample showing mostly angular particles composed of mainly chalcopyrite and pyrite embedded in mounting resin (black). Chalcopyrite grains are mostly liberated (SEM, backscattered electron image).
Chalcopyrite grains in bioleaching residues showed a divergent liberation. While chalcopyrite grains in the residue with 1.0% PD were marked by a higher liberation degree, 74% of chalcopyrite grains were mainly liberated (>60% free grain surface); the liberation of chalcopyrite grains in the bioleaching residues with 2.5 and 5% PD was lower. The results showed that only 36.5% of chalcopyrite grains in the bioleaching test at 2.5% PD had a mainly liberated grain surface (>60% free surface), whereas 43% of chalcopyrite grains were mainly liberated in the residue from the test conducted with 5.0% PD.
Microscopic examinations of bioleaching residues showed that both chalcopyrite and other mineral grains exhibited coatings and overgrowths of secondary sulfate minerals (Figure 6).

Figure 6.
Particle composed of chalcopyrite (Ccp) overgrown and attached by secondary formed jarosite (Jar). A particle composed of galena (Gn) and sphalerite (Sp) exhibits an alteration rim composed of anglesite (Ang). (SEM, backscattered electron image).
Their formation is related to the bioleaching process and is linked with the dissolution of minerals, mainly base metal sulfides, and the release of metal ions. Sulfuric acid, used to maintain low pH conditions in the leaching media, supplies sulfur dioxide, which readily reacts with metal ions, such as iron and lead, to form sulfate minerals, such as jarosite (KFe3(SO4)2(OH)6) and anglesite (PbSO4). While fine-grained acicular jarosite crystals are mainly precipitated in the leaching media, anglesite is predominantly formed in situ on the surfaces of galena grains and replaces the latter with a progressive leaching duration. Due to the amouring effect of anglesite concerning galena, the content of anglesite in the bioleaching residues is comparatively low. Chalcopyrite grains remaining in bioleaching residues are marked by textural features, such as intense fractures and corrosion embayments, and exhibit various stages of decomposition (Figure 7 and Figure 8).
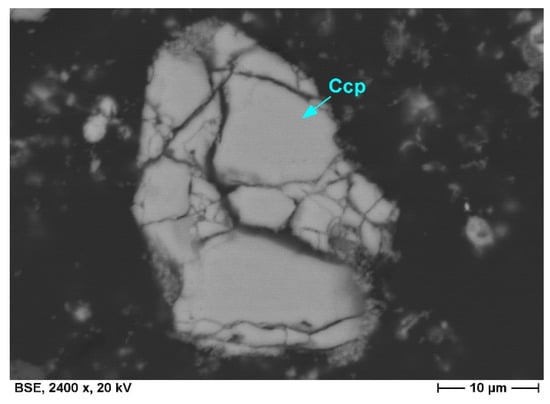
Figure 7.
Chalcopyrite grain in decomposition shows irregular cracks. Surface and areas along fissures are partly oxidized to iron sulfate. (SEM, backscattered electron image).
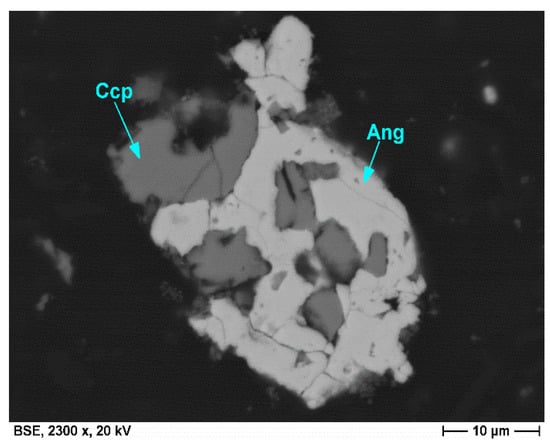
Figure 8.
Particle initially composed of galena and chalcopyrite (Ccp) exhibits the in situ replacement of galena by anglesite (Ang). Note the highly corroded chalcopyrite rims. (SEM, backscattered electron image).
3.5. Bioleaching of Armanis Copper Concentrate by Pure Cultures and Associations
Comparative studies of copper bioleaching by pure and mixed cultures of A. caldus, L. ferriphilum CC, S. thermosulfidooxidans 6, and their association, as well as the indigenous consortium of iron-oxidizing bacteria Arm, were performed (Figure 9).

Figure 9.
Extraction of copper in g/L (a) and % (b,c) during the bioleaching of Armanis concentrate by pure cultures A. caldus, L. ferriphilum CC, S. thermosulfidooxidans 6, their mixed culture (a,b), and indigenous consortium Arm-12 (c) in the presence of Fe2+ (PD −2.5%, Fe2+ −1.0 g/L, 170 rpm, 40 °C).
As can be seen from Figure 8, the extractions of copper by L. ferriphilum CC, S. thermosulfidooxidans 6, and A. caldus were 2.6, 2.4, and 1.9 g/L (Figure 9a), respectively, for the bioleaching of Armanis copper concentrate during the 27 days. Only 1.6 g/L (Figure 9a) or 58% (Figure 9b) of copper was recovered in the same period in the case of uninoculated control. Obviously, among tested cultures, L. ferriphilum CC showed a higher activity of copper extraction (84%) (Figure 9b). However, the highest amount of copper recovery (2.8 g/L or 94%) was observed in the case of the application of the tested mixed culture (Figure 9a,b). This finding corresponds with the studies of Sajjad et al., 2018 [42].
At 27 h, on the day of processing, the copper recovery rate was 100% (Figure 9b) using indigenous Arm. Mixed cultures of acidophiles are typically considered to be more effective than pure cultures for dissolving metals [36,55,56,57,58], as the consortium has a higher capability to resist copper, zinc, arsenic, and chloride ions, compared to pure cultures. In this study, consortia of bacterial strains were found to be very effective in copper dissolution under optimized conditions.
Studies have shown that the indigenous consortium of Arm is more active than the mixed cultures above (Figure 9c). Bryan et al., 2011 [38], Liu et al., 2011 [59], and Ma et al., 2017 [37] demonstrated that the indigenous strains had a stronger colonization ability and were more suitable for leaching high-oxidative oxide–sulfide copper minerals.
Additionally, data presented in Figure 9c show that the addition of ferrous iron (Fe2+) in the concentration of 1.0 g/L stimulated the extraction of copper using a mixed culture comprising A. caldus, L. ferriphilum CC, and S. thermosulfidooxidans 6. Thus, copper recoveries using this mixed culture for 27 days were 94 and 91%, with and without Fe2+ in the medium, respectively (Figure 9c). This result is in agreement with those obtained by Hiroyoshi et al., 1997 [60] and Third et al., 2002 [61], who reported that ferrous iron is effective in dissolving chalcopyrite. On the contrary, in the case of indigenous association, the Arm recovery of copper in the same period increased from 88% to 100% in the absence of Fe2+.
During the first 11 days, the mixed bacterial culture (36.3%), in terms of copper extraction, exceeded Arm association (24.0%) about 1.5 times. On the 17th day, the amounts of extracted copper by mixed culture and association Arm were equal; afterward, between 20–27 days, the Arm association showed slightly more activity in copper extraction. Copper recoveries by association Arm and constructed bacterial association in 27 days were 88% and 100% and 94 and 91%, with and without Fe2+, respectively (Figure 9c).
Microbiological analysis carried out at the end of the bioleaching experiment showed that the association Arm consisted of sulfur-oxidizing A. caldus (108 cells/mL) and L. ferriphilum (104 cells/mL). Moderate thermophilic iron- and sulfur-oxidizing S. thermosulfidooxidans were not found in the association Arm.
Interestingly, S. thermosulfidooxidans 6 was not detected in bioleaching pulp with the constructed association of iron- and sulfur-oxidizing bacteria. At the end of the bioleaching experiment, only L. ferriphilum CC (103 cells/mL) and A. caldus (107 cells/mL) were observed in the constructed association. It could be concluded that the conditions generated during the bioleaching of Armanis concentrate were not favorable for S. thermosulfidooxidans 6. In contrast to L. ferriphilum CC and A. caldus, S. thermosulfidooxidans 6 was not viable in the tested association and disappeared after a short time.
3.6. Eh and pH Monitoring
The redox potential (ORP) of the inoculated systems increased rapidly from 400 to 650 mV in the first two days, indicating oxidative processes, consistent with the iron ratio results. The sterilized control, on the other hand, maintained a constant potential of 400 mV throughout the assay.
It has widely been reported that pyrite can enhance chalcopyrite dissolution in ferric sulfate media, and a technique process named Galvanox™ has been developed to process chalcopyrite [62,63]. The catalytic effect of pyrite on chalcopyrite dissolution was mainly attributed to the galvanic current. In a galvanic cell consisting of pyrite as the cathodic part and chalcopyrite as the anodic part, the oxidation of chalcopyrite was intensified. However, it has been extensively reported that chalcopyrite dissolution can be accelerated when redox potential (ORP) is controlled at a relatively low value, where chalcopyrite can be reduced to Cu2S, whose rapid dissolution then causes a higher copper extraction. On the contrary, the direct oxidation of chalcopyrite at a relatively high redox potential (ORP) value can rapidly cause passivation, due to the formation of the passivation layer [64,65,66]. Wang et al., 2014 [67] suggested that the decrease in pH could be due to the RISCs, which might have accumulated during this phase and might have stimulated the growth of oxidizers of RISCs, such as A. caldus. The Arm consortium may contribute to the removal of the passivation layer, due to the activities of sulfur-oxidizing bacteria. As for sphalerite, it is well known that in the mixture of sulfide minerals (in the case of tested concentrate), sphalerite is oxidized first, due to its lowest rest potential [68].
The pH evolution during the bioleaching process is an important consideration for its feasibility. If the global pH changes significantly, it will be necessary to adjust it with external agents regularly during the facility’s operation, implying economic costs. Notably, the global pH remained constant in all samples at less than 2 units.
4. Conclusions
This work was aimed at studying the bioleaching of the copper concentrate of the Armanis gold-polymetallic mine using pure cultures of iron- and sulfur-oxidizing bacteria and association constructed on their basis, as well as using indigenous iron-oxidizing consortia, obtained using a button-up approach. A comparative study of the effects of PD and Fe2+ on the growth of bacteria and in the process of copper bioleaching was also performed. Monitoring the microbial community in bioleaching processes in Armanis Cu concentrate is essential to control process parameters and enhance leaching efficiency. It has been shown that the mixed inoculum of pure cultures exhibits about 94% copper recovery when compared to pure strains.
The native indigenous consortium was used to demonstrate the viability of these processes. At 40 °C, copper could be completely dissolved in 27 days. Using indigenous moderate thermophilic strains that originated from the same ore, biotop was proven to be a sustainable copper extraction method.
More research will be carried out to assess the copper recovery using a consortium of mesophilic and moderately thermophilic microorganisms. A mixed consortium could improve the process efficiency by allowing the microbial succession of leaching microorganisms.
Mineralogical analysis showed that the dissolution of chalcopyrite was below 20% at all tested PDs. Galena was generally resistant to acid leaching, due to its known amouring by lead sulfate. The dissolution of pyrite was about 22.7% at maximum. Sphalerite showed nearly complete dissolution (>95%) at 2.5% and 5% PD.
Author Contributions
Conceptualization, A.V., R.Z., S.W., A.K. (Andreas Kamradt), A.H. and N.V.; methodology, A.V., R.Z., S.W., A.K. (Andreas Kamradt), A.H. and N.V.; software, A.V.; validation, A.V., R.Z., A.K. (Anna Khachatryan), Z.M., S.W., A.K. (Andreas Kamradt), M.J., A.H., Y.Z., C.W. and N.V.; formal analysis, A.K. (Anna Khachatryan), Z.M., M.J. and C.W.; investigation, A.V., R.Z., A.K. (Anna Khachatryan), Z.M., S.W., A.K. (Andreas Kamradt), M.J., A.H., Y.Z., C.W. and N.V.; resources, A.V., R.Z., A.K. (Andreas Kamradt) and N.V.; data curation, A.V., R.Z., S.W., A.K. (Andreas Kamradt), A.H., Y.Z. and N.V.; writing—original draft preparation A.V., A.K. (Anna Khachatryan) and A.K. (Andreas Kamradt); writing—review and editing, R.Z., S.W., A.H. and N.V.; visualization, A.V., R.Z., A.K. (Anna Khachatryan), Z.M., S.W., A.K. (Andreas Kamradt), M.J., A.H., Y.Z., C.W. and N.V.; supervision, A.V., R.Z., N.V. and A.K. (Andreas Kamradt); project administration, A.V. and N.V.; funding acquisition, A.V. and R.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Higher Education and Science Committee of the Ministry of Science, Education, Culture, and Sports of the Republic of Armenia, research project grant number No. 22rl-031, and The APC was funded by the Institute of Oceanology, Chinese Academy of Sciences, China.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no roles in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Amar, A.; Massello, F.L.; Costa, C.S.; Castro, C.; Donati, E.R. Bioleaching of a chalcocite-dominant copper ore from Salta, Argentina, by mesophilic and thermophilic microorganisms. Minerals 2023, 13, 52. [Google Scholar] [CrossRef]
- Brar, K.K.; Magdouli, S.; Etteieb, S.; Zolfaghari, M.; Fathollahzadeh, H.; Calugaru, L.; Komtchou, S.P.; Tanabene, R.; Brar, S.K. Integrated bioleaching-electrometallurgy for copper recovery—A critical review. J. Clean. Prod. 2021, 291, 125257. [Google Scholar] [CrossRef]
- Yu, S.; Liao, R.; Yang, B.; Fang, C.; Wang, Z.; Liu, Y.; Wu, B.; Wang, J.; Qiu, G. Chalcocite (bio)hydrometallurgy—Current state, mechanism, and future directions: A review. Chin. J. Chem. Eng. 2022, 41, 109–120. [Google Scholar] [CrossRef]
- Kaksonen, A.H.; Boxall, N.J.; Gumulya, Y.; Khaleque, H.N.; Morris, C.; Bohu, T.; Cheng, K.Y.; Usher, K.M.; Lakaniemi, A.M. Recent progress in biohydrometallurgy and microbial characterisation. Hydrometallurgy 2018, 180, 7–25. [Google Scholar] [CrossRef]
- Mishra, S.; Panda, S.; Akcil, A.; Dembele, S. Biotechnological avenues in mineral processing: Fundamentals, applications and advances in bioleaching and bio-beneficiation. Miner. Process. Extr. Metall. Rev. 2022, 44, 22–51. [Google Scholar] [CrossRef]
- Nkuna, R.; Ijoma, G.N.; Matambo, T.S.; Chimwani, N. Accessing metals from low-grade ores and the environmental impact considerations: A review of the perspectives of conventional versus bioleaching strategies. Minerals 2022, 12, 506. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Yaghmaei, S.; Vossoughi, M.; Jafari, A.; Roostaazad, R.; Turunen, I. Bacterial leaching of low-grade ZnS concentrate using indigenous mesophilic and thermophilic strains. Hydrometallurgy 2007, 85, 59–65. [Google Scholar] [CrossRef]
- Deveci, H.; Akcil, A.; Alp, I. Bioleaching of complex zinc sulphides using mesophilic and thermophilic bacteria: Comparative importance of pH and iron. Hydrometallurgy 2004, 73, 293–303. [Google Scholar] [CrossRef]
- Liao, M.X.; Deng, T.L. Zinc and lead extraction from complex raw sulfides by sequential bioleaching and acidic brine leach. Miner. Eng. 2004, 17, 17–22. [Google Scholar] [CrossRef]
- Qiu, M.Q.; Xiong, S.Y.; Zhang, W.M.; Wang, G.X. A comparison of bioleaching of chalcopyrite using pure culture or a mixed culture. Miner. Eng. 2005, 18, 987–990. [Google Scholar] [CrossRef]
- Bosecker, K. Bioleaching: Metal solubilization by microorganisms. FEMS Microbiol. Rev. 1997, 20, 591–604. [Google Scholar] [CrossRef]
- Baba, A.; Ezekafor, E.; Adekola, F.; Ahmed, R.; Panda, S. Bio-oxidation of a low grade chalcopyrite ore by mixed culture of acidophilic bacteria. J. Ecobiotechnol. 2011, 3, 01–06. [Google Scholar]
- Vera, M.; Schippers, A.; Hedrich, S.; Sand, W. Progress in bioleaching: Fundamentals and mechanisms of microbial metal sulfide oxidation-part A. Appl. Microbiol. Biotechnol. 2022, 106, 6933–6952. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, D.E.; Johnson, D.B. The microbiology of biomining: Development and optimization of mineral-oxidizing microbial consortia. Microbiology 2007, 153, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Conić, V.T.; Rajčić Vujasinović, M.M.; Trujić, V.K.; Cvetkovski, V.B. Copper, zinc, and iron bioleaching from polymetallic sulphide concentrate. Trans. Nonferrous Met. Soc. China 2014, 24, 3688–3695. [Google Scholar] [CrossRef]
- Dopson, M.; Baker-Austin, C.; Koppineedi, P.R.; Bond, P.L. Growth in sulfidic mineral environments: Metal resistance mechanisms in acidophilic micro-organisms. Microbiology 2003, 149, 1959–1970. [Google Scholar] [CrossRef] [PubMed]
- Dopson, M.; Holmes, D.S. Metal resistance in acidophilic microorganisms and its significance for biotechnologies. Appl. Microbiol. Biotechnol. 2014, 98, 8133–8144. [Google Scholar] [CrossRef] [PubMed]
- Quatrini, R.; Jedlicki, E.; Holmes, D.S. Genomic insights into the iron uptake mechanisms of the biomining microorganism Acidithiobacillus ferrooxidans. J. Ind. Microbiol. Biotechnol. 2005, 32, 606–614. [Google Scholar] [CrossRef]
- Dong, Y.; Lin, H.; Xu, X.; Zhou, S. Bioleaching of different copper sulfides by Acidithiobacillus ferrooxidans and its adsorption on minerals. Hydrometallurgy 2013, 140, 42–47. [Google Scholar] [CrossRef]
- Zhang, S.; Yan, L.; Xing, W.; Chen, P.; Zhang, Y.; Wang, W. Acidithiobacillus ferrooxidans and its potential application. Extremophiles 2018, 22, 563–579. [Google Scholar] [CrossRef]
- Coram, N.J.; Rawlings, D.E. Molecular relationship between two groups of the genus leptospirillum and finding that Leptospirillum ferriphilum sp. nov. dominates South African the commercial biooxidation tanks that operate at 40 °C. Appl. Environ. Microbial. 2002, 68, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Olson, G.; Brierly, J.; Brierly, C. Bioleaching review part B. Appl. Microbiol. Biotechnol. 2003, 63, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Karavaiko, G.; Dubinina, G.; Kondrat’eva, T. Lithotrophic microorganisms of the oxidative cycles of sulfur and iron. Microbiology 2006, 75, 512–545. [Google Scholar] [CrossRef]
- Bryant, R.D.; McGroarty, K.M.; Costerton, J.W. Isolation and characterization of a new acidophilic Thiobacillus species (T. albertis). Can. J. Microbiol. 1983, 23, 1159–1170. [Google Scholar] [CrossRef]
- Laishly, E.J.; Rae, K.; Dillman, A.M.; Bryant, R.D. Characterization of a new acidophilic Thiobacillus isolate (Thiobacillus capsulatus). Can. J. Microbiol. 1988, 34, 960–966. [Google Scholar] [CrossRef]
- Hallberg, K.B.; Johnson, D.B. Biodiversity of acidophilic prokaryotes. Adv. Appl. Microbiol. 2001, 49, 37–84. [Google Scholar] [PubMed]
- Hallberg, K.B.; Lindström, E.B. Characterization of Thiobacillus caldus sp. nov., a moderately thermophilic acidophile. Microbiology 1994, 140, 3451–3456. [Google Scholar] [CrossRef] [PubMed]
- Goebel, B.M.; Stackebrandt, E. Cultural and phylogenetic analysis of mixed microbial populations found in natural and commercial bioleaching environments. Appl. Environ. Microbiol. 1994, 60, 1614–1621. [Google Scholar] [CrossRef] [PubMed]
- Dopson, M.; Lindstrom, E.B. Analysis of Community Composition during Moderately Thermophilic Bioleaching of Pyrite, Arsenical pyrite, and Chalcopyrite. Microb. Ecol. 2004, 48, 19–28. [Google Scholar] [CrossRef]
- Dopson, M.; Lindstrom, E.B. Potential role of Thiobacillus caldus in Arsenopyrite Leaching. Appl. Environ. Microbiol. 1999, 65, 36–40. [Google Scholar] [CrossRef]
- Xia, L.; Liu, J.; Xiao, L.; Zeng, J.; Li, B.; Geng, M.; Qiu, G. Single and cooperative bioleaching of sphalerite by two kinds of bacteria—Acidithiobacillus ferriooxidans and Acidithiobacillus thiooxidans. Trans. Nonferrous Met. Soc. China 2008, 18, 190–195. [Google Scholar] [CrossRef]
- Zeng, W.; Qiu, G.; Zhou, H.; Peng, J.; Chen, M.; Tan, S.N.; Chao, W.; Liu, X.; Zhang, Y. Community structure and dynamics of the free and attached microorganisms during moderately thermophilic bioleaching of chalcopyrite concentrate. Bioresour. Technol. 2010, 101, 7068–7075. [Google Scholar] [CrossRef]
- Panda, S.; Pradhan, N.; Mohapatra, U.B.; Panda, S.K.; Rath, S.S.; Nayak, B.D.; Sukla, L.B.; Mishra, B.K. Bioleaching of copper from pre and post thermally activated low grade chalcopyrite contained ball mill spillage. Front. Environ. Sci. Eng. China 2013, 7, 281–293. [Google Scholar] [CrossRef]
- Li, S.; Zhong, H.; Hu, Y.; Zhao, J.; He, Z.; Gu, G. Bioleaching of a low-grade nickel–copper sulfide by mixture of four thermophiles. Bioresour. Technol. 2014, 153, 300–306. [Google Scholar] [CrossRef]
- Panda, S.; Sanjay, K.; Sukla, L.B.; Pradhan, N.; Subbaiah, T.; Mishra, B.K.; Prasad, M.S.R.; Ray, S.K. Insights into heap bioleaching of lowgrade chalcopyrite ores: A pilot scale study. Hydrometallurgy 2012, 125–126, 157–165. [Google Scholar] [CrossRef]
- Panda, S.; Biswal, A.; Mishra, S.; Panda, P.K.; Pradhan, N.; Mohapatra, U.; Akcil, A. Reductive dissolution by waste newspaper for enhanced meso-acidophilic bioleaching of copper from low grade chalcopyrite: A new concept of biohydrometallurgy. Hydrometallurgy 2015, 153, 98–105. [Google Scholar] [CrossRef]
- Ma, L.; Wang, X.; Tao, J.; Feng, X.; Zou, K.; Xiao, Y.; Liang, Y.; Yin, H.; Liu, X. Bioleaching of the mixed oxide-sulfide copper ore by artificial indigenous and exogenous microbial community. Hydrometallurgy 2017, 169, 41–46. [Google Scholar] [CrossRef]
- Bryan, C.G.; Joulian, C.; Spolaore, P.; El Achbouni, H.; Challan-Belval, S.; Morin, D.; d’Hugues, P. The efficiency of indigenous and designed consortia in bioleaching stirred tank reactors. Miner. Eng. 2011, 24, 1149–1156. [Google Scholar] [CrossRef]
- Giaveno, A.; Lavalle, L.; Chiacchiarini, P.; Donati, E. Bioleaching of zinc from low-grade complex sulphide ores in an airlift by isolated Leptospirillum ferrooxidans. Hydrometallurgy 2007, 89, 117–126. [Google Scholar] [CrossRef]
- Wakeman, K.; Auvinen, H.; Johnson, D.B. Microbiological and geochemical dynamics in simulated heap leaching of a polymetallic sulfide ore. Biotechnol. Bioeng. 2008, 101, 739–750. [Google Scholar] [CrossRef]
- Watling, H. The bioleaching of sulphide minerals with emphasis on copper sulphides—A review. Hydrometallurgy 2006, 84, 81–108. [Google Scholar] [CrossRef]
- Sajjad, W.; Zheng, G.; Zhang, G.; Ma, X.; Xu, W.; Khan, S. Bioleaching of copper- and zinc-bearing ore using consortia of indigenous iron-oxidizing bacteria. Extremophiles 2018, 22, 851–863. [Google Scholar] [CrossRef]
- Okibe, N.; Johnson, D.B. Biooxidation of pyrite by defined mixed cultures of moderately thermophilic acidophiles in pH controlled bioreactors: Significance of microbial interactions. Biotechnol. Bioeng. 2004, 87, 574–583. [Google Scholar] [CrossRef]
- Yin, S.; Wang, L.; Kabwe, E.; Chen, X.; Yan, R.; An, K.; Zhang, L.; Wu, A. Copper Bioleaching in China: Review and Prospect. Minerals 2018, 8, 32. [Google Scholar] [CrossRef]
- Roberto, F.F.; Schippers, A. Progress in bioleaching: Part B, applications of microbial processes by the minerals industries. Appl. Microbiol. Biotechnol. 2022, 106, 5913–5928. [Google Scholar] [CrossRef]
- Mackintosh, M.E. Nitrogen fixation by Thiobacillus ferrooxidans. J. Gen. Microbiol. 1987, 105, 215–218. [Google Scholar] [CrossRef]
- Erkmen, O. Parctice 4—Most probable number technique. In Microbiological Analysis of Foods and Food Processing Environments; Academic Press: Cambridge, MA, USA, 2022; pp. 31–37. [Google Scholar] [CrossRef]
- Lucchesi, C.A.; Hirn, C.F. EDTA Titration of total Iron in Iron(II) and Iron(III) mixtures. Application to Iron driers. Anal. Chem. 1960, 32, 1191–1193. [Google Scholar] [CrossRef]
- Fandrich, R.; Gu, Y.; Burrows, D.; Moeller, K. Modern SEM-based mineral liberation analysis. Int. J. Miner. Process. 2007, 84, 310–320. [Google Scholar] [CrossRef]
- Pirrie, D.; Rollinson, G.K. Unlocking the applications of automated mineral analysis. Geol. Today 2011, 27, 226–235. [Google Scholar] [CrossRef]
- Olubambi, P.A.; Ndlovu, S.; Potgieter, J.H.; Borode, J.O. Role of ore mineralogy in optimizing conditions for bioleaching low-grade complex sulphide ores. Trans. Nonferrous Met. Soc. China 2008, 18, 1234–1246. [Google Scholar] [CrossRef]
- Rouchalova, D.; Rouchalova, K.; Janakova, I.; Cablik, V.; Janstova, S. Bioleaching of Iron, Copper, Lead, and Zinc from the Sludge Mining Sediment at Different Particle Sizes, pH, and Pulp Density Using Acidithiobacillus ferrooxidans. Minerals 2020, 10, 1013. [Google Scholar] [CrossRef]
- Makita, M.; Esperón, M.; Pereyra, B.; López, A.; Orrantia, E. Reduction of arsenic content in a complex galena concentrate by Acidithiobacillus ferrooxidans. BMC Biotechnol. 2004, 4, 22. [Google Scholar] [CrossRef]
- Abhilash; Mehta, K.D.; Pandey, B.D. Bacterial leaching kinetics for copper dissolution from a lowgrade Indian chalcopyrite ore. Rem Rev. Esc. Minas 2013, 66, 245–250. [Google Scholar] [CrossRef]
- Ciftci, H.; Akcil, A. Effect of biooxidation conditions on cyanideconsumption and gold recovery from a refractory flotation gold concentrate. Hydrometallurgy 2010, 104, 142–149. [Google Scholar] [CrossRef]
- Yang, Y.; Diao, M.; Liu, K.; Qian, L.; Nguyen, A.V.; Qiu, G. Column bioleaching of low-grade copper ore by Acidithiobacillus ferrooxidans in pure and mixed cultures with a heterotrophic acidophile Acidiphilium sp. Hydrometallurgy 2013, 131, 93–98. [Google Scholar] [CrossRef]
- Utimura, S.K.; Rosario, C.G.A.; Botelho, A.B.; Tenório, J.A.S.; Espinosa, D.C.R. Bioleaching Process for Metal Recovery from Waste Materials. In Energy Technol., 2nd ed.; Zhang, L., Drelich, J.W., Neelameggham, N.R., Guillen, D.P., Haque, N., Zhu, J., Sun, Z., Wang, T., Howarter, J.A., Tesfaye, F., et al., Eds.; The Minerals, Metals & Materials Series; Springer: Cham, Switzerland, 2017; pp. 283–290. [Google Scholar]
- Deng, Y.; Liu, X.D.; Liu, H.W.; Jiang, H.D.; Xu, L.F.; Xiao, Y.H.; Liang, Y.L. Bioleaching of cadmium from contaminated paddy felds by consortium of autotrophic and indigenous cadmium-tolerant bacteria. Solid State Phenom. 2017, 262, 617–621. [Google Scholar] [CrossRef]
- Liu, Y.; Yin, H.; Zeng, W.; Liang, Y.; Liu, Y.; Baba, N.; Qiu, G.; Shen, L.; Fu, X.; Liu, X. The effect of the introduction of exogenous strain Acidithiobacillus thiooxidans A01 on functional gene expression, structure and function of indigenous consortium during pyrite bioleaching. Bioresour. Technol. 2011, 102, 8092–8098. [Google Scholar] [CrossRef]
- Hiroyoshi, N.; Hirota, M.; Hirajima, T.; Tsunekawa, M. A case of ferrous sulfate addition enhancing chalcopyrite leaching. Hydrometallurgy 1997, 47, 37–45. [Google Scholar] [CrossRef]
- Third, K.A.; Cord-Ruwisch, R.; Watling, H.R. Control of the redox potential by oxygen limitation improves bacterial leaching of chalcopyrite. Biotechnol. Bioeng. 2002, 78, 433–441. [Google Scholar] [CrossRef]
- Dixon, D.G.; Mayne, D.D.; Baxter, K.G. Galvanox™—A novel process for recovery of copper from primary copper concentrates. Can. Metall. Q. 2008, 47, 327–336. [Google Scholar] [CrossRef]
- Nazari, G.; Dixon, D.G.; Dreisinger, D.B. Enhancing the kinetics of chalcopyrite leaching in the Galvanox™ process. Hydrometallurgy 2011, 105, 251–258. [Google Scholar] [CrossRef]
- Sandström, Å.; Shchukarev, A.; Paul, J. XPS characterisation of chalcopyrite chemically and bio-leached at high and low redox potential. Miner. Eng. 2005, 18, 505–515. [Google Scholar] [CrossRef]
- Petersen, J.; Dixon, D.G. Competitive bioleaching of pyrite and chalcopyrite. Hydrometallurgy 2006, 83, 40–49. [Google Scholar] [CrossRef]
- Córdoba, E.; Munoz, J.; Blázquez, M.; González, F.; Ballester, A. Leaching of chalcopyrite with ferric ion. Part IV: The role of redox potential in the presence of mesophilic and thermophilic bacteria. Hydrometallurgy 2008, 93, 106–115. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, W.; Qiu, G.; Chen, X.; Zhou, H. A moderately thermophilic mixed microbial culture for bioleaching of chalcopyrite concentrate at high pulp density. Appl. Environ. Microbiol. 2014, 80, 741–750. [Google Scholar] [CrossRef]
- Fu, K.; Ning, Y.; Chen, S.; Wang, Z. Bioleaching of different copper sulphide minerals and their physicochemical properties dependence. Miner. Process. Extr. Metall. 2016, 125, 1–4. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).