Structure and Activity of β-Oligosaccharides Obtained from Lentinus edodes (Shiitake)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Isolation and Purification of LEOPs from LF3
2.3. β-Glucan Content Determination
2.4. Polysaccharide Content Determination
2.5. Determination of Molecular Weight Distribution Using HPLC
2.6. Infrared Spectroscopy Analysis
2.7. Determination of Monosaccharide Composition via Anion Chromatography
2.8. Methylation Analysis
2.9. NMR Analysis
2.10. Prophagocytic Effect of LEOPs on Macrophages Determined via Flow Cytometry
2.11. DPPH Radical Scavenging Activity of LEOPs
2.12. In Vitro Inducing Effect of LF3 and LEOPs on NF-κB Activation via Dectin-1 Receptor
2.13. Binding Analysis of LEOPs and Dectin-1 Receptors Using Surface Plasmon Resonance (SPR)
2.14. Hepatoprotective Activity of LEOPs on Hepatic Damage
2.14.1. Alcohol-Induced Liver Injury Experiments
2.14.2. H2O2-Induced Liver Injury Experiments
2.15. Data Statistics
3. Results
3.1. Elucidation of Properties and Structure of LEOPs Obtained from L. edodes
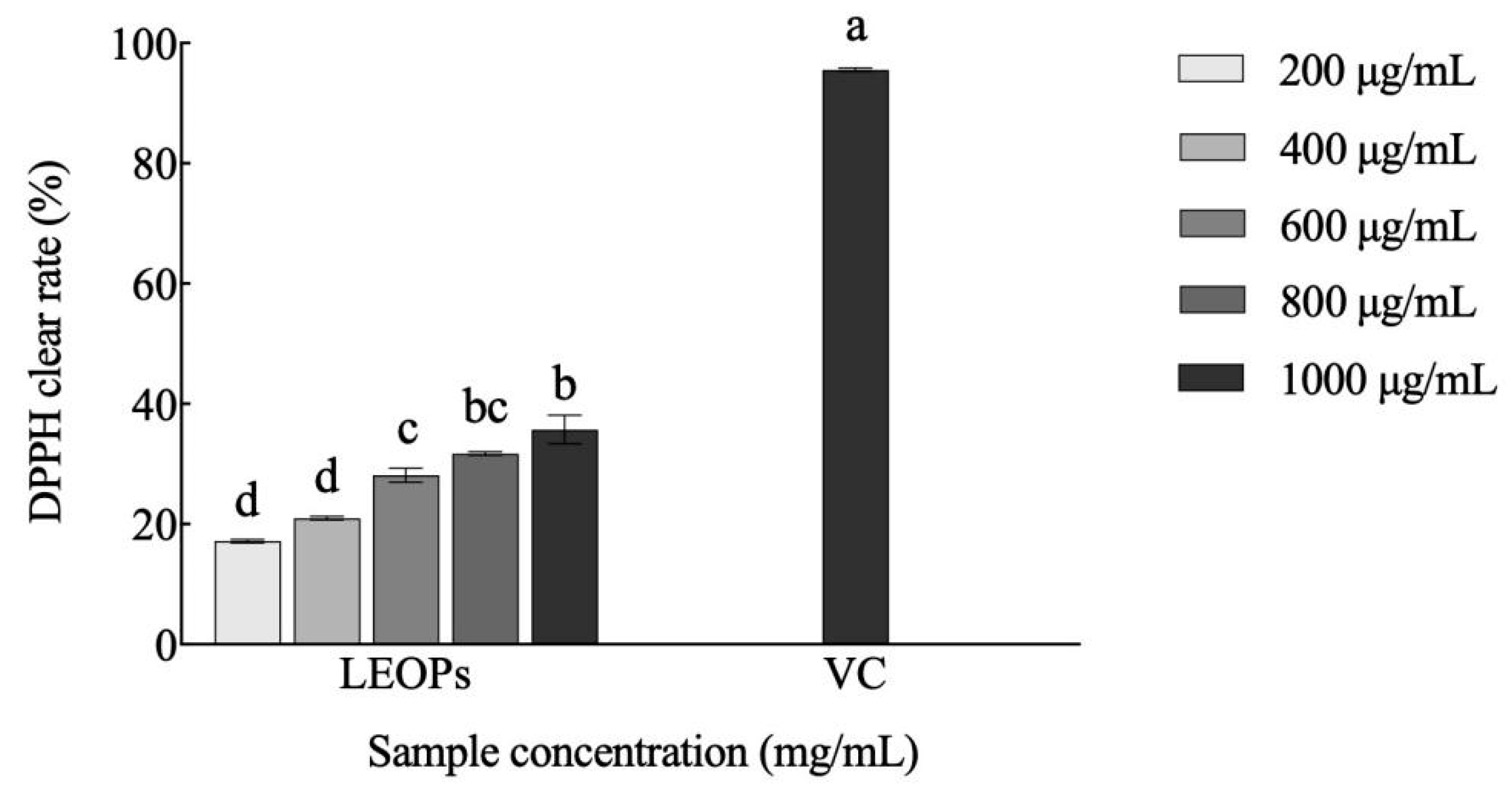
3.2. Antioxidant Activity of LEOPs Obtained from L. edodes
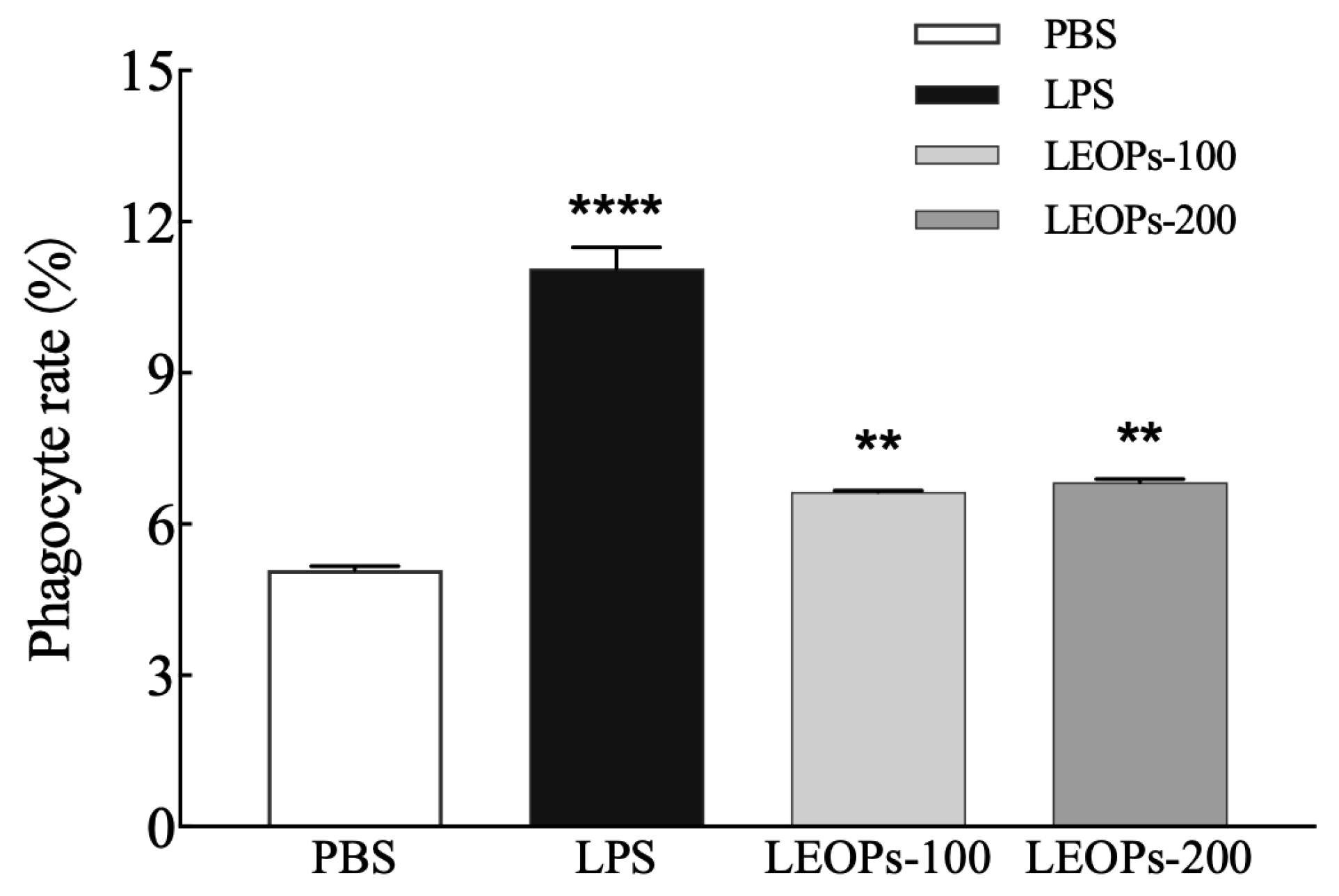
3.3. LEOPs Promote the Phagocytic Function of RAW264.7 Macrophages
3.4. LEOPs Mediate NF-κB Activation via Dectin-1 Receptor
3.5. LEOPs Bind to Dectin-1 Receptors
3.6. Reparative Effect of LEOPs on Liver Injury
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sasaki, T.; Takasuka, N. Further study of the structure of lentinan, an anti-tumor polysaccharide from Lentinus edodes. Carbohydr. Res. 1976, 47, 99–104. [Google Scholar] [CrossRef]
- Xu, X.; Chen, P.; Zhang, L.; Ashida, H. Chain structures of glucans from Lentinus edodes and their effects on NO production from RAW 264.7 macrophages. Carbohydr. Polym. 2012, 87, 1855–1862. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, S.; Zhang, L. Aggregation Behavior of Triple Helical Polysaccharide with Low Molecular Weight in Diluted Aqueous Solution. J. Phys. Chem. B 2010, 114, 4945–4954. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, O.; Nakashima, H.; Yoshida, T.; Kaneko, Y.; Yamamoto, I.; Matsuzaki, K.; Uryu, T.; Yamamoto, N. Sulfation of the immunomodulating polysaccharide lentinan: A novel strategy for antivirals to human immunodeficiency virus (HIV). Biochem. Pharmacol. 1988, 37, 2887–2891. [Google Scholar] [CrossRef] [PubMed]
- CZOP, J.K. Isolation of a yeast heptaglucoside that inhibits monocyte phagocytosis of zymosan particles. J. Immunol. 1989, 142, 959–965. [Google Scholar]
- Kataoka, K.; Muta, T.; Yamazaki, S.; Takeshige, K. Activation of macrophages by linear (1→3)-β-D-glucans. J. Biol. Chem. 2002, 277, 36825–36831. [Google Scholar] [CrossRef]
- Klarzynski, O.; Plesse, B.; Joubert, J.M.; Yvin, J.C.; Kopp, M.; Kloareg, B.; Fritig, B. Linear β-1,3-glucans are elicitors of defense responses in tobacco. Plant Physiol. 2000, 124, 1027–1037. [Google Scholar] [CrossRef]
- Khan, A.A.; Gani, A.; Masoodi, F.A.; Amin, F.; Wani, I.A.; Khanday, F.A.; Gani, A. Structural, thermal, functional, antioxidant & antimicrobial properties of β-D-glucan extracted from baker’s yeast (Saccharomyces cerevisiae)-Effect of γ-irradiation. Carbohydr. Polym. 2016, 140, 442–450. [Google Scholar]
- Jia, W.; Yu, Y.Z.; Liu, H.L.; Liu, Y.F.; Zhang, M.Y.; Li, Q.Z.; Zhang, J.S.; Wang, W.H. Preparation and In Vitro Activity of β-Glucan-Rich Polysaccharide Extracts from Lentinula edodes Fruiting Bodies. Acta Edulis Fungi 2023, 30, 51–55. [Google Scholar]
- Yan, J.K.; Wang, Y.Y.; Ma, H.L.; Wang, Z.-B. Ultrasonic effects on the degradation kinetics, preliminary characterization and antioxidant activities of polysaccharides from Phellinus linteus mycelia. Ultrason. Sonochem. 2016, 29, 251–257. [Google Scholar] [CrossRef]
- Wang, D.; Dai, L.; Gao, Y.X. Research progress on enzymaticmodification of polysaccharides. J. Chin. Cereals Oils Assoc. 2017, 32, 134–140. (In Chinese) [Google Scholar]
- Sinurat, E.; Saepudin, E.; Hudiyono, S. Immunostimulatory activity of brown seaweed-derived fucoidans at different molecular weights and purity levels towards white spot syndrome virus (WSSV) in shrimp Litopenaeus vanname. J. Appl. Pharm. Sci. 2016, 6, 82–91. [Google Scholar] [CrossRef]
- Xue, W.; Li, L.C.; Qi, M.X.; Wu, W.H.; Chi, L.L.; Wang, P.P. Structural characteristic of fucoidans from Sargassum pallidum and their anti-influenza virus activities. J. Shanghai Ocean. Uni. 2023, 32, 227–233. (In Chinese) [Google Scholar]
- Li, J.; Zhu, L.; Zheng, Z.Y.; Zhan, X.-B.; Lin, C.-C.; Zong, Y.; Li, W.-J. A new effective process for production of curdlan oligosaccharides based on alkali-neutralization treatment and acid hydrolysis of curdlan particles in water suspension. Appl. Microbiol. Biotechnol. 2013, 97, 8495–8503. [Google Scholar] [CrossRef] [PubMed]
- Nitschke, J.; Modick, H.; Busch, E.; Von Rekowski, R.W.; Altenbach, H.J.; Mölleken, H. A new colorimetric method to quantify β-1, 3-1, 6-glucans in comparison with total β-1, 3-glucans in edible mushrooms. Food Chem. 2011, 127, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Nowotny, A. Carbohydrate Determination by Phenol-Sulfuric Acid; Springer: Berlin/Heidelberg, Germany, 1979; pp. 171–173. [Google Scholar]
- Jia, W.; Zhang, J.S.; Jiang, Y.; Zheng, Z.-Y.; Zhan, X.-B.; Lin, C.-C. Structure of oligosaccharide F21 derived from exopolysaccharide WL-26 produced by Sphingomonas sp. ATCC 31555. Carbohydr. Polym. 2012, 90, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Anumula, K.R.; Taylor, P.B. A comprehensive procedure for preparation of partially methylated alditol acetates from glycoprotein carbohydrates. Anal. Biochem. 1992, 203, 101–108. [Google Scholar] [CrossRef]
- Zhang, A.Q.; Zhang, J.S.; Tang, Q.J.; Jia, W.; Yang, Y.; Liu, Y.F.; Fan, J.M.; Pan, Y.J. Structural elucidation of a novel fucogalactanthat contains 3-O-methyl rhamnose isolated from the fruiting bodies of the fungus. Hericium Erinaceus. Carbohydr. Res. 2006, 341, 645–649. [Google Scholar] [CrossRef]
- Tatarczak, M.M.; Flieger, J. Application of high-performance liquid chromatography with diode array detection to simultaneous analysis of reference antioxidants and 1,1-diphenyl-2-picrylhydrazyl (DPPH) in free radical scavenging test. Int. J. Environ. Res. Public Health 2022, 19, 8288. [Google Scholar] [CrossRef]
- Liu, L.P.; Feng, J.; Gao, K.; Zhou, S.; Yan, M.; Tang, C.; Zhou, J.; Liu, Y.; Zhang, J. Influence of carbon and nitrogen sources on structural features and immunomodulatory activity of exopolysaccharides from Ganoderma lucidum. Process Biochem. 2022, 119, 96–105. [Google Scholar] [CrossRef]
- Madushani, H.K.; Bing, S.J.; Cho, J.; Kim, A.; Kim, G.; Kim, J.S.; Kim, J.B.; Doh, Y.H.; Jee, Y. Sasa quelpaertensis leaves ameliorate alcohol-induced liver injury by attenuating oxidative stress in HepG2 cells and mice. Acta Histochemica 2018, 120, 477–489. [Google Scholar] [CrossRef]
- Wang, M.; Ma, H.L.; Liu, B.; Wang, H.B.; Xie, H.; Li, R.D.; Wang, J.F. Pinus massoniana bark extract protects against oxidative damage in LO2 hepatic cells and mice. Am. J. Chin. Med. 2010, 38, 909–919. [Google Scholar] [CrossRef]
- Lee, S.L.; Hsu, W.H.; Tu, C.M.; Wang, W.H.; Yang, C.Y.; Lee, H.K.; Chin, T.Y. The effects of freshwater clam (Corbicula fluminea) extract on activated hepatic stellate cells. Evid.-Based Complement. Altern. Med. 2021, 2021, 6065168. [Google Scholar] [CrossRef]
- Lim, H.; Oh, J.S.; Kang, K.R.; Seo, J.Y.; Kim, D.K.; Yu, S.K.; Kim, H.J.; Park, J.C.; Kim, J.S. 25-Hydroxycholesterol induces odontoclastic differentiation through RANK-RANKL upregulation and NF-κB activation in odontoblast-like MDPC-23 cells: An in vitro study. Int. Endod. J. 2023, 56, 432–446. [Google Scholar] [CrossRef]
- Bubb, W.A. NMR spectroscopy in the study of carbohydrates: Characterizing the structural complexity. Concept. Magn. Reson. A 2003, 19, 1–19. [Google Scholar] [CrossRef]
- Lehtovaara, B.C.; Gu, F.X. Pharmacological, structural, and drug delivery properties and applications of 1, 3-beta-glucans. J. Agric. Food Chem. 2011, 59, 6813–6828. [Google Scholar] [CrossRef] [PubMed]
- Duus, J.; Gotfredsen, C.H.; Bock, K. Carbohydrate structural determination by NMR spectroscopy: Modern methods and limitations. Chem. Rev. 2000, 100, 4589–4614. [Google Scholar] [CrossRef] [PubMed]
- Hounsell, E.F. 1H NMR in the structural and conformational analysis of oligosaccharides and glycoconjugates. Prog. Nucl. Magn. Reson. Spectrosc. 1995, 27, 445–474. [Google Scholar] [CrossRef]
- Fhernanda, R.S.; Elaine, R.C.; Caroline, G.M.; Sassaki, G.L.; Gorin, P.A.; Iacomini, M. Structural characterization of a polysaccharide and a β-glucan isolated from the edible mushroom Flammulina velutipes. Phytochemistry 2006, 67, 2189–2196. [Google Scholar]
- Rout, D.; Mondal, S.; Chakraborty, I.; Pramanik, M.; Islam, S.S. Chemical analysis of a new (1→3)-(1→6)-branched glucan from an edible mushroom, Pleurotus florida. Carbohydr. Res. 2005, 340, 2533–2539. [Google Scholar] [CrossRef]
- Chen, H.; Ju, Y.; Li, J.; Yu, M. Antioxidant activities of polysaccharides from Lentinus edodes and their significance for disease prevention. Int. J. Biol. Macromol. 2012, 50, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cai, C.; Zheng, M.; Hao, J.; Wang, Y.; Hu, M.; Fan, L.; Yu, G. Alkaline Extraction, Structural Characterization, and Bioactivities of (1→6)-β-d-Glucan from Lentinus edodes. Molecules 2019, 24, 1610. [Google Scholar] [CrossRef] [PubMed]
- Jeff, I.B.; Fan, E.; Tian, M.; Song, C.; Yan, J.; Zhou, Y. In vivo anticancer and immunomodulating activities of Manno galactoglucan-type polysaccharides from Lentinus edodes (Berkeley) Singer. Cent. Eur. J. Immunol. 2016, 41, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yang, J.; Luo, Z.; Zhang, X. Lentinula edodes-derived polysaccharide enhances systemic and mucosal immunity by spatial modulation of intestinal gene expression in mice. Food Funct. 2015, 6, 2068–2080. [Google Scholar] [CrossRef]
- Son, S.U.; Kim, T.E.; Park, J.H.; Suh, H.J.; Shin, K.-S. Immunostimulating effects of ulvan type polysaccharide isolated from Korean Ulva pertusa in cyclophosphamide-induced immunosuppressed BALB/c mice. Int. J. Biol. Macromol. 2024, 275 Pt 1, 133518. [Google Scholar] [CrossRef]
- Saito, H.; Ohki, T.; Takasuka, N.; Sasaki, T. 13C N.M.R spectral study of a gel-forming (1,3)-β-D-glucan, (lentinan) from Lentinus edodes, and its acid degraded fraction’s structure, and dependence of conformation on the molecular weight. Carbohydr. Res. 1977, 58, 293–305. [Google Scholar] [CrossRef]
- Hua, Y.; Zhang, H.; Fu, Q.; Feng, Y.; Duan, Y.; Ma, H. Effects of ultrasound modification with different frequency modes on the structure, chain conformation, and immune activity of polysaccharides from Lentinus edodes. Foods 2022, 11, 2470. [Google Scholar] [CrossRef]





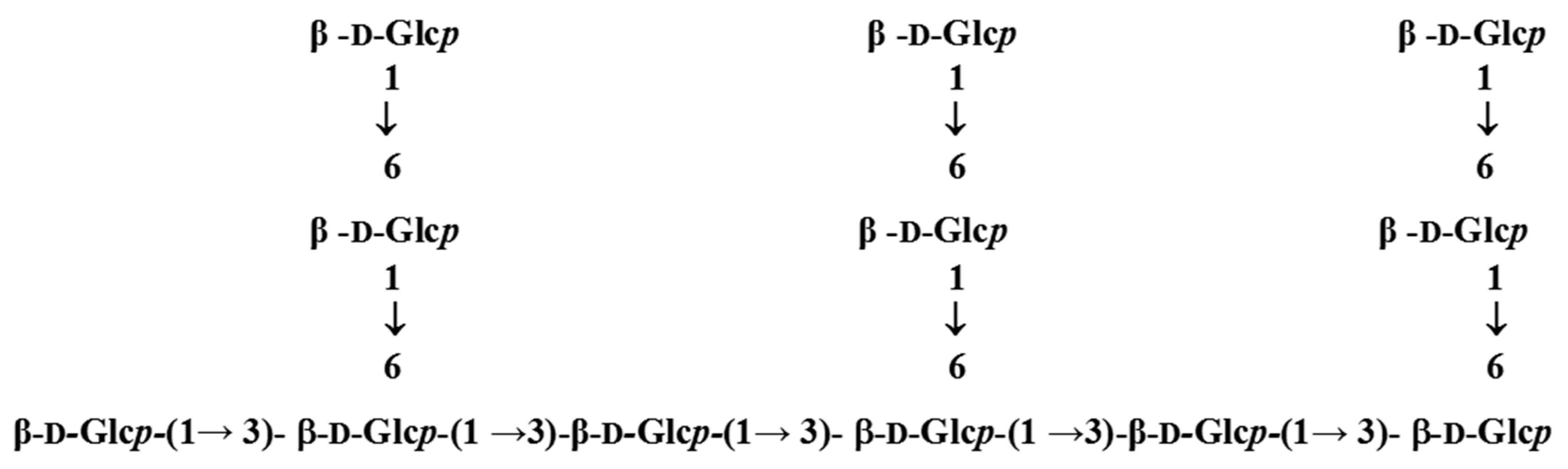



| Methylated Sugars | Linkages | Molar Ratios | Retention Time (s) |
|---|---|---|---|
| 2,3,4,6-Me4-Glcp | T-Glcp | 1.66 | 12.27 |
| 2,4,6-Me3-Glcp | 1,3-Glcp | 1.81 | 13.43 |
| 2,3,4-Me3-Glcp | 1,6-Glcp | 3.47 | 13.88 |
| 2,4-Me2-Glcp | 1,3,6-Glcp | 1.00 | 15.30 |
| Residue | Proton or Carbon | |||||
|---|---|---|---|---|---|---|
| H-1/C-1 | H-2/C-2 | H-3/C-3 | H-4/C-4 | H-5/C-5 | H-6/C-6 | |
| A β-D-Glcp- (1 | 4.02 | 3.13 | 3.30 | 3.18 | 3.49 | 3.48 a,3.69 b |
| 103.09 | 76.40 | 71.86 | 71.23 | 77.03 | 61.36 | |
| B →6)-β-D-Glcp- (1→ | 4.00 | 3.39 | 3.62 | 3.50 | 3.38 | 3.18 a, 3.12 b |
| 103.16 | 79.36 | 76.13 | 71.86 | 75.23 | 69.92 | |
| C →3,6)-β-D-Glcp- (1→ | 3.99 | 3.61 | 3.47 | 3.24 | 3.19 | 3.38 a, 3.49 b |
| 103.23 | 74.58 | 85.96 | 70.70 | 75.31 | 69.85 | |
| D →3)-β-D-Glcp- (1→ | 3.93 | 3.60 | 3.48 | 3.37 | 3.17 | 3.70 a,3.49 b |
| 102.94 | 74.80 | 86.95 | 75.33 | 77.01 | 61.11 | |
| Residue | Proton | Intra-Correlation |
|---|---|---|
| A β-D-Glcp- (1→ | 4.02 (H-1) | 3.12 (B:H-6) |
| B →6)-β-D-Glcp- (1→ | 4.00 (H-1) | 3.62 (B:H-3),3.38 (C:H-6) |
| 3.12 (H-6) | 4.02 (A:H-1) | |
| C →3,6-β-D-Glcp- (1→ | 3.99 (H-1) | 3.61 (C:H-2),3.48 (D H-3),3.38 (C:H-6),3.24 (C:H-4) |
| 3.38,3.49 (H-6) | 3.93 (D:H-1) | |
| 3.47 (H-3) | 3.93 (D:H-1) | |
| D →3)-β-D-Glcp- (1→ | 3.93 (H-1) | 3,37 (D:H-4) |
| 3.48 (D:H-3) | 3.99 (C:H-1) |
| Residue | Proton | Proton Correlation |
|---|---|---|
| A β-D-Glcp- (1→ | 4.02 (H-1) | 69.92 (B:C-6) |
| B →6)-β-D-Glcp- (1→ | 4.00 (H-1) | 69.85 (C:C-6) |
| C →3,6)-β-D-Glcp- (1→ | 3.99 (H-1) | 86.95 (D:C-3) |
| 3.47 (H-3) | 102.94 (D:C-1),103.23 (C:C-1) | |
| 3.38 (H-6) | 103.16 (B:C-1) | |
| D →3)-β-D-Glcp- (1→ | 3.93 (H-1) | 86.36 (D:C-3) |
| 3.48 (H-3) | 103.23 (C:C-1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, W.; Wang, W.; Yu, Y.; Wang, H.; Zhang, H.; Liu, P.; Zhang, M.; Li, Q.; Zhang, H.; Li, H.; et al. Structure and Activity of β-Oligosaccharides Obtained from Lentinus edodes (Shiitake). Separations 2024, 11, 326. https://doi.org/10.3390/separations11110326
Jia W, Wang W, Yu Y, Wang H, Zhang H, Liu P, Zhang M, Li Q, Zhang H, Li H, et al. Structure and Activity of β-Oligosaccharides Obtained from Lentinus edodes (Shiitake). Separations. 2024; 11(11):326. https://doi.org/10.3390/separations11110326
Chicago/Turabian StyleJia, Wei, Wenhan Wang, Yanzhen Yu, Huimin Wang, Hongtao Zhang, Peng Liu, Meiyan Zhang, Qiaozhen Li, Henan Zhang, Huaxiang Li, and et al. 2024. "Structure and Activity of β-Oligosaccharides Obtained from Lentinus edodes (Shiitake)" Separations 11, no. 11: 326. https://doi.org/10.3390/separations11110326
APA StyleJia, W., Wang, W., Yu, Y., Wang, H., Zhang, H., Liu, P., Zhang, M., Li, Q., Zhang, H., Li, H., & Zhang, J. (2024). Structure and Activity of β-Oligosaccharides Obtained from Lentinus edodes (Shiitake). Separations, 11(11), 326. https://doi.org/10.3390/separations11110326







