Exploration of the Functional Constituents of the Substrate of Flammulina velutipes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Extraction Procedures
2.2.1. Preparation of Extracts
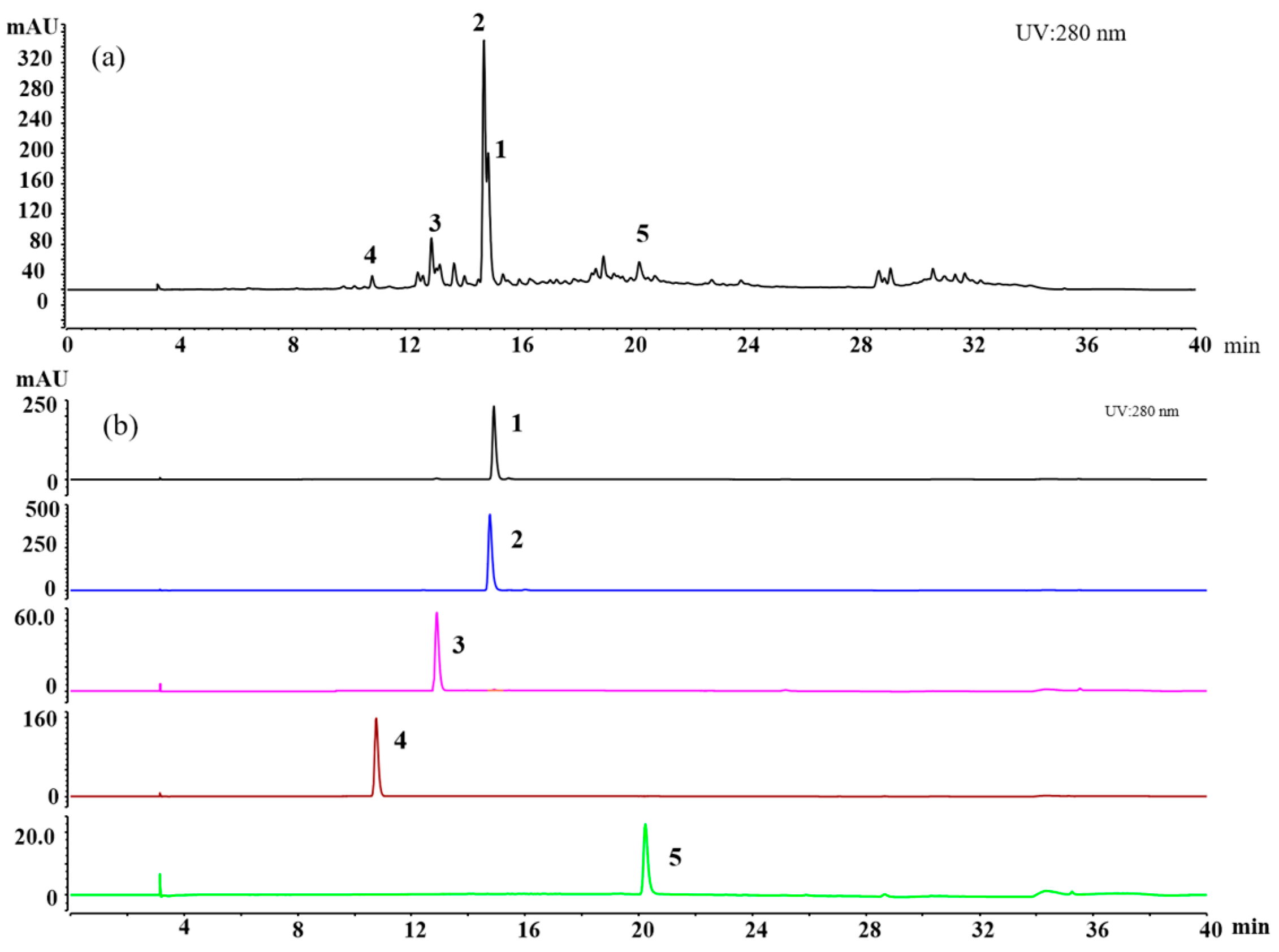
2.2.2. Isolation and Purification of Compounds 1–5
2.3. NMR Analysis
2.4. DPPH Radical Scavenging Capacity
2.5. Quantitative Analysis of Chemical Constituents
2.6. Statistical Analysis
3. Results and Discussion
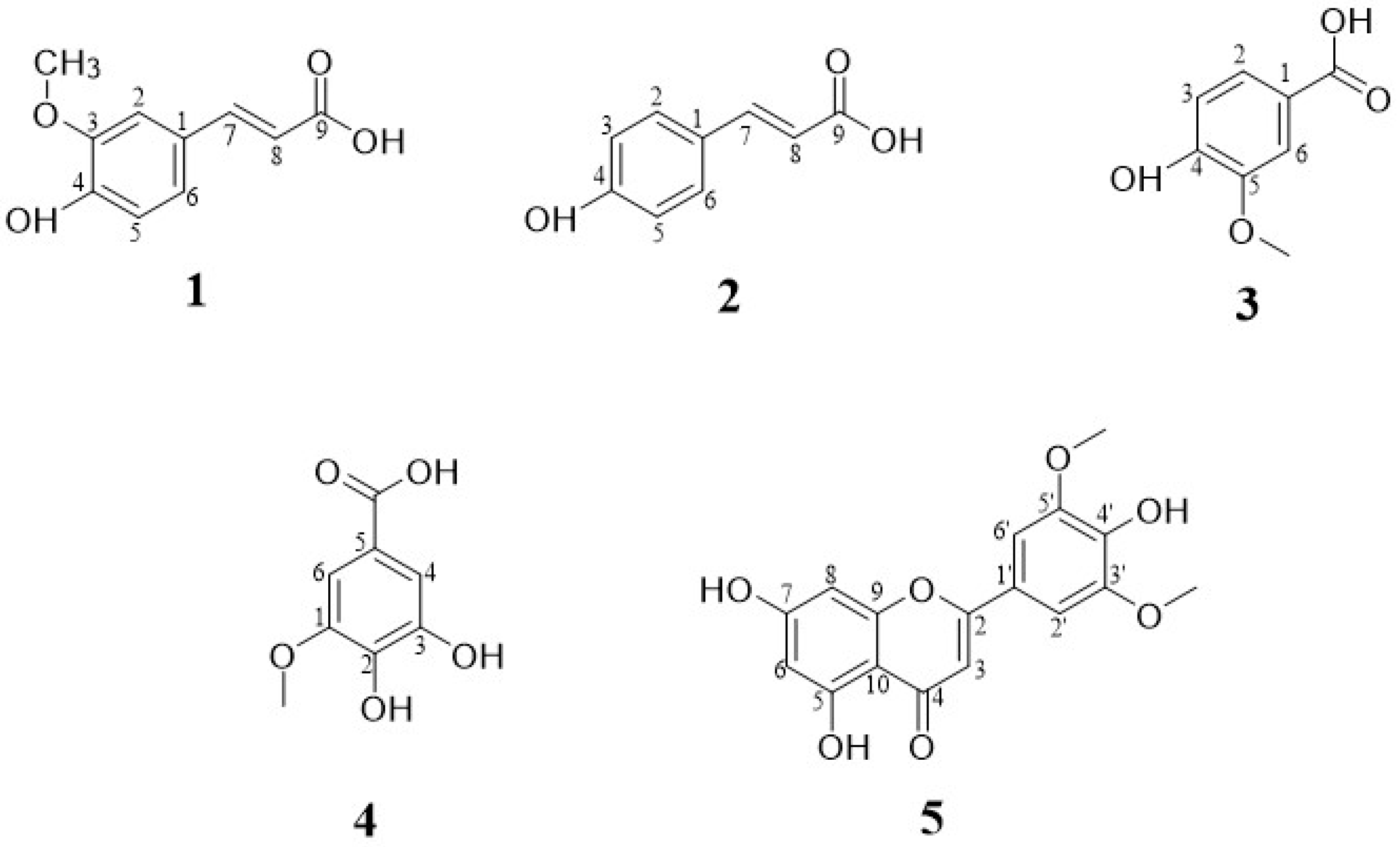
3.1. Structure Identification
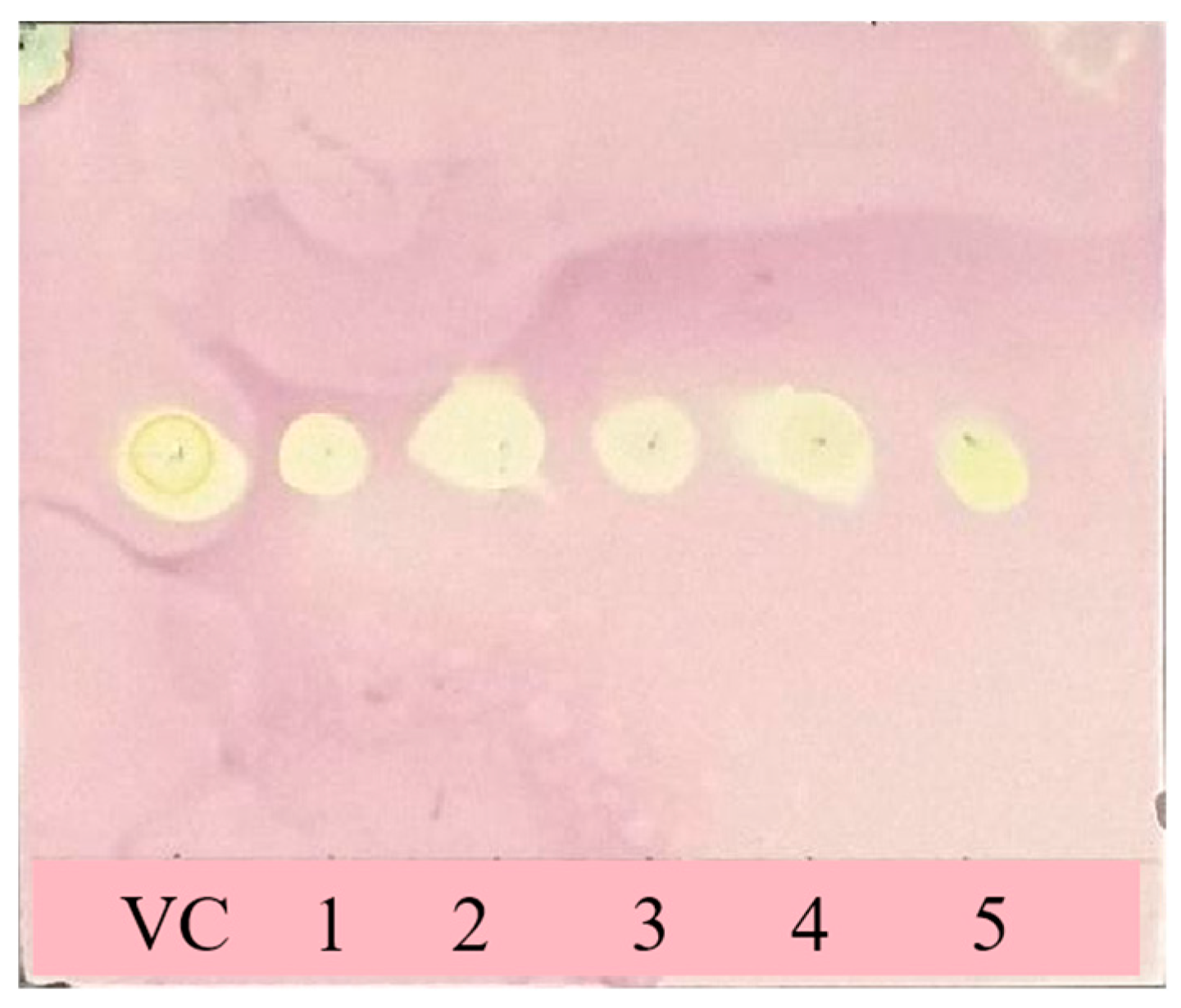
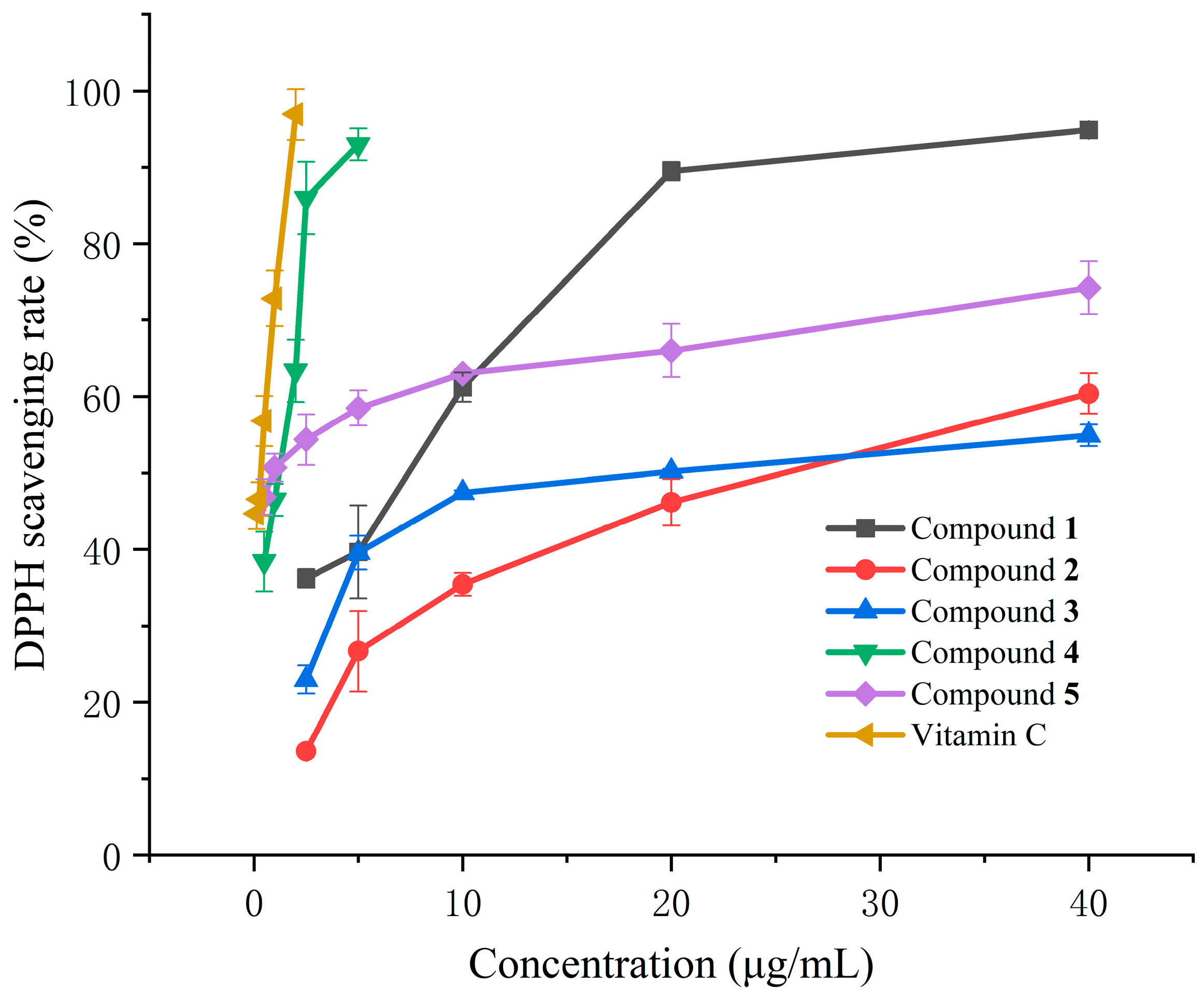
3.2. DPPH Free Radical Scavenging Activity
3.3. The Amount of the Isolated Chemical Ingredient
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Qin, P.; Li, T.; Liu, C.; Liang, Y.; Sun, H.; Chai, Y.; Yang, T.; Gong, X.; Wu, Z. Extraction and utilization of active substances from edible fungi substrate and residue: A review. Food Chem. 2023, 398, 133872. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, S.; Yamauchi, M.; Watari, T.; Hatamoto, M.; Yamada, M.; Maki, S.; Hara, H.; Yamaguchi, T. Development of Enokitake (Flammulina velutipes) mushroom cultivation technology using spent mushroom substrate anaerobic digestion residue. Environ. Technol. Innov. 2021, 24, 102046. [Google Scholar] [CrossRef]
- Luo, J.; Chen, L. Status and development of spent mushroom substrate recycling: A review. J. Air Waste Manag. Assoc. 2024, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Umor, N.A.; Ismail, S.; Abdullah, S.; Huzaifah, M.H.R.; Huzir, N.M.; Mahmood, N.A.N.; Zahrim, A.Y. Zero waste management of spent mushroom compost. J. Mater. Cycles Waste Manag. 2021, 23, 1726–1736. [Google Scholar] [CrossRef]
- Pérez-Chávez, A.M.; Mayer, L.; Albertó, E. Mushroom cultivation and biogas production: A sustainable reuse of organic resources. Energy Sustain. Dev. 2019, 50, 50–60. [Google Scholar] [CrossRef]
- Kulshreshtha, S. Removal of pollutants using spent mushrooms substrates. Environ. Chem. Lett. 2018, 17, 833–847. [Google Scholar] [CrossRef]
- Jia, C.-W.; Li, C.-T.; Tian, F.-H.; Sun, B.; Tang, L.-D. Research on the Application Effects of Flammulina velutipes Waste and Chicken Embryos in the High-yield and High-quality Cultivation of Pleurotus ostreatus. Mod. Agric. Sci. Technol. 2018, 22, 66–67. [Google Scholar]
- Lin, L.; Cui, F.; Zhang, J.; Gao, X.; Zhou, M.; Xu, N.; Zhao, H.; Liu, M.; Zhang, C.; Jia, L. Antioxidative and renoprotective effects of residue polysaccharides from Flammulina velutipes. Carbohydr. Polym. 2016, 146, 388–395. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, X.; Zhang, J.; Wang, T.; Liu, S.; Ma, H.; Inam, M.; Guan, L. Green ultrasonic-assisted enzymatic extraction of polysaccharides from Flammulina velutipes residues by response surface methodology. Sustain. Chem. Pharm. 2024, 41, 101690. [Google Scholar] [CrossRef]
- Tso, K.H.; Lumsangkul, C.; Ju, J.C.; Fan, Y.K.; Chiang, H.I. The Potential of Peroxidases Extracted from the Spent Mushroom (Flammulina velutipes) Substrate Significantly Degrade Mycotoxin Deoxynivalenol. Toxins 2021, 13, 72. [Google Scholar] [CrossRef]
- Heleno, S.A.; Prieto, M.A.; Barros, L.; Rodrigues, A.; Barreiro, M.F.; Ferreira, I.C.F.R. Optimization of microwave-assisted extraction of ergosterol from Agaricus bisporus L. by-products using response surface methodology. Food Bioprod. Process. 2016, 100, 25–35. [Google Scholar] [CrossRef]
- Liu, X.; Hou, R.; Wang, D.; Mai, M.; Wu, X.; Zheng, M.; Fu, J. Comprehensive utilization of edible mushroom Auricularia auricula waste residue-Extraction, physicochemical properties of melanin and its antioxidant activity. Food Sci. Nutr. 2019, 7, 3774–3783. [Google Scholar] [CrossRef] [PubMed]
- Sato, N.; Shinji, K.; Mizuno, M.; Nozaki, K.; Suzuki, M.; Makishima, S.; Shiroishi, M.; Onoda, T.; Takahashi, F.; Kanda, T.; et al. Improvement in the productivity of xylooligosaccharides from waste medium after mushroom cultivation by hydrothermal treatment with suitable pretreatment. Bioresour. Technol. 2010, 101, 6006–6011. [Google Scholar] [CrossRef] [PubMed]
- Kassim, N.K.; Lim, P.C.; Ismail, A.; Awang, K. Isolation of antioxidative compounds from Micromelum minutum guided by preparative thin layer chromatography-2,2-diphenyl-1-picrylhydrazyl (PTLC-DPPH) bioautography method. Food Chem. 2019, 272, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Prachayasittikul, S.; Suphapong, S.; Worachartcheewan, A.; Lawung, R.; Ruchirawat, S.; Prachayasittikul, V. Bioactive metabolites from Spilanthes acmella Murr. Molecules 2009, 14, 850–867. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.; Luo, S. The mechanism for enhancing extraction of ferulic acid from Radix Angelica sinensis by high hydrostatic pressure. Sep. Purif. Technol. 2016, 165, 208–213. [Google Scholar] [CrossRef]
- Li, M.; Jia, Z.; Wan, G.; Wang, S.; Min, D. Enhancing isolation of p-coumaric and ferulic acids from sugarcane bagasse by sequential hydrolysis. Chem. Pap. 2019, 74, 499–507. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, H.; Lin, L.; Zhao, M.; Zhang, L.; Zhang, Y.; Wu, Y. Application of natural deep eutectic solvents to extract ferulic acid from Ligusticum chuanxiong Hort with microwave assistance. RSC Adv. 2019, 9, 22677–22684. [Google Scholar] [CrossRef]
- Shon, Y.J.; Kim, W.C.; Lee, S.-H.; Hong, S.; Kim, S.-Y.; Park, M.H.; Lee, P.; Lee, J.; Park, K.H.; Lim, W.; et al. Antimelanogenic potential of brewer’s spent grain extract through modulation of the MAPK/MITF axis. Sustain. Mater. Technol. 2023, 38, e00721. [Google Scholar] [CrossRef]
- Johnson, E.M.; Jayabalan, L.R.; Patra, S.K.; Suh, J.-W. Self-assembled fructo-oligosaccharide conjugated ferulic acid microparticle: Anticancer, anti-inflammatory and immunomodulatory effects. J. Drug Deliv. Sci. Technol. 2024, 92, 105339. [Google Scholar] [CrossRef]
- Alam, M.A. Anti-hypertensive Effect of Cereal Antioxidant Ferulic Acid and Its Mechanism of Action. Front. Nutr. 2019, 6, 121. [Google Scholar] [CrossRef] [PubMed]
- Bami, E.; Ozakpinar, O.B.; Ozdemir-Kumral, Z.N.; Koroglu, K.; Ercan, F.; Cirakli, Z.; Sekerler, T.; Izzettin, F.V.; Sancar, M.; Okuyan, B. Protective effect of ferulic acid on cisplatin induced nephrotoxicity in rats. Environ. Toxicol. Pharmacol. 2017, 54, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Bergman, M.; Varshavsky, L.; Gottlieb, H.E.; Grossman, S. The antioxidant activity of aqueous spinach extract: Chemical identification of active fractions. Phytochemistry 2001, 58, 143–152. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Nakamura, K.; Ohno, O.; Suenaga, K.; Okuda, N. Asparagus decline: Autotoxicity and autotoxic compounds in asparagus rhizomes. J. Plant Physiol. 2017, 213, 23–29. [Google Scholar] [CrossRef]
- Cha, H.; Lee, S.; Lee, J.H.; Park, J.W. Protective effects of p-coumaric acid against acetaminophen-induced hepatotoxicity in mice. Food Chem. Toxicol. 2018, 121, 131–139. [Google Scholar] [CrossRef]
- Sheng, K.; Li, Y.; Wang, Z.; Hang, K.; Ye, Z. p-Coumaric acid suppresses reactive oxygen species-induced senescence in nucleus pulposus cells. Exp. Ther. Med. 2022, 23, 183. [Google Scholar] [CrossRef]
- Guo, J.; Su, K.; Wang, L.; Feng, B.; You, X.; Deng, M.; Toh, W.S.; Wu, J.; Cheng, B.; Xia, J. Poly(p-coumaric acid) nanoparticles alleviate temporomandibular joint osteoarthritis by inhibiting chondrocyte ferroptosis. Bioact. Mater. 2024, 40, 212–226. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, N.; Xu, R.; Li, G.; Dong, H.; Wang, B.; Li, Z.; Fan, M.; Wei, X. Deciphering the antibacterial activity and mechanism of p-coumaric acid against Alicyclobacillus acidoterrestris and its application in apple juice. Int. J. Food Microbiol. 2022, 378, 109822. [Google Scholar] [CrossRef]
- Yoon, D.S.; Cho, S.Y.; Yoon, H.J.; Kim, S.R.; Jung, U.J. Protective effects of p-coumaric acid against high-fat diet-induced metabolic dysregulation in mice. Biomed. Pharmacother. 2021, 142, 111969. [Google Scholar] [CrossRef]
- Boo, Y.C. p-Coumaric Acid as An Active Ingredient in Cosmetics: A Review Focusing on its Antimelanogenic Effects. Antioxidants 2019, 8, 275. [Google Scholar] [CrossRef]
- Chen, X.; Yu, F.; Li, Y.; Lou, Z.; Toure, S.L.; Wang, H. The inhibitory activity of p-coumaric acid on quorum sensing and its enhancement effect on meat preservation. CyTA—J. Food 2020, 18, 61–67. [Google Scholar] [CrossRef]
- Gutierrez-Lugo, M.T.; Woldemichael, G.M.; Singh, M.P.; Suarez, P.A.; Maiese, W.M.; Montenegro, G.; Timmermann, B.N. Isolation of three new naturally occurring compounds from the culture of Micromonospora sp. P1068. Nat. Prod. Res. 2005, 19, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Kwon, S.P.; Park, S.; Cho, S.H.; Oh, Y.; Kim, S.H.; Park, Y.H.; Jung, H.S.; Lee, D.C.; Jeong, H.; et al. Effect of Pinoresinol and Vanillic Acid Isolated from Catalpa bignonioides on Mouse Myoblast Proliferation via the Akt/mTOR Signaling Pathway. Molecules 2022, 27, 5397. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.L.; Hassan, O. Bioconversion of ferulic acid attained from pineapple peels and pineapple crown leaves into vanillic acid and vanillin by Aspergillus niger I-1472. BMC Chem. 2020, 14, 7. [Google Scholar] [CrossRef]
- Vinothiya, K.; Ashokkumar, N. Modulatory effect of vanillic acid on antioxidant status in high fat diet-induced changes in diabetic hypertensive rats. Biomed. Pharmacother. 2017, 87, 640–652. [Google Scholar] [CrossRef]
- Khoshnam, S.E.; Sarkaki, A.; Rashno, M.; Farbood, Y. Memory deficits and hippocampal inflammation in cerebral hypoperfusion and reperfusion in male rats: Neuroprotective role of vanillic acid. Life Sci. 2018, 211, 126–132. [Google Scholar] [CrossRef]
- Matejczyk, M.; Ofman, P.; Juszczuk-Kubiak, E.; Swislocka, R.; Shing, W.L.; Kesari, K.K.; Prakash, B.; Lewandowski, W. Biological effects of vanillic acid, iso-vanillic acid, and orto-vanillic acid as environmental pollutants. Ecotoxicol. Environ. Saf. 2024, 277, 116383. [Google Scholar] [CrossRef]
- Li, D.; Ding, P.; Liu, S.; Yang, X.; Rufeng, W. Screening of An Antibacterial Strain from Human Intestinal Microbes and Investigation on Its Active Metabolites. Mod. Chin. Med. 2020, 22, 26–34. [Google Scholar] [CrossRef]
- Forester, S.C.; Choy, Y.Y.; Waterhouse, A.L.; Oteiza, P.I. The anthocyanin metabolites gallic acid, 3-O-methylgallic acid, and 2,4,6-trihydroxybenzaldehyde decrease human colon cancer cell viability by regulating pro-oncogenic signals. Mol. Carcinog. 2014, 53, 432–439. [Google Scholar] [CrossRef]
- Donnelly, M.I.; Dagley, S. Bacterial degradation of 3,4,5-trimethoxycinnamic acid with production of methanol. J. Bacteriol. 1981, 147, 471–476. [Google Scholar] [CrossRef]
- Kuwabara, H.; Mouri, K.; Otsuka, H.; Kasai, R.; Yamasaki, K. Tricin from a malagasy connaraceous plant with potent antihistaminic activity. J. Nat. Prod. 2003, 66, 1273–1275. [Google Scholar] [CrossRef] [PubMed]
- Moheb, A.; Agharbaoui, Z.; Kanapathy, F.; Ibrahim, R.K.; Roy, R.; Sarhan, F. Tricin biosynthesis during growth of wheat under different abiotic stresses. Plant Sci. 2013, 201–202, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Ajitha, M.J.; Mohanlal, S.; Suresh, C.H.; Jayalekshmy, A. DPPH radical scavenging activity of tricin and its conjugates isolated from “Njavara” rice bran: A density functional theory study. J. Agric. Food Chem. 2012, 60, 3693–3699. [Google Scholar] [CrossRef]
- Santos, A.L.; Yamamoto, E.S.; Passero, L.F.D.; Laurenti, M.D.; Martins, L.F.; Lima, M.L.; Uemi, M.; Soares, M.G.; Lago, J.H.G.; Tempone, A.G.; et al. Antileishmanial Activity and Immunomodulatory Effects of Tricin Isolated from Leaves of Casearia arborea (Salicaceae). Chem. Biodivers. 2017, 14, e1600458. [Google Scholar] [CrossRef] [PubMed]
- Han, J.M.; Kwon, H.J.; Jung, H.J. Tricin, 4′,5,7-trihydroxy-3′,5′-dimethoxyflavone, exhibits potent antiangiogenic activity in vitro. Int. J. Oncol. 2016, 49, 1497–1504. [Google Scholar] [CrossRef] [PubMed]
- Li, J.X.; Li, R.Z.; Sun, A.; Zhou, H.; Neher, E.; Yang, J.S.; Huang, J.M.; Zhang, Y.Z.; Jiang, Z.B.; Liang, T.L.; et al. Metabolomics and integrated network pharmacology analysis reveal Tricin as the active anti-cancer component of Weijing decoction by suppression of PRKCA and sphingolipid signaling. Pharmacol. Res. 2021, 171, 105574. [Google Scholar] [CrossRef]
- An, M.; Kim, H.; Moon, J.M.; Ko, H.S.; Clayton, P.; Lim, Y.H. Enzyme-Treated Zizania latifolia Ethanol Extract Protects from UVA Irradiation-Induced Wrinkle Formation via Inhibition of Lysosome Exocytosis and Reactive Oxygen Species Generation. Antioxidants 2020, 9, 912. [Google Scholar] [CrossRef]
- Akuzawa, K.; Yamada, R.; Li, Z.; Li, Y.; Sadanari, H.; Matsubara, K.; Watanabe, K.; Koketsu, M.; Tuchida, Y.; Murayama, T. Inhibitory effects of tricin derivative from Sasa albo-marginata on replication of human cytomegalovirus. Antivir. Res. 2011, 91, 296–303. [Google Scholar] [CrossRef]
- Doring, K.; Langeder, J.; Duwe, S.; Tahir, A.; Grienke, U.; Rollinger, J.M.; Schmidtke, M. Insights into the direct anti-influenza virus mode of action of Rhodiola rosea. Phytomedicine 2022, 96, 153895. [Google Scholar] [CrossRef]
- Wang, X.; Hu, W.; Qu, L.; Wang, J.; Wu, A.; Lo, H.H.; Ng, J.P.L.; Tang, Y.; Yun, X.; Wu, J.; et al. Tricin promoted ATG-7 dependent autophagic degradation of alpha-synuclein and dopamine release for improving cognitive and motor deficits in Parkinson’s disease. Pharmacol. Res. 2023, 196, 106874. [Google Scholar] [CrossRef]
- Kim, Y.J.; Jeong, S.-J.; Seo, C.-S.; Lim, H.-S.; Sohn, E.; Yun, J.; Kim, B.-Y. Simultaneous Determination of the Traditional Herbal Formula Ukgansan and the In Vitro Antioxidant Activity of Ferulic Acid as an Active Compound. Molecules 2018, 23, 1659. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.A.; Jumbri, K.; Ramli, A.; Abd Ghani, N.; Ahmad, H.; Lim, J.W. A Kinetic Approach of DPPH Free Radical Assay of Ferulate-Based Protic Ionic Liquids (PILs). Molecules 2018, 23, 3201. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhu, J.; Xu, J.R.; Ji, D.D. Synthesis of N-hydroxycinnamoyl amide derivatives and evaluation of their anti-oxidative and anti-tyrosinase activities. Bioorg. Med. Chem. 2019, 27, 114918. [Google Scholar] [CrossRef] [PubMed]
- Benbettaieb, N.; Nyagaya, J.; Seuvre, A.-M.; Debeaufort, F. Antioxidant Activity and Release Kinetics of Caffeic and p-Coumaric Acids from Hydrocolloid-Based Active Films for Healthy Packaged Food. J. Agric. Food Chem. 2018, 66, 6906–6916. [Google Scholar] [CrossRef] [PubMed]
- Kilic, I.; Yesiloglu, Y. Spectroscopic studies on the antioxidant activity of p-coumaric acid. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 115, 719–724. [Google Scholar] [CrossRef]
- Akbar, S.; Ishtiaq, S.; Jahangir, M.; Elhady, S.S.; Bogari, H.A.; Alahdal, A.M.; Ashour, M.L.; Youssef, F.S. Evaluation of The Antioxidant, Antimicrobial, and Anticancer Activities of Dicliptera bupleuroides Isolated Compounds Using In Vitro and In Silico Studies. Molecules 2021, 26, 7196. [Google Scholar] [CrossRef]
- Huyut, Z.; Beydemir, Ş.; Gülçin, İ. Antioxidant and Antiradical Properties of Selected Flavonoids and Phenolic Compounds. Biochem. Res. Int. 2017, 2017, 7616791. [Google Scholar] [CrossRef]
- Renuka Devi, R.; Arumughan, C. Antiradical efficacy of phytochemical extracts from defatted rice bran. Food Chem. Toxicol. 2007, 45, 2014–2021. [Google Scholar] [CrossRef]
- Cheng, H.-T.; Li, X.-L.; Li, X.-R.; Li, Y.-H.; Wang, L.-J.; Xue, M. Simultaneous quantification of selected compounds from Salvia herbs by HPLC method and their application. Food Chem. 2012, 130, 1031–1035. [Google Scholar] [CrossRef]
- Zavala-Lopez, M.; Garcia-Lara, S. An improved microscale method for extraction of phenolic acids from maize. Plant Methods 2017, 13, 81. [Google Scholar] [CrossRef]
- Muti, H.Y.; Olimat, S. HPLC Method of Analysis for Determination and Standardization of Luteolin and Vanillic Acid in Dry Extract of Paronychia Argentea Lam. Orient. J. Chem. 2018, 34, 2721–2727. [Google Scholar] [CrossRef]
- Armijos, C.; Ponce, J.; Ramírez, J.; Gozzini, D.; Vidari, P.V.F. An Unprecedented High Content of the Bioactive Flavone Tricin in Huperzia Medicinal Species Used by the Saraguro in Ecuador. Nat. Prod. Commun. 2016, 11, 1934578X1601100301. [Google Scholar] [CrossRef]
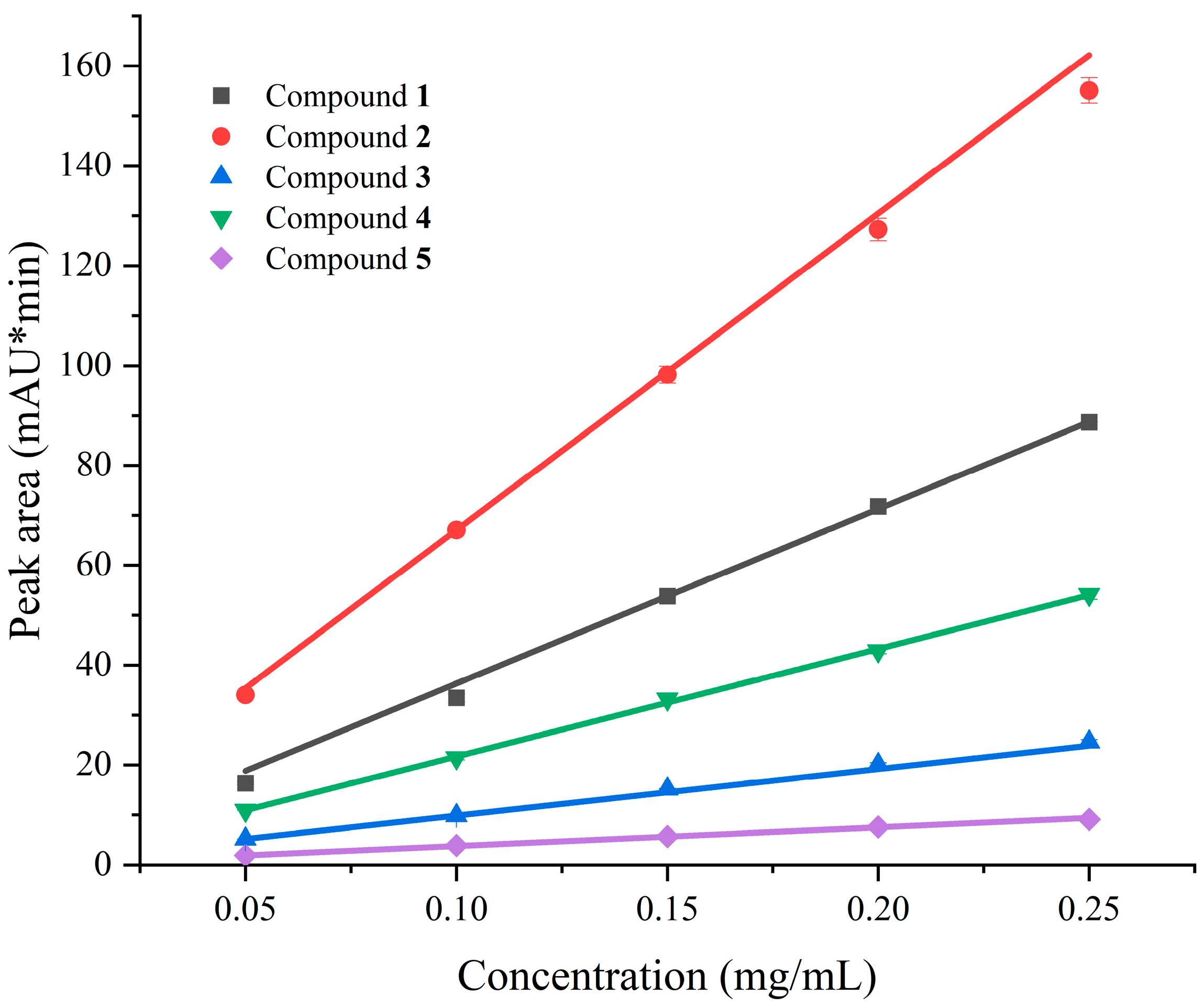
| Compounds | Retention Time (min) | Linear Regression | R2 | Content in Organic Extract (mg/mL) | Content in SFV (mg/kg) |
|---|---|---|---|---|---|
| 1 | 14.90 | y = 349.63x + 1.35 | 0.9998 | 0.0679 | 218.88 |
| 2 | 14.75 | y = 633.68x + 3.68 | 0.9964 | 0.0448 | 157.31 |
| 3 | 12.89 | y = 93.39x + 0.53 | 0.9993 | 0.0873 | 281.54 |
| 4 | 10.75 | y = 215.34x + 0.18 | 0.9996 | 0.0105 | 33.85 |
| 5 | 20.24 | y = 37.45x + 0.04 | 0.9985 | 0.2213 | 713.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, R.; Zheng, R.; Yuan, B.; Li, B.; Chen, Y.; Zheng, Y. Exploration of the Functional Constituents of the Substrate of Flammulina velutipes. Separations 2024, 11, 311. https://doi.org/10.3390/separations11110311
Jiang R, Zheng R, Yuan B, Li B, Chen Y, Zheng Y. Exploration of the Functional Constituents of the Substrate of Flammulina velutipes. Separations. 2024; 11(11):311. https://doi.org/10.3390/separations11110311
Chicago/Turabian StyleJiang, Ruixiang, Renqing Zheng, Bin Yuan, Bo Li, Yiyong Chen, and Yongbiao Zheng. 2024. "Exploration of the Functional Constituents of the Substrate of Flammulina velutipes" Separations 11, no. 11: 311. https://doi.org/10.3390/separations11110311
APA StyleJiang, R., Zheng, R., Yuan, B., Li, B., Chen, Y., & Zheng, Y. (2024). Exploration of the Functional Constituents of the Substrate of Flammulina velutipes. Separations, 11(11), 311. https://doi.org/10.3390/separations11110311






