Study on an Integrated Water Treatment System by Simultaneously Coupling Granular Activated Carbon (GAC) and Powdered Carbon with Ultrafiltration
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
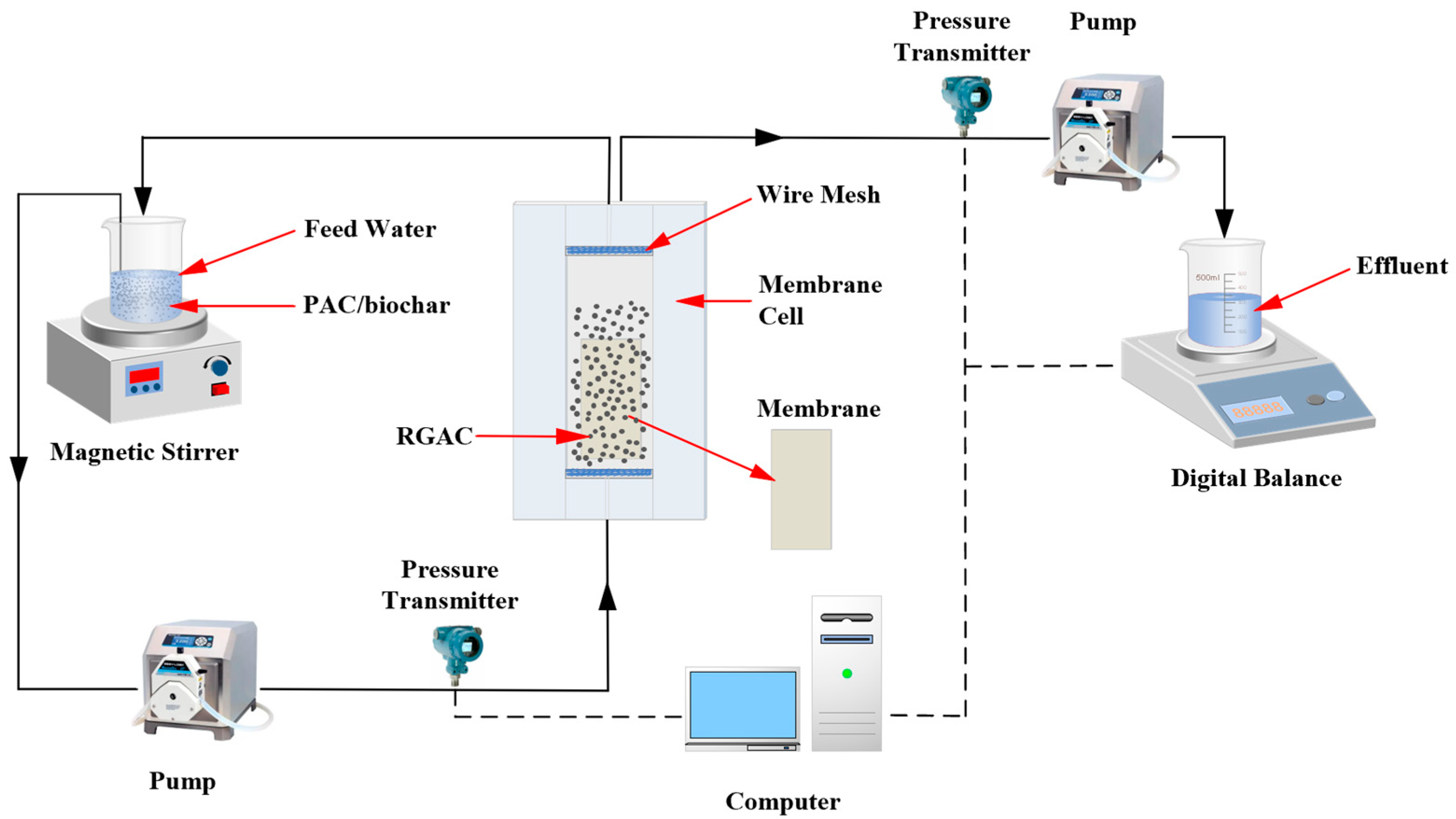
2.2. Experiment Setup and Operation
2.3. Measurement of Membrane Resistances
2.4. Determination of PAC/Biochar Amounts and Extraction of Membrane Foulants
2.5. Analytical Methods for Membrane Foulants Samples and Water Samples
3. Results and Discussion
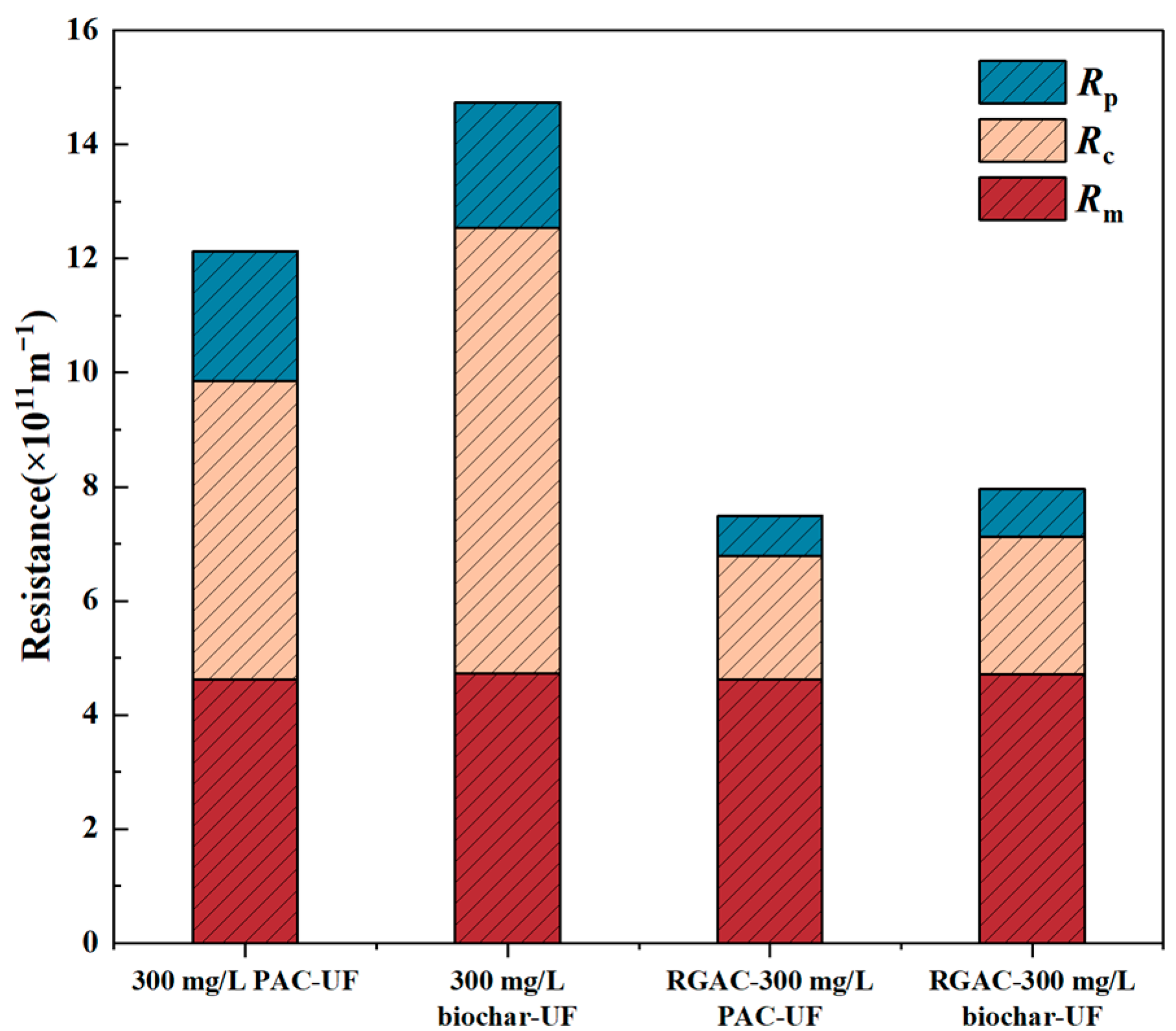
3.1. Membrane Fouling Tendency
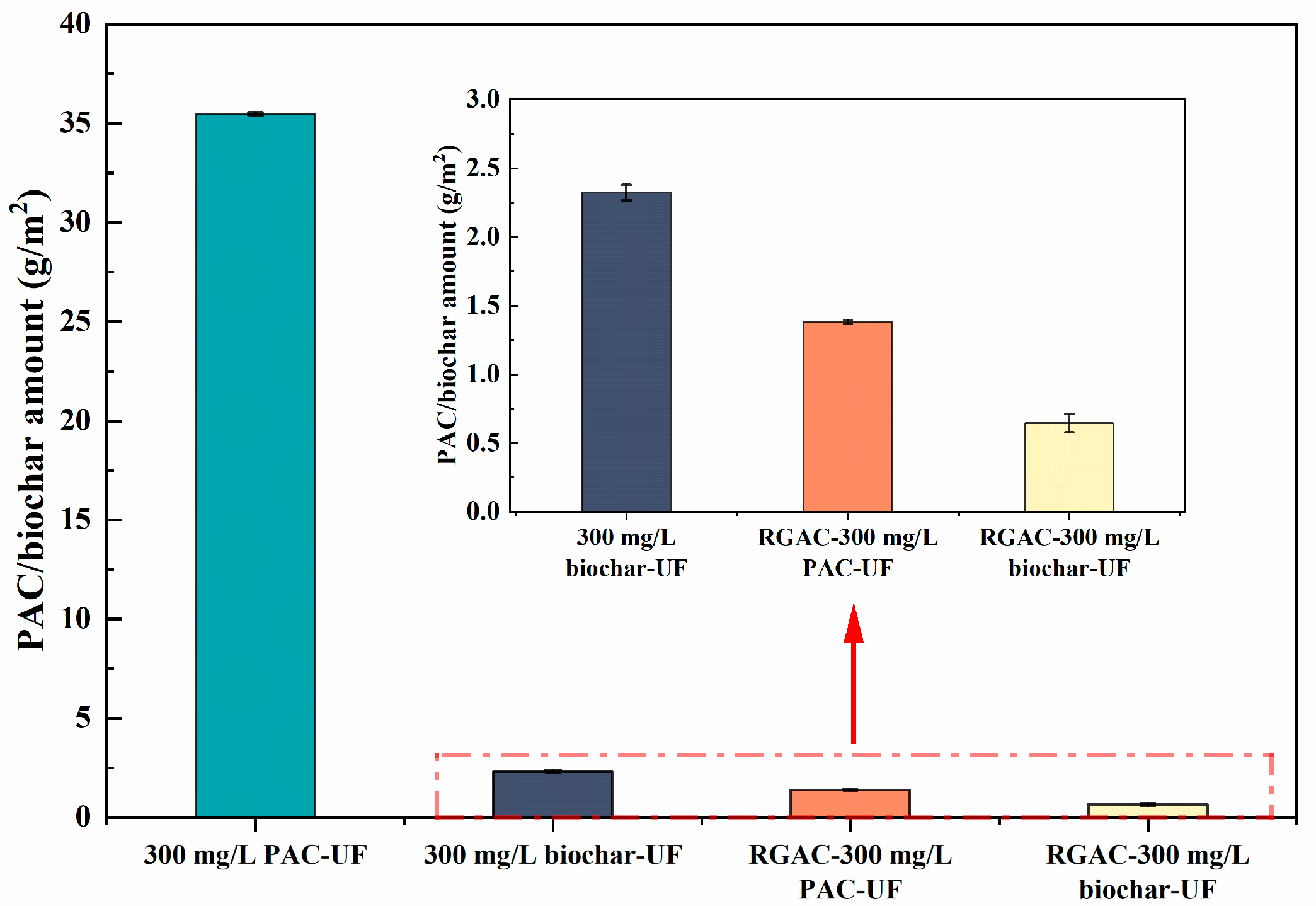
3.2. PAC/Biochar Attachment on Membrane Surface
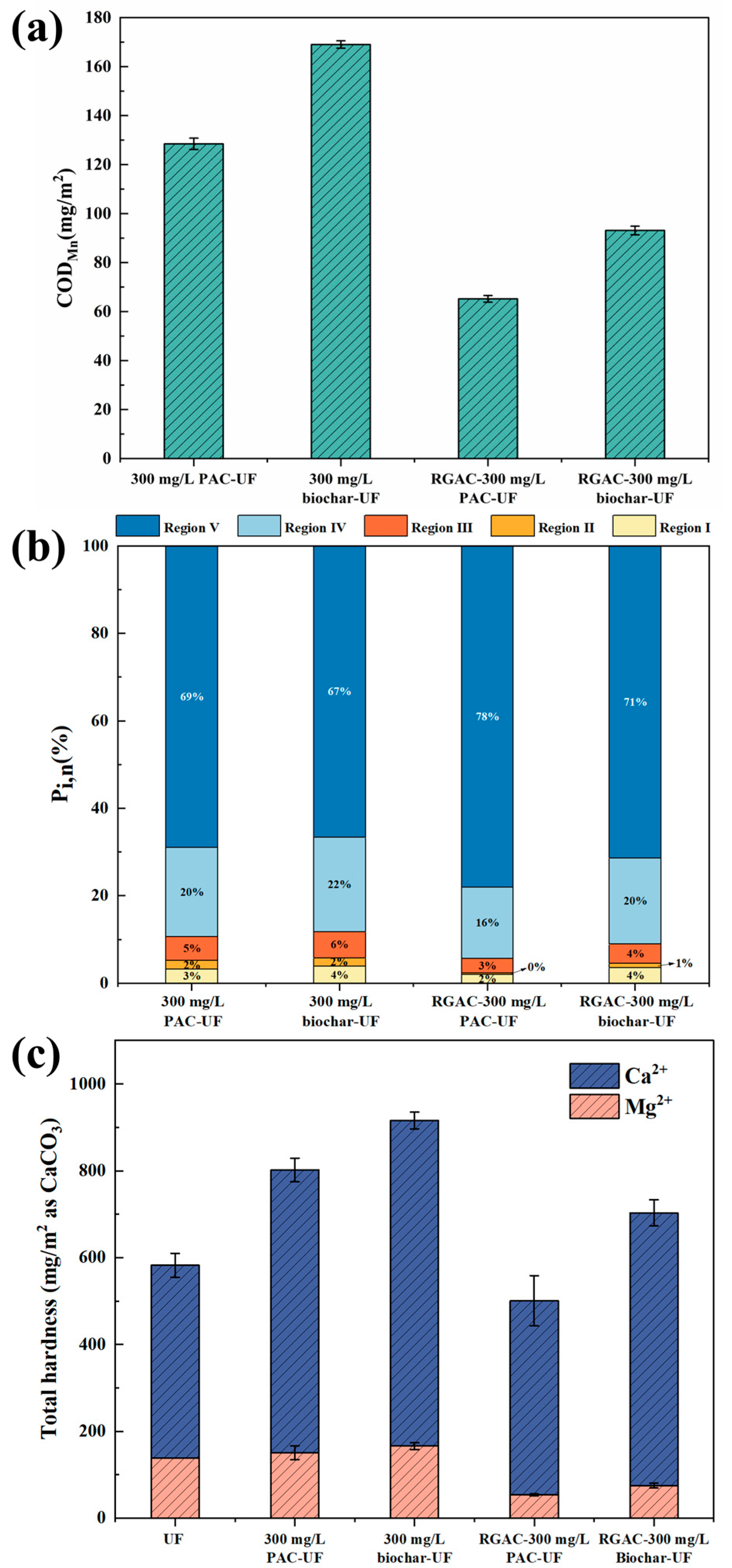
3.3. Componential Analysis of Membrane Foulants
3.4. Water Quality Analysis
3.5. Performance Comparison of Different-Sized Biochar in the Integrated Water Treatment System
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shi, Y.; Zhang, Z.; Zhang, M.; Ding, G.; Zhao, B.; Wang, L.; Zhang, H. Biological membrane fouling control with the integrated and separated processes of MIEX and UF. Sep. Purif. Technol. 2021, 259, 118151. [Google Scholar] [CrossRef]
- Moslehyani, A.; Ismail, A.F.; Matsuura, T.; Rahman, M.A.; Goh, P.S. Chapter 3—Recent Progresses of Ultrafiltration (UF) Membranes and Processes in Water Treatment. In Membrane Separation Principles and Applications; Ismail, A.F., Rahman, M.A., Othman, M.H.D., Matsuura, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 85–110. [Google Scholar]
- Chang, H.; Liang, H.; Qu, F.; Liu, B.; Yu, H.; Du, X.; Li, G.; Snyder, S.A. Hydraulic backwashing for low-pressure membranes in drinking water treatment: A review. J. Membr. Sci. 2017, 540, 362–380. [Google Scholar] [CrossRef]
- Khanzada, N.K.; Farid, M.U.; Kharraz, J.A.; Choi, J.; Tang, C.Y.; Nghiem, L.D.; Jang, A.; An, A.K. Removal of organic micropollutants using advanced membrane-based water and wastewater treatment: A review. J. Membr. Sci. 2020, 598, 117672. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, J.; Shao, L.; Wang, L.; Yang, X.; Zhao, B.; Li, J.; Ma, C.; Chu, X.; Zhang, P. Comparison between integrated MIEX/UF and PAC/UF in long-term irreversible membrane fouling reduction: Effectiveness and mechanism analysis. Process Saf. Environ. Prot. 2022, 168, 1179–1187. [Google Scholar] [CrossRef]
- Mozia, S.; Tomaszewska, M. Treatment of surface water using hybrid processes—Adsorption on PAC and ultrafiltration. Desalination 2004, 162, 23–31. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Yang, Y.; Liang, S. Fouling control of PAC/UF process for treating algal-rich water. Desalination 2015, 355, 75–82. [Google Scholar] [CrossRef]
- Ivančev-Tumbas, I.; Hoffmann, G.; Hobby, R.; Kerkez, Đ.; Tubić, A.; Babić-Nanić, S.; Panglisch, S. Removal of diclofenac from water by in/out PAC/UF hybrid process. Environ. Technol. 2018, 39, 2315–2320. [Google Scholar] [CrossRef]
- Löwenberg, J.; Zenker, A.; Baggenstos, M.; Koch, G.; Kazner, C.; Wintgens, T. Comparison of two PAC/UF processes for the removal of micropollutants from wastewater treatment plant effluent: Process performance and removal efficiency. Water Res. 2014, 56, 26–36. [Google Scholar] [CrossRef]
- Campinas, M.; Rosa, M.J. Assessing PAC contribution to the NOM fouling control in PAC/UF systems. Water Res. 2010, 44, 1636–1644. [Google Scholar] [CrossRef]
- Tomaszewska, M.; Mozia, S. Removal of organic matter from water by PAC/UF system. Water Res. 2002, 36, 4137–4143. [Google Scholar] [CrossRef]
- Shao, S.; Cai, L.; Li, K.; Li, J.; Du, X.; Li, G.; Liang, H. Deposition of powdered activated carbon (PAC) on ultrafiltration (UF) membrane surface: Influencing factors and mechanisms. J. Membr. Sci. 2017, 530, 104–111. [Google Scholar] [CrossRef]
- Tanudjaja, H.J.; Anantharaman, A.; Ng, A.Q.Q.; Ma, Y.; Tanis-Kanbur, M.B.; Zydney, A.L.; Chew, J.W. A review of membrane fouling by proteins in ultrafiltration and microfiltration. J. Water Process Eng. 2022, 50, 103294. [Google Scholar] [CrossRef]
- Oh, H.; Yu, M.; Takizawa, S.; Ohgaki, S. Evaluation of PAC behavior and fouling formation in an integrated PAC–UF membrane for surface water treatment. Desalination 2006, 192, 54–62. [Google Scholar] [CrossRef]
- Li, Q.; Snoeyink, V.L.; Mariñas, B.J.; Campos, C. Pore blockage effect of NOM on atrazine adsorption kinetics of PAC: The roles of PAC pore size distribution and NOM molecular weight. Water Res. 2003, 37, 4863–4872. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Zhao, Z.-P.; Cai, W. Chemically induced alteration in PAC characteristics and its influences on PAC/UF water treatment: Implications for on-line membrane cleaning with NaClO. Sep. Purif. Technol. 2022, 294, 121130. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, S.; Wang, J.; Zhao, Z.-P.; Cai, W. Advanced water treatment process by simultaneous coupling granular activated carbon (GAC) and powdered carbon with ultrafiltration: Role of GAC particle shape and powdered carbon type. Water Res. 2023, 231, 119606. [Google Scholar] [CrossRef]
- Yu, S.; Wang, J.; Zhao, Z.; Cai, W. Simultaneous coupling of fluidized granular activated carbon (GAC) and powdered activated carbon (PAC) with ultrafiltration process: A promising synergistic alternative for water treatment. Sep. Purif. Technol. 2022, 282, 120085. [Google Scholar] [CrossRef]
- Wu, B.; Zamani, F.; Lim, W.; Liao, D.; Wang, Y.; Liu, Y.; Chew, J.W.; Fane, A.G. Effect of mechanical scouring by granular activated carbon (GAC) on membrane fouling mitigation. Desalination 2017, 403, 80–87. [Google Scholar] [CrossRef]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef]
- Wang, X.; Guo, Z.; Hu, Z.; Zhang, J. Recent advances in biochar application for water and wastewater treatment: A review. PeerJ 2020, 8, e9164. [Google Scholar] [CrossRef]
- Shankar, V.; Heo, J.; Al-Hamadani, Y.A.J.; Park, C.M.; Chu, K.H.; Yoon, Y. Evaluation of biochar-ultrafiltration membrane processes for humic acid removal under various hydrodynamic, pH, ionic strength, and pressure conditions. J. Environ. Manag. 2017, 197, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.; Boateng, L.K.; Flora, J.R.V.; Oh, J.; Braswell, M.C.; Son, A.; Yoon, Y. Competitive adsorption of selected non-steroidal anti-inflammatory drugs on activated biochars: Experimental and molecular modeling study. Chem. Eng. J. 2015, 264, 1–9. [Google Scholar] [CrossRef]
- Cheng, X.; Liang, H.; Ding, A.; Qu, F.; Shao, S.; Liu, B.; Wang, H.; Wu, D.; Li, G. Effects of pre-ozonation on the ultrafiltration of different natural organic matter (NOM) fractions: Membrane fouling mitigation, prediction and mechanism. J. Membr. Sci. 2016, 505, 15–25. [Google Scholar] [CrossRef]
- Cai, W.; Liu, J.; Zhang, X.; Ng, W.J.; Liu, Y. Generation of dissolved organic matter and byproducts from activated sludge during contact with sodium hypochlorite and its implications to on-line chemical cleaning in MBR. Water Res. 2016, 104, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Rice, E.W.; Baird, R.B.; Eaton, A.D.; American Public Health Association. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2012; Volume 10. [Google Scholar]
- Jiang, Y.; Liu, Y.; Shi, D.; Fu, W.; Sun, P.-F.; Li, J.; Shao, S. Membrane fouling in a powdered activated carbon—Membrane bioreactor (PAC-MBR) for micro-polluted water purification: Fouling characteristics and the roles of PAC. J. Clean. Prod. 2020, 277, 122341. [Google Scholar] [CrossRef]
- Zhang, J.N.; Lü, F.; Luo, C.H.; Shao, L.M.; He, P.J. Humification characterization of biochar and its potential as a composting amendment. J. Environ. Sci. 2014, 26, 390–397. [Google Scholar] [CrossRef]
- Tang, C.Y.; Chong, T.H.; Fane, A.G. Colloidal interactions and fouling of NF and RO membranes: A review. Adv. Colloid Interface Sci. 2011, 164, 126–143. [Google Scholar] [CrossRef]
- Seidel, A.; Elimelech, M. Coupling between chemical and physical interactions in natural organic matter (NOM) fouling of nanofiltration membranes: Implications for fouling control. J. Membr. Sci. 2002, 203, 245–255. [Google Scholar] [CrossRef]
- Ahn, W.-Y.; Kalinichev, A.G.; Clark, M.M. Effects of background cations on the fouling of polyethersulfone membranes by natural organic matter: Experimental and molecular modeling study. J. Membr. Sci. 2008, 309, 128–140. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Yu, S.; Cai, W. Study on an Integrated Water Treatment System by Simultaneously Coupling Granular Activated Carbon (GAC) and Powdered Carbon with Ultrafiltration. Separations 2024, 11, 312. https://doi.org/10.3390/separations11110312
Wang Y, Yu S, Cai W. Study on an Integrated Water Treatment System by Simultaneously Coupling Granular Activated Carbon (GAC) and Powdered Carbon with Ultrafiltration. Separations. 2024; 11(11):312. https://doi.org/10.3390/separations11110312
Chicago/Turabian StyleWang, Yi, Sijia Yu, and Weiwei Cai. 2024. "Study on an Integrated Water Treatment System by Simultaneously Coupling Granular Activated Carbon (GAC) and Powdered Carbon with Ultrafiltration" Separations 11, no. 11: 312. https://doi.org/10.3390/separations11110312
APA StyleWang, Y., Yu, S., & Cai, W. (2024). Study on an Integrated Water Treatment System by Simultaneously Coupling Granular Activated Carbon (GAC) and Powdered Carbon with Ultrafiltration. Separations, 11(11), 312. https://doi.org/10.3390/separations11110312






