Retention Mechanisms of Basic Compounds in Liquid Chromatography with Sodium Dodecyl Sulfate and 1-Hexyl-3-Methylimidazolium Chloride as Mobile Phase Reagents in Two C18 Columns
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Mobile Phases
2.2. Chromatographic Instrumentation and Columns
3. Results and Discussion
3.1. Retention Behaviour of β-Adrenoceptor Antagonists with Mobile Phases Containing Ionic Additives
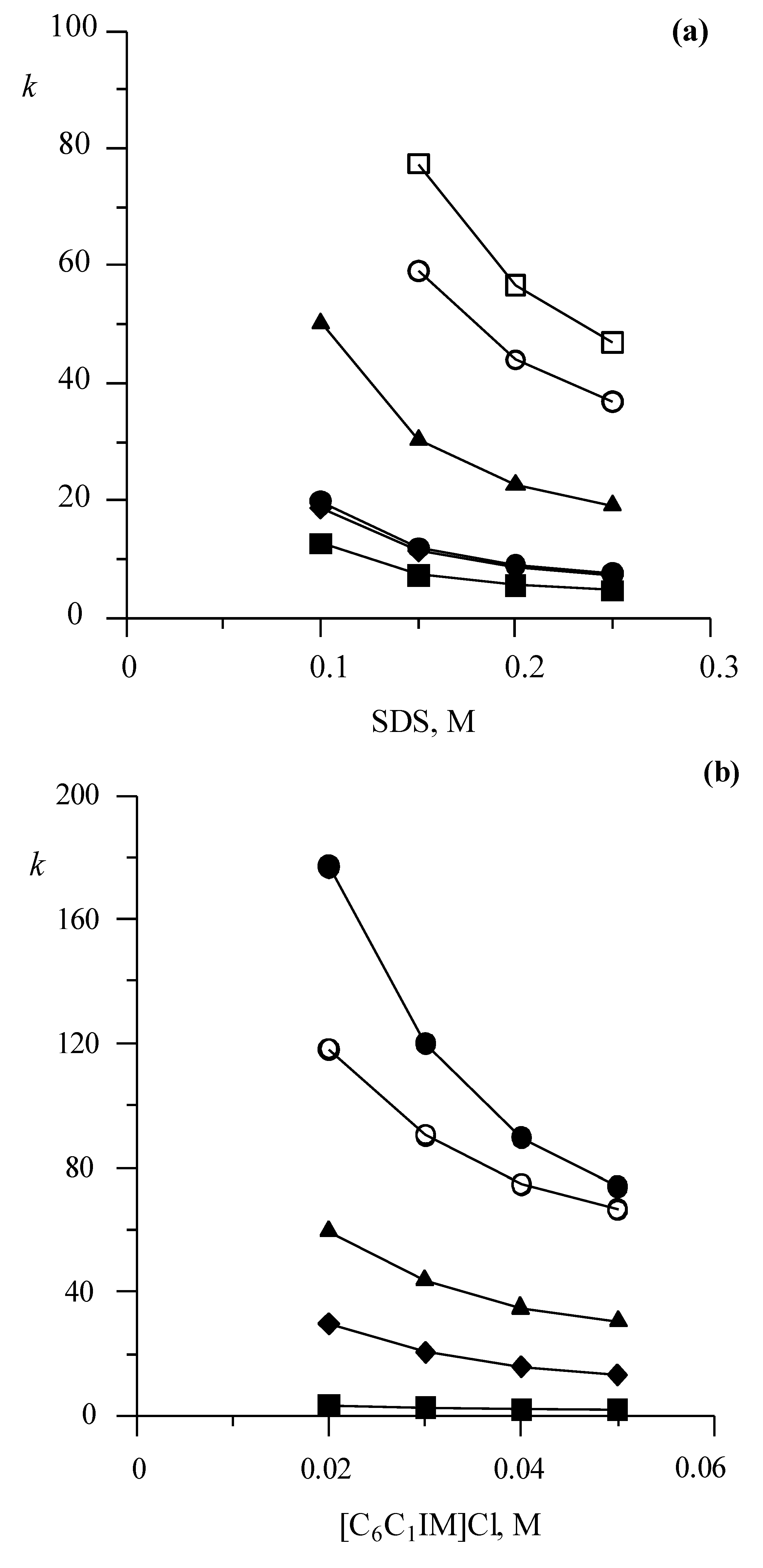
3.1.1. Effect on Retention of Mobile Phases Containing Only SDS or [C6C1IM][Cl]
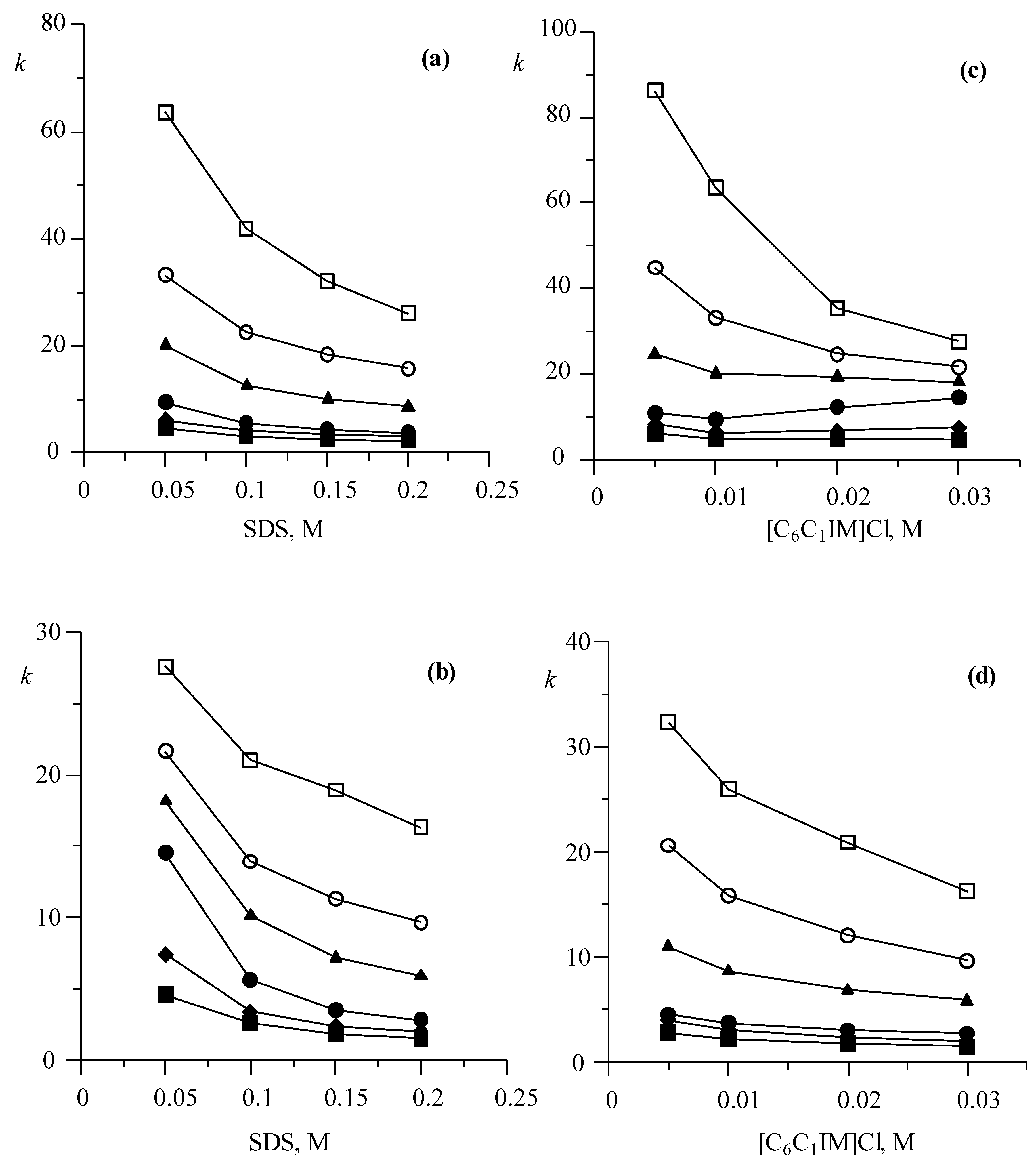
3.1.2. Effect on Retention of Mobile Phases Containing Both SDS and [C6C1IM][Cl]
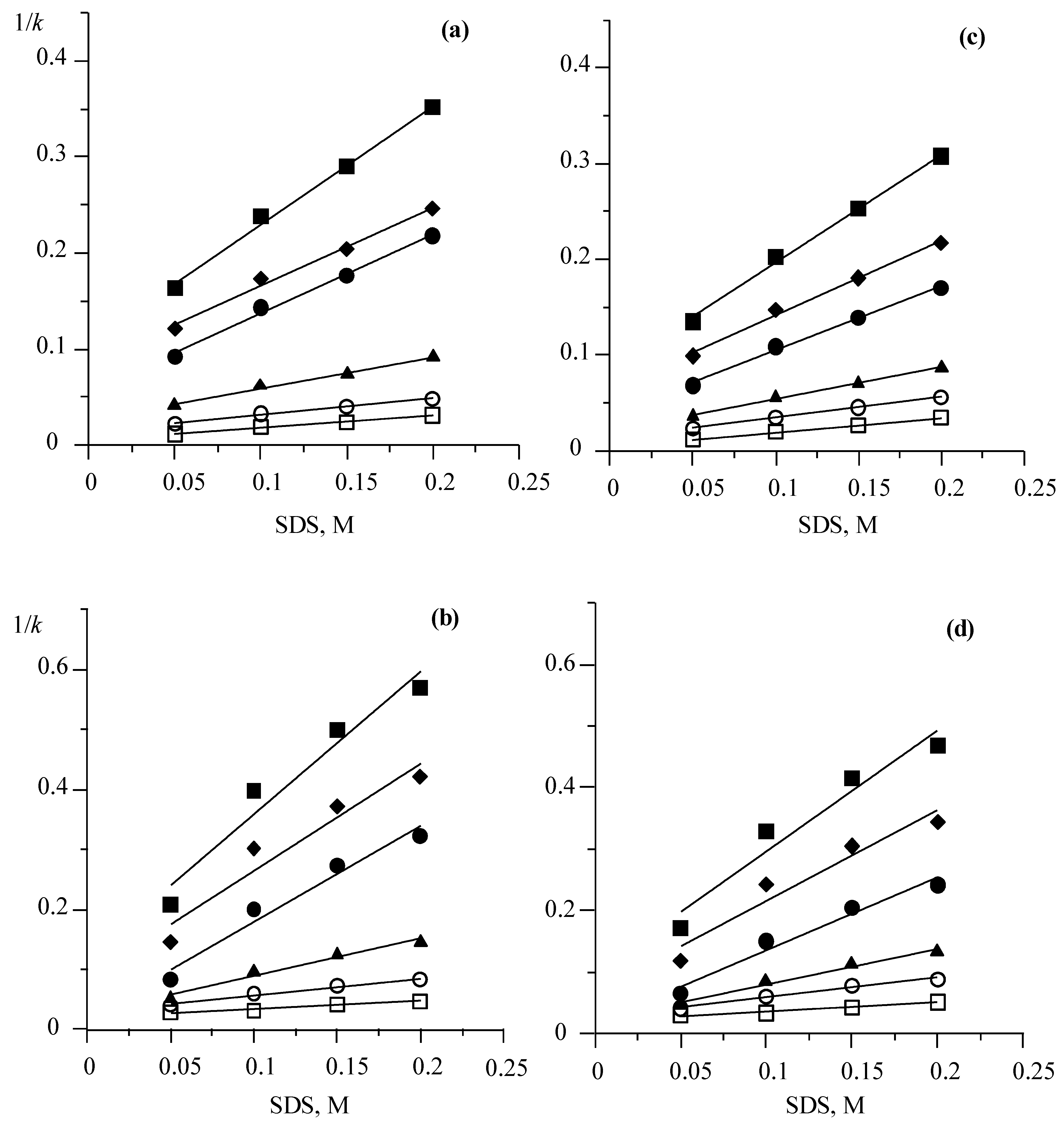
3.2. Solute–Stationary Phase and Solute–Mobile Phase Interactions
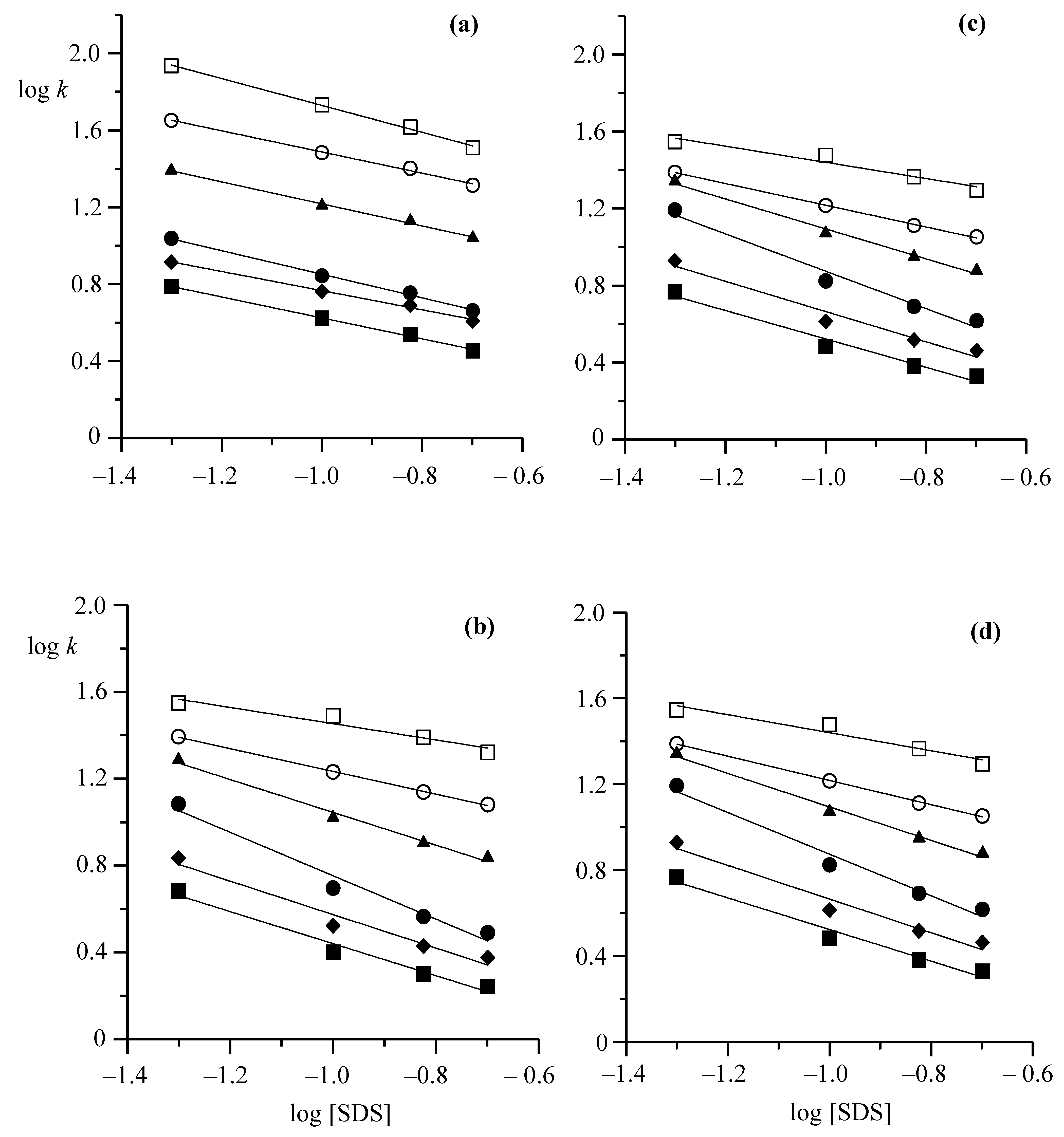
3.3. Estimation of Hydrophobic and Ionic Interactions
- (i)
- The hydrocarbon chains of the stationary phase, which provide a site for hydrophobic interactions.
- (ii)
- The C18 chains modified through the adsorption of anionic SDS monomers and IL cations, enabling ionic interactions with the cationic solutes.
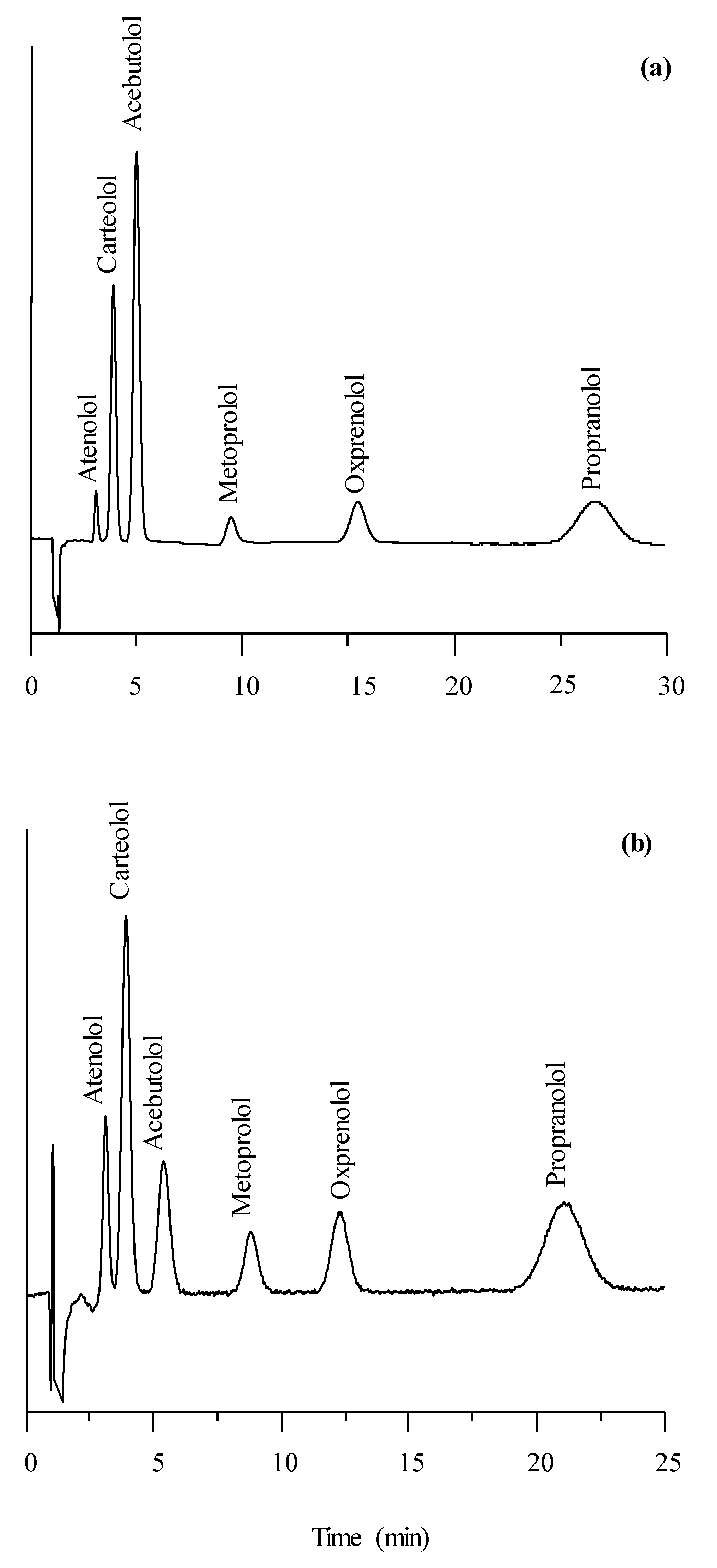
3.4. Separation of β-Adrenoceptor Antagonists with Aqueous Mobile Phases in RPLC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| β-Adrenoceptor Antagonists | Structure | pKa a | log Po/w a |
|---|---|---|---|
| Atenolol | 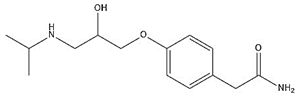 | 9.17 | 0.25 |
| Carteolol | 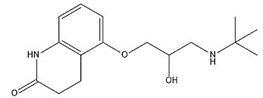 | 9.24 | 1.49 |
| Acebutolol | 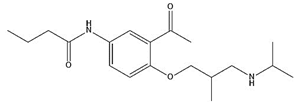 | 9.24 | 1.83 |
| Metoprolol |  | 9.31 | 1.90 |
| Oxprenolol |  | 9.08 | 2.30 |
| Propranolol | 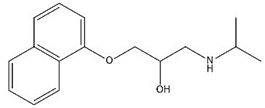 | 9.25 | 3.41 |
References
- Peng, L.Q.; Cao, J.; Du, L.J.; Zhang, Q.D.; Shi, Y.T.; Xu, J.J. Analysis of phenolic acids by ionic liquid-in-water microemulsion liquid chromatography coupled with ultraviolet and electrochemical detector. J. Chromatogr. A 2017, 1499, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Pankajkumar-Patel, N.; Peris-García, E.; Ruiz-Ángel, M.J.; García-Alvarez-Coque, M.C. Interactions of basic compounds with ionic liquids used as oils in microemulsion liquid chromatography. J. Chromatogr. A 2022, 1674, 463142. [Google Scholar] [CrossRef] [PubMed]
- García-Alvarez-Coque, M.C.; Ruiz-Angel, M.J.; Carda-Broch, S. Micellar liquid Chromatography: Method Development and Applications. In Analytical Separation Science Series; Anderson, J.L., Berthod, A., Pino, V., Stalcup, A., Eds.; Wiley-VCH: New York, NY, USA, 2015; Volume 2, pp. 407–460. [Google Scholar]
- Berthod, A.; García-Alvarez-Coque, M.C. Micellar Liquid Chromatography. In Chromatographic Science Series; Cazes, J., Ed.; Marcel Dekker: New York, NY, USA, 2000. [Google Scholar]
- Wang, J.; Qiao, J.Q.; Zheng, W.J.; Lian, H.Z. Effect of ionic liquids as mobile phase additives on retention behaviors of G-quadruplexes in reversed-phase high performance liquid chromatography. J. Chromatogr. A 2024, 1715, 464604. [Google Scholar] [CrossRef] [PubMed]
- Axente, R.E.; Stan, M.; Chitescu, C.L.; Nitescu, V.G.; Vlasceanu, A.M.; Baconi, D.L. Application of ionic liquids as mobile phase additives for simultaneous analysis of nicotine and its metabolite cotinine in human plasma by HPLC-DAD. Molecules 2023, 28, 1563. [Google Scholar] [CrossRef]
- Treder, N.; Olędzka, I.; Roszkowska, A.; Kowalski, P.; Bączek, T.; Plenis, A. Practical and theoretical considerations of the effects of ionic liquids on the separation properties of phenyl-based stationary phases in reversed-phase liquid chromatography. Michrochem. J. 2022, 178, 107396. [Google Scholar] [CrossRef]
- Sun, P.; Armstrong, D.W. Ionic liquids in analytical chemistry. Anal. Chim. Acta 2010, 661, 1–16. [Google Scholar] [CrossRef]
- Domańska, U. General review of ionic liquids and their properties. In Ionic Liquids in Chemical Analysis; Koel, M., Ed.; CRC Press: New York, NY, USA, 2009; pp. 1–71. [Google Scholar]
- Buettnera, C.S.; Cognignia, A.; Schröder, C.; Bica-Schröder, K. Surface-active ionic liquids. J. Mol. Liq. 2022, 347, 118160. [Google Scholar] [CrossRef]
- Brown, P.; Butts, C.; Eastoe, J.; Fermin, D.; Grillo, I.; Lee, H.-C.; Parke, D. Anionic surfactant ionic liquids with 1-butyl-3-methyl-imidazolium cations: Characterization and application. Langmuir 2012, 28, 2502–2509. [Google Scholar] [CrossRef]
- Berthod, A.; Ruiz-Angel, M.J.; Huguet, S. Non molecular solvents in separation methods: The dual nature of room temperature ionic liquids. Anal. Chem. 2005, 77, 4071–4080. [Google Scholar] [CrossRef]
- Tereba-Mamani, C.J.; Blázquez-Mateu, M.; Ruiz-Angel, M.J.; García-Alvarez-Coque, M.C. The role of the cation and anion in aqueous liquid chromatography with sodium dodecyl sulphate and imidazolium-based ionic liquids as mobile phase reagents. Anal. Chim. Acta 2024, 1318, 342942. [Google Scholar] [CrossRef]
- Tereba-Mamani, C.J.; Janczuk, M.A.; Ruiz-Angel, M.J.; García-Alvarez-Coque, M.C. Aqueous liquid chromatography with mobile phases of sodium dodecyl sulphate and ionic liquid. J. Chromatogr. A 2023, 1689, 463740. [Google Scholar] [CrossRef] [PubMed]
- Ubeda-Torres, M.T.; Ortiz-Bolsico, C.; García-Alvarez-Coque, M.C.; Ruiz-Angel, M.J. Gaining insight in the behaviour of imidazolium-based ionic liquids as additives in reversed-phase liquid chromatography for the analysis of basic compounds. J. Chromatogr. A 2015, 1380, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Angel, M.J.; Carda-Broch, S.; Torres-Lapasió, J.R.; García-Alvarez-Coque, M.C. Retention mechanisms for basic drugs in the submicellar and micellar reversed-phase liquid chromatography modes. Anal. Chem. 2008, 80, 9705–9713. [Google Scholar] [CrossRef] [PubMed]
- Foley, J.P.; May, W.E. Optimization of secondary chemical equilibria in liquid chromatography: Theory and verification. Anal. Chem. 1987, 59, 102–109. [Google Scholar] [CrossRef]
- Olives, A.I.; González-Ruiz, V.; Martin, M.A. Sustainable and eco-friendly alternatives for liquid chromatographic analysis. ACS Sust. Chem. Eng. 2017, 5, 5618–5634. [Google Scholar] [CrossRef]
- Treder, N.; Oledzka, I.; Roszkowska, A.; Baczek, T.; Plenis, A. Control of retention mechanisms on an octadecyl-bonded silica column using ionic liquid-based mobile phase in analysis of cytostatic drugs by liquid chromatography. J. Chromatogr. A 2021, 1651, 462257. [Google Scholar] [CrossRef]
- Mehvar, R.; Brocks, D.R. Stereospecific pharmacokinetics and pharmacodynamics of beta-adrenergic blockers in humans. J. Pharm. Sci. 2001, 4, 185–200. [Google Scholar]
- Torres-Lapasió, J.R. MICHROM Software; Marcel Dekker: New York, NY, USA, 2000. [Google Scholar]
- Torres-Lapasió, J.R.; García-Alvarez-Coque, M.C.; Baeza-Baeza, J.J. Global treatment of chromatographic data with MICHROM. Anal. Chim. Acta 1997, 348, 187–196. [Google Scholar] [CrossRef]
- Ruiz-Angel, M.J.; García-Alvarez-Coque, M.C. Micellar liquid chromatography: How to start. LC-GC Eur. 2008, 21, 420–429. [Google Scholar]
- Peris-García, E.; García-Alvarez-Coque, M.C.; Carda-Broch, S.; Ruiz-Angel, M.J. Effect of buffer nature and concentration on the chromatographic performance of basic compounds in the absence and presence of 1-hexyl-3-methylimidazolium chloride. J. Chromatogr. A 2019, 1602, 397–408. [Google Scholar] [CrossRef]
- Arunyanart, M.; Cline-Love, L.J. Model for micellar effects on liquid chromatography capacity factors and for determination of micelle-solute equilibrium constants. Anal. Chem. 1984, 56, 1557–1561. [Google Scholar] [CrossRef]
- Armstrong, D.W.; Nome, F. Partitioning behavior of solutes eluted with micellar mobile phases in liquid chromatography. Anal. Chem. 1981, 53, 1662–1666. [Google Scholar] [CrossRef]
- Ruiz-Angel, M.J.; Peris-García, E.; García-Alvarez-Coque, M.C. Reversed-phase liquid chromatography with mixed micellar mobile phases of Brij-35 and sodium dodecyl sulphate: A method for the analysis of basic compounds. Green Chem. 2015, 17, 3561–3570. [Google Scholar] [CrossRef]
- Fernández-Navarro, J.J.; García-Alvarez-Coque, M.C.; Ruiz-Angel, M.J. The role of the dual nature of ionic liquids in the reversed-phase liquid chromatographic separation of basic drugs. J. Chromatogr. A 2011, 1218, 398–407. [Google Scholar] [CrossRef]
- Sokolowski, A.; Wahlund, K.G. Peak tailing and retention behaviour of tricyclic antidepressant amines and related hydrophobic ammonium compounds in reversed-phase ion-pair liquid chromatography on alkyl-bonded phases. J. Chromatogr. A 1980, 189, 299–316. [Google Scholar] [CrossRef]
- Nahum, A.; Horváth, C. Surface silanols in silica-bonded hydrocarbonaceous stationary phases: I. Dual retention mechanism in reversed-phase chromatography. J. Chromatogr. 1981, 203, 53–63. [Google Scholar] [CrossRef]
- Bij, K.E.; Horváth, C.; Melander, W.R.; Nahum, A. Surface silanols in silica-bonded hydrocarbonaceous stationary phases: II. Irregular retention behavior and effect of silanol masking. J. Chromatogr. 1981, 203, 65–84. [Google Scholar] [CrossRef]
- Carda-Broch, S.; Berthod, A. pH dependence of the hydrophobicity of beta-blocker amine compounds measured by counter-current chromatography. J. Chromatogr. A 2003, 995, 55–66. [Google Scholar] [CrossRef]
| Compound | 0.005 M [C6C1IM][Cl] | 0.01 M [C6C1IM][Cl] | ||
|---|---|---|---|---|
| KAS | KAM | KAS | KAM | |
| Acebutolol | 18.3 ± 2.3 | 15.1 ± 2.1 | 16 ± 4 | 17 ± 5 |
| Atenolol | 9.4 ± 0.7 | 11.6 ± 1.1 | 6.8 ± 0.9 | 10.6 ± 1.7 |
| Carteolol | 11.8 ± 1.0 | 9.6 ± 1.0 | 8.4 ± 1.2 | 8.8 ± 1.6 |
| Metoprolol | 39 ± 5 | 12.7 ± 1.7 | 32 ± 6 | 14 ± 3 |
| Oxprenolol | 69 ± 6 | 11.8 ± 1.3 | 49 ± 6 | 10.7 ± 1.5 |
| Propranolol | 186 ± 16 | 23.6 ± 2.1 | 119 ± 6 | 17.9 ± 1.0 |
| 0.02 M [C6C1IM][Cl] | 0.03 M [C6C1IM][Cl] | |||
| KAS | KAM | KAS | KAM | |
| Acebutolol | 50 ± 70 | 80 ± 110 | – | – |
| Atenolol | 8 ± 4 | 20 ± 9 | 13 ± 5 | 40 ± 15 |
| Carteolol | 12 ± 6 | 21 ± 12 | 30 ± 40 | 80 ± 100 |
| Metoprolol | 38 ± 14 | 23 ± 9 | 51 ± 15 | 39 ± 12 |
| Oxprenolol | 35 ± 4 | 10.0 ± 1.5 | 33 ± 5 | 12.5 ± 2.3 |
| Propranolol | 49 ± 4 | 6.5 ± 0.8 | 34 ± 3 | 5.5 ± 0.7 |
| Compound | 0.005 M [C6C1IM][Cl] | 0.01 M [C6C1IM][Cl] | ||
|---|---|---|---|---|
| KAS | KAM | KAS | KAM | |
| Acebutolol | 27 ± 3 | 17.8 ± 2.4 | 23 ± 6 | 19 ± 5 |
| Atenolol | 12.1 ± 1.0 | 13.8 ± 1.3 | 8.4 ± 1.4 | 11.4 ± 2.2 |
| Carteolol | 15.6 ± 1.4 | 12.1 ± 1.2 | 11.4 ± 1.8 | 10.9 ± 2.0 |
| Metoprolol | 50 ± 5 | 17.1 ± 2.0 | 40 ± 8 | 17 ± 4 |
| Oxprenolol | 79 ± 6 | 17.3 ± 1.4 | 53 ± 7 | 14.1 ± 2.0 |
| Propranolol | 220 ± 40 | 34.0 ± 2.1 | 130 ± 170 | 23.1 ± 1.8 |
| 0.02 M [C6C1IM][Cl] | 0.03 M [C6C1IM][Cl] | |||
| KAS | KAM | KAS | KAM | |
| Acebutolol | 50 ± 60 | 60 ± 70 | – | – |
| Atenolol | 10 ± 5 | 19 ± 9 | 16 ± 6 | 39 ± 16 |
| Carteolol | 15 ± 8 | 22 ± 13 | 40 ± 40 | 80 ± 90 |
| Metoprolol | 46 ± 18 | 27 ± 11 | 70 ± 30 | 49 ± 18 |
| Oxprenolol | 37 ± 5 | 12 ± 2 | 35 ± 6 | 15 ± 3 |
| Propranolol | 50 ± 30 | 7.8 ± 1.0 | 40 ± 70 | 6.6 ± 0.9 |
| Compound | Without IL | 0.005 M [C6C1IM][Cl] | 0.01 M [C6C1IM][Cl] | |||
|---|---|---|---|---|---|---|
| m | R2 | m | R2 | m | R2 | |
| Acebutolol | −1.07 ± 0.08 | 0.9899 | −0.613 ± 0.022 | 0.9973 | −0.67 ± 0.04 | 0.9942 |
| Atenolol | −1.10 ± 0.09 | 0.9857 | −0.545 ± 0.018 | 0.9978 | −0.538 ± 0.013 | 0.9988 |
| Carteolol | −1.04 ± 0.07 | 0.9919 | −0.498 ± 0.023 | 0.9957 | −0.497 ± 0.020 | 0.9968 |
| Metoprolol | −1.07 ± 0.07 | 0.9905 | −0.571 ± 0.023 | 0.9966 | −0.61 ± 0.03 | 0.9956 |
| Oxprenolol | – | – | −0.549 ± 0.023 | 0.9966 | −0.536 ± 0.007 | 0.9996 |
| Propranolol | – | – | −0.697 ± 0.024 | 0.9977 | −0.641 ± 0.018 | 0.9984 |
| 0.02 M [C6C1IM][Cl] | 0.03 M [C6C1IM][Cl] | |||||
| m | R2 | m | R2 | |||
| Acebutolol | −1.00 ± 0.12 | 0.9727 | −1.21 ± 0.08 | 0.9920 | ||
| Atenolol | −0.74 ± 0.08 | 0.9762 | −0.83 ± 0.03 | 0.9975 | ||
| Carteolol | −0.77 ± 0.11 | 0.9623 | −0.97 ± 0.07 | 0.9884 | ||
| Metoprolol | −0.75 ± 0.05 | 0.9897 | −0.820 ± 0.019 | 0.9989 | ||
| Oxprenolol | −0.523 ± 0.011 | 0.9992 | −0.583 ± 0.021 | 0.9975 | ||
| Propranolol | −0.36 ± 0.07 | 0.9321 | −0.367 ± 0.024 | 0.9914 | ||
| Compound | Without IL | 0.005 M [C6C1IM][Cl] | 0.01 M [C6C1IM][Cl] | |||
|---|---|---|---|---|---|---|
| m | R2 | m | R2 | m | R2 | |
| Acebutolol | −1.14 ± 0.11 | 0.9824 | −0.588 ± 0.012 | 0.9992 | −0.562 ± 0.022 | 0.9971 |
| Atenolol | −1.22 ± 0.13 | 0.9786 | −0.558 ± 0.012 | 0.9990 | −0.551 ± 0.020 | 0.9974 |
| Carteolol | −1.16 ± 0.12 | 0.9793 | −0.654 ± 0.011 | 0.9994 | −0.70 ± 0.03 | 0.9956 |
| Metoprolol | −1.23 ± 0.13 | 0.9771 | −0.641 ± 0.012 | 0.9993 | −0.659 ± 0.021 | 0.9979 |
| Oxprenolol | −1.25 ± 0.13 | 0.9801 | −0.636 ± 0.015 | 0.9989 | −0.602 ± 0.006 | 0.9998 |
| Propranolol | −1.29 ± 0.13 | 0.9807 | −0.770 ± 0.014 | 0.9994 | −0.695 ± 0.023 | 0.9979 |
| 0.02 M [C6C1IM][Cl] | 0.03 M [C6C1IM][Cl] | |||||
| m | R2 | m | R2 | |||
| Acebutolol | −0.74 ± 0.09 | 0.9736 | −0.83 ± 0.03 | 0.9969 | ||
| Atenolol | −0.78 ± 0.11 | 0.9644 | −0.96 ± 0.08 | 0.9879 | ||
| Carteolol | −0.97 ± 0.11 | 0.9768 | −1.13 ± 0.06 | 0.9946 | ||
| Metoprolol | −0.78 ± 0.05 | 0.9916 | −0.857 ± 0.024 | 0.9984 | ||
| Oxprenolol | −0.563 ± 0.012 | 0.9990 | −0.619 ± 0.022 | 0.9974 | ||
| Propranolol | −0.419 ± 0.072 | 0.9440 | −0.415 ± 0.022 | 0.9944 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tereba-Mamani, C.J.; Garcia-Alvarez-Coque, M.C.; Ruiz-Ángel, M.J. Retention Mechanisms of Basic Compounds in Liquid Chromatography with Sodium Dodecyl Sulfate and 1-Hexyl-3-Methylimidazolium Chloride as Mobile Phase Reagents in Two C18 Columns. Separations 2024, 11, 300. https://doi.org/10.3390/separations11100300
Tereba-Mamani CJ, Garcia-Alvarez-Coque MC, Ruiz-Ángel MJ. Retention Mechanisms of Basic Compounds in Liquid Chromatography with Sodium Dodecyl Sulfate and 1-Hexyl-3-Methylimidazolium Chloride as Mobile Phase Reagents in Two C18 Columns. Separations. 2024; 11(10):300. https://doi.org/10.3390/separations11100300
Chicago/Turabian StyleTereba-Mamani, Carlos Josué, Maria Celia Garcia-Alvarez-Coque, and María José Ruiz-Ángel. 2024. "Retention Mechanisms of Basic Compounds in Liquid Chromatography with Sodium Dodecyl Sulfate and 1-Hexyl-3-Methylimidazolium Chloride as Mobile Phase Reagents in Two C18 Columns" Separations 11, no. 10: 300. https://doi.org/10.3390/separations11100300
APA StyleTereba-Mamani, C. J., Garcia-Alvarez-Coque, M. C., & Ruiz-Ángel, M. J. (2024). Retention Mechanisms of Basic Compounds in Liquid Chromatography with Sodium Dodecyl Sulfate and 1-Hexyl-3-Methylimidazolium Chloride as Mobile Phase Reagents in Two C18 Columns. Separations, 11(10), 300. https://doi.org/10.3390/separations11100300







