Research Progress on Physical and Chemical Remediation Methods for the Removal of Cadmium from Soil
Abstract
1. Introduction
2. Typical Remediation Methods and Mechanisms for Cadmium Pollution in Soil
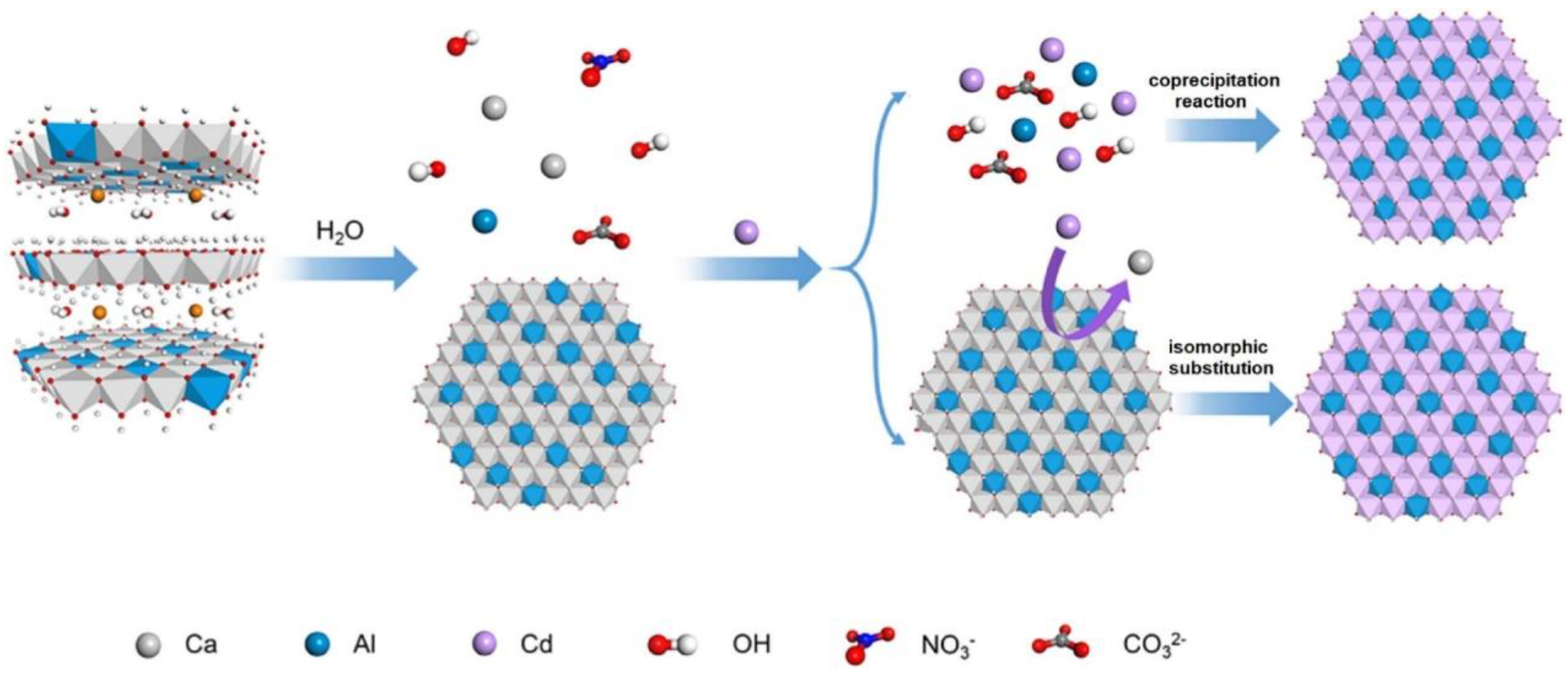
2.1. Study on the CaAl-LDH Remediation Method and Mechanism
2.1.1. CaAl-LDH Remediation Method
2.1.2. Mechanism of CaAl-LDH Remediation Method
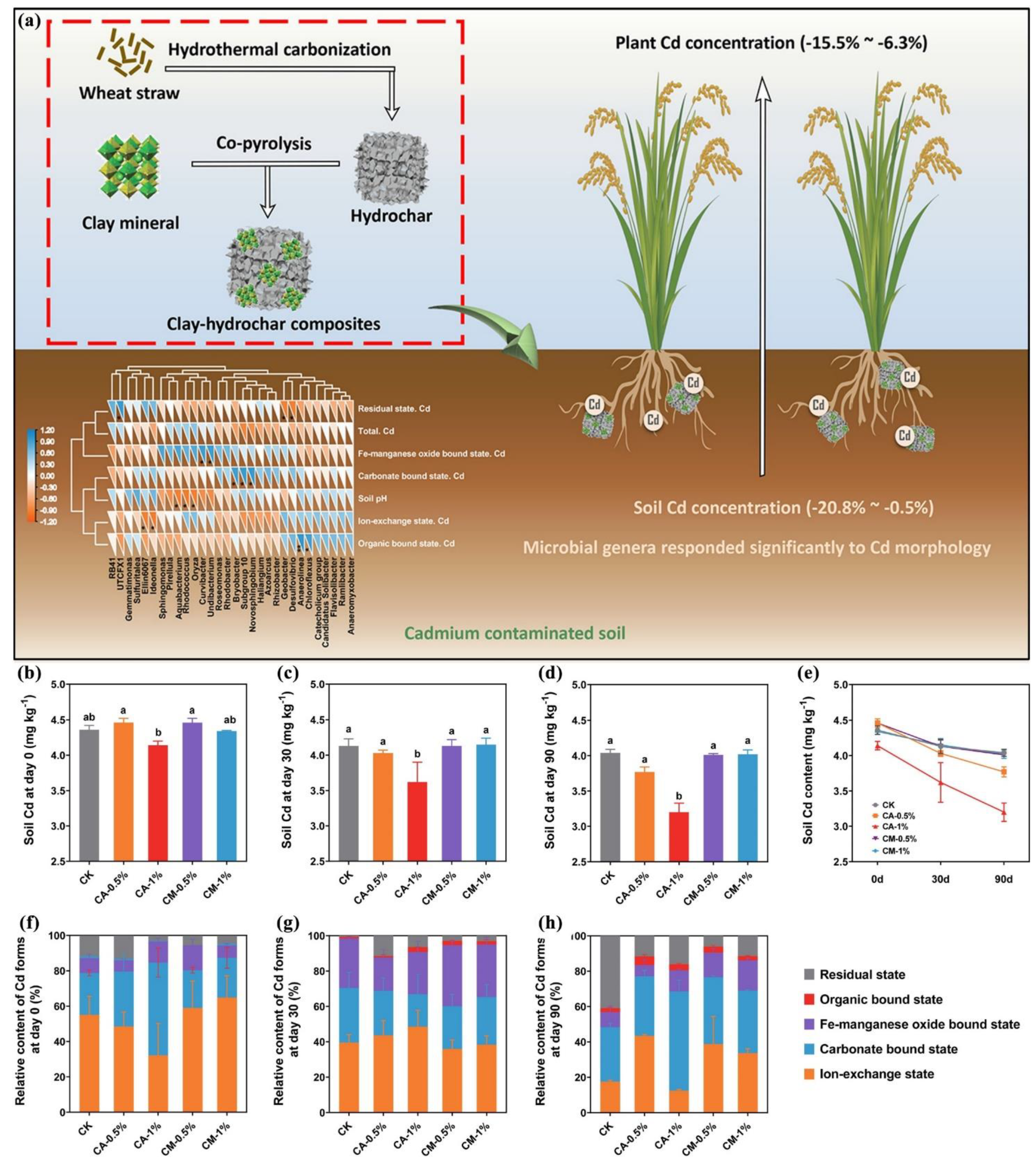
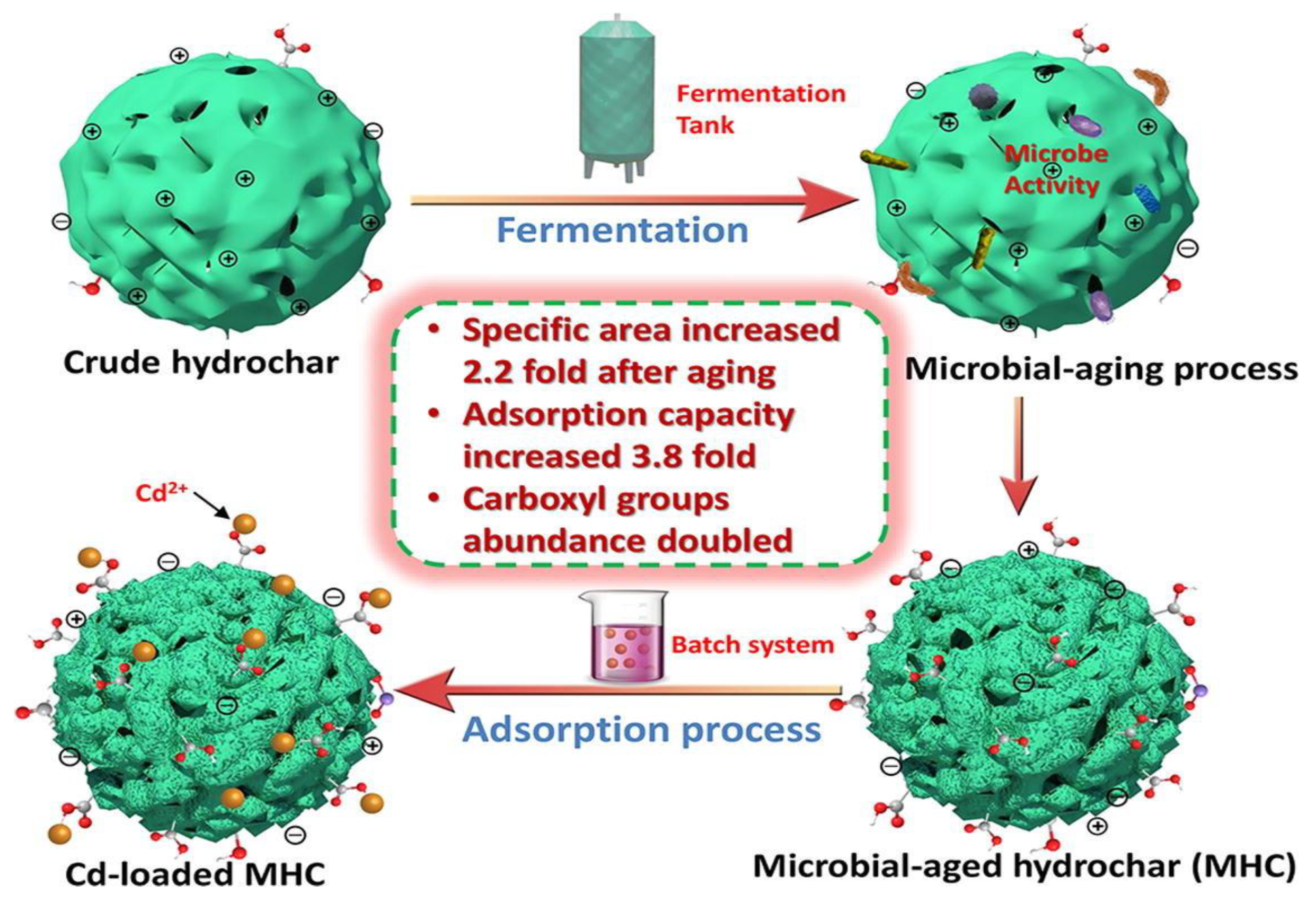
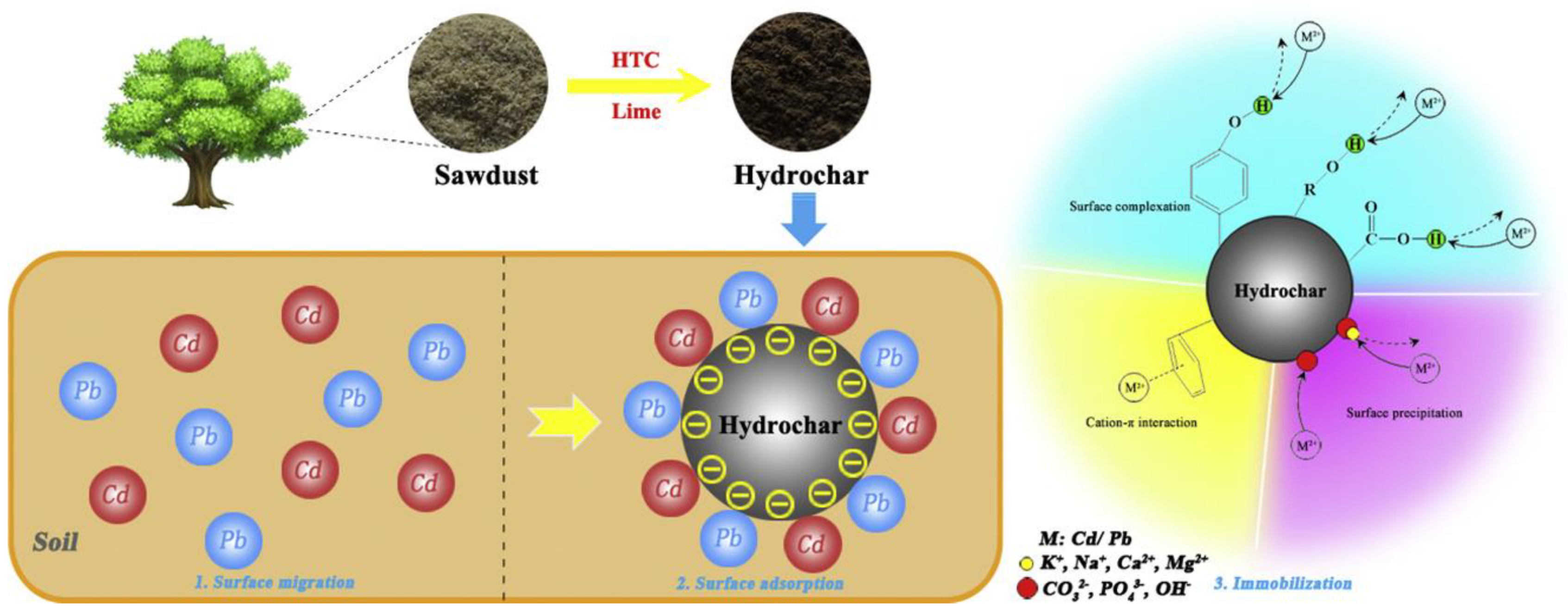
2.2. Study on the Hydrochar Remediation Methods
2.2.1. Remediation Methods Based on Hydrochar
2.2.2. Mechanism of Hydrochar Remediation Methods
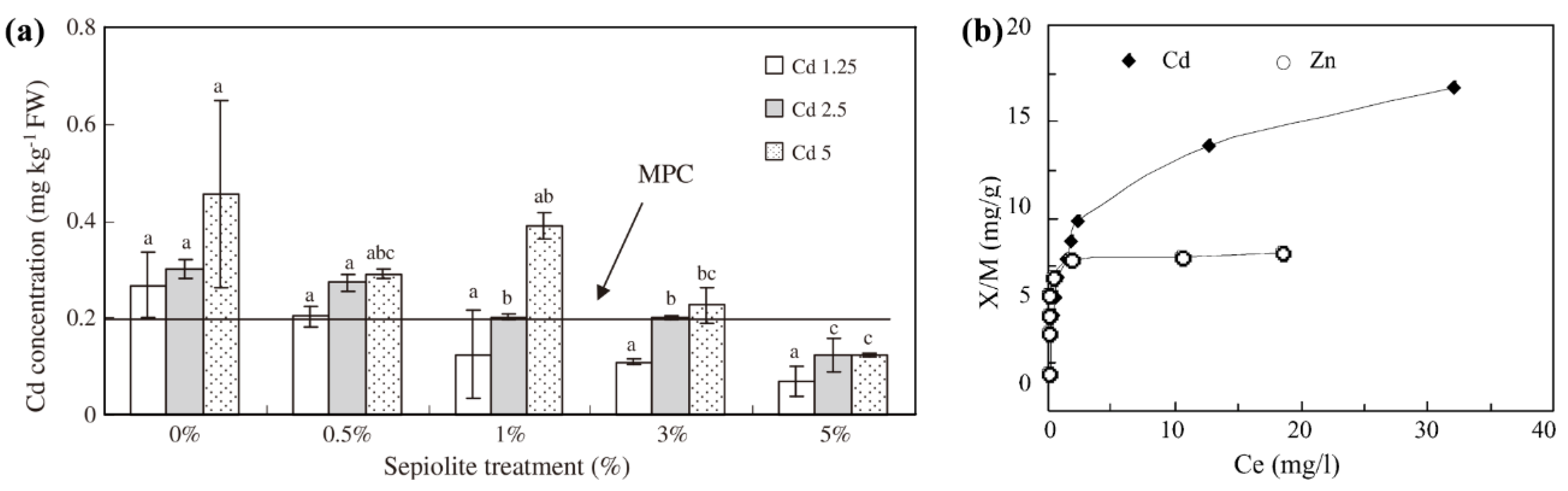
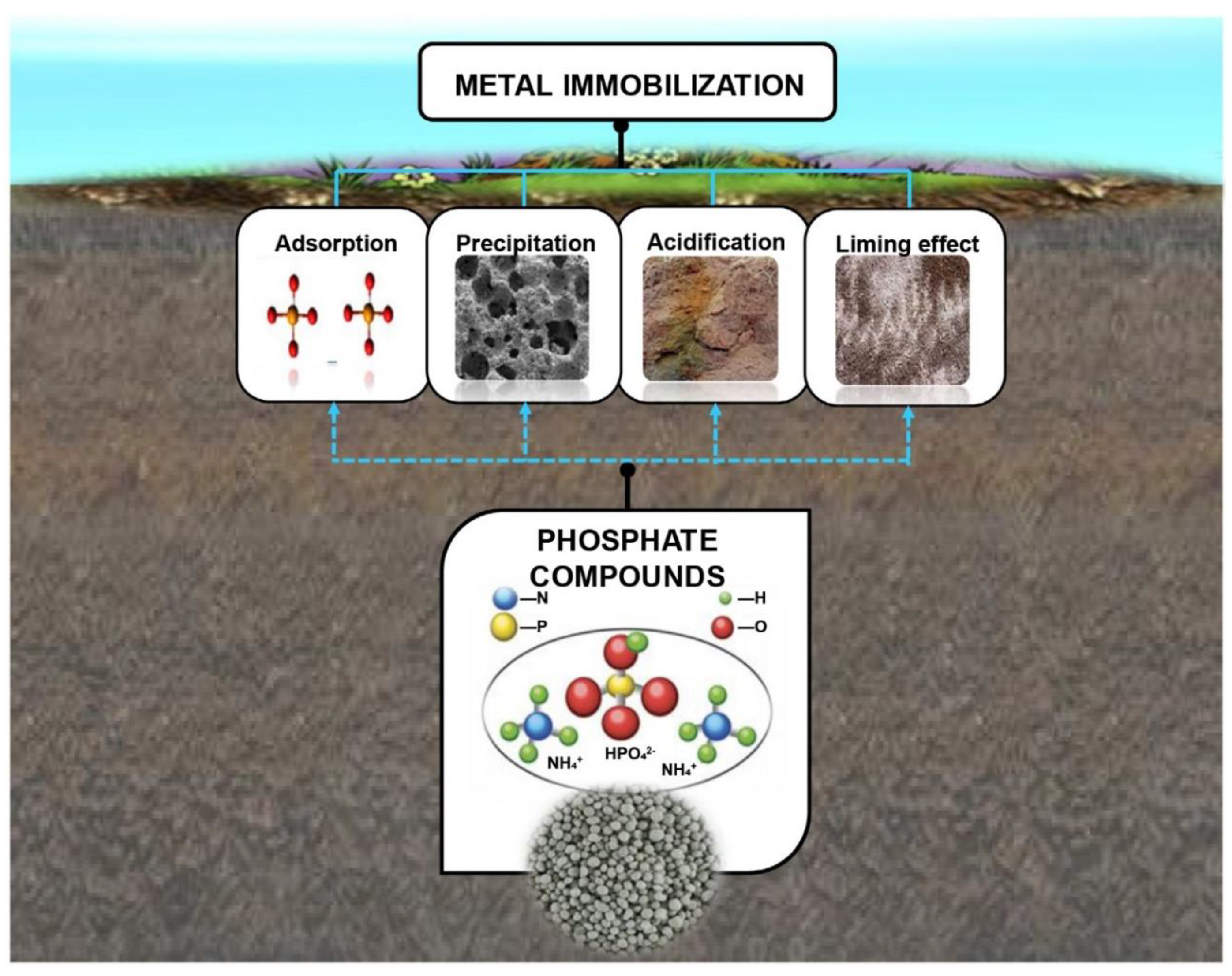
2.3. Methods and Mechanisms of Co-Application of Phosphate Fertilizer and Sepiolite
2.3.1. Co-Application of Phosphate Fertilizer and Sepiolite Method
2.3.2. Mechanisms of Co-Application of Phosphate Fertilizer and Sepiolite Method
2.4. Other Treatment Techniques for Cadmium Pollution in Soil
3. Factors That May Affect the Stabilization of Cadmium Fixation in Soil
3.1. Soil pH
3.2. Organic Matter in Soil
3.3. Soil Bacteria
3.4. Other Factors
4. Problems and Future Prospects of Remediation of Soil Cadmium Pollution
4.1. Main Problems to Be Faced
4.2. Future Prospects
4.2.1. Material Innovation
4.2.2. Original Technological Improvement
4.2.3. Policy Guidance
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vikrant, K.; Kumar, V.; Vellingiri, K.; Kim, K.H. Nanomaterials for the abatement of cadmium (II) ions from water/wastewater. Nano Res. 2019, 12, 1489–1507. [Google Scholar] [CrossRef]
- Chen, C.M.; Liu, M.C. Ecological risk assessment on a cadmium contaminated soil landfill—A preliminary evaluation based on toxicity tests on local species and site-specific information. Sci. Total Environ. 2006, 359, 120–129. [Google Scholar] [CrossRef]
- Nabulo, G.; Young, S.D.; Black, C.R. Assessing risk to human health from tropical leafy vegetables grown on contaminated urban soils. Sci. Total Environ. 2010, 408, 5338–5351. [Google Scholar] [CrossRef]
- Aoshima, K. Itai-itai disease: Renal tubular osteomalacia induced by environmental exposure to cadmium—Historical review and perspectives. Soil Sci. Plant Nutr. 2016, 62, 319–326. [Google Scholar] [CrossRef]
- Hu, Y.; Cheng, H.; Tao, S. The Challenges and Solutions for Cadmium-contaminated Rice in China: A Critical Review. Environ. Int. 2016, 92–93, 515–532. [Google Scholar] [CrossRef]
- Kubier, A.; Wilkin, R.T.; Pichler, T. Cadmium in soils and groundwater: A review. Appl. Geochem. 2019, 108, 104388. [Google Scholar] [CrossRef]
- Adriano, D.C.; Wenzel, W.W.; Vangronsveld, J.; Bolan, N.S. Role of assisted natural remediation in environmental cleanup. Geoderma 2004, 122, 121–142. [Google Scholar] [CrossRef]
- Khan, M.A.; Khan, S.; Khan, A.; Alam, M. Soil contamination with cadmium, consequences and remediation using organic amendments. Sci. Total Environ. 2017, 601, 1591–1605. [Google Scholar] [CrossRef]
- Kubier, A.; Hamer, K.; Pichler, T. Cadmium Background Levels in Groundwater in an Area Dominated by Agriculture. Integr. Environ. Assess. Manag. 2020, 16, 103–113. [Google Scholar] [CrossRef]
- Acosta, J.A.; Faz, A.; Martinez-Martinez, S.; Zornoza, R.; Carmona, D.M.; Kabas, S. Multivariate statistical and GIS-based approach to evaluate heavy metals behavior in mine sites for future reclamation. J. Geochem. Explor. 2011, 109, 8–17. [Google Scholar] [CrossRef]
- Liu, J.; Pan, Y.; Shi, H. The source of Cadmium in farmland soil and its contribution to atmospheric sedimentation in north china. J. Agro-Environ. Sci. 2022, 41, 1698–1708. (In Chinese) [Google Scholar] [CrossRef]
- Li, J.; Xu, Y. Evaluation of palygorskite for remediation of Cd-polluted soil with different water conditions. J. Soils Sediments 2018, 18, 526–533. [Google Scholar] [CrossRef]
- Zhao, F.; Ma, Y.; Zhu, Y.; Tang, Z.; McGrath, S.P. Soil Contamination in China: Current Status and Mitigation Strategies. Environ. Sci. Technol. 2015, 49, 750–759. [Google Scholar] [CrossRef]
- Perez-de-Mora, A.; Burgos, P.; Madejon, E.; Cabrera, F.; Jaeckel, P.; Schloter, M. Microbial community structure and function in a soil contaminated by heavy metals: Effects of plant growth and different amendments. Soil Biol. Biochem. 2006, 38, 327–341. [Google Scholar] [CrossRef]
- Kumpiene, J.; Lagerkvist, A.; Maurice, C. Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments—A review. Waste Manag. 2008, 28, 215–225. [Google Scholar] [CrossRef]
- Strobel, B.W.; Borggaard, O.K.; Hansen, H.C.B.; Andersen, M.K.; Raulund-Rasmussen, K. Dissolved organic carbon and decreasing pH mobilize cadmium and copper in soil. Eur. J. Soil Sci. 2005, 56, 189–196. [Google Scholar] [CrossRef]
- Chavez, E.; He, Z.L.; Stoffella, P.J.; Mylavarapu, R.S.; Li, Y.C.; Baligar, V.C. Chemical speciation of cadmium: An approach to evaluate plant-available cadmium in Ecuadorian soils under cacao production. Chemosphere 2016, 150, 57–62. [Google Scholar] [CrossRef]
- Hamid, Y.; Tang, L.; Yaseen, M.; Hussain, B.; Zehra, A.; Aziz, M.Z.; He, Z.; Yang, X. Comparative efficacy of organic and inorganic amendments for cadmium and lead immobilization in contaminated soil under rice-wheat cropping system. Chemosphere 2019, 214, 259–268. [Google Scholar] [CrossRef]
- Kumpiene, J.; Antelo, J.; Brannvall, E.; Carabante, I.; Ek, K.; Komarek, M.; Soderberg, C.; Warell, L. In situ chemical stabilization of trace element-contaminated soil—Field demonstrations and barriers to transition from laboratory to the field—A review. Appl. Geochem. 2019, 100, 335–351. [Google Scholar] [CrossRef]
- Lim, J.E.; Ahmad, M.; Usman, A.R.A.; Lee, S.S.; Jeon, W.T.; Oh, S.E.; Yang, J.E.; Ok, Y.S. Effects of natural and calcined poultry waste on Cd, Pb and As mobility in contaminated soil. Environ. Earth Sci. 2013, 69, 11–20. [Google Scholar] [CrossRef]
- Bian, R.; Li, L.; Bao, D.; Zheng, J.; Zhang, X.; Zheng, J.; Liu, X.; Cheng, K.; Pan, G. Cd immobilization in a contaminated rice paddy by inorganic stabilizers of calcium hydroxide and silicon slag and by organic stabilizer of biochar. Environ. Sci. Pollut. Res. Int. 2016, 23, 10028–10036. [Google Scholar] [CrossRef]
- Pardo, T.; Clemente, R.; Alvarenga, P.; Bernal, M.P. Efficiency of soil organic and inorganic amendments on the remediation of a contaminated mine soil: II. Biological and ecotoxicological evaluation. Chemosphere 2014, 107, 101–108. [Google Scholar] [CrossRef]
- Song, P.P.; Xu, D.; Yue, J.Y.; Ma, Y.C.; Dong, S.J.; Feng, J. Recent advances in soil remediation technology for heavy metal contaminated sites: A critical review. Sci. Total Environ. 2022, 838, 156417. [Google Scholar] [CrossRef]
- Chen, X.; Gao, L.; Zhuo, L. Research Progress of Phytoremediation in Mine Subsidence Area Governance. In Proceedings of the 4th International Conference on Environmental Technology and Knowledge Transfer, Hefei, China, 24–25 May 2012; pp. 791–794. [Google Scholar]
- Wu, C.; Zhang, X.; Deng, Y. Review in Strengthening Technology for Phytoremediation of Soil Contaminated by Heavy Metals. In Proceedings of the 8th International Conference on Environmental Science and Technology (ICEST 2017), Madrid, Spain, 12–14 June 2017. [Google Scholar]
- Wang, J.; Delavar, M.A. Techno-economic analysis of phytoremediation: A strategic rethinking. Sci. Total Environ. 2023, 902, 165949. [Google Scholar] [CrossRef]
- Raicevic, S.; Perovic, V.; Zouboulis, A.I. Theoretical assessment of phosphate amendments for stabilization of (Pb + Zn) in polluted soil. Waste Manag. 2008, 29, 1779–1784. [Google Scholar] [CrossRef]
- Kulikowska, D.; Gusiatin, Z.M.; Bulkowska, K.; Kierklo, K. Humic substances from sewage sludge compost as washing agent effectively remove Cu and Cd from soil. Chemosphere 2015, 136, 42–49. [Google Scholar] [CrossRef]
- Mulligan, C.N.; Yong, R.N.; Gibbs, B.F. Remediation technologies for metal-contaminated soils and groundwater: An evaluation. Eng. Geol. 2001, 60, 193–207. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, G.; Xu, Y.; Liu, W.; Liang, X.; Wang, L. Evaluation of the effectiveness of sepiolite, bentonite, and phosphate amendments on the stabilization remediation of cadmium-contaminated soils. J. Environ. Manag. 2016, 166, 204–210. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, G.; Xu, Y.; Wang, L.; Liang, X.; Lin, D. Assessment of sepiolite for immobilization of cadmium-contaminated soils. Geoderma 2013, 193, 149–155. [Google Scholar] [CrossRef]
- Liang, X.; Qin, X.; Huang, Q.; Huang, R.; Yin, X.; Wang, L.; Sun, Y.; Xu, Y. Mercapto functionalized sepiolite: A novel and efficient immobilization agent for cadmium polluted soil. RSC Adv. 2017, 7, 39955–39961. [Google Scholar] [CrossRef]
- Zhu, Q.-H.; Huang, D.-Y.; Liu, S.-L.; Zhou, B.; Luo, Z.-C.; Zhu, H.-H. Flooding-enhanced immobilization effect of sepiolite on cadmium in paddy soil. J. Soils Sediments 2012, 12, 169–177. [Google Scholar] [CrossRef]
- Egbosiuba, T.C.; Abdulkareem, A.S.; Kovo, A.S.; Afolabi, E.A.; Tijani, J.O.; Auta, M.; Roos, W.D. Ultrasonic enhanced adsorption of methylene blue onto the optimized surface area of activated carbon: Adsorption isotherm, kinetics and thermodynamics. Chem. Eng. Res. Des. 2020, 153, 315–336. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Chen, B.; Xu, Y.; Wang, H.; Jin, F.; Shen, Z.; Hou, D. Green synthesis of layered double hydroxides (LDH) for the remediation of As and Cd in water and soil. Appl. Clay Sci. 2024, 249, 107262. [Google Scholar] [CrossRef]
- Van Minh, D.; Huu Tap, V.; Vinh, N.D.; Thi Minh Hoa, D.; Thi Bich Hanh, N.; Thi Tuyet, N.; Thi Ngoc Ha, T.; Trung Kien, H.; Thi Pha, T.; Lan Huong, N.; et al. Enhancement of exchangeable Cd and Pb immobilization in contaminated soil using Mg/Al LDH-zeolite as an effective adsorbent. RSC Adv. 2021, 11, 17019. [Google Scholar]
- Egbosiuba, T.C.; Egwunyenga, M.C.; Tijani, J.O.; Mustapha, S.; Abdulkareem, A.S.; Kovo, A.S.; Krikstolaityte, V.; Veksha, A.; Wagner, M.; Lisak, G. Activated multi-walled carbon nanotubes decorated with zero valent nickel nanoparticles for arsenic, cadmium and lead adsorption from wastewater in a batch and continuous flow modes. J. Hazard. Mater. 2022, 423, 126993. [Google Scholar] [CrossRef]
- Egbosiuba, T.C.; Abdulkareem, A.S.; Kovo, A.S.; Afolabi, E.A.; Tijani, J.O.; Bankole, M.T.; Bo, S.; Roos, W.D. Adsorption of Cr(VI), Ni(II), Fe(II) and Cd(II) ions by KIAgNPs decorated MWCNTs in a batch and fixed bed process. Sci. Rep. 2021, 11, 75. [Google Scholar] [CrossRef]
- Xia, Y.; Meng, Y.; Sun, Y.; Deng, X.; Li, K.; Liu, Z.; Miao, W. Facile one-step synthesis of attapulgite-hydrochar composite for in situ remediation of cadmium contaminated soils: Characterization, performance and mechanisms. J. Environ. Chem. Eng. 2021, 9, 106841. [Google Scholar] [CrossRef]
- Ge, Q.; Tian, Q.; Wang, S.; Zhu, F. Synergistic effects of phosphoric acid modified hydrochar and coal gangue-based zeolite on bioavailability and accumulation of cadmium and lead in contaminated soil. Chin. J. Chem. Eng. 2022, 46, 150–160. [Google Scholar] [CrossRef]
- He, L.; Wang, B.; Cui, H.; Yang, S.; Wang, Y.; Feng, Y.; Sun, X.; Feng, Y. Clay-hydrochar composites return to cadmium contaminated paddy soil: Reduced Cd accumulation in rice seed and affected soil microbiome. Sci. Total Environ. 2022, 835, 155542. [Google Scholar] [CrossRef]
- Maziarz, P.; Matusik, J.; Straczek, T.; Kapusta, C.; Woch, W.M.; Tokarz, W.; Radziszewska, A.; Leiviska, T. Highly effective magnet-responsive LDH-Fe oxide composite adsorbents for As(V) removal. Chem. Eng. J. 2019, 362, 207–216. [Google Scholar] [CrossRef]
- Gao, R.; Yan, D. Ordered assembly of hybrid room-temperature phosphorescence thin films showing polarized emission and the sensing of VOCs. Chem. Commun. 2017, 53, 5408–5411. [Google Scholar] [CrossRef]
- Kong, X.; Ge, R.; Liu, T.; Xu, S.; Hao, P.; Zhao, X.; Li, Z.; Lei, X.; Duan, H. Super-stable mineralization of cadmium by calcium-aluminum layered double hydroxide and its large-scale application in agriculture soil remediation. Chem. Eng. J. 2021, 407, 127178. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, Y.; Li, J.; Li, Y.; Song, W.; Li, X.; Yan, L.; Yu, H. Highly efficient removal of aqueous Cu(II) and Cd(II) by hydrothermal synthesized CaAl-layered double hydroxide. Colloids Surf. A-Physicochem. Eng. Asp. 2022, 641, 128584. [Google Scholar] [CrossRef]
- Liu, Q.; Li, Y.; Zhang, J.; Chi, Y.; Ruan, X.; Liu, J.; Qian, G. Effective removal of zinc from aqueous solution by hydrocalumite. Chem. Eng. J. 2011, 175, 33–38. [Google Scholar] [CrossRef]
- Kumar, R.; Chawla, J.; Kaur, I. Removal of cadmium ion from wastewater by carbon-based nanosorbents: A review. J. Water Health 2015, 13, 33. [Google Scholar] [CrossRef]
- Li, H.; Li, N.; Zuo, P.; Qu, S.; Qin, F.; Shen, W. Utilization of nitrogen, sulfur co-doped porous carbon micron spheres as bifunctional electrocatalysts for electrochemical detection of cadmium, lead and mercury ions and oxygen evolution reaction. J. Colloid Interface Sci. 2023, 640, 404. [Google Scholar] [CrossRef]
- Dai, L.; Wu, B.; Tan, F.; He, M.; Wang, W.; Qin, H.; Tang, X.; Zhu, Q.; Pan, K.; Hu, Q. Engineered hydrochar composites for phosphorus removal/recovery: Lanthanum doped hydrochar prepared by hydrothermal carbonization of lanthanum pretreated rice straw. Bioresour. Technol. 2014, 161, 327–332. [Google Scholar] [CrossRef]
- Egbosiuba, T.C. Biochar and bio-oil fuel properties from nickel nanoparticles assisted pyrolysis of cassava peel. Heliyon 2022, 8, e10114. [Google Scholar] [CrossRef]
- George, C.; Wagner, M.; Kucke, M.; Rillig, M.C. Divergent consequences of hydrochar in the plant-soil system: Arbuscular mycorrhiza, nodulation, plant growth and soil aggregation effects. Appl. Soil Ecol. 2012, 59, 68–72. [Google Scholar] [CrossRef]
- Fuertes, A.B.; Arbestain, M.C.; Sevilla, M.; Macia-Agullo, J.A.; Fiol, S.; Lopez, R.; Smernik, R.J.; Aitkenhead, W.P.; Arce, F.; Macias, F. Chemical and structural properties of carbonaceous products obtained by pyrolysis and hydrothermal carbonisation of corn stover. Aust. J. Soil Res. 2010, 48, 618–626. [Google Scholar] [CrossRef]
- Xia, Y.; Liu, H.; Guo, Y.; Liu, Z.; Jiao, W. Immobilization of heavy metals in contaminated soils by modified hydrochar: Efficiency, risk assessment and potential mechanisms. Sci. Total Environ. 2019, 685, 1201–1208. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, C.; Ai, S.; Wang, H.; Gao, Y.; Yan, L.; Mei, Z.; Wang, W. Biological pretreatment enhances the activity of functional microorganisms and the ability of methanogenesis during anaerobic digestion. Bioresour. Technol. 2019, 290, 121660. [Google Scholar] [CrossRef]
- Hua, Y.; Zheng, X.; Xue, L.; Han, L.; He, S.; Mishra, T.; Feng, Y.; Yang, L.; Xing, B. Microbial aging of hydrochar as a way to increase cadmium ion adsorption capacity: Process and mechanism. Bioresour. Technol. 2020, 300, 122708. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, Y.; Li, L.; Shi, Q.; Hou, J.; Zhang, R.; Zhang, S.; Chen, J. Nonthermal air plasma dehydration of hydrochar improves its carbon sequestration potential and dissolved organic matter molecular characteristics. Sci. Total Environ. 2019, 659, 655–663. [Google Scholar] [CrossRef]
- Jian, X.; Zhuang, X.; Li, B.; Xu, X.; Wei, Z.; Song, Y.; Jiang, E. Comparison of characterization and adsorption of biochars produced from hydrothermal carbonization and pyrolysis. Environ. Technol. Innov. 2018, 10, 27–35. [Google Scholar] [CrossRef]
- Kalsoom; Ali, A.; Khan, S.; Ali, N.; Khan, M.A. Enhanced ultrasonic adsorption of pesticides onto the optimized surface area of activated carbon and biochar: Adsorption isotherm, kinetics, and thermodynamics. Biomass Convers. Biorefin. 2024, 14, 15519–15534. [Google Scholar] [CrossRef]
- Ekpo, U.; Ross, A.B.; Camargo-Valero, M.A.; Fletcher, L.A. Influence of pH on hydrothermal treatment of swine manure: Impact on extraction of nitrogen and phosphorus in process water. Bioresour. Technol. 2016, 214, 637–644. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, F.; Wu, J. Characterization and application of chars produced from pinewood pyrolysis and hydrothermal treatment. Fuel 2010, 89, 510–514. [Google Scholar] [CrossRef]
- Li, J.; Yu, G.; Xie, S.; Pan, L.; Li, C.; You, F.; Wang, Y. Immobilization of heavy metals in ceramsite produced from sewage sludge biochar. Sci. Total Environ. 2018, 628–629, 131–140. [Google Scholar] [CrossRef]
- Liu, L.; Sun, J.; Li, M.; Wang, S.; Pei, H.; Zhang, J. Enhanced enzymatic hydrolysis and structural features of corn stover by FeCl3 pretreatment. Bioresour. Technol. 2009, 100, 5853–5858. [Google Scholar] [CrossRef]
- Li, M.; Liu, Q.; Guo, L.; Zhang, Y.; Lou, Z.; Wang, Y.; Qian, G. Cu(II) removal from aqueous solution by Spartina alterniflora derived biochar. Bioresour. Technol. 2013, 141, 83–88. [Google Scholar] [CrossRef]
- Lu, H.; Dong, Y.; Feng, Y.; Bai, Y.; Tang, X.; Li, Y.; Yang, L.; Liu, J. Paddy periphyton reduced cadmium accumulation in rice (Oryza sativa) by removing and immobilizing cadmium from the water–soil interface. Environ. Pollut. 2020, 261, 114103. [Google Scholar] [CrossRef]
- Song, H.; Peng, L.; Li, Z.; Deng, X.; Shao, J.; Gu, J.-D. Metal distribution and biological diversity of crusts in paddy fields polluted with different levels of cadmium. Ecotoxicol. Environ. Saf. 2019, 184, 109620. [Google Scholar] [CrossRef]
- Dedysh, S.N.; Kulichevskaya, I.S.; Huber, K.J.; Overmann, J. Defining the taxonomic status of described subdivision 3 Acidobacteria: Proposal of Bryobacteraceae fam. nov. Int. J. Syst. Evol. Microbiol. 2017, 498–501, 501. [Google Scholar] [CrossRef]
- Liu, H.; Wang, C.; Xie, Y.; Luo, Y.; Sheng, M.; Xu, F.; Xu, H. Ecological responses of soil microbial abundance and diversity to cadmium and soil properties in farmland around an enterprise-intensive region. J. Hazard. Mater. 2020, 392, 122478. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Y.; Zhang, X.; Shi, J. Effects of biochar on bacterial genetic diversity in soil contaminated with Cadmium. Soil Use Manag. 2021, 37, 298. [Google Scholar]
- Hou, P.; Feng, Y.; Wang, N.; Petropoulos, E.; Li, D.; Yu, S.; Xue, L.; Yang, L. Win-win: Application of sawdust-derived hydrochar in low fertility soil improves rice yield and reduces greenhouse gas emissions from agricultural ecosystems. Sci. Total Environ. 2020, 748, 142457. [Google Scholar] [CrossRef]
- Kim, H.-S.; Lee, S.-H.; Jo, H.Y.; Finneran, K.T.; Kwon, M.J. Diversity and composition of soil Acidobacteria and Proteobacteria communities as a bacterial indicator of past land-use change from forest to farmland. Sci. Total Environ. 2021, 797, 148944. [Google Scholar] [CrossRef]
- Zhan, F.; Zeng, W.; Yuan, X.; Li, B.; Li, T.; Zu, Y.; Jiang, M.; Li, Y. Field experiment on the effects of sepiolite and biochar on the remediation of Cd and Pb polluted farmlands around a Pb-Zn mine in Yunnan Province, China. Environ. Sci. Pollut. Res. 2019, 26, 7743–7751. [Google Scholar] [CrossRef]
- Su, C.; Li, W.; Chen, M. Effects of acid and heating treatments on the structure of sepiolite and its adsorption of lead and cadmium. Adv. Mater. Res. 2012, 518–523, 728–731. [Google Scholar] [CrossRef]
- Kocaoba, S. Adsorption of Cd(II), Cr(III) and Mn(II) on natural sepiolite. Desalination 2009, 244, 24–30. [Google Scholar] [CrossRef]
- Alvarezayuso, E.; Garciasanchez, A. Sepiolite as a feasible soil additive for the immobilization of cadmium and zinc. Sci. Total Environ. 2003, 305, 1–12. [Google Scholar] [CrossRef]
- Qayyum, M.F.; Rehman, M.Z.U.; Ali, S.; Rizwan, M.; Naeem, A.; Maqsood, M.A.; Khalid, H.; Rinklebe, J.; Ok, Y.S. Residual effects of monoammonium phosphate, gypsum and elemental sulfur on cadmium phytoavailability and translocation from soil to wheat in an effluent irrigated field. Chemosphere 2017, 174, 515–523. [Google Scholar] [CrossRef]
- Gupta, D.K.; Chatterjee, S.; Datta, S.; Veer, V.; Walther, C. Role of phosphate fertilizers in heavy metal uptake and detoxification of toxic metals. Chemosphere 2014, 108, 134–144. [Google Scholar] [CrossRef]
- Seshadri, B.; Bolan, N.S.; Choppala, G.; Kunhikrishnan, A.; Sanderson, P.; Wang, H.; Currie, L.D.; Tsang, D.C.W.; Ok, Y.S.; Kim, G. Potential value of phosphate compounds in enhancing immobilization and reducing bioavailability of mixed heavy metal contaminants in shooting range soil. Chemosphere 2017, 184, 197–206. [Google Scholar] [CrossRef]
- Huang, R.; Li, Y.; Li, F.; Yin, X.; Li, R.; Wu, Z.; Liang, X.; Li, Z. Phosphate fertilizers facilitated the Cd contaminated soil remediation by sepiolite: Cd mobilization, plant toxicity, and soil microbial community. Ecotoxicol. Environ. Saf. 2022, 234, 113388. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, Y.; Xu, Y.; Wang, L.; Liang, X.; Li, Y. Reliability and stability of immobilization remediation of Cd polluted soils using sepiolite under pot and field trials. Environ. Pollut. 2016, 208, 739–746. [Google Scholar] [CrossRef]
- Wu, L.; Zhou, J.; Zhou, T.; Li, Z.; Jiang, J.; Zhu, D.; Hou, J.; Wang, Z.; Luo, Y.; Christie, P. Estimating cadmium availability to the hyperaccumulator Sedum plumbizincicola in a wide range of soil types using a piecewise function. Sci. Total Environ. 2018, 637, 1342–1350. [Google Scholar] [CrossRef]
- Yuan, Z.; Luo, Z.; Lei, T.; Zhou, J.; Zhuang, X. A Special Issue on Bioenergy Research and Development in China: Bio-Based Materials and Bioenergy. J. Biobased Mater. Bioenergy 2016, 10, 403–404. [Google Scholar] [CrossRef]
- Simmler, M.; Ciadamidaro, L.; Schulin, R.; Madejon, P.; Reiser, R.; Clucas, L.; Weber, P.; Robinson, B. Lignite Reduces the Solubility and Plant Uptake of Cadmium in Pasturelands. Environ. Sci. Technol. 2013, 47, 4497–4504. [Google Scholar] [CrossRef]
- Sun, L.; Chen, S.; Chao, L.; Sun, T. Effects of flooding on changes in Eh, pH and speciation of cadmium and lead in contaminated soil. Bull. Environ. Contam. Toxicol. 2007, 79, 514–518. [Google Scholar] [CrossRef]
- Loganathan, P.; Vigneswaran, S.; Kandasamy, J.; Naidu, R. Cadmium Sorption and Desorption in Soils: A Review. Crit. Rev. Environ. Sci. Technol. 2012, 42, 489–533. [Google Scholar] [CrossRef]
- Li, W.; Ni, P.; Yi, Y. Comparison of reactive magnesia, quick lime, and ordinary Portland cement for stabilization/solidification of heavy metal-contaminated soils. Sci. Total Environ. 2019, 671, 741–753. [Google Scholar] [CrossRef]
- Wei, L.; Huang, Y.; Huang, L.; Huang, Q.; Li, Y.; Li, X.; Yang, S.; Liu, C.; Liu, Z. Combined biochar and soda residues increases maize yields and decreases grain Cd/Pb in a highly Cd/Pb-polluted acid Udults soil. Agric. Ecosyst. Environ. 2021, 306, 107198. [Google Scholar] [CrossRef]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef]
- Cui, L.Q.; Pan, G.X.; Li, L.Q.; Bian, R.J.; Liu, X.Y.; Yan, J.L.; Quan, G.X.; Ding, C.; Chen, T.M.; Liu, Y.; et al. Continuous immobilization of cadmium and lead in biochar amended contaminated paddy soil: A five-year field experiment. Ecol. Eng. 2016, 93, 1–8. [Google Scholar] [CrossRef]
- Rees, F.; Simonnot, M.O.; Morel, J.L. Short-term effects of biochar on soil heavy metal mobility are controlled by intra-particle diffusion and soil pH increase. Eur. J. Soil Sci. 2014, 65, 149–161. [Google Scholar] [CrossRef]
- Li, H.; Ye, X.; Geng, Z.; Zhou, H.; Guo, X.; Zhang, Y.; Zhao, H.; Wang, G. The influence of biochar type on long-term stabilization for Cd and Cu in contaminated paddy soils. J. Hazard. Mater. 2016, 304, 40–48. [Google Scholar] [CrossRef]
- Gwenzi, W.; Muzava, M.; Mapanda, F.; Tauro, T.P. Comparative short-term effects of sewage sludge and its biochar on soil properties, maize growth and uptake of nutrients on a tropical clay soil in Zimbabwe. J. Integr. Agric. 2016, 15, 1395–1406. [Google Scholar] [CrossRef]
- Cheng, J.Z.; Li, Y.L.; Gao, W.C.; Chen, Y.; Pan, W.J.; Lee, X.Q.; Tang, Y. Effects of biochar on Cd and Pb mobility and microbial community composition in a calcareous soil planted with tobacco. Biol. Fertil. Soils 2018, 54, 373–383. [Google Scholar] [CrossRef]
- Nie, C.; Yang, X.; Niazi, N.K.; Xu, X.; Wen, Y.; Rinklebe, J.; Ok, Y.S.; Xu, S.; Wang, H. Impact of sugarcane bagasse-derived biochar on heavy metal availability and microbial activity: A field study. Chemosphere 2018, 200, 274–282. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Abbas, T.; Adrees, M.; Zia-ur-Rehman, M.; Ibrahim, M.; Abbas, F.; Qayyum, M.F.; Nawaz, R. Residual effects of biochar on growth, photosynthesis and cadmium uptake in rice (Oryza sativa L.) under Cd stress with different water conditions. J. Environ. Manag. 2018, 206, 676–683. [Google Scholar] [CrossRef]
- Katoh, M.; Moriguchi, S.; Takagi, N.; Akashi, Y.; Sato, T. Simultaneous control of cadmium release and acidic pH neutralization in excavated sedimentary rock with concurrent oxidation of pyrite using steel slag. J. Soils Sediments 2018, 18, 1204. [Google Scholar] [CrossRef]
- Song, G.; Cao, L.; Chen, X.; Hou, W.; Wang, Q. Heavy metal adsorption changes of EAF steel slag after phosphorus adsorption. Water Sci. Technol. 2012, 65, 1576. [Google Scholar] [CrossRef]
- Bashir, S.; Rizwan, M.S.; Salam, A.; Fu, Q.; Zhu, J.; Shaaban, M.; Hu, H. Cadmium Immobilization Potential of Rice Straw-Derived Biochar, Zeolite and Rock Phosphate: Extraction Techniques and Adsorption Mechanism. Bull. Environ. Contam. Toxicol. 2018, 100, 727–732. [Google Scholar] [CrossRef]
- Bashir, S.; Salam, A.; Chhajro, M.A.; Fu, Q.; Khan, M.J.; Zhu, J.; Shaaban, M.; Kubar, K.A.; Ali, U.; Hu, H. Comparative efficiency of rice husk-derived biochar (RHB) and steel slag (SS) on cadmium (Cd) mobility and its uptake by Chinese cabbage in highly contaminated soil. Int. J. Phytoremediat. 2018, 20, 1221–1228. [Google Scholar] [CrossRef]
- Hou, D.; Wang, K.; Liu, T.; Wang, H.; Lin, Z.; Qian, J.; Lu, L.; Tian, S. Unique Rhizosphere Micro-characteristics Facilitate Phytoextraction of Multiple Metals in Soil by the Hyperaccumulating Plant Sedum alfredii. Environ. Sci. Technol. 2017, 51, 5675–5684. [Google Scholar] [CrossRef]
- Spark, K.M.; Wells, J.D.; Johnson, B.B. Characterizing trace metal adsorption on kaolinite. Eur. J. Soil. Sci. 1995, 46, 633–640. [Google Scholar] [CrossRef]
- Buerge Weirich, D.; Hari, R.; Xue, H.; Behra, P.; Sigg, L. Adsorption of Cu, Cd, and Ni on goethite in the presence of natural groundwater ligands. Environ. Sci. Technol. 2002, 36, 328–336. [Google Scholar] [CrossRef]
- Braissant, O.; Decho, A.W.; Dupraz, C.; Glunk, C.; Przekop, K.M.; Visscher, P.T. Exopolymeric substances of sulfate-reducing bacteria: Interactions with calcium at alkaline pH and implication for formation of carbonate minerals. Geobiology 2007, 5, 401–411. [Google Scholar] [CrossRef]
- Su, P.; Zhang, J.; Li, Y. Investigation of chemical associations and leaching behavior of heavy metals in sodium sulfide hydrate stabilized stainless steel pickling sludge. Process Saf. Environ. Prot. 2019, 123, 79–86. [Google Scholar] [CrossRef]
- Karlsson, T.; Persson, P.; Skyllberg, U. Complexation of copper (II) in organic soils and in dissolved organic matter—EXAFS evidence for chelate ring structures. Environ. Sci. Technol. 2006, 40, 2623–2628. [Google Scholar] [CrossRef]
- Manzoor, M.; Gul, I.; Manzoor, A.; Kallerhoff, J.; Arshad, M. Optimization of integrated phytoremediation system (IPS) for enhanced lead removal and restoration of soil microbial activities. Chemosphere 2021, 277, 130243. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, C.; Liu, Z.; Quan, B.; Lu, W.; Li, X.; Su, P.; Tang, Y.; Bu, Y.; Zhou, R. The efficient treatment of pickling wastewater using a self-assembled in situ polymerized ceramic membrane with graphene/carbon nanotubes/polypyrrole. Environ. Sci.-Water Res. Technol. 2023, 9, 1238–1253. [Google Scholar] [CrossRef]
- Chavez, E.; He, Z.L.; Stoffella, P.J.; Mylavarapu, R.S.; Li, Y.C.; Moyano, B.; Baligar, V.C. Concentration of cadmium in cacao beans and its relationship with soil cadmium in southern Ecuador. Sci. Total Environ. 2015, 533, 205–214. [Google Scholar] [CrossRef]
- Qaswar, M.; Liu, Y.; Huang, J.; Liu, K.; Mudasir, M.; Lv, Z.; Hou, H.; Lan, X.; Ji, J.; Ahmed, W.; et al. Soil nutrients and heavy metal availability under long-term combined application of swine manure and synthetic fertilizers in acidic paddy soil. J. Soils Sediments 2020, 20, 2093–2106. [Google Scholar] [CrossRef]
- Shan, H.; Liu, R.; Li, S. The effect of applying organic materials on the forms of Cadmium in soil. J. Plant Nutr. Fertil. 2010, 16, 136–144. (In Chinese) [Google Scholar]
- Blanco-Canqui, H.; Lal, R. Soil structure and organic carbon relationships following 10 years of wheat straw management in no-till. Soil. Tillage Res. 2007, 95, 240–254. [Google Scholar] [CrossRef]
- Yang, B.; Jiang, S.; Zhang, C.; Zhao, G.; Wu, M.; Xiao, N.; Su, P. Recovery of iron from iron-rich pickling sludge for preparing P-doped polyferric chloride coagulant. Chemosphere 2021, 283, 131216. [Google Scholar] [CrossRef]
- Su, P.; Zhang, J.; Xiao, K.; Zhao, S.; Djellabi, R.; Li, X.; Yang, B.; Zhao, X. C3N4 modified with single layer ZIF67 nanoparticles for efficient photocatalytic degradation of organic pollutants under visible light. Chin. J. Catal. 2020, 41, 1894–1905. [Google Scholar] [CrossRef]
- Sheoran, A.S.; Sheoran, V.; Choudhary, R.P. Bioremediation of acid-rock drainage by sulphate-reducing prokaryotes: A review. Miner. Eng. 2010, 23, 1073–1100. [Google Scholar] [CrossRef]
- Cheng, L.; Qian, C.X.; Wang, R.X.; Wang, J.Y. Study on the mechanism of calcium carbonate formation induced by carbonate-mineralization microbe. Acta Chim. Sin. 2007, 65, 2133–2138. [Google Scholar]
- Tian, Z.; Tang, X.; Xiu, Z.; Xue, Z. Effect of different biological solutions on microbially induced carbonate precipitation and reinforcement of sand. Mar. Georesour. Geotechnol. 2020, 38, 450–460. [Google Scholar] [CrossRef]
- Zhang, J.; Su, P.; Li, L. Bioremediation of stainless steel pickling sludge through microbially induced carbonate precipitation. Chemosphere 2022, 298, 134213. [Google Scholar] [CrossRef]
- Huang, R.; Cui, X.; Luo, X.; Mao, P.; Zhuang, P.; Li, Y.; Li, Y.; Li, Z. Effects of plant growth regulator and chelating agent on the phytoextraction of heavy metals by Pfaffia glomerata and on the soil microbial community*. Environ. Pollut. 2021, 283, 117159. [Google Scholar] [CrossRef]
- Bolan, N.S.; Adriano, D.C.; Duraisamy, P.; Mani, A.; Arulmozhiselvan, K. Immobilization and phytoavailability of cadmium in variable charge soils. I. Effect of phosphate addition. Plant Soil 2003, 250, 83–94. [Google Scholar] [CrossRef]
- Jiang, Q.; He, Y.; Wu, Y.; Dian, B.; Zhang, J.; Li, T.; Jiang, M. Solidification/stabilization of soil heavy metals by alkaline industrial wastes: A critical review. Environ. Pollut. 2022, 312, 120094. [Google Scholar] [CrossRef]
- Pomies, M.P.; Lequeux, N.; Boch, P. Speciation of cadmium in cement Part I. Cd2+ uptake by C-S-H. Cem. Concr. Res. 2001, 31, 563–569. [Google Scholar] [CrossRef]
- Su, P.; Zhang, J.; Li, Y. Chemical fixation of toxic metals in stainless steel pickling residue by Na2S center dot xH2O, FeSO4 center dot 6H2O and phosphoric acid for beneficial uses. J. Environ. Sci. 2020, 90, 364–374. [Google Scholar] [CrossRef]
- Yin, P.; Shi, L. Remediation of Cd, Pb, and Cu-Contaminated Agricultural Soil Using Three Modified Industrial By-products. Water Air Soil Pollut. 2014, 225, 2194. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Naik, S.N.; Khare, S.K. Harnessing the bio-mineralization ability of urease producing Serratia marcescens and Enterobacter cloacae EMB19 for remediation of heavy metal cadmium (II). J. Environ. Manag. 2018, 215, 143–152. [Google Scholar] [CrossRef]
- Su, P.; Zhang, J.; Tang, J.; Zhang, C. Preparation of nitric acid modified powder activated carbon to remove trace amount of Ni(II) in aqueous solution. Water Sci. Technol. 2019, 80, 86–97. [Google Scholar] [CrossRef]
- Su, P.; Liu, Y.; Zhang, J.; Chen, C.; Yang, B.; Zhang, C.; Zhao, X. Pb-Based Perovskite Solar Cells and the Underlying Pollution behind Clean Energy: Dynamic Leaching of Toxic Substances from Discarded Perovskite Solar Cells. J. Phys. Chem. Lett. 2020, 11, 2812–2817. [Google Scholar] [CrossRef]
- Zhang, J.; Su, P.; Xu, T.; Yuan, L.; Qiao, M.; Yang, B.; Zhao, X. Comprehensive study on the role of reactive oxygen species and active chlorine species on the inactivation and subcellular damage of E.coli in electrochemical disinfection. Sep. Purif. Technol. 2023, 304, 122408. [Google Scholar] [CrossRef]
- Zhang, J.; Su, P.; Li, L. Microbial induced carbonate precipitation modified steel slag: Mechanical improvement and erosion resistance to sulfate attack. J. Clean. Prod. 2023, 405, 136982. [Google Scholar] [CrossRef]
- Ahmad, M.; Usman, A.R.A.; Faraj, A.S.; Ahmad, M.; Sallam, A.; Wabel, M.I. Phosphorus-loaded biochar changes soil heavy metals availability and uptake potential of maize (Zea mays L.) plants. Chemosphere 2018, 194, 327–339. [Google Scholar] [CrossRef]
- Huang, L.; Li, Y.; Zhao, M.; Chao, Y.; Qiu, R.; Yang, Y.; Wang, S. Potential of Cassia alata L. Coupled with Biochar for Heavy Metal Stabilization in Multi-Metal Mine Tailings. Int. J. Environ. Res. Public Health 2018, 15, 494. [Google Scholar] [CrossRef]
- Zhao, X.; Cao, D.; Su, P.; Guan, X. Efficient recovery of Sb(V) by hydrated electron reduction followed by cathodic deposition in a photoelectrochemical process. Chem. Eng. J. 2020, 395, 124153. [Google Scholar] [CrossRef]
- Huang, M.; Zhang, J.; Quan, G.; Guo, J. Review on the current status of research and utilization of soil heavy metal hyperaccumulation plants and the prospects of invasive plant remediation. Ecol. Sci. 2018, 37, 194–203. (In Chinese) [Google Scholar] [CrossRef]
- Alidoust, D.; Kawahigashi, M.; Yoshizawa, S.; Sumida, H.; Watanabe, M. Mechanism of cadmium biosorption from aqueous solutions using calcined oyster shells. J. Environ. Manag. 2015, 150, 103–110. [Google Scholar] [CrossRef]



| 0.025 mol·L−1 HCl | DTPA | 0.025 mol·L−1 HCl | DTPA | ||
|---|---|---|---|---|---|
| Methods | mg·kg−1 | Methods | mg·kg−1 | ||
| CK | 0.65 ± 0.09 a | 0.42 ± 0.02 a | S | 0.44 ± 0.09 b | 0.38 ± 0.01 bc |
| P1.L | 0.51 ± 0.08 b | 0.42 ± 0.02 a | S.P1.L | 0.26 ± 0.06 cd | 0.35 ± 0.02 bc |
| P1.M | 0.49 ± 0.05 b | 0.41 ± 0.02 a | S.P1.M | 0.17 ± 0.01 de | 0.32 ± 0.07 c |
| P1.H | 0.35 ± 0.06 c | 0.39 ± 0.02 ab | S.P1.H | 0.12 ± 0.03 e | 0.35 ± 0.02 bc |
| P2.L | 0.45 ± 0.04 bc | 0.40 ± 0.02 ab | S.P2.L | 0.27 ± 0.04 de | 0.29 ± 0.01 d |
| P2.M | 0.38 ± 0.05 c | 0.36 ± 0.01 c | S.P2.M | 0.19 ± 0.05 e | 0.28 ± 0.01 d |
| P2.H | 0.36 ± 0.03 cd | 0.38 ± 0.03 bce | S.P2.H | 0.18 ± 0.02 e | 0.30 ± 0.01 d |
| Method | Advantage | Disadvantage | Economic Performance |
|---|---|---|---|
| CaAl-LDH remediation | The treatment cost with CaAl-LDH is less than 4000 RMB/ha per year, significantly lower than biochar (8250 RMB/ha per year). CdAl-LDH demonstrates superior thermal stability compared to CaAl-LDH, ensuring long-term remediation effects. | There is still room for improvement in the repair effectiveness time. | Cost-effective, as raw materials are easy to access, resulting in treatment costs as low as 4000 RMB/ha per year, which is 50% less than conventional biochar remediation |
| Hydrocar remediation | Simple preparation process, environmentally friendly, and pollution-free | Poor heat resistance and low porosity; needs to be adjusted depending on soil quality | 2000–4000 RMB/ha for initial treatment, but additional costs may be incurred for modifications, potentially raising the cost to 6000–7000 RMB/ha depending on the soil requirements. |
| Co-application of phosphate fertilizer and sepiolite remediation | The combination of sepiolite with CMP or SSP demonstrated greater cadmium-removal efficiency than sepiolite alone, emphasizing their synergistic effect. | Limited application range; more effective in acidic soils | The cost of combining sepiolite with phosphate fertilizers (CMP/SSP) is approximately 3000–4500 RMB/ha, which is cost-effective due to the easy availability of both materials. |
| Factor | The Impact of Factor | Impact Mode |
|---|---|---|
| pH | The adsorption of cadmium is influenced by pH value and exhibits an “S” curve. | The acidic environment makes it difficult for the sediment of Cd2+ to reach saturation state. When the pH is between 4–8, the adsorption of cadmium by the soil increases. |
| Organic matter | The microorganisms and organic matter in organic fertilizers affect the properties of soil. | The increase in organic matter affects the recombination and chelation reactions of cadmium, and its decomposition products also affect the remediation of cadmium. |
| Microbiota matter | Microorganisms in the soil have an adsorption effect on heavy metals. | The concentration and type of bacteria in soil affect the composition and size of biocrystals in the soil, thereby affecting the efficiency of cadmium remediation. |
| Non-metallic element | The influence of non-metallic elements such as phosphorus and silicon on the fixation of cadmium | Silicon can form calcium silicate hydrates, promoting the remediation of cadmium. Phosphorus can form stable phosphate compounds, which help fix cadmium. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mu, Y.; Zhang, C.; Li, Y.; Zhou, W.; Li, Y.; Zhao, G.; Su, P. Research Progress on Physical and Chemical Remediation Methods for the Removal of Cadmium from Soil. Separations 2024, 11, 299. https://doi.org/10.3390/separations11100299
Mu Y, Zhang C, Li Y, Zhou W, Li Y, Zhao G, Su P. Research Progress on Physical and Chemical Remediation Methods for the Removal of Cadmium from Soil. Separations. 2024; 11(10):299. https://doi.org/10.3390/separations11100299
Chicago/Turabian StyleMu, Yonglin, Chunhui Zhang, Yiyun Li, Weilong Zhou, Yanxin Li, Guifeng Zhao, and Peidong Su. 2024. "Research Progress on Physical and Chemical Remediation Methods for the Removal of Cadmium from Soil" Separations 11, no. 10: 299. https://doi.org/10.3390/separations11100299
APA StyleMu, Y., Zhang, C., Li, Y., Zhou, W., Li, Y., Zhao, G., & Su, P. (2024). Research Progress on Physical and Chemical Remediation Methods for the Removal of Cadmium from Soil. Separations, 11(10), 299. https://doi.org/10.3390/separations11100299








