Treatment and Recycling of Tungsten Beneficiation Wastewater: A Review
Abstract
1. Introduction
2. Sources and Transformation of Typical Pollutants in Tungsten Beneficiation Wastewater
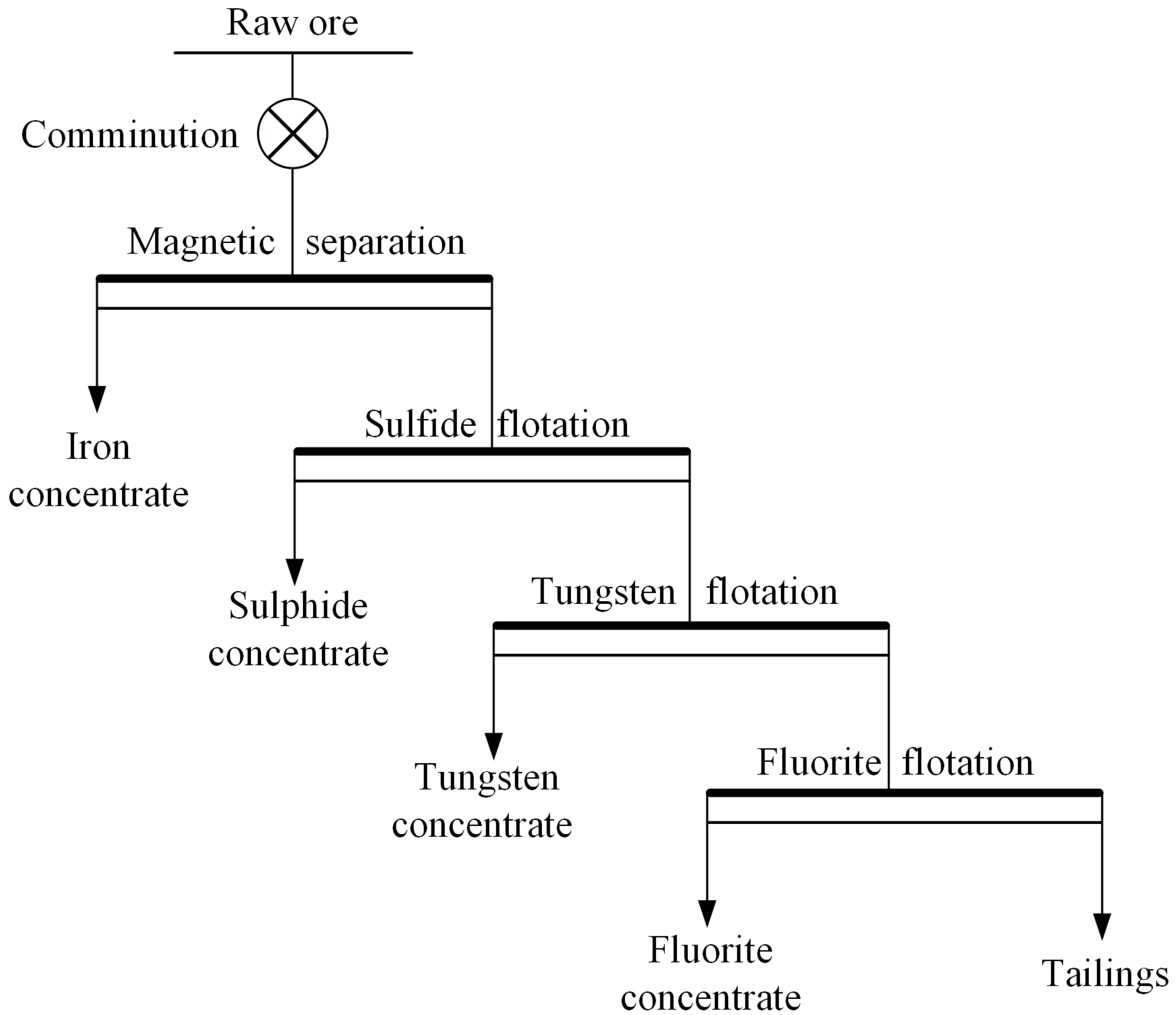
2.1. Sources and Characteristics of Typical Pollutants
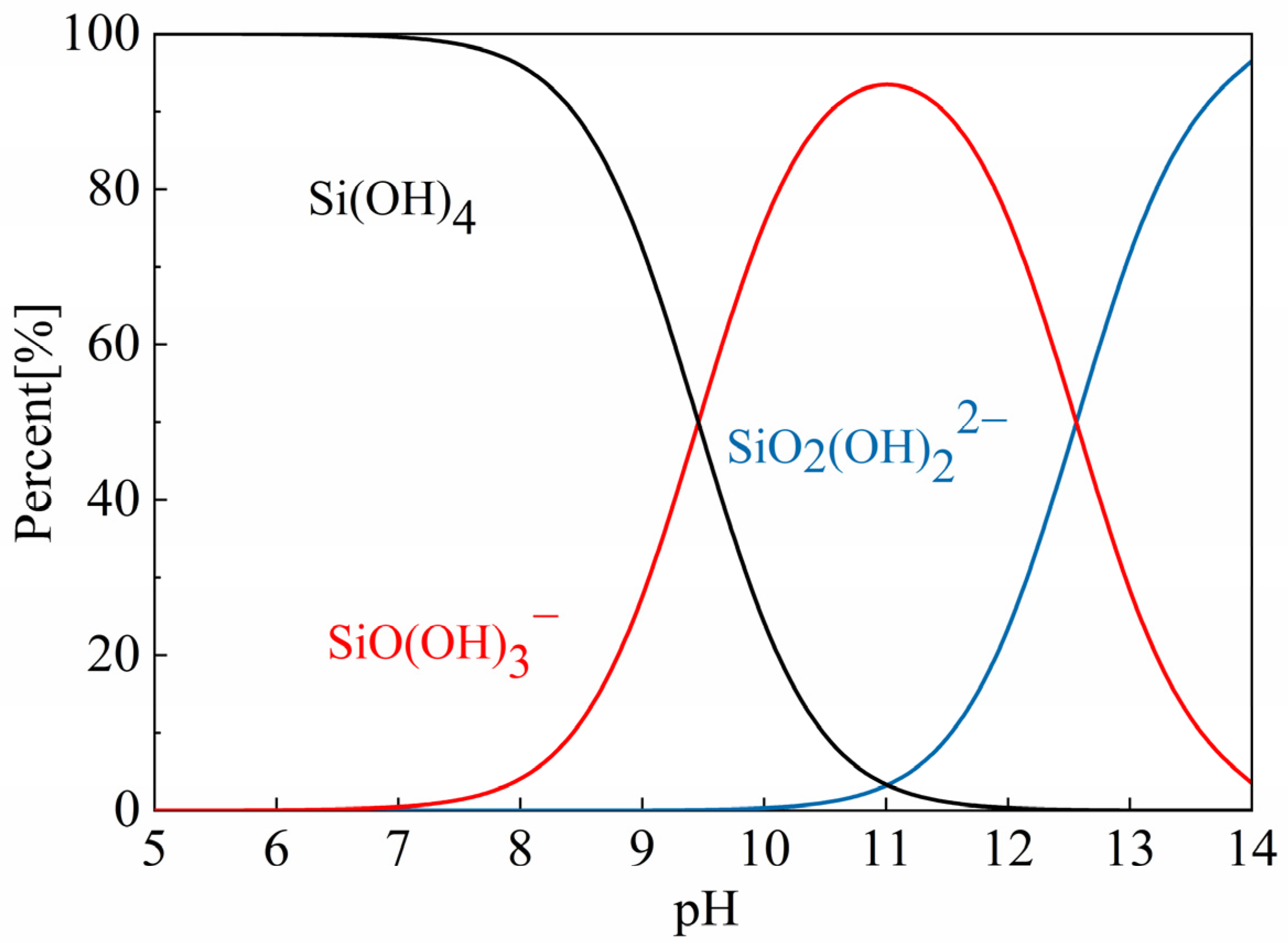
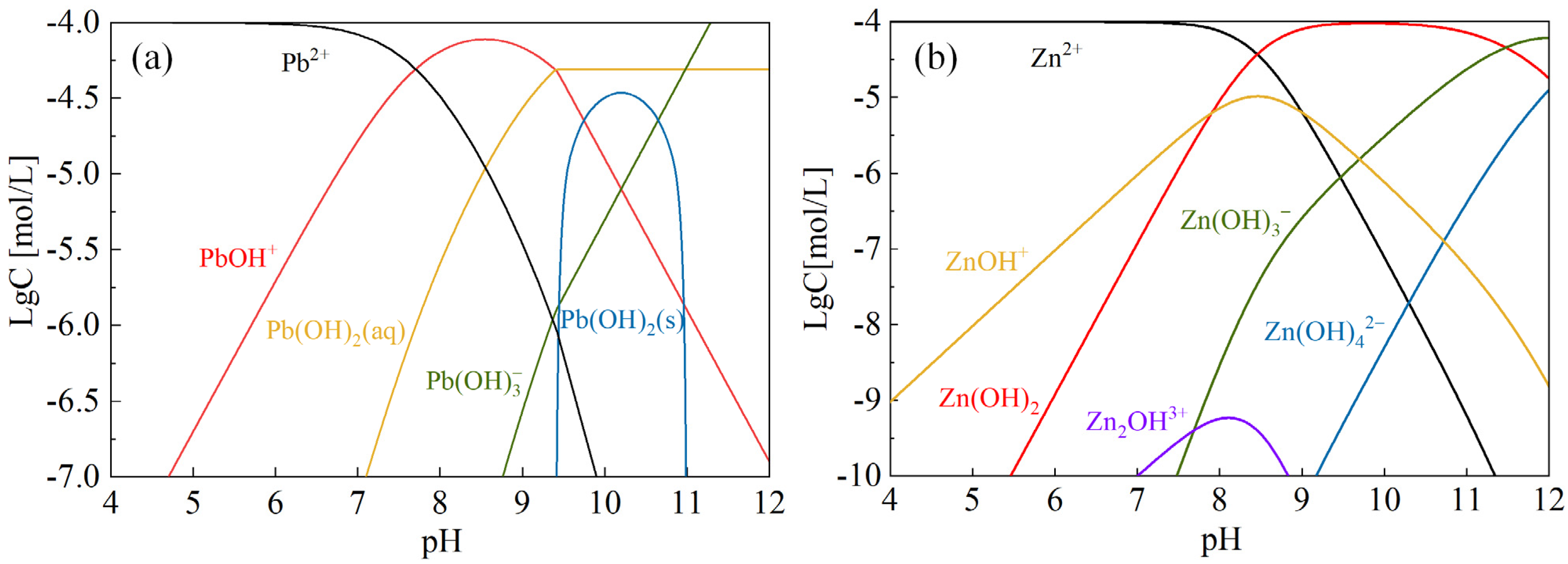
2.2. Occurrence State and Transformation Forms of Typical Pollutants
3. Purification Technologies of Tungsten Beneficiation Wastewater
3.1. Coagulation of SSs
3.2. Adsorption and Chemical Precipitation of Soluble Ions
3.3. Oxidation and Biological Treatment of Organics
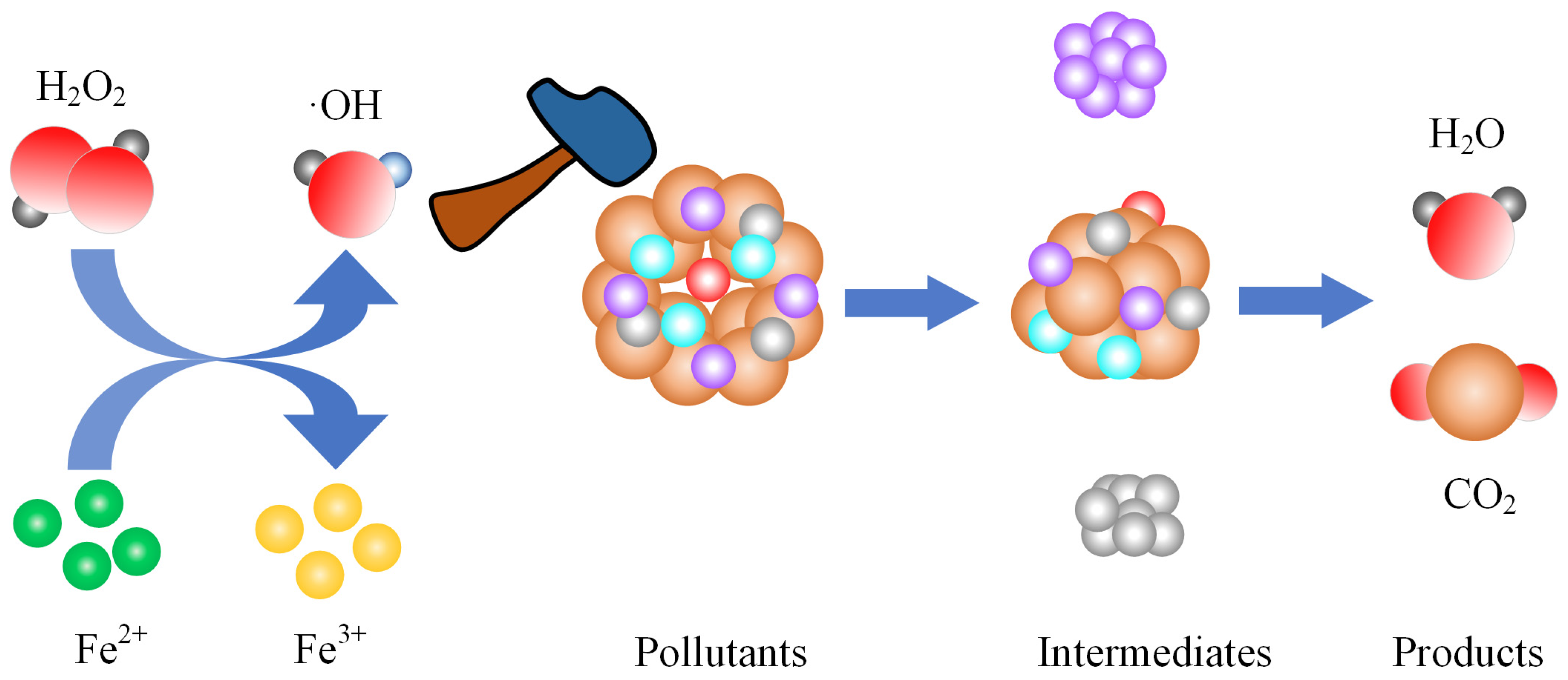
3.3.1. Advanced Oxidation Treatment of Organics
3.3.2. Biological Treatment of Organics
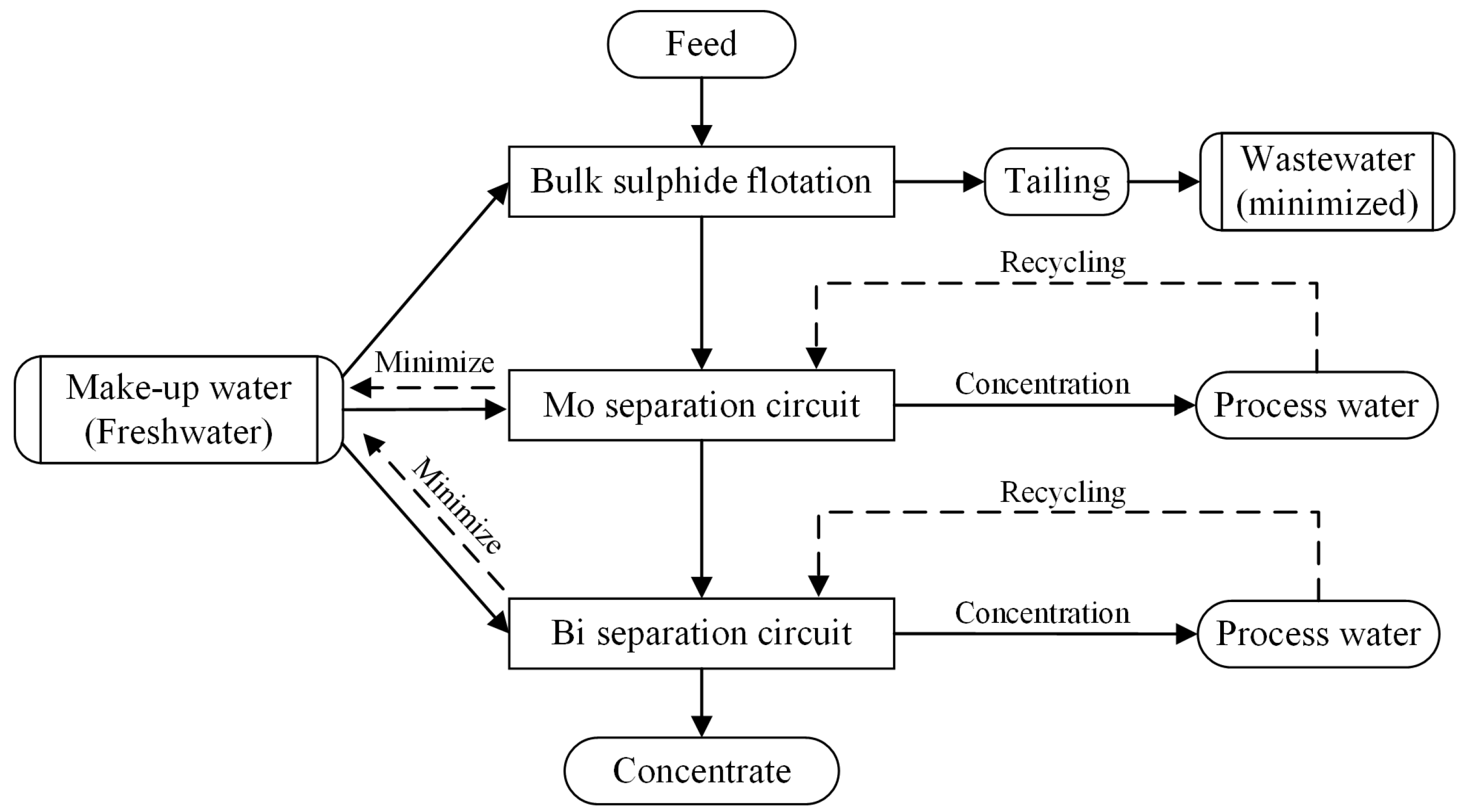
4. Recycling Methods of Tungsten Beneficiation Wastewater
4.1. Centralized Recycling
4.2. Internal Recycling at Certain Stages
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Yang, C.; Huang, K.; Gui, W. Non-ferrous metals price forecasting based on variational mode decomposition and LSTM network. Knowl. Based Syst. 2020, 188, 105006. [Google Scholar] [CrossRef]
- Dyussenova, S.; Abdulvaliyev, R.; Akcil, A.; Gladyshev, S.; Manapova, A. Gravity beneficiation of low quality gibbsite-kaolinite bauxite. J. Mater. Res. Technol. 2022, 20, 1802–1813. [Google Scholar] [CrossRef]
- Wen, X.; Bao, Q.; Guo, L.; Guo, Z. The introduction of super-gravity into optimization separation of bismuth and zinc from crude bismuth melt. Chem. Eng. Process. 2021, 160, 108266. [Google Scholar] [CrossRef]
- Wei, Q.; Dong, L.; Qin, W.; Jiao, F.; Qi, Z.; Feng, C.; Sun, D.; Wang, L.; Xiao, S. Efficient flotation recovery of lead and zinc from refractory lead-zinc ores under low alkaline conditions. Geochemistry 2021, 81, 125769. [Google Scholar] [CrossRef]
- Han, G.; Du, Y.; Huang, Y.; Yang, S.; Wang, W.; Su, S.; Liu, B. Efficient removal of hazardous benzohydroxamic acid (BHA) contaminants from the industrial beneficiation wastewaters by facile precipitation flotation process. Sep. Purif. Technol. 2021, 279, 119718. [Google Scholar] [CrossRef]
- Zhu, J.; Wu, F.; Pan, X.; Guo, J.; Wen, D. Removal of antimony from antimony mine flotation wastewater by electrocoagulation with aluminum electrodes. J. Environ. Sci. 2011, 23, 1066–1071. [Google Scholar] [CrossRef]
- Lu, H.; Qian, C.; Luo, S.; Zhu, Y.; Liu, R.; Wu, M. Study on the influence and mechanism of polyferric sulfate on COD removal and reuse of scheelite flotation wastewater. Miner. Eng. 2023, 191, 107940. [Google Scholar] [CrossRef]
- Zhao, Z.; Fu, T.-Y.; Gan, J.-W.; Liu, C.; Wang, D.-H.; Sheng, J.-F.; Li, W.-B.; Wang, P.-A.; Yu, Z.-F.; Chen, Y.-C. A synthesis of mineralization style and regional distribution and a proposed new metallogenic model of Mesozoic W-dominated polymentallic deposits in South China. Ore Geol. Rev. 2021, 133, 104008. [Google Scholar] [CrossRef]
- Wu, S.; Mao, J.; Ireland, T.R.; Zhao, Z.; Yao, F.; Yang, Y.; Sun, W. Comparative geochemical study of scheelite from the Shizhuyuan and Xianglushan tungsten skarn deposits, South China: Implications for scheelite mineralization. Ore Geol. Rev. 2019, 109, 448–464. [Google Scholar] [CrossRef]
- Wang, L.; Li, B.; Wang, Y.; Gao, T.; He, L. Tracing the Fe-enriched quartz vein-type wolframite mineralization: Wolframite U-Pb dating and compositions of wolframite and scheelite from the Anglonggangri and Jiaoxi areas, Xizang, China. Ore Geol. Rev. 2024, 170, 106154. [Google Scholar] [CrossRef]
- Wu, S.; Sun, W.; Wang, X. A new model for porphyry W mineralization in a world-class tungsten metallogenic belt. Ore Geol. Rev. 2019, 107, 501–512. [Google Scholar] [CrossRef]
- Zhang, Z.; Xie, G.; Olin, P. Tungsten mineralization in the Caojiaba tungsten deposit, southern China: Constraints from scheelite in-situ trace elements and Sr isotope evidence. Ore Geol. Rev. 2023, 158, 105496. [Google Scholar] [CrossRef]
- Di, H.; Shao, Y.-J.; Xiong, Y.-Q.; Zheng, H.; Fang, X.; Fang, W. Scheelite as a microtextural and geochemical tracer of multistage ore-forming processes in skarn mineralization: A case study from the giant Xintianling W deposit, South China. Gondwana Res. 2024, 136, 104–125. [Google Scholar] [CrossRef]
- Yang, X. Beneficiation studies of tungsten ores—A review. Miner. Eng. 2018, 125, 111–119. [Google Scholar] [CrossRef]
- Wang, X.; Qin, W.-q.; Jiao, F.; Dong, L.-y.; Guo, J.-g.; Zhang, J.; Yang, C.-r. Review of tungsten resource reserves, tungsten concentrate production and tungsten beneficiation technology in China. Trans. Nonferrous Met. Soc. China 2022, 32, 2318–2338. [Google Scholar] [CrossRef]
- Foucaud, Y.; Collet, A.; Filippova, I.V.; Badawi, M.; Filippov, L.O. Synergistic effects between fatty acids and non-ionic reagents for the selective flotation of scheelite from a complex tungsten skarn ore. Miner. Eng. 2022, 182, 107566. [Google Scholar] [CrossRef]
- Lotter, N.O.; Bradshaw, D.J. The formulation and use of mixed collectors in sulphide flotation. Miner. Eng. 2010, 23, 945–951. [Google Scholar] [CrossRef]
- Huang, X.; Huang, K.; Jia, Y.; Wang, S.; Cao, Z.; Zhong, H. Investigating the selectivity of a xanthate derivative for the flotation separation of chalcopyrite from pyrite. Chem. Eng. Sci. 2019, 205, 220–229. [Google Scholar] [CrossRef]
- Hao, H.; Li, L.; Yuan, Z.; Liu, J. Comparative effects of sodium silicate and citric acid on the dispersion and flotation of carbonate-bearing iron ore. J. Mol. Liq. 2018, 271, 16–23. [Google Scholar] [CrossRef]
- Zhang, L.; Jiao, X.; Wu, S.; Song, X.; Yao, R. Study on Optimization of Tungsten Ore Flotation Wastewater Treatment by Response Surface Method (RSM). Minerals 2021, 11, 184. [Google Scholar] [CrossRef]
- Molaei, N.; Forster, J.; Shoaib, M.; Wani, O.; Khan, S.; Bobicki, E. Efficacy of sustainable polymers to mitigate the negative effects of anisotropic clay minerals in flotation and dewatering operations. Clean. Eng. Technol. 2022, 8, 100470. [Google Scholar] [CrossRef]
- Wang, L.; Peng, Y.; Runge, K. Entrainment in froth flotation: The degree of entrainment and its contributing factors. Powder Technol. 2016, 288, 202–211. [Google Scholar] [CrossRef]
- Li, H.; Feng, Q.; Yang, S.; Ou, L.; Lu, Y. The entrainment behaviour of sericite in microcrystalline graphite flotation. Int. J. Miner. Process. 2014, 127, 1–9. [Google Scholar] [CrossRef]
- Hu, S.; Huang, T.; Zhang, N.; Lei, Y.; Wang, Y. Chitosan-assisted MOFs dispersion via covalent bonding interaction toward highly efficient removal of heavy metal ions from wastewater. Carbohydr. Polym. 2022, 277, 118809. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ren, L.; Zhang, Y.; Bao, S. Effect of aluminum ion on rutile flotation. Miner. Eng. 2023, 198, 108083. [Google Scholar] [CrossRef]
- Huang, W.; Liu, W.; Zhong, W.; Chi, X.; Rao, F. Effects of common ions on the flotation of fluorapatite and dolomite with oleate collector. Miner. Eng. 2021, 174, 107213. [Google Scholar] [CrossRef]
- Meng, X.; Jiang, M.; Lin, S.; Gao, Z.; Han, H.; Tian, M.; Zhang, C.; Liu, R.; Wu, M.; Bao, H.; et al. Removal of residual benzohydroxamic acid-lead complex from mineral processing wastewater by metal ion combined with gangue minerals. J. Clean. Prod. 2023, 396, 136578. [Google Scholar] [CrossRef]
- Jing, G.; Wang, J.; Sun, W.; Pooley, S.; Liao, D.; Shi, Z.; Chen, Q.; Gao, Z. Reuse of mine and ore washing wastewater in scheelite flotation process to save freshwater: Lab to industrial scale. J. Water Process Eng. 2023, 53, 103674. [Google Scholar] [CrossRef]
- Kupka, N.; Rudolph, M. Froth flotation of scheelite—A review. Int. J. Min. Sci. Technol. 2018, 28, 373–384. [Google Scholar] [CrossRef]
- Corin, K.C.; Charamba, A.; Manono, M.S. Water quality impact on flotation Response: A focus on specific ions and temperature. Miner. Eng. 2024, 207, 108549. [Google Scholar] [CrossRef]
- Abujazar, M.S.S.; Karaağaç, S.U.; Abu Amr, S.S.; Alazaiza, M.Y.D.; Bashir, M.J.K. Recent advancement in the application of hybrid coagulants in coagulation-flocculation of wastewater: A review. J. Clean. Prod. 2022, 345, 131133. [Google Scholar] [CrossRef]
- Daud, Z.; Awang, H.; Latif, A.A.A.; Nasir, N.; Ridzuan, M.B.; Ahmad, Z. Suspended Solid, Color, COD and Oil and Grease Removal from Biodiesel Wastewater by Coagulation and Flocculation Processes. Procedia Soc. Behav. Sci. 2015, 195, 2407–2411. [Google Scholar] [CrossRef]
- Han, H.; Hu, Y.; Sun, W.; Li, X.; Cao, C.; Liu, R.; Yue, T.; Meng, X.; Guo, Y.; Wang, J.; et al. Fatty acid flotation versus BHA flotation of tungsten minerals and their performance in flotation practice. Int. J. Miner. Process. 2017, 159, 22–29. [Google Scholar] [CrossRef]
- Yao, W.; Li, M.; Zhang, M.; Cui, R.; Shi, J.; Ning, J. Effect of Zn2+ and its addition sequence on flotation separation of scheelite from calcite using water glass. Colloids Surf. A 2020, 588, 124394. [Google Scholar] [CrossRef]
- Kang, J.; Fan, R.; Hu, Y.; Sun, W.; Liu, R.; Zhang, Q.; Liu, H.; Meng, X. Silicate removal from recycled wastewater for the improvement of scheelite flotation performance. J. Clean. Prod. 2018, 195, 280–288. [Google Scholar] [CrossRef]
- Zhu, W.; Kang, J.; Zhang, D. Treatment and prevention of pollutants in the wastewater from a scheelite and fluorite processing plant. J. Water Process Eng. 2023, 53, 103763. [Google Scholar] [CrossRef]
- Chen, Q.; Wu, W.; Qi, D.; Ding, Y.; Zhao, Z. Review on microaeration-based anaerobic digestion: State of the art, challenges, and prospectives. Sci. Total Environ. 2020, 710, 136388. [Google Scholar] [CrossRef]
- Shi, K.; Liang, B.; Cheng, H.-Y.; Wang, H.-C.; Liu, W.-Z.; Li, Z.-L.; Han, J.-L.; Gao, S.-H.; Wang, A.-J. Regulating microbial redox reactions towards enhanced removal of refractory organic nitrogen from wastewater. Water Res. 2024, 258, 121778. [Google Scholar] [CrossRef]
- Zhang, X.; Gu, X.; Lu, S.; Miao, Z.; Xu, M.; Fu, X.; Qiu, Z.; Sui, Q. Degradation of trichloroethylene in aqueous solution by calcium peroxide activated with ferrous ion. J. Hazard. Mater. 2015, 284, 253–260. [Google Scholar] [CrossRef]
- Oturan, M.A.; Aaron, J.-J. Advanced Oxidation Processes in Water/Wastewater Treatment: Principles and Applications. A Review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2577–2641. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, S.; Xiang, J.; Jiao, X.; Wang, J. Research on treatment and mechanism of salicylhydroxamic acid flotation wastewater by O3-BAF process. Water Sci. Technol. 2020, 82, 861–876. [Google Scholar] [CrossRef] [PubMed]
- Raj, S.; Singh, H.; Bhattacharya, J. Treatment of textile industry wastewater based on coagulation-flocculation aided sedimentation followed by adsorption: Process studies in an industrial ecology concept. Sci. Total Environ. 2023, 857, 159464. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Xie, Q.; Wu, Z.; Ji, L.; Li, Y.; Cai, Y.; Jiang, P.; Yu, B. Mechanistic insights into the selective adsorption of phosphorus from wastewater by MgO(100)-functionalized cellulose sponge. Sci. Total Environ. 2023, 868, 161646. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.; Lee, K.; Jeon, M.K.; Foster, R.I.; Lee, C.-H. Chemical precipitation–based treatment of acidic wastewater generated by chemical decontamination of radioactive concrete. J. Environ. Chem. Eng. 2023, 11, 110306. [Google Scholar] [CrossRef]
- Bao, H.; Wu, M.; Meng, X.; Han, H.; Zhang, C.; Sun, W. Application of electrochemical oxidation technology in treating high-salinity organic ammonia-nitrogen wastewater. J. Environ. Chem. Eng. 2023, 11, 110608. [Google Scholar] [CrossRef]
- Sarkar, M. Light-responsive biodegradation of wastewater pollutants: New developments and potential perspectives. J. Hazard. Mater. Adv. 2023, 10, 100281. [Google Scholar] [CrossRef]
- Jin, K.; Hou, K.; Wang, J.; Zhai, S.; Fan, Z.; Zhao, Y.; Xie, K.; Cai, Z.; Feng, X. Composite membranes with multifunctionalities for processing textile wastewater: Simultaneous oil/water separation and dye adsorption/degradation. Sep. Purif. Technol. 2023, 320, 124176. [Google Scholar] [CrossRef]
- Ajibade, T.F.; Tian, H.; Lasisi, K.H.; Zhang, K. Bio-inspired PDA@WS2 polyacrylonitrile ultrafiltration membrane for the effective separation of saline oily wastewater and the removal of soluble dye. Sep. Purif. Technol. 2022, 299, 121711. [Google Scholar] [CrossRef]
- Owodunni, A.A.; Ismail, S.; Kurniawan, S.B.; Ahmad, A.; Imron, M.F.; Abdullah, S.R.S. A review on revolutionary technique for phosphate removal in wastewater using green coagulant. J. Water Process Eng. 2023, 52, 103573. [Google Scholar] [CrossRef]
- Georgiou, D.; Aivazidis, A.; Hatiras, J.; Gimouhopoulos, K. Treatment of cotton textile wastewater using lime and ferrous sulfate. Water Res. 2003, 37, 2248–2250. [Google Scholar] [CrossRef]
- Ali, M.E.M.; Moniem, S.M.A.; Hemdan, B.A.; Ammar, N.S.; Ibrahim, H.S. Innovative polymeric inorganic coagulant-flocculant for wastewater purification with simultaneous microbial reduction in treated effluent and sludge. S. Afr. J. Chem. Eng. 2022, 42, 127–137. [Google Scholar] [CrossRef]
- Li, B.; Zhao, J.; Ge, W.; Li, W.; Yuan, H. Coagulation-flocculation performance and floc properties for microplastics removal by magnesium hydroxide and PAM. J. Environ. Chem. Eng. 2022, 10, 107263. [Google Scholar] [CrossRef]
- Nath, A.; Mishra, A.; Pande, P.P. A review natural polymeric coagulants in wastewater treatment. Mater. Today Proc. 2021, 46, 6113–6117. [Google Scholar] [CrossRef]
- Zhai, Q.; Lu, H.; Liu, R.; He, D.; Wang, C.; Sun, W. Removal of suspended solids from weathered tungsten-ore beneficiation wastewater by electroneutralization and chemical precipitation. Miner. Eng. 2021, 173, 107167. [Google Scholar] [CrossRef]
- Mweene, L.; Khanal, G.P.; Kashinga, R.J. Experimental and theoretical investigation on the separation of chalcopyrite from biotite using xanthan gum as a selective depressant. Sep. Purif. Technol. 2021, 274, 119012. [Google Scholar] [CrossRef]
- Suresh, C.H.; Remya, G.S.; Anjalikrishna, P.K. Molecular electrostatic potential analysis: A powerful tool to interpret and predict chemical reactivity. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2022, 12, e1601. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Sumathi, S.; Hameed, B.H. Coagulation of residue oil and suspended solid in palm oil mill effluent by chitosan, alum and PAC. Chem. Eng. J. 2006, 118, 99–105. [Google Scholar] [CrossRef]
- Bahrodin, M.; Zaidi, N.S.; Hussein, N.; Sillanpää, M.; Prasetyo, D.; Syafiuddin, A. Recent Advances on Coagulation-Based Treatment of Wastewater: Transition from Chemical to Natural Coagulant. Curr. Pollut. Rep. 2021, 7, 379–391. [Google Scholar] [CrossRef]
- Lee, C.S.; Robinson, J.; Chong, M.F. A review on application of flocculants in wastewater treatment. Process Saf. Environ. Prot. 2014, 92, 489–508. [Google Scholar] [CrossRef]
- Wu, H.; Lin, G.; Liu, C.; Chu, S.; Mo, C.; Liu, X. Progress and challenges in molecularly imprinted polymers for adsorption of heavy metal ions from wastewater. Trends Environ. Anal. Chem. 2022, 36, e00178. [Google Scholar] [CrossRef]
- Jing, G.; Meng, X.; Zheng, R.; Chen, J.; Sun, W.; Gao, Z. Efficient removal of NaOl from mineral processing wastewater using Al-electrocoagulation. J. Environ. Manag. 2023, 338, 117817. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zeng, L.; Wang, Y.; Tian, J.; Wang, J.; Sun, W.; Han, H.; Yang, Y. Selective separation of metals from wastewater using sulfide precipitation: A critical review in agents, operational factors and particle aggregation. J. Environ. Manag. 2023, 344, 118462. [Google Scholar] [CrossRef] [PubMed]
- AlJaberi, F.Y.; Hawaas, Z.A. Electrocoagulation removal of Pb, Cd, and Cu ions from wastewater using a new configuration of electrodes. MethodsX 2023, 10, 101951. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.-J.; Jiang, S.-K.; Chao, X.-Y.; Zhang, C.-X.; Shi, Q.; Wang, Z.-Y.; Liu, M.-L.; Sun, S.-P. Removing miscellaneous heavy metals by all-in-one ion exchange-nanofiltration membrane. Water Res. 2022, 222, 118888. [Google Scholar] [CrossRef] [PubMed]
- Ha, T.-H.; Mahasti, N.N.N.; Lu, M.-C.; Huang, Y.-H. Application of low-solubility dolomite as seed material for phosphorus recovery from synthetic wastewater using fluidized-bed crystallization (FBC) technology. Sep. Purif. Technol. 2022, 303, 122192. [Google Scholar] [CrossRef]
- Wang, L.; Fu, P.; Ma, Y.; Zhang, X.; Zhang, Y.; Yang, X. Steel slag as a cost-effective adsorbent for synergic removal of collectors, Cu(II) and Pb(II) ions from flotation wastewaters. Miner. Eng. 2022, 183, 107593. [Google Scholar] [CrossRef]
- Chen, P.; Wu, J.; Li, L.; Yang, Y.; Cao, J. Modified fly ash as an effect adsorbent for simultaneous removal of heavy metal cations and anions in wastewater. Appl. Surf. Sci. 2023, 624, 157165. [Google Scholar] [CrossRef]
- Rostamnezhad, N.; Kahforoushan, D.; Sahraei, E.; Ghanbarian, S.; Shabani, M. A method for the removal of Cu(II) from aqueous solutions by sulfide precipitation employing heavy oil fly ash. Desalin. Water Treat. 2016, 57, 17593–17602. [Google Scholar] [CrossRef]
- Wu, H.; Wang, W.; Huang, Y.; Han, G.; Yang, S.; Su, S.; Sana, H.; Peng, W.; Cao, Y.; Liu, J. Comprehensive evaluation on a prospective precipitation-flotation process for metal-ions removal from wastewater simulants. J. Hazard. Mater. 2019, 371, 592–602. [Google Scholar] [CrossRef]
- Cao, Z.; Cheng, Z.; Wang, J.; Cao, Y. Synergistic depression mechanism of Ca2+ ions and sodium silicate on bastnaesite flotation. J. Rare Earths 2022, 40, 988–995. [Google Scholar] [CrossRef]
- Chen, Q.; Yao, Y.; Li, X.; Lu, J.; Zhou, J.; Huang, Z. Comparison of heavy metal removals from aqueous solutions by chemical precipitation and characteristics of precipitates. J. Water Process Eng. 2018, 26, 289–300. [Google Scholar] [CrossRef]
- Fei, Y.; Hu, Y.H. Recent progress in removal of heavy metals from wastewater: A comprehensive review. Chemosphere 2023, 335, 139077. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, S.; Malik, W.; Annachhatre, A.P. Heavy metal precipitation from sulfide produced from anaerobic sulfidogenic reactor. Mater. Today. Proc. 2020, 32, 936–942. [Google Scholar] [CrossRef]
- Alvarez, M.T.; Crespo, C.; Mattiasson, B. Precipitation of Zn(II), Cu(II) and Pb(II) at bench-scale using biogenic hydrogen sulfide from the utilization of volatile fatty acids. Chemosphere 2007, 66, 1677–1683. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xu, L.; Wu, H.; Wang, Z.; Shu, K.; Fang, S.; Zhang, Z. Flotation and co–adsorption of mixed collectors octanohydroxamic acid/sodium oleate on bastnaesite. J. Alloys Compd. 2020, 819, 152948. [Google Scholar] [CrossRef]
- Zhen, H.; Xu, Q.; Hu, Y.; Cheng, J. Characteristics of heavy metals capturing agent dithiocarbamate (DTC) for treatment of ethylene diamine tetraacetic acid–Cu (EDTA–Cu) contaminated wastewater. Chem. Eng. J. 2012, 209, 547–557. [Google Scholar] [CrossRef]
- Thomas, M.; Zdebik, D.; Białecka, B. Using Sodium Trithiocarbonate to Precipitate Heavy Metals from Industrial Wastewater-from the Laboratory to Industrial Scale. Pol. J. Environ. Stud. 2018, 27, 1753–1763. [Google Scholar] [CrossRef]
- Fu, F.; Chen, R.; Xiong, Y. Application of a novel strategy—Coordination polymerization precipitation to the treatment of Cu2+-containing wastewaters. Sep. Purif. Technol. 2006, 52, 388–393. [Google Scholar] [CrossRef]
- Ayalew, Z.M.; Zhang, X.; Guo, X.; Ullah, S.; Leng, S.; Luo, X.; Ma, N. Removal of Cu, Ni and Zn directly from acidic electroplating wastewater by Oligo-Ethyleneamine dithiocarbamate (OEDTC). Sep. Purif. Technol. 2020, 248, 117114. [Google Scholar] [CrossRef]
- Titchou, F.E.; Zazou, H.; Afanga, H.; El Gaayda, J.; Ait Akbour, R.; Nidheesh, P.V.; Hamdani, M. Removal of organic pollutants from wastewater by advanced oxidation processes and its combination with membrane processes. Chem. Eng. Process. 2021, 169, 108631. [Google Scholar] [CrossRef]
- Costa, S.I.G.; Ferreira, F.L.; Weschenfelder, S.E.; Fuck, J.V.R.; da Cunha, M.d.F.R.; Marinho, B.A.; Mazur, L.P.; da Silva, A.; de Souza, S.M.A.G.U.; de Souza, A.A.U. Towards the removal of soluble organic compounds present in oilfield produced water by advanced oxidation processes: Critical review and future directions. Process Saf. Environ. Prot. 2023, 174, 608–626. [Google Scholar] [CrossRef]
- Mahmoudi, F.; Saravanakumar, K.; Maheskumar, V.; Njaramba, L.K.; Yoon, Y.; Park, C.M. Application of perovskite oxides and their composites for degrading organic pollutants from wastewater using advanced oxidation processes: Review of the recent progress. J. Hazard. Mater. 2022, 436, 129074. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhu, R.; Xi, Y.; Zhu, J.; Zhu, G.; He, H. Strategies for enhancing the heterogeneous Fenton catalytic reactivity: A review. Appl. Catal. B 2019, 255, 117739. [Google Scholar] [CrossRef]
- Zhang, M.; Dong, H.; Zhao, L.; Wang, D.; Meng, D. A review on Fenton process for organic wastewater treatment based on optimization perspective. Sci. Total Environ. 2019, 670, 110–121. [Google Scholar] [CrossRef]
- Meng, X.; Wu, J.; Kang, J.; Gao, J.; Liu, R.; Gao, Y.; Wang, R.; Fan, R.; Khoso, S.A.; Sun, W.; et al. Comparison of the reduction of chemical oxygen demand in wastewater from mineral processing using the coagulation–flocculation, adsorption and Fenton processes. Miner. Eng. 2018, 128, 275–283. [Google Scholar] [CrossRef]
- Guo, Y.; Xue, Q.; Zhang, H.; Wang, N.; Chang, S.; Wang, H.; Pang, H.; Chen, H. Treatment of real benzene dye intermediates wastewater by the Fenton method: Characteristics and multi-response optimization. RSC Adv. 2018, 8, 80–90. [Google Scholar] [CrossRef]
- Çalık, Ç.; Çifçi, D.İ. Comparison of kinetics and costs of Fenton and photo-Fenton processes used for the treatment of a textile industry wastewater. J. Environ. Manag. 2022, 304, 114234. [Google Scholar] [CrossRef]
- Nidheesh, P.V. Heterogeneous Fenton catalysts for the abatement of organic pollutants from aqueous solution: A review. RSC Adv. 2015, 5, 40552–40577. [Google Scholar] [CrossRef]
- Tian, K.; Hu, L.; Li, L.; Zheng, Q.; Xin, Y.; Zhang, G. Recent advances in persulfate-based advanced oxidation processes for organic wastewater treatment. Chin. Chem. Lett. 2022, 33, 4461–4477. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.-G.; Hu, Y.; Gao, M.; Guo, L.; Ji, J. Insight in degradation of tetracycline in mariculture wastewater by ultraviolet/persulfate advanced oxidation process. Environ. Res. 2022, 212, 113324. [Google Scholar] [CrossRef]
- Li, N.; Wu, S.; Dai, H.; Cheng, Z.; Peng, W.; Yan, B.; Chen, G.; Wang, S.; Duan, X. Thermal activation of persulfates for organic wastewater purification: Heating modes, mechanism and influencing factors. Chem. Eng. J. 2022, 450, 137976. [Google Scholar] [CrossRef]
- Zhang, L.; Ding, W.; Qiu, J.; Jin, H.; Ma, H.; Li, Z.; Cang, D. Modeling and optimization study on sulfamethoxazole degradation by electrochemically activated persulfate process. J. Clean. Prod. 2018, 197, 297–305. [Google Scholar] [CrossRef]
- Su, S.; Guo, W.; Yi, C.; Leng, Y.; Ma, Z. Degradation of amoxicillin in aqueous solution using sulphate radicals under ultrasound irradiation. Ultrason. Sonochem. 2012, 19, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wang, P.; Yang, X.; Wei, G.; Zhang, W.; Shan, L. A novel advanced oxidation process to degrade organic pollutants in wastewater: Microwave-activated persulfate oxidation. J. Environ. Sci. 2009, 21, 1175–1180. [Google Scholar] [CrossRef]
- Zhu, L.; Ai, Z.; Ho, W.; Zhang, L. Core–shell Fe–Fe2O3 nanostructures as effective persulfate activator for degradation of methyl orange. Sep. Purif. Technol. 2013, 108, 159–165. [Google Scholar] [CrossRef]
- Karim, A.V.; Jiao, Y.; Zhou, M.; Nidheesh, P.V. Iron-based persulfate activation process for environmental decontamination in water and soil. Chemosphere 2021, 265, 129057. [Google Scholar] [CrossRef]
- Liu, S.; Zeng, G.-m.; Niu, Q.; Liu, Y.; Zhou, L.; Jiang, L.; Tan, X.; Xu, P.; Zhang, C.; Cheng, M. Bioremediation mechanisms of combined pollution of PAHs and heavy metals by bacteria and fungi: A mini review. Bioresour. Technol. 2017, 224, 25–33. [Google Scholar] [CrossRef]
- Patel, A.K.; Singhania, R.R.; Albarico, F.P.J.B.; Pandey, A.; Chen, C.-W.; Dong, C.-D. Organic wastes bioremediation and its changing prospects. Sci. Total Environ. 2022, 824, 153889. [Google Scholar] [CrossRef]
- Han, X.; Zuo, Y.-T.; Hu, Y.; Zhang, J.; Zhou, M.-X.; Chen, M.; Tang, F.; Lu, W.-Q.; Liu, A.-L. Investigating the performance of three modified activated sludge processes treating municipal wastewater in organic pollutants removal and toxicity reduction. Ecotoxicol. Environ. Saf. 2018, 148, 729–737. [Google Scholar] [CrossRef]
- Wang, H.; Cui, H.; Ma, X.; Cornell, C.R.; Zhang, L.; Ren, Y.; Li, M.; Liu, Y.; Gao, S.; Li, Z.; et al. Molecular mechanism of adsorbing triclocarban by the activated sludge in wastewater treatment systems. Chem. Eng. J. 2023, 463, 142431. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.; Zhang, Q.; Peng, Q.; Zhang, W.; Gao, F. New insight into the biological treatment by activated sludge: The role of adsorption process. Bioresour. Technol. 2014, 153, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Vila-Tojo, S.; Sabucedo, J.-M.; Andrade, E.; Gómez-Román, C.; Alzate, M.; Seoane, G. From scarcity problem diagnosis to recycled water acceptance: A perceptive-axiological model (PAM) of low and high contact uses. Water Res. 2022, 217, 118380. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Herrera, A.A.; Hernández-Montoya, V.; Castillo-Borja, F.; Pérez-Cruz, M.A.; Montes-Morán, M.A.; Cervantes, F.J. Competitive adsorption of pollutants from anodizing wastewaters to promote water reuse. J. Environ. Manag. 2021, 293, 112877. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Liu, R.; Wu, M.; Hu, Y.; Sun, W.; Shi, Z.; Han, H.; Li, W. Minimizing beneficiation wastewater through internal reuse of process water in flotation circuit. J. Clean. Prod. 2020, 245, 118898. [Google Scholar] [CrossRef]
- Santos, M.A.; Capponi, F.; Ataíde, C.H.; Barrozo, M.A.S. Wastewater treatment using DAF for process water reuse in apatite flotation. J. Clean. Prod. 2021, 308, 127285. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, Q.; Wu, G.; Hu, H.-Y. Centralized water reuse system with multiple applications in urban areas: Lessons from China’s experience. Resour. Conserv. Recycl. 2017, 117, 125–136. [Google Scholar] [CrossRef]
- Wu, M.; Sun, W.; Meng, X.; Kang, J.; Yang, Y. Natural marmatite photocatalyst for treatment of mineral processing wastewater to help zero wastewater discharge. J. Environ. Sci. 2023, 142, 83–91. [Google Scholar] [CrossRef]
- Kang, J.; Chen, C.; Sun, W.; Tang, H.; Yin, Z.; Liu, R.; Hu, Y.; Nguyen, A.V. A significant improvement of scheelite recovery using recycled flotation wastewater treated by hydrometallurgical waste acid. J. Clean. Prod. 2017, 151, 419–426. [Google Scholar] [CrossRef]
- Ihle, C.F.; Kracht, W. The relevance of water recirculation in large scale mineral processing plants with a remote water supply. J. Clean. Prod. 2018, 177, 34–51. [Google Scholar] [CrossRef]
- Azevedo, A.; Oliveira, H.A.; Rubio, J. Treatment and water reuse of lead-zinc sulphide ore mill wastewaters by high rate dissolved air flotation. Miner. Eng. 2018, 127, 114–121. [Google Scholar] [CrossRef]
- Shen, Y.; Peng, X.; Ouvang, K.; Shi, S. Research on the treatment and reuse of the dressing wastewater from tungsten anomolybdenum copper poly-metallic ore. Ind. Water Treat. 2018, 38, 62–64. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, W.; Kang, J.; Zhang, D.; Sun, W.; Gao, Z.; Han, H.; Liu, R. Treatment and Recycling of Tungsten Beneficiation Wastewater: A Review. Separations 2024, 11, 298. https://doi.org/10.3390/separations11100298
Zhu W, Kang J, Zhang D, Sun W, Gao Z, Han H, Liu R. Treatment and Recycling of Tungsten Beneficiation Wastewater: A Review. Separations. 2024; 11(10):298. https://doi.org/10.3390/separations11100298
Chicago/Turabian StyleZhu, Wenxia, Jianhua Kang, Danxian Zhang, Wei Sun, Zhiyong Gao, Haisheng Han, and Runqing Liu. 2024. "Treatment and Recycling of Tungsten Beneficiation Wastewater: A Review" Separations 11, no. 10: 298. https://doi.org/10.3390/separations11100298
APA StyleZhu, W., Kang, J., Zhang, D., Sun, W., Gao, Z., Han, H., & Liu, R. (2024). Treatment and Recycling of Tungsten Beneficiation Wastewater: A Review. Separations, 11(10), 298. https://doi.org/10.3390/separations11100298









